Abstract
Background
Inflammatory-mediated pathological processes in the endothelium arise as a consequence of the dysregulation of vascular homeostasis. Of particular importance are mediators produced by stimulated monocytes/macrophages inducing activation of endothelial cells (ECs). This is manifested by excessive soluble pro-inflammatory mediator production and cell surface adhesion molecule expression. Nitro-fatty acids are endogenous products of metabolic and inflammatory reactions that display immuno-regulatory potential and may represent a novel therapeutic strategy to treat inflammatory diseases. The purpose of our study was to characterize the effects of nitro-oleic acid (OA-NO2) on inflammatory responses and the endothelial-mesenchymal transition (EndMT) in ECs that is a consequence of the altered healing phase of the immune response.
Methods
The effect of OA-NO2 on inflammatory responses and EndMT was determined in murine macrophages and murine and human ECs using Western blotting, ELISA, immunostaining, and functional assays.
Results
OA-NO2 limited the activation of macrophages and ECs by reducing pro-inflammatory cytokine production and adhesion molecule expression through its modulation of STAT, MAPK and NF-κB-regulated signaling. OA-NO2 also decreased transforming growth factor-β-stimulated EndMT and pro-fibrotic phenotype of ECs. These effects are related to the downregulation of Smad2/3.
Conclusions
The study shows the pleiotropic effect of OA-NO2 on regulating EC-macrophage interactions during the immune response and suggests a role for OA-NO2 in the regulation of vascular endothelial immune and fibrotic responses arising during chronic inflammation.
General significance
These findings propose the OA-NO2 may be useful as a novel therapeutic agent for treatment of cardiovascular disorders associated with dysregulation of the endothelial immune response.
Keywords: nitro-fatty acids, nitro-oleic acid, endothelial cells, macrophages, vascular inflammation, endothelial-mesenchymal transition
Graphical abstract
1. Introduction
Vascular inflammation is one of the key pathological processes involved in the development of cardiovascular disorders, the major cause of mortality in developed countries. Understanding that inflammation contributes to the progression of various pathological states related to cardiovascular diseases including atherosclerosis, thrombosis, endothelial dysfunction, and fibrosis is crucial for gaining an insight into the mechanisms underlying these conditions and for identifying new pharmacological targets [1].
Innate immune cell activation is a characteristic feature of inflammatory vascular disorders. In particular, the activation of monocytes/macrophages plays a significant role in chronic inflammation inception in vessels. During the early phase of inflammation, T-cells and NK-cells predominantly secrete interferon-γ (IFN-γ), macrophages release tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and reactive oxygen and nitrogen oxide-derived species, inducing pro-inflammatory phenotypes in both immune and endothelial cells (ECs). In the later phase, macrophage-derived transforming growth factor (TGF-β) is produced [2].
The activation of endothelium is a hallmark of vascular inflammatory disorders and plays a central role in mediating structural changes in the vasculature [3]. ECs represent a heterogeneous population that actively participates in both innate and adaptive immune responses, being one of the first cell types to encounter foreign pathogens and endogenous danger signals [4]. During acute and chronic inflammation, pro-inflammatory signaling pathways are triggered in ECs. Subsequently, secreted pro-inflammatory cytokines and chemokines, adhesion molecules (such as intercellular adhesion molecule-1, ICAM-1, and vascular cell adhesion molecule-1) on the surface of ECs, and the increased permeability of the vessel wall facilitate leukocyte transmigration to the site of inflamed or damaged tissue [5, 6]. This effect was shown to be time-dependent [4]. While Regulated on Activation Normal T cell Expressed and Secreted (RANTES) and interleukin-6 (IL-6) are potent chemoattractants for T lymphocytes and neutrophil granulocytes, murine monocyte chemoattractant protein (MCP-5, the structural and functional homologue of human MCP-1) is a chemoattractant for blood monocytes [6, 7]. Granulocyte-macrophage colony-stimulating factor (GM-CSF) stimulates pro-inflammatory M1 macrophages to induce fibrosis progression [8]. Moreover, during the activation of ECs, nitric oxide (·NO) production is also dysregulated [9].
