Abstract
Objective
To determine expression of stanniocalcin-1 (STC1) in human endometrium with and without endometriosis and its regulation by steroid hormones.
Design
Laboratory study.
Setting
University.
Patients
19 women with endometriosis and 33 control women.
Intervention(s)
Endometrial biopsy and fluid sampling
Main Outcome Measure(s)
Analysis of early secretory (ESE) and mid-secretory (MSE) endometrial secretomes from fertile women with nano-LC/MS/MS; real-time Q-PCR and immunohistochemistry for STC1 and its receptor CASR mRNA and proteins in endometrium with and without endometriosis; evaluation of STC1 and CASR mRNA expression in endometrial stromal fibroblasts (eSF) from women with and without endometriosis decidualized with E2P4 or 8-bromo-cAMP.
Results
STC1 protein was strongly upregulated (p=0.0004) in MSE versus ESE in endometrial fluid of fertile women. STC1 mRNA significantly increased in MSE from women with, but not without endometriosis, compared to proliferative endometrium (PE) or ESE, with no significant difference throughout the menstrual cycle between groups. STC1 mRNA in eSF from control women increased >230-fold upon decidualization with cAMP versus 45-fold from women with endometriosis, which was not seen upon decidualization with E2/P4. CASR mRNA did not exhibit significant differences in any condition, and was not expressed in isolated eSF. STC1 protein immunoexpression in eSF was significantly lower in women with endometriosis compared to controls.
Conclusions
STC1 protein is significantly upregulated in MSE endometrial fluid, and is dysregulated in eutopic endometrial tissue from women with endometriosis. It is likely regulated by cAMP and may be involved in the pathogenesis of decidualization defects.
Keywords: stanniocalcin-1, human endometrium, endometriosis, stromal fibroblasts, decidualization, proteomics
Introduction
Human endometrium is a dynamic tissue that undergoes cyclic morphological and molecular changes under a changing hormonal milieu and plays a central role in human implantation. Endometrial tissue in humans and other mammals is a target of extensive research with the goal to increase understanding of its physiology and pathophysiology and improve treatment of gynecological pathologies such as endometriosis, adenomyosis, recurrent pregnancy loss, as well as implantation failure and unexplained infertility.
High-throughput ‘omics’ studies of global gene expression profiling in human endometrium have identified stanniocalcin-1 (STC1) as an important player in normal and diseased endometrial functions. STC is expressed in pre-pregnancy endometrium and early pregnancy decidua in rats and pigs, is regulated by estrogen and progesterone, and has been suggested as an implantation marker in pig endometrium (1, 2). In humans, consistent mid-secretory endometrial (MSE) STC1 gene expression was demonstrated in patients who conceived with the help of assisted reproductive technologies (ART) (3). STC1 was significantly upregulated in microarray analysis of MSE of the normal menstrual cycle compared to early secretory endometrium (ESE) (4), and was down-regulated in microarray analysis of MSE from women with unexplained infertility versus fertile control subjects (5), suggesting a possible role in human endometrial receptivity and implantation. Placental expression of STC1 was documented in a well-designed microarray study of women with pregnancy complications, which demonstrated an increased STC1 in placenta and serum at term in women with pregnancy complications, particularly pre-eclampsia and small for gestational age babies (6).
STC1 is a glycoprotein phosphorylated by protein kinase C (7) initially described in a bony fish produced by unique endocrine glands, the corpuscles of Stannius, eliciting antihypercalcemic and antihypophosphatemic responses in various tissues (8, 9). In mammals, STC1 is expressed in a wide variety of tissues, and interestingly is not detected in the circulation under normal conditions, except pregnancy (9-11), suggesting an autocrine/paracrine rather than an endocrine function. STC1 in mammals is not necessarily directly linked to calcium/phosphorus pathways, but rather is regulated by multiple factors (reviewed in (9)). Its roles in calcium homeostasis, bone and muscle formation, angiogenesis and reproduction were demonstrated in experiments with transgenic mice that experienced growth retardation and small litter size (11, 12).
The STC receptor, Ca-sensing receptor (CASR), is a G-protein-coupled receptor, initially identified in bovine parathyroid cells and expressed in wide variety of tissues including ovaries and uterus in human and rodents (13-15). Its major physiologic role is believed to be maintenance of calcium homeostasis, including regulation of secretion, gene expression, cell proliferation, differentiation and apoptosis (13, 16). CASR was found to mediate STC1 secretion in response to extracellular calcium fluctuation in fish (17). It is expressed in first trimester and term human placenta (18, 19), and is induced during implantation and decidualization in rat uterus (15).
Based on the above, there is yet limited data on human endometrial STC1 expression, regulation and signaling through its receptor. We, therefore, aimed to investigate expression of STC1 and its receptor in human endometrium and regulation of STC1 in human endometrial stromal fibroblasts in women without and with endometriosis in an effort to determine potential roles for this protein/receptor complex in normal and abnormal endometrial function.
Materials and Methods
Study subjects and materials
Endometrial samples were obtained through the UCSF NIH Human Endometrial Tissue and DNA Bank and from healthy volunteers at the Department of Obstetrics and Gynecology, Karolinska University Hospital, Huddinge, Sweden and Tartu University Women's Clinic, Tartu, Estonia with appropriate IRB (UCSF) and The Ethics Committees (Karolinska Institute and University of Tartu) approvals. Written informed consents were obtained from all participating subjects. Table 1 summarizes characteristics of all participating women and the techniques applied for analyzing the samples herein.
Table 1.
Characteristics of patients donated endometrial biopsy or fluid samples for the study.
| Patient | Cycle phase | Type of experiment | Diagnosis at laparoscopy | Age | Ethnicity |
|---|---|---|---|---|---|
| Endometriosis | |||||
| 233 | MSE | Cell culture and decidualization, Q-PCR | Mild endometriosis, pelvic pain | 31 | Caucasian |
| 243 | ESE | Cell culture and decidualization, Q-PCR | Minimal endometriosis, bilateral ovarian cyst, intramural myoma | 46 | Caucasian |
| 267 | LSE | Cell culture and decidualization, Q-PCR | Mild endometriosis, pelvic pain | 32 | Caucasian |
| 268 | Not Evaluated | Cell culture and decidualization, Q-PCR | Mild endometriosis, intramural myoma | 38 | Asian |
| 288 | PE | Cell culture and decidualization, Q-PCR | Severe endometriosis | 22 | Caucasian |
| 270 | PE | Cell culture and decidualization, Q-PCR | Mild endometriosis | 49 | Caucasian |
| 651 | PE | Tissue Q-PCR | Severe endometriosis/endometrioma, chronic pelvic pain, fibroid uterus | 37 | Caucasian |
| 575 | PE | Tissue Q-PCR | Severe endometriosis/endometrioma, chronic pelvic pain, | 26 | Unknown |
| 587 | PE | Tissue Q-PCR | Severe peritoneal, rectovaginal endometriosis, chronic pelvic pain | 36 | Caucasian |
| ST90 | PE | Tissue Q-PCR | Severe pelvic endometriosis, chronic pelvic pain | 42 | Caucasian |
| 607 | ESE | Tissue Q-PCR | Severe peritoneal and rectovaginal endometriosis, chronic pelvic pain | 24 | Asian |
| 517 | ESE | Tissue Q-PCR | Severe peritoneal endometriosis, chronic pelvic pain, fibroids, infertility | 35 | Unknown |
| ST112 | ESE | Tissue Q-PCR | Severe peritoneal endometriosis, chronic pelvic pain | 38 | Caucasian |
| 645 | MSE | Tissue Q-PCR | Severe peritoneal endometriosis, chronic pelvic pain | 39 | Asian Indian |
| 678 | MSE | Tissue Q-PCR, immunohistochemistry | Severe endometriosis, fibroid uterus, chronic pelvic pain | 44 | Caucasian |
| ST96 | MSE | Tissue Q-PCR | Severe peritoneal endometriosis, chronic pelvic pain | 31 | Caucasian |
| 635 | MSE | Immunohistochemistry | Mild endometriosis, bleeding, intramural myoma | 42 | Caucasian |
| 516 | MSE | Immunohistochemistry | Severe endometriosis, intramural myoma | 34 | Asian |
| 540 | MSE | Immunohistochemistry | Severe rectovaginal endometriosis | 37 | Caucasian |
| No Endometriosis | |||||
| 229 | LSE | Cell culture and decidualization, Q-PCR | Pelvic pain (no endometriosis at laparoscopy) | 47 | Caucasian |
| 236 | PE | Cell culture and decidualization, Q-PCR | Symptomatic pelvic prolapse | 47 | Caucasian |
| 237 | ESE | Cell culture and decidualization, Q-PCR | Intramural myoma, left paratubal cyst | 39 | Caucasian |
| 238 | ESE | Cell culture and decidualization, Q-PCR | Endometrial polyp | 41 | Black |
| 285 | PE | Cell culture and decidualization, Q-PCR | Intramural myoma, pelvic adhesions | 37 | Caucasian |
| 293 | PE | Cell culture and decidualization, Q-PCR | Intramural myoma | 49 | Caucasian |
| 310 | PE | Cell culture and decidualization, Q-PCR | Intramural myoma, enterocele | 41 | Asian |
| ME 09 | PE | Tissue, Q-PCR | Undesired fertility | 37 | Caucasian |
| M182 | PE | Tissue, Q-PCR | Healthy volunteer | 34 | Caucasian |
| M169 | PE | Tissue, Q-PCR | Healthy volunteer | 32 | Caucasian |
| ETB038 | PE | Tissue, Q-PCR | Egg donor, natural cycle biopsy | 23 | Caucasian |
| ETB048 | PE | Tissue, Q-PCR | Egg donor, natural cycle biopsy | 28 | Black |
| ETB065 | PE | Tissue, Q-PCR | Egg donor, natural cycle biopsy | 29 | Caucasian |
| ME 34 | ESE | Tissue, Q-PCR | Undesired fertility | 33 | Caucasian |
| ME 13 | ESE | Tissue, Q-PCR | Pelvic pain (no endometriosis at laparoscopy) | 33 | Black |
| ME 42 | ESE | Tissue, Q-PCR | Undesired fertility | 37 | Caucasian |
| ME 36 | MSE | Tissue, Q-PCR | Undesired fertility | 35 | Black |
| ME 50 | MSE | Tissue, Q-PCR | Undesired fertility | 35 | Caucasian |
| 16 | MSE | Tissue, Q-PCR | Healthy fertile volunteer | 42 | Unknown |
| 22 | MSE | Tissue, Q-PCR | Healthy fertile volunteer | 40 | Unknown |
| 30 | MSE | Tissue, Q-PCR | Healthy fertile volunteer | 35 | Unknown |
| MM1 | MSE | Immunohistochemistry | Healthy fertile volunteer | 36 | Caucasian |
| MM2 | MSE | Immunohistochemistry | Healthy fertile volunteer | 41 | Caucasian |
| MM5 | MSE | Immunohistochemistry | Healthy fertile volunteer | 30 | Caucasian |
| MM16 | MSE | Immunohistochemistry | Healthy fertile volunteer | 35 | Caucasian |
| ST18 | MSE | Immunohistochemistry | Healthy fertile volunteer | 37 | Caucasian |
| ST21 | MSE | Immunohistochemistry | Healthy fertile volunteer | 35 | Caucasian |
| Secr1 | ESE, MSE | Mass-spectrometry, histology | Healthy fertile volunteer | 30 | Caucasian |
| Secr2 | ESE, MSE | Mass-spectrometry, histology | Healthy fertile volunteer | 32 | Caucasian |
| Secr3 | ESE, MSE | Mass-spectrometry, histology | Healthy fertile volunteer | 32 | Caucasian |
| Secr4 | ESE, MSE | Mass-spectrometry, histology | Healthy fertile volunteer | 33 | Caucasian |
| Secr5 | ESE, MSE | Mass-spectrometry, histology | Healthy fertile volunteer | 30 | Caucasian |
| Secr6 | ESE, MSE | Mass-spectrometry, histology | Healthy fertile volunteer | 29 | Caucasian |
PE=proliferative endometrium, ESE=early secretory endometrium, MSE=mid-secretory endometrium, LSE=late secretory endometrium, Q-PCR=quantitative polymerase chain reaction.
Endometrial secretome samples for proteomics analysis were collected from early secretory (ESE, LH+2) and mid-secretory (MSE, LH+8) phase endometria during the same menstrual cycle from fertile healthy volunteers (n=6) (age 31±1.5 years). The day of the LH surge (LH+0) was determined with a urinary ovulation prediction test (Kaigert, Estonia), as it is an accepted and validated method for predicting ovulation in the clinical and translational research setting; it is also patient friendly, as it is a non-invasive test applicable for self-use at home. However, being aware of substantial inter-patient variability, we supported our endometrial dating with endometrial histology. Histology samples were collected for confirmation of endometrial dating according to the criteria of Noyes et al. (20) and were found to correspond to days 19-24 of the 28-day cycle.