TGF-β is a multifunctional modulator of physiologic processes such as wound healing, with its overproduction triggering pathological vascular remodeling and fibrotic changes through the induction of EndMT [10-13]. In fibrotic tissue, fibroblasts are of different origin [14]. For example, cardiac and renal fibrosis is associated with the emergence of fibroblasts originating from ECs (ECs are estimated to participate in more than 25% of fibroblast formation), supporting that EndMT could be involved in fibrosis development [11, 14]. Interestingly, macrophages releasing TGF-β (regulatory macrophages) promote fibroblast/myofibroblast switch as well as EndMT [15-18]. While in the early phase of fibrotic changes, cytoplasmic protein fibroblast-specific protein 1 (FSP1, also known as S100A-4) is produced, in latter phases, structural proteins including α-smooth muscle actin (α-SMA) are overexpressed [11]. Smads are one of the major downstream cytoplasmic mediators of TGF-β signaling in ECs [10-13]. The role of Smad3 in TGF-β-induced EndMT was also documented in different types of ECs, whereas the function of Smad2 in fibrosis induction in response to TGF-β is still not clear [10, 19].
Fatty acid nitroalkene derivatives (NO2-FAs) are endogenously generated by nitrating species formed by the acidic conditions of digestion and as a consequence of oxidative inflammatory reactions of nitrogen oxides. This latter mechanism can serve as an adaptive response to oxidative stress, since the physiological concentrations (~1 μM) that are generated clinically [20-22] are sufficient to mediate pleiotropic signaling responses that promote the resolution of inflammation [23]. Recent evidence suggests that these pluripotent signaling mediators can, as a consequence of post-translational protein modification, regulate many physiological and pathological processes by affecting a whole range of mammalian cell functions (e.g. the activation of macrophages and neutrophils, vasorelaxation, angiogenesis and platelet aggregation) [2, 3, 22, 24].
Placing special focus on the modulation of macrophage functions [2, 25] we now elucidate the effects of nitro-oleic acid (OA-NO2) on inflammation and the EndMT responses of ECs. We hypothesize that OA-NO2 inhibits pathological tissue remodeling induced by the activation of macrophages and ECs during chronic inflammation. Thus the effects of OA-NO2 on macrophage-mediated EC inflammatory responses and EC-derived EndMT were studied in murine ECs stimulated by IFN-γ, TNF-α, IL-1β TGF-β, human ECs stimulated by TNF-α, and in bacterial endotoxin-activated macrophages.
2. Materials and methods
2.1 Materials
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA). OA-NO2 ((E)-9- and 10-nitro-octadec-9-enoic acid) was diluted to a 100 mM concentration in methanol and stored at −80°C. Before each experiment, a 10 mM solution of OA-NO2 in methanol was prepared from the stock solution and diluted in Dulbecco's Modified Eagle's Medium (DMEM; PAN-Biotech, Aidenbach, Germany) to obtain 100 μM of OA-NO2, which was used immediately for cell culture experiments. All stock solutions were prepared and stored in sterile, low-binding tubes [2].
2.2 Cell culture and treatment
Murine pancreatic endothelial MS-1 cells and peritoneal RAW 264.7 macrophages (ATCC, Manassas, VA, USA) were grown in DMEM with 10% low endotoxin fetal bovine serum (FBS; PAA, Pasching, Austria) and 1% Penicillin/Streptomycin. Human umbilical vein endothelial cells (HUVEC) from Lonza [26] were cultivated in EGM Bullet Kit medium (Lonza).
ECs were treated with 1.0 μM OA-NO2 with or without cytokines – IFN-γ (50 ng/ml), IL-1β (5 ng/ml), TNF-α (10 ng/ml), or TGF-β (10 ng/ml) – for different time periods (20 min - 48 h). The inflammatory phenotype in macrophages was induced using bacterial lipopolysaccharide (LPS, 100 ng/ml) treated for 24 h. Before each experiment, ECs and macrophages were cultured in complete media as indicated above. Two hours before the start of experiments, the complete medium was replaced with DMEM with 2% of FBS or FBS-free DMEM. In long-term experiments (6 d), medium and treatments were renewed regularly after 2 days. The final concentration of OA-NO2 used for all experiments (1.0 μM) was selected on the basis of its physiological relevance and our previous experience [2, 20]. OA-NO2 was applied alone or together with cytokines. Cell viability was measured by ATP Cell Viability test (BioThema, Handen, Sweden) [27]; no effect of cytokines or OA-NO2 exposure was detected (data not shown).