Endometrial biopsies for PCR, immunohistochemistry and cell culture experiments were obtained from subjects with and without endometriosis (n=19 and n=27, respectively). Menstrual cycle phase was assigned by the day of LH surge and endometrial histology as above. In addition, all samples used for mRNA expression analysis were assigned cycle phase using bioinformatics methods (21). Only samples, where all evaluation criteria were in agreement, were used in the study.
Controls (no endometriosis) were healthy 35.6±0.86 years old (range 23-49) women undergoing gynecologic surgery for pelvic pain (no endometriosis found during laparoscopy) or management of fibroids, healthy volunteers without uterine pathology or women undergoing laparoscopic tubal ligation. Controls had regular menstrual cycles, were not pregnant, had no history of endometriosis, and had not been on hormonal treatment for at least 3 months before tissue sampling.
Women with endometriosis participating in the study were 35.9±1.65 years old (range 22-49), were not pregnant, and did not use any hormonal medication within at least 3 months before surgery. The diagnosis of endometriosis was based on visualization of lesions during laparoscopy and confirmed by histology. Staging of endometriosis was defined according to the revised American Fertility Society classification system (22).
Out of 19 samples from women with endometriosis and 27 samples from control women (without endometriosis), 14 and 10 whole tissue samples respectively were used for real-time qRT-PCR (n=6 and n=4 in proliferative phase (PE) from women with and without endometriosis, respectively, n=3 early secretory phase endometrium (ESE) in each group, and n=5 and n=3 in mid-secretory endometrium (MSE) from women with and without endometriosis, respectively). Immunohistochemistry (IHC) was performed on MSE samples, n=4 and n=6 for endometriosis and no endometriosis groups, respectively.
Isolated endometrial stromal fibroblasts (eSF) from women with (n=6) and without endometriosis (n=7) were used in cell culture experiments (as shown previously, cycle phase does not confound response, (23)).
Early secretory and mid-secretory phase endometrial fluid proteomics analysis
The secretomes were obtained by uterine flushing – i.e., injecting 0.5 ml of PBS into the uterine cavity, followed by aspiration of the fluid. Prior to protein extraction the samples were precleared with centrifugation at 450 g for 5 minutes. The collected secretomes were separated into six fractions according to molecular weight using SDS-PAGE (Invitrogen-Thermo Fisher Scientific, Grand Island, NY, USA). Proteins were reduced, alkylated and in-gel digested with dimethylated porcine trypsin (Sigma) followed by analysis with nano-LC/MS/MS (Dionex Ultimate 3000 RSLC and Q Exactive MS/MS, Thermo Fisher Scientific). The label-free peptide elution profiles of different study subjects were identified and quantified with MaxQuant software package (UniProtKB human reference proteome database, 2014 September version) (24). Label-free data were normalized with MaxLFQ (25) algorithm and compared with paired t-test statistics.
Total RNA isolation and real time RT-PCR
Total RNA from endometrial biopsies and cells was purified using Qiagen RNeasy Plus Mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. Samples were stored in RNase-free H2O and quantified by spectroscopy, and the purity was analyzed by the 260/280 absorbance ratio. RNA quality and integrity was analyzed with Agilent Bioanalyser 2100 (Agilent Technologies, Santa Clara, CA, USA) with all samples having high quality RNA (RIN=9.7-10.0). For real-time qRT-PCR analysis, 1 μg of RNA was converted to cDNA using the iScript cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA). Duplicate mRNAs were pooled from each set of treatments. The real-time RT-PCR reaction was carried out for 40 cycles with primers to insulin growth factor-binding protein 1 (IGFBP1) (eSF only), prolactin (PRL) (eSF only), STC1 (eSF+tissue) and calcium-sensing receptor (CASR) (eSF+tissue). Relative gene expression was normalized with RPL19 as the internal reference, which has been shown to have reproducibility and stable expression in endometrial tissue (23, 26). The following primer sequences were used: IGFBP1 forward, 5′-CTATGATGGCTCGAAGGCTC-3′, reverse, 5′-TTCTTGTTGCAGTTTGGCAG-3′; PRL forward, 5′-CATCAACAGCTGCCACACTT-3′, reverse, 5′-CGTTTGGTTTGCTCCTCAAT-3′; STC1 forward, 5′-GCAGGAAGAGTGCTACAGCAAG-3′, reverse, 5′-CATTCCAGCAGGCTTCGGACAA-3′; CASR forward, 5′-CTCTTCACCAATGGCTCCTGT-3′, reverse, 5′-CCACACTCATCAAAGGTCACCTG-3′; RPL19 forward, 5′-GCAGATAATGGGAGGAGCC-3′, and reverse, 5′-GCCCATCTTTGATGAGCTTC-3′.
Immunohistochemistry
Paraffin-embedded sections (4 μm) of endometrial biopsies were deparaffinized and washed. Antigen retrieval was performed by submerging slides in citrate buffer (1x Citra Plus, Vector Laboratories Inc, Burlingame, CA) at 90°C for 10 minutes. Endogenous peroxidase was blocked by 3% H2O2 in methanol for 10 minutes. Sections were then incubated with 10% blocking normal goat or horse serum in phosphate-buffered saline (Vector Laboratories) for 30 minutes for anti-STC1 (rabbit anti-human polyclonal, Santa Cruz Biotechnology, Santa Cruz, CA) or CASR (mouse anti-human monoclonal, Abcam, Cambridge, MA) antibodies, respectively. Thereafter, STC1 and CASR primary antibodies were applied at dilutions of 1:150 and 1:200 respectively at 4°C overnight. Non-immune IgG of equivalent concentration from the same species was used as negative control.
The binding of primary antibodies was detected using secondary antibodies: goat anti-rabbit and horse anti-mouse (Vector Laboratories) for 1 hour at room temperature, at a 1:300 dilution for both. After 30 minutes incubation with ABC complex (Vectastain Elite ABC immunoperoxidase detection kit, Vector Laboratories,), diaminobenzidine-hydrogen peroxide substrate (DAB kit, Vector Laboratories) was added to the slides; slides were rinsed with distilled water and counterstained with haematoxylin (Vector Laboratories) and mounted with mounting medium. A Leica microscope was used to evaluate and photograph the slides.
Semi-quantitative evaluation of IHC was performed by two observers blinded to the identity of the slides by using a grading system; each sample was analyzed twice. Staining intensity and the number of stained cells were graded on a following numeric scale: 0 = no staining, +/- = few stained cells, + = faint staining, ++ = moderate staining, and +++ = strong staining. Average value from two observers is presented.
Endometrial stromal cells isolation and culture
Fresh endometrial tissue was digested with collagenase as described (27). Human eSFs were separated from epithelium based on size and plated with DMEM/MCDB-105 medium containing 10% charcoal-stripped fetal bovine serum (FBS), insulin (5 μg/ml), gentamicin, penicillin, and streptomycin, as described (23, 26). At passage 2, cells were cultured to near confluence in the same media, followed by changing to low-serum medium (2% FBS) and cultured for 24 hours prior to the onset of treatment. All cell culture experiments were conducted using 2nd to 4th cell passages; cell purity (99% stromal fibroblasts) was determined by immunostaining with cytokeratin, vimentin and CD45 antibodies, as described (23).
Decidualization of human endometrial stromal fibroblasts
Human eSFs were treated with 0.5mM 8-Br-cAMP (hereafter referred to as cAMP) for 96 hours or with estradiol (E2) (10 nM) alone or E2 (10 nM) plus progesterone (P4) (1 μM) (E2/P4) for 14 days or vehicle. The duration and hormone concentrations were optimized previously (23). 8-Br-cAMP and steroid hormones were obtained from Sigma-Aldrich, St Louis, MO, USA. All cultures were performed in duplicate. Culture media were changed every other day. Time “zero” (t=0) controls were samples collected before initiation of treatment. Cells lysed in RLT lysis buffer (Qiagen, Valencia, CA) containing 0.1% β-mercaptoethanol and culture media were collected after 96 hours of incubation (cAMP-treated samples) or 14 days (E2/P4-treated samples).
Enzyme-Linked ImmunoSorbent Assay (ELISA)
IGFBP1 and prolactin proteins in the culture media were measured to assess the decidualization status of eSFs using ELISA, according to the manufacturer's instructions (Alpha Diagnostic Int., Inc, San Antonio, TX, USA and Diagnostic Systems Labs, Webster, TX, USA, respectively). All samples were assayed in duplicate. A standard curve was run in each experiment. Levels of IGFBP1 and prolactin for each sample were normalized to total RNA. Inter- and intra-assay coefficients of variation for the IGFBP1 ELISA were 5-7.4% and 2.4-3.4%, respectively, and for prolactin were 6.7-10.4% and 7.8-8.2%, respectively. Minimum detection limits for PRL and IGFBP1 assays were 0.14 ng/ml and 0.4 ng/ml, respectively.
Statistical evaluation
Statistical analysis for the qRT-PCR and immunohistochemistry were performed using the non-parametric one-way ANOVA Kruskal-Wallis test. Results from the ELISA analysis demonstrated a normal distribution, and a two-tailed Student's t-test assuming unequal variances was applied. Significance was determined at p≤0.05.
Results
Stanniocalcin-1 protein is up-regulated in mid-secretory phase endometrial secretome
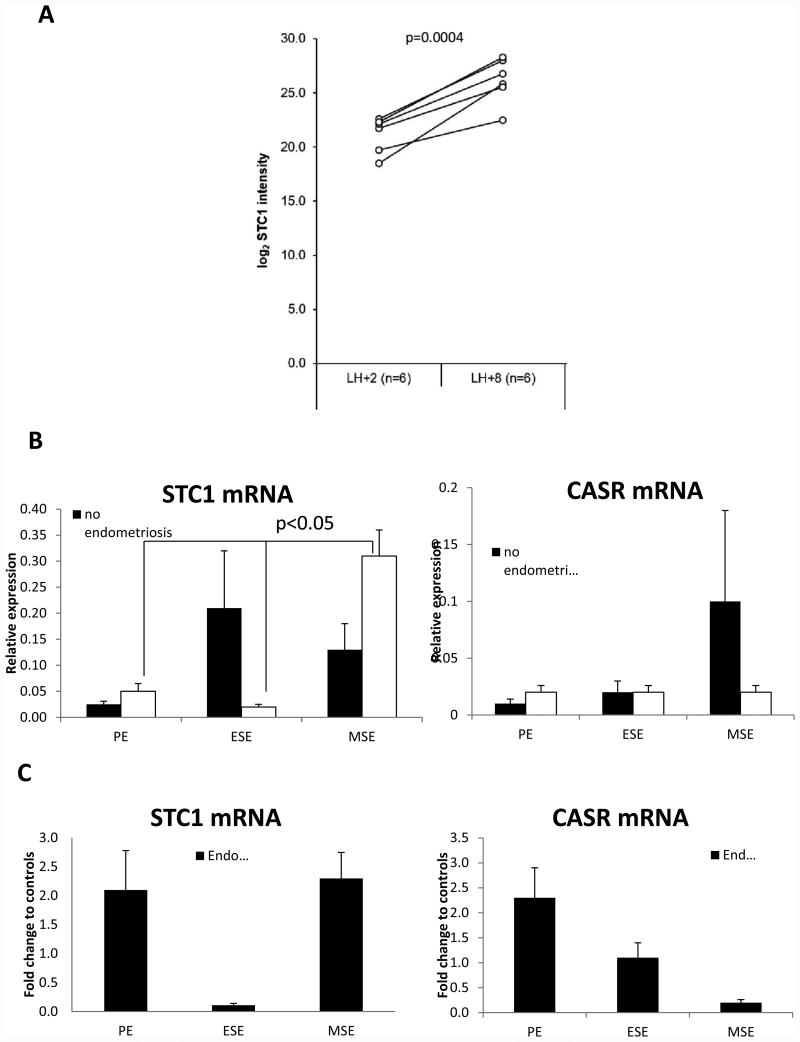
We identified STC1 protein with eight peptides (Supplemental Table 1) in endometrial aspirates from fertile women without endometriosis. Using label-free proteomics data we observed STC1 protein to be significantly (p=0.0004) up-regulated (mean fold change, FC=+39.6) in MSE (LH+8) versus ESE (LH+2) endometrial fluid or secretome (Figure 1A).
Figure 1.
A. Stanniocalcin-1 (STC1) protein level is increased in receptive (LH+8) phase endometrial secretome. Paired data from six (n=6) fertile women are presented. X-axis values have been obtained by taking log2 from summed peptide peak areas of STC1 protein in different samples. Paired t-test p-value indicated with significance accepted at p≤0.05. B. Relative expression of STC1 and CASR mRNA in human endometrial tissue throughout the menstrual cycle in women with and without endometriosis. C. Expression of STC1 and CASR mRNA in human endometrial tissue throughout the menstrual cycle in women with endometriosis normalized to samples from women without endometriosis and expressed as fold change. Significance accepted at p≤0.05. PE: proliferative phase endometrium, ESE: early secretory phase endometrium, and MSE: mid-secretory phase endometrium. Non-parametric one-way ANOVA Kruskal-Wallis test was used for statistical analysis, and significance was accepted at p≤0.05.