2.3 Determination of relative cytokine and chemokine levels
Commercially available mouse cytokine ELISA kits (R&D Systems, Minneapolis, MN, USA; eBioscience, San Diego, CA, USA) were used for the determination of cytokines in cell supernatants of ECs as well as cytokines produced by macrophages. These analyses were performed according to the supplier's instructions.
2.4 Detection of protein expression
The expression of proteins was detected in cell lysates after 20 min - 24 h treatments. Primary rabbit antibodies against ICAM-1, Smad2, phospho-Smad2 (Ser465/467), Smad3, phospho-Smad3 (Ser423/425), signal transducer and activator of transcription 1 (STAT1), phospho-STAT1 (Tyr701), STAT3, phospho-STAT3 (Tyr705), c-Jun N-terminal kinase (SAPK/JNK), phospho-SAPK/JNK (Thr183/Tyr185), extracellular signal-regulated kinase (ERK1/2), phospho-ERK1/2 (Thr202/Tyr204), p38 mitogen-activated protein kinase (MAPK), phospho-p38 MAPK (Thr180/Tyr182), nuclear factor-κB (NF-kB) p65, phospho-NF-kB p65 (Ser536), endothelial nitric oxide synthase (eNOS), phospho-eNOS (Ser1777), GAPDH; primary mouse antibodies against α-SMA, inducible nitric oxide synthase (iNOS), β-actin; and appropriate secondary IgG antibodies (Santa Cruz Biotechnology, Dallas, TX, USA and Cell Signaling Technology, Danvers, MA, USA) were used. Relative levels of proteins were quantified by scanning densitometry using the ImageJ program (National Institutes of Health, Bethesda, MD, USA) with the individual band density value expressed in arbitrary units (optical density, OD). The data in graphs represents the ratio between the individual values of OD for detected protein, β-actin, or GAPDH.
2.5 Immunostaining of inflammatory and fibrotic markers
For the immunostaining of MS-1, BD Falcon CultureSlides were used. Cells were seeded evenly. After treatments, cells were fixed with 4% formaldehyde, and blocked with 10% goat serum. Permeabilization using 0.1% Triton X-100 was performed after fixation step for FSP1 and actin visualization. Primary rabbit antibodies against ICAM-1 (Santa Cruz, USA) and FSP1 (S100A4; Cell Signalling, USA) in combination with goat anti-mouse and anti-rabbit IgG (H+L) secondary antibody, DyLight 488 conjugate (Thermo Scientific, USA), were used. Alexa Fluor 488 phalloidin was used for actin filaments staining. Nuclei were stained with DAPI. Glass slides were washed and mounted in Mowiol (Calbiochem, USA) solution. Images were acquired on a confocal microscope (TCS SP5, Leica, Germany) using a 63 × 1.4 oil immersion objective in identical settings. Negative controls obtained by omitting primary antibodies revealed no autofluorescence or nonspecific fluorescence (data not shown). The mean value of the optical intensity of the green component (495 - 672 nm) of each image was calculated using the ImageJ program (National Institutes of Health, Bethesda, MD, USA).
2.6 Adhesion of macrophages to endothelial cells
For the adhesion experiments, MS-1 ECs were cultured in 96-well tissue culture plates (Genetix, USA) until confluent. ECs were treated for 24 h with or without IFN-γ (50 μg/ml) and/or OA-NO2 (1.0 μM). RAW 264.7 macrophages were fluorescently labeled with calcein-AM (1 μM) for 20 min at 37°C in the dark and washed. The adhesion of macrophages to EC cultures was performed under static conditions. Before the adhesion assay was performed, ECs were washed and DMEM plus 2% FBS was added to cells. Labeled macrophages were applied at a density of 5×103 cells/well to the EC surface and allowed to adhere for 1 h at 37°C with gentle rocking. Non-adherent cells were removed by washing two times with HBSS. The relative fluorescence intensity of adherent macrophages was analyzed by an Infinite M200 microplate spectrofluorimeter with excitation and emission wavelengths of 480 and 530 nm, respectively.
2.8 Data analysis
Data were statistically analyzed using a t-test (GraphPad Prism 5.01). All data are reported as means ± SEM. A *p value of less than 0.05 was considered significant.