Stanniocalcin-1 and CASR expression in human endometrial tissue, mRNA and protein
In women without endometriosis, endometrial STC1 mRNA expression fluctuated throughout the menstrual cycle; however the differences did not reach statistical significance (p=0.088, Figure 1B). In endometrium from women with endometriosis, STC1 mRNA expression significantly increased in MSE compared to PE or ESE (p=0.043, Figure 1B). However, when comparing STC1 mRNA expression in non-endometriosis to endometriosis samples, there was no statistically significant difference in its expression throughout the menstrual cycle (p>0.1, Figure 1C). On the other hand, STC1 receptor CASR mRNA did not change significantly throughout the menstrual cycle in both women with and without endometriosis (p>0.1, Figure 1B), and there was no significant difference when its expression was compared between subjects with versus without endometriosis (p>0.1, Figure 1C).
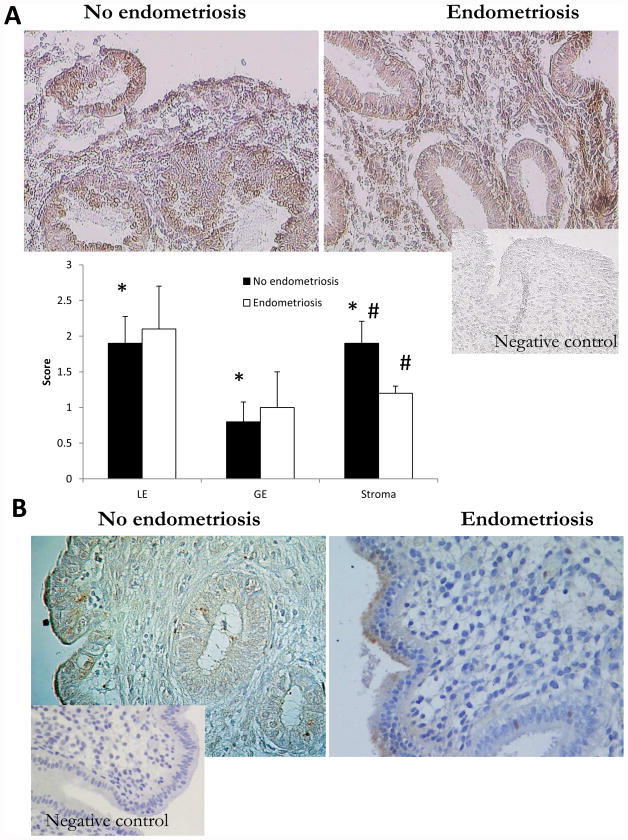
Immunohistochemistry was used to evaluate the specific cell-type expression of STC1 protein in mid-secretory human endometrium. We observed cytoplasmic STC1 protein expression in both epithelial and stromal cells from women with and without endometriosis (Figure 2A). STC1 immunoexpression in disease-free women was significantly higher in luminal epithelium and stroma compared to glandular epithelium (p<0.02). In endometriosis, there was no significant difference in STC1 protein expression in epithelial cells; however, its expression in endometrial stromal cells was significantly decreased in women with endometriosis compared to controls (p=0.04, Figure 2A). When analyzing CASR protein immunoexpression, no stromal expression of CASR was observed, while it had faint cytoplasmic expression in epithelial cells, both luminal and glandular, which was not different between specimens from women with versus without endometriosis (Figure 2B).
Figure 2.
A. Immunochemical evaluation of STC1 protein expression in mid-secretory human endometrium from women without and with endometriosis. LE=luminal epithelium and GE=glandular epithelium. * - statistically significant difference between LE and GE, and between GE and stroma, p<0.02; # - statistically significant difference in stromal staining between no endometriosis and endometriosis samples, p=0.04. Magnification ×200. One-way Kruskal-Wallis test was used for statistical analysis, with significance accepted at p≤0.05. B. Immunochemical evaluation of CASR protein expression in mid-secretory human endometrium from women without and with endometriosis. No stromal expression of CASR was observed, and no difference in epithelial (luminal and glandular) staining was noted between specimens from women with versus without endometriosis. Magnification ×200.
Stanniocalcin-1 and CASR expression in cultured human endometrial stromal fibroblasts and effect of decidualization stimuli
Next we investigated the expression of STC1 and CASR mRNA in cultured eSF and their response to decidualization stimuli in vitro. We subjected eSF to short-term decidualization with cAMP for 96 hours or long-term decidualization with P4 (with estrogen priming) for 14 days, as described in Materials and Methods. Decidualization of eSF was assessed by IGFBP1 and prolactin mRNA and protein secretion (data not shown, but significance reported earlier (23)).
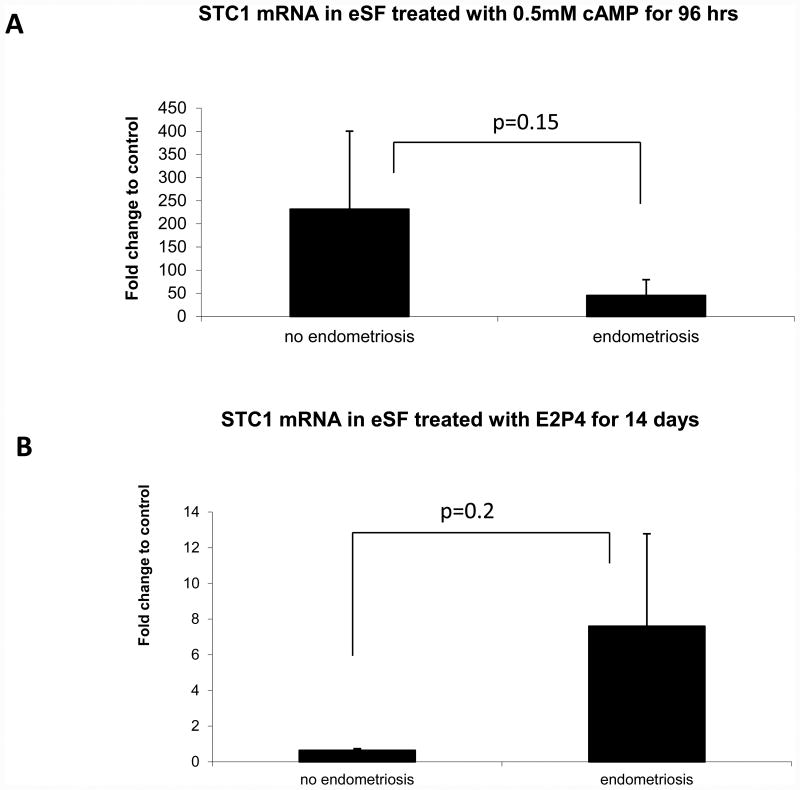
STC1 mRNA expression was dramatically up-regulated by over 230-fold by cAMP in control women (p=0.02 vs. vehicle control) compared to 45-fold in women with endometriosis (p=0.001 vs. vehicle control). However, the difference in degree of decidualization between samples from subjects with or without endometriosis did not reach statistical significance due to large variability between samples (p=0.15, Figure 3A). In contrast, there was no significant change in STC1 mRNA in response to E2/P4 stimulus by eSF from women without endometriosis, which did not differ significantly from the response of eSF from women with endometriosis (p=0.2, Figure 3B). CASR mRNA was not detected in any of the eSF samples analyzed in both groups.