3. Results
3.1 OA-NO2 downregulates the development of EC pro-inflammatory phenotypes
To elucidate the role of OA-NO2 in regulating the development of macrophage-derived EC phenotypes, TNF-α, IL-1β, IFN-γ, and TGF-β were selected as exemplary mediators [4]. Experiments with RAW 264.7 cells exposed to LPS (100 ng/ml) for 24 h revealed that OA-NO2 (1.0 μM) significantly decreased the production of TNF-α, IL-1β, and TGF-β (Suppl. Fig. 1), but not IFN-γ (data not shown).
Next, the effect of OA-NO2 on the induction of a pro-inflammatory phenotype in mouse MS-1 ECs was reflected by changes in the production of RANTES, IL-6, MCP-5, and GM-CSF in response to the inflammatory mediators IFN-γ (50 ng/ml), IL-1β (5 ng/ml), TNF-α (10 ng/ml), and TGF-β (10 ng/ml) after exposure for 24 h (Fig. 1). RANTES (Fig. 1a-c) and IL-6 levels (Fig. 1d-f) were significantly elevated in IFN-γ-, IL-1β- and TNF-α-treated ECs; TNF-α was the weakest inducer of both RANTES and IL-6 production (Fig. 1c,f). All of these responses were significantly inhibited by 1.0 μM OA-NO2 (Fig. 1a-f). MCP-5 production was elevated only in IFN-γ-stimulated ECs, with this effect significantly reversed by OA-NO2 (Fig. 1g). In addition, GM-CSF was significantly enhanced only after IL-1β treatment (Fig. 1h). OA-NO2 also downregulated GM-CSF levels (Fig. 1h). TGF-β did not enhance production of any cytokine detected (data not shown). Similarly, OA-NO2 (1.0 μM) limited ICAM-1 expression induced in response to IFN-γ (Fig. 2a,b).
Fig. 1. OA-NO2 regulates cytokine production in activated ECs.
MS-1 cells were treated with or without OA-NO2 (1.0 μM) and the following cytokines for 24 h: IFN-γ (50 ng/ml), IL-1β (5 ng/ml), and TNF-α (10 ng/ml). RANTES (A-C), IL-6 (D-F), MCP-5 (G), and GM-CSF (H) levels were detected by ELISA. The data represent 3-6 independent experiments (each determined in duplicate). A *p value of less than 0.05 was considered significant when evaluating differences between the individual bars.
Fig. 2. OA-NO2 decreases IFN-γ-induced ICAM-1 expression and adhesive properties in ECs.
MS-1 cells were treated with or without OA-NO2 (1.0 μM) and IFN-γ (50 ng/ml) for 24 h. ICAM-1 expression (green) was detected by immunostaining (A), nuclei were stained with DAPI (blue) [45]. Typical representative figures are shown from n=3 independent replicates. The expression of ICAM-1 was detected also by WB (B). Adhesion of Calcein AM-stained RAW 264.7 macrophages to OA-NO2 (1.0 μM)- and IFN-γ (50 ng/ml)-treated MS-1 ECs was observed after 1 h co-incubation (C). Relative fluorescence intensity of Calcein AM was measured (ex. 480, em. 530 nm, determined in duplicates). The data represent the ratios between individual values for the optical densities of bands determined for ICAM-1 and housekeeping protein (WB) or means from fluorescence measurements (adherence assay), n=3-4. A *p value of less than 0.05 was considered significant when evaluating differences between the individual bars.
To help to elucidate the impact of OA-NO2 on macrophage-induced endothelial inflammatory responses, we performed chemotactic and cell adhesion assays. IFN-γ significantly activated RAW 264.7 macrophage adhesion to MS-1 ECs, with OA-NO2 inhibiting this effect (Fig. 2c, Suppl. Fig. 2). Additionally, conditioned media from IFN-γ-treated MS-1 ECs induced migration of RAW 264.7 macrophages, with OA-NO2 inhibiting this IFN-γ-induced macrophage chemotactic response (Suppl. Fig. 3).
To confirm the clinical relevance of these observations in a more human-related inflammatory model system, additional experiments were performed with HUVECs. Consistent with the responses of MS-1 ECs, IL-6 production and ICAM-1 expression was enhanced by the activation of HUVECs, and OA-NO2 significantly inhibited these responses (Suppl. Fig. 4a,b).
Since the activation of ECs is connected with the dysregulation of eNOS function and ·NO signaling, the impact of OA-NO2 on ·NO synthesis was determined. ·NO levels were enhanced only in IFN-γ-stimulated MS-1 cells (Suppl. Fig. 5a). Neither iNOS (data not shown) nor total eNOS expression (Suppl. Fig. 5b) were affected by IFN-γ. However, the phosphorylation of eNOS was slightly enhanced in IFN-γ-treated ECs (Suppl. Fig. 5b). OA-NO2 did not affect ·NO production (Suppl. Fig. 5).
3.2 OA-NO2 downregulates activation of different signaling pathways in ECs in response to pro-inflammatory mediators
To help to clarify the signaling pathways responsible for OA-NO2 immunomodulation of ECs, the activation state of STATs, MAPKs, and NF-κB was determined in ECs treated for 30 or 20 min, respectively. The phosphorylation of both STAT1 and STAT3 was significantly stimulated only by IFN-γ and was significantly inhibited by OA-NO2 (Fig. 3a, b). Concerning the MAPK signaling pathways, IL-1β was the only mediator that strongly enhanced phosphorylation of all three kinases (p38 MAPK, ERK, and JNK) and OA-NO2 significantly diminished these IL-1β actions (Fig. 3c-e). ERK was also activated by TNF-α and IFN-γ, however OA-NO2-induced downregulation of ERK phosphorylation bordered on significance (Fig. 3d). Phosphorylation of NF-κB was markedly enhanced in TNF-α and IL-1β-treated ECs, and OA-NO2 significantly downregulated their effects (Fig. 3f). TGF-β did not enhance activation of any of signaling pathways detected (Fig. 3a-f).
Fig. 3. Signaling pathways affected by OA-NO2 in activated MS-1 ECs.
MS-1 cells were treated with or without OA-NO2 (1.0 μM) and selected inflammatory mediators IFN-γ (50 ng/ml), IL-1β (5 ng/ml), TNF-α (10 ng/ml), and TGF-β (10 ng/ml). STAT1 (A) and STAT3 (B) phosphorylation was detected after 30 min, MAPK (C-E) and NF-κB (F) phosphorylation after 20 min. The data represent the ratios between individual values for the optical densities of bands determined for the phosphorylated and total forms of each protein (n=3-5). A *p value of less than 0.05 was considered significant when evaluating differences between the individual bars.
3.3 OA-NO2 downregulates the development of EndMT in ECs
Further, the effect of OA-NO2 on morphological changes and EndMT following inflammatory activation of ECs was analyzed. Structural changes were identified in MS-1 cells by monitoring the development of filamentous actin stress fibers (Fig. 4). After 48 h, TGF-β significantly shifted actin cytoskeletal appearance to a more fibrotic and filamentous structure (Fig. 4, Suppl. Fig. 6). OA-NO2 partially prevented these TGF-β-triggered changes (Fig. 4, Suppl. Fig. 6). Further, FSP1 as the marker of EC conversion towards the pro-fibrotic phenotype was detected in the cytosol of MS-1 cells after 48 h of TGF-β (10 ng/ml) treatment (Fig. 5a,b). OA-NO2 also reversed the TGF-β effect on FSP1 expression (Fig. 5a,b). Although we did not detect the upregulation of α-SMA expression in MS-1 cells after 24 or 48 h of EC stimulation with TGF-β (data not shown), we observed a significant increase in α-SMA expression after 6 d of TGF-β treatment of ECs (Fig. 5c). OA-NO2 significantly decreased this TGF-β-induced α-SMA expression (Fig. 5c). The phosphorylation of transduction proteins Smad2/3 was markedly elevated in TGF-β-treated ECs after 30 min of incubation (Fig. 6). Importantly, OA-NO2 significantly reduced the activation of both Smad2 (Fig. 6a) and Smad3 (Fig. 6b) in MS-1 cells.