Figure 3.
Expression of STC1 mRNA in decidualized human endometrial stromal fibroblasts A. with 0.5 mM cAMP for 96 hours, normalized to 96 hour control, or B. with 10 nM E2 / 1 μM P4 for 14 days, normalized to 14 day control. One-way Kruskal-Wallis test was used for statistical analysis, with significance accepted at p≤0.05.
Discussion
Endometrial STC1 is dysregulated in endometriosis
Herein we demonstrate that STC1 and CASR are expressed in human endometrial tissue. Despite STC1 mRNA detection during various microarray studies on human endometrium under different conditions, there were no attempts to characterize its expression in endometrial cell types and involvement in the decidualization process until now. Yet, the very robust upregulation of STC1 protein in endometrial fluid secretome in mid-secretory versus early secretory phase of healthy subjects reported here can potentially be a clinically relevant finding. Unfortunately, we did not have endometrial fluid secretome available from subjects with endometriosis, which would have made the analysis more robust.
Interestingly, the current data showed a trend to upregulation of STC1 mRNA in ESE versus PE or MSE in control samples in contrast to microarray results from an earlier study (4), and a trend to downregulation of STC1 mRNA in ESE from endometriosis versus no endometriosis samples, which correlated to modest downregulation in a microarray study (28), but was different from upregulation of STC1 in MSE in women with endometriosis herein. It may be suggested that the increase in STC1 mRNA expression in women with endometriosis is an attempt to compensate the simultaneously low receptor expression during MSE in women with endometriosis. These differences could be explained by the fact that in the current study we used samples from an entirely different cohort of patients and our control subjects for this part of the study were carefully selected to not have any uterine or endometrial pathology. In addition, the samples used for mRNA analyses in our study were entirely from subjects with severe endometriosis based on availability of tissue, which, while decreasing heterogeneity of the current results, may explain the discrepancy with earlier reports.
Correlation between mRNA and protein
Significant abundance of STC1 protein in the secretome of receptive mid-secretory endometrium from healthy fertile volunteers is a strong indicator of its involvement in the critical process of implantation. The changes on mRNA level from PE/ESE to MSE were not significant, although there was a trend towards upregulation in control subjects. The discrepancy between transcript and protein expression is not surprising, as the poor correlation between transcript level and the amount of corresponding protein has been shown, specifically in endometrial research (29-31). When analyzing proteomics data in the endometrium from women with versus without endometriosis, Stephens et al (31) did not find a correlation between changes in protein abundance with published transcriptome data (31), suggesting that extensive post-translation control of gene expression occurs in the tissue and is an important factor directly linked to the phenotype. Moreover, Fassbender et al. (29) did not find differences on mRNA level in the secretory endometrium from women with versus without endometriosis, although proteomic analysis of the same samples allowed the diagnosis of endometriosis with high sensitivity and specificity (29).
STC1 regulation via cAMP-dependent protein kinase A (PKA) pathway
On the levels of isolated pure human endometrial stromal fibroblasts (eSF) on the other hand, STC1 was significantly and strongly upregulated by cAMP, but not E2/P4, in control subjects, suggesting regulation likely mediated via the phosphokinase A (PKA) pathway. In vitro, cAMP was shown to upregulate STC1 gene expression in human mesenchymal stem cells (hMSC) and rat neuroblastoma cells (32, 33) but downregulated it in rat Sertoli and Leydig cells (34). In the rodent male reproductive system the effect of cAMP on STC expression was mediated by PKA pathway, as the phenotype could be rescued by H89, a PKA-pathway inhibitor (34). In rat cortical neurons however, the cAMP effect was mediated through the ERK1/2, not the PKA, pathway (35). In equine endometrium, STC1 was highly up-regulated in pregnant endometrium and expressed exclusively by endometrial glands, although it was not significantly regulated by E2/P4 treatment of cultured endometrial explants (36), with the latter being in line with our findings.
STC1 as possible new marker of decidualization
Some inconsistency between MSE whole tissue STC1 qRT-PCR and eSF decidualization results in the present study could derive from differences in tissue cell type composition. However, the PCR results in eSF culture experiments supported to some degree the IHC results, with higher STC1 eSF expression in control samples compared to endometriosis. Dysregulation of STC1 in eutopic endometrium and stromal cells from subjects with endometriosis suggests its involvement in the pathogenesis of decidualization defects. These observations have led us to suggest that STC1 is a potentially new marker of decidualization, with more studies needed to elaborate on that novel concept and the roles of progesterone signaling and the PKA pathway and other pathways in this regard.
CASR in human endometrium
To the best of our knowledge, this is the first study reporting on the expression of CASR in human endometrium. Calcium homeostasis has been suggested to play a critical role in embryo implantation in rodents and humans, as calcium transporter genes are abundantly expressed in reproductive tissues including endometrium in a cyclic manner, are regulated by ovarian steroid hormones, and their dysregulation affects the expression of some important markers of endometrial receptivity and decreased embryo implantation (37, 38).
As mentioned, CASR, a known mediator of a wide range of calcium-dependent physiological responses in various tissues, mediates STC1 transcription in response to extracellular calcium in fish and is induced in rat endometrium during implantation and subsequent decidualization, particularly in luminal and glandular epithelial cells (15). Herein, CASR did not exhibit variation in its mRNA expression under cyclic hormone influence or in endometriosis on the tissue level. In addition, we did not observe stromal expression of CASR in the samples analyzed, in contrast to its high expression in rat endometrium during implantation and decidualization (15). In our study, absence of amplification of the CASR transcript in eSFs was confirmed by negative stromal immunoexpression of CASR in all samples, in line with an early study on CASR expression in rat luminal epithelium but not stroma (39). In addition, the CASR transcript was not detected in any of the array studies reviewed. This pattern of expression suggests paracrine actions of stromal cell-derived STC1 on endometrial epithelial cells.
Strengths, limitations and conclusions
The strengths of the current study include its novelty, obtaining endometrial flushings and biopsies from well characterized subjects. The limitations are the descriptive nature of the study and relatively small samples size.
Thus, we reported herein on the pattern of STC1 and CASR mRNA and protein expression in endometrial tissue and endometrial stromal cells from women with and without endometriosis. Whether STC1 affects endometrial steroidogenesis, similar to reports in ovarian tissue (40-42), is an appealing question for future investigation.
Further characterization of STC1 expression and function in human endometrium in pathological conditions, such as unexplained infertility, is ongoing, as well as the analysis of secretome in women with unexplained infertility.
Supplementary Material
Supplementary Table 1. Identified Stanniocalcin-1 peptides. Sequences along with peptide identification and quantification parameters have been indicated. Note, that when the peptide signal has fallen below the limit of peak integration (limit of quantification), the intensity values are presented as zeros.
Acknowledgments
Financial support: Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award P50HD055764 (LCG); Marie Curie post-doctoral fellowship (FP7, no. 329812, NutriOmics) (SA), Enterprise Estonia (grant no. EU48695), Estonian Ministry of Education and Research (grant IUT34-16), the EU-FP7 Eurostars Program (grant NOTED, EU41564), H2020-TWINN-2015 project WIDENLIFE (grant no. 692056) (SA, SK, AS), the EU-FP7 IAPP Project (grant SARM, EU324509) (SA, SK, AS), Uppsala University and the Swedish society of medicine (ASE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xiao LJ, Yuan JX, Song XX, Li YC, Hu ZY, Liu YX. Expression and regulation of stanniocalcin 1 and 2 in rat uterus during embryo implantation and decidualization. Reproduction. 2006;131(6):1137–49. doi: 10.1530/rep.1.01100. [DOI] [PubMed] [Google Scholar]
- 2.Song G, Dunlap KA, Kim J, Bailey DW, Spencer TE, Burghardt RC, et al. Stanniocalcin 1 is a luminal epithelial marker for implantation in pigs regulated by progesterone and estradiol. Endocrinology. 2009;150(2):936–45. doi: 10.1210/en.2008-1026. [DOI] [PubMed] [Google Scholar]
- 3.Allegra A, Marino A, Coffaro F, Lama A, Rizza G, Scaglione P, et al. Is there a uniform basal endometrial gene expression profile during the implantation window in women who became pregnant in a subsequent ICSI cycle? Hum Reprod. 2009;24(10):2549–57. doi: 10.1093/humrep/dep222. [DOI] [PubMed] [Google Scholar]
- 4.Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147(3):1097–121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- 5.Altmae S, Martinez-Conejero JA, Salumets A, Simon C, Horcajadas JA, Stavreus-Evers A. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod. 2010;16(3):178–87. doi: 10.1093/molehr/gap102. Epub 2009/11/26 doi:10.1093/molehr/gap102 gap102. [pii] [DOI] [PubMed] [Google Scholar]
- 6.Uuskula L, Mannik J, Rull K, Minajeva A, Koks S, Vaas P, et al. Mid-gestational gene expression profile in placenta and link to pregnancy complications. PLoS One. 2012;7(11):e49248. doi: 10.1371/journal.pone.0049248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jellinek DA, Chang AC, Larsen MR, Wang X, Robinson PJ, Reddel RR. Stanniocalcin 1 and 2 are secreted as phosphoproteins from human fibrosarcoma cells. Biochem J. 2000;350 Pt 2:453–61. [PMC free article] [PubMed] [Google Scholar]
- 8.Wendelaar Bonga SE, Pang PK. Control of calcium regulating hormones in the vertebrates: parathyroid hormone, calcitonin, prolactin, and stanniocalcin. Int Rev Cytol. 1991;128:139–213. doi: 10.1016/s0074-7696(08)60499-4. [DOI] [PubMed] [Google Scholar]
- 9.Yoshiko Y, Aubin JE. Stanniocalcin 1 as a pleiotropic factor in mammals. Peptides. 2004;25(10):1663–9. doi: 10.1016/j.peptides.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Deol HK, Varghese R, Wagner GF, Dimattia GE. Dynamic regulation of mouse ovarian stanniocalcin expression during gestation and lactation. Endocrinology. 2000;141(9):3412–21. doi: 10.1210/endo.141.9.7658. [DOI] [PubMed] [Google Scholar]
- 11.Varghese R, Gagliardi AD, Bialek PE, Yee SP, Wagner GF, Dimattia GE. Overexpression of human stanniocalcin affects growth and reproduction in transgenic mice. Endocrinology. 2002;143(3):868–76. doi: 10.1210/endo.143.3.8671. [DOI] [PubMed] [Google Scholar]
- 12.Filvaroff EH, Guillet S, Zlot C, Bao M, Ingle G, Steinmetz H, et al. Stanniocalcin 1 alters muscle and bone structure and function in transgenic mice. Endocrinology. 2002;143(9):3681–90. doi: 10.1210/en.2001-211424. [DOI] [PubMed] [Google Scholar]
- 13.Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81(1):239–97. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 14.McNeil L, Hobson S, Nipper V, Rodland KD. Functional calcium-sensing receptor expression in ovarian surface epithelial cells. Am J Obstet Gynecol. 1998;178(2):305–13. doi: 10.1016/s0002-9378(98)80017-3. [DOI] [PubMed] [Google Scholar]
- 15.Xiao LJ, Yuan JX, Li YC, Wang R, Hu ZY, Liu YX. Extracellular Ca2+-sensing receptor expression and hormonal regulation in rat uterus during the peri-implantation period. Reproduction. 2005;129(6):779–88. doi: 10.1530/rep.1.00621. [DOI] [PubMed] [Google Scholar]
- 16.Hendy GN, D'Souza-Li L, Yang B, Canaff L, Cole DE. Mutations of the calcium-sensing receptor (CASR) in familial hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia. Hum Mutat. 2000;16(4):281–96. doi: 10.1002/1098-1004(200010)16:4<281∷AID-HUMU1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Radman DP, McCudden C, James K, Nemeth EM, Wagner GF. Evidence for calcium-sensing receptor mediated stanniocalcin secretion in fish. Mol Cell Endocrinol. 2002;186(1):111–9. doi: 10.1016/s0303-7207(01)00643-8. [DOI] [PubMed] [Google Scholar]
- 18.Bradbury RA, Cropley J, Kifor O, Lovicu FJ, de Iongh RU, Kable E, et al. Localization of the extracellular Ca(2+)-sensing receptor in the human placenta. Placenta. 2002;23(2-3):192–200. doi: 10.1053/plac.2001.0765. [DOI] [PubMed] [Google Scholar]
- 19.Bradbury RA, Sunn KL, Crossley M, Bai M, Brown EM, Delbridge L, et al. Expression of the parathyroid Ca(2+)-sensing receptor in cytotrophoblasts from human term placenta. J Endocrinol. 1998;156(3):425–30. doi: 10.1677/joe.0.1560425. [DOI] [PubMed] [Google Scholar]
- 20.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1951;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 21.Tamaresis JS, Irwin JC, Goldfien GA, Rabban JT, Burney RO, Nezhat C, et al. Molecular classification of endometriosis and disease stage using high-dimensional genomic data. Endocrinology. 2014;155(12):4986–99. doi: 10.1210/en.2014-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Society TAF. Revised American Fertility Society classification of endometriosis. Fertil Steril. 1985;43:351–2. doi: 10.1016/s0015-0282(16)48430-x. [DOI] [PubMed] [Google Scholar]
- 23.Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod. 2009;80(1):105–14. doi: 10.1095/biolreprod.108.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26(12):1367–72. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 25.Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014;13(9):2513–26. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aghajanova L, Horcajadas JA, Weeks JL, Esteban FJ, Nezhat CN, Conti M, et al. The protein kinase A pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology. 2010;151(3):1341–55. doi: 10.1210/en.2009-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC. Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab. 2006;91(4):1453–61. doi: 10.1210/jc.2005-0769. [DOI] [PubMed] [Google Scholar]
- 28.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 29.Fassbender A, Verbeeck N, Bornigen D, Kyama CM, Bokor A, Vodolazkaia A, et al. Combined mRNA microarray and proteomic analysis of eutopic endometrium of women with and without endometriosis. Hum Reprod. 2012;27(7):2020–9. doi: 10.1093/humrep/des127. [DOI] [PubMed] [Google Scholar]
- 30.Ning K, Fermin D, Nesvizhskii AI. Comparative analysis of different label-free mass spectrometry based protein abundance estimates and their correlation with RNA-Seq gene expression data. J Proteome Res. 2012;11(4):2261–71. doi: 10.1021/pr201052x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stephens AN, Hannan NJ, Rainczuk A, Meehan KL, Chen J, Nicholls PK, et al. Post-translational modifications and protein-specific isoforms in endometriosis revealed by 2D DIGE. J Proteome Res. 2010;9(5):2438–49. doi: 10.1021/pr901131p. [DOI] [PubMed] [Google Scholar]
- 32.Aghajanova L, Horcajadas JA, Esteban FJ, Giudice LC. The Bone Marrow-Derived Human Mesenchymal Stem Cell: Potential Progenitor of the Endometrial Stromal Fibroblast. Biol Reprod. 2010 doi: 10.1095/biolreprod.109.082867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong CK, Yeung HY, Mak NK, DiMattia GE, Chan DK, Wagner GF. Effects of dibutyryl cAMP on stanniocalcin and stanniocalcin-related protein mRNA expression in neuroblastoma cells. J Endocrinol. 2002;173(1):199–209. doi: 10.1677/joe.0.1730199. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Wong CK. Effects of dexamethasone and dibutyryl cAMP on stanniocalcin-1 mRNA expression in rat primary Sertoli and Leydig cells. Mol Cell Endocrinol. 2008;283(1-2):96–103. doi: 10.1016/j.mce.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 35.Holighaus Y, Weihe E, Eiden LE. STC1 induction by PACAP is mediated through cAMP and ERK1/2 but not PKA in cultured cortical neurons. J Mol Neurosci. 2012;46(1):75–87. doi: 10.1007/s12031-011-9653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kikuchi M, Nakano Y, Nambo Y, Haneda S, Matsui M, Miyake Y, et al. Production of calcium maintenance factor Stanniocalcin-1 (STC1) by the equine endometrium during the early pregnant period. J Reprod Dev. 2011;57(2):203–11. doi: 10.1262/jrd.10-079k. [DOI] [PubMed] [Google Scholar]
- 37.Choi KC, An BS, Yang H, Jeung EB. Regulation and molecular mechanisms of calcium transport genes: do they play a role in calcium transport in the uterine endometrium? J Physiol Pharmacol. 2011;62(5):499–504. [PubMed] [Google Scholar]
- 38.Zhang RJ, Zou LB, Zhang D, Tan YJ, Wang TT, Liu AX, et al. Functional expression of large-conductance calcium-activated potassium channels in human endometrium: a novel mechanism involved in endometrial receptivity and embryo implantation. J Clin Endocrinol Metab. 2012;97(2):543–53. doi: 10.1210/jc.2011-2108. [DOI] [PubMed] [Google Scholar]
- 39.Bernadotte F, Holmdahl R, Juhlin C, Mattsson R. Expression of a cell surface antigen with potential Ca2+-sensor/receptor function in rat placenta and uterus. J Reprod Immunol. 1989;16(2):199–205. doi: 10.1016/0165-0378(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 40.Varghese R, Wong CK, Deol H, Wagner GF, DiMattia GE. Comparative analysis of mammalian stanniocalcin genes. Endocrinology. 1998;139(11):4714–25. doi: 10.1210/endo.139.11.6313. [DOI] [PubMed] [Google Scholar]
- 41.Paciga M, McCudden CR, Londos C, DiMattia GE, Wagner GF. Targeting of big stanniocalcin and its receptor to lipid storage droplets of ovarian steroidogenic cells. J Biol Chem. 2003;278(49):49549–54. doi: 10.1074/jbc.M307302200. [DOI] [PubMed] [Google Scholar]
- 42.Luo CW, Kawamura K, Klein C, Hsueh AJ. Paracrine regulation of ovarian granulosa cell differentiation by stanniocalcin (STC) 1: mediation through specific STC1 receptors. Mol Endocrinol. 2004;18(8):2085–96. doi: 10.1210/me.2004-0066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Identified Stanniocalcin-1 peptides. Sequences along with peptide identification and quantification parameters have been indicated. Note, that when the peptide signal has fallen below the limit of peak integration (limit of quantification), the intensity values are presented as zeros.