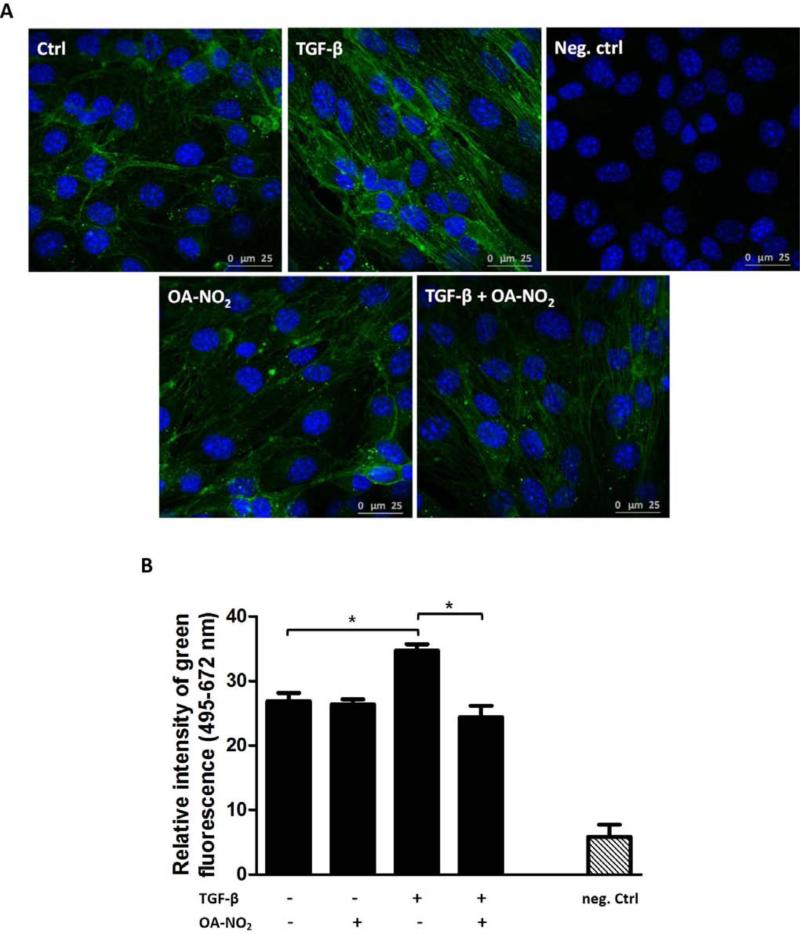
Fig. 4. OA-NO2 regulates TGF-β-triggered changes in actin cytoskeleton in ECs.
MS-1 cells were treated with or without OA-NO2 (1.0 μM) and TGF-β (10 ng/ml) for 48 h. Immunostaining was used to identify filamentous actin stress fibers (green) characteristic of active fibroblasts (A). Nuclei were stained with DAPI (blue) [45]. Typical representative figures are shown from n=3-6 independent replicates. The mean value of the optical intensity of the green component (ex. 495, em. 672 nm) of each image was calculated (B). A *p value of less than 0.05 was considered significant when evaluating differences between the individual bars.
Fig. 5. OA-NO2 decreases the TGF-β-induced FSP1 and α-SMA expression in ECs.
MS-1 cells were treated with or without OA-NO2 (1.0 μM) and TGF-β (10 ng/ml). The expression of fibroblast marker FSP-1 (green) was detected by immunostaining after 48 h of treatment (A). Nuclei were stained with DAPI (blue) [45]. Typical representative figures are shown from n=3 independent replicates. The mean value of the optical intensity of the green component (ex. 495, em. 672 nm) of each image was calculated (B). The expression of α-SMA was determined in MS-1 cells treated for 6 d by WB (C). The data represent the ratios between individual values for the optical densities of bands determined for α-SMA and housekeeping protein (n=4). A *p value of less than 0.05 was considered significant when evaluating differences between the individual bars.
Fig. 6. OA-NO2 decreases the TGF-β-induced Smad2/3 activation in ECs.
MS-1 cells were treated with or without OA-NO2 (1.0 μM) and TGF-β (10 ng/ml). The expressions of p-Smad2/t-Smad2 (A) and p-Smad3/t-Smad3 (B) were detected after 30 min of treatment. The data displayed in graphs (n=3-5) represent the ratios between individual values for the optical densities of bands determined for the phosphorylated and total forms of each protein. A *p value of less than 0.05 was considered significant when evaluating differences between the individual bars.
As for MS-1 cells, TGF-β induced both α-SMA expression and Smad2/3 phosphorylation in human ECs. OA-NO2 significantly reduced α-SMA expression as well as activation of both Smad2 and Smad3 in HUVECs (Suppl. Fig. 4c-e).
4. Discussion
The present study defines the role of OA-NO2 in the treatment of pathological processes instigated by the production of pro-inflammatory mediators in ECs and macrophages. We demonstrate that OA-NO2 decreased macrophage-derived production of TNF-α, IL-1β, and TGF-β, as previously [2, 28]. On the basis of these results, TNF-α, IL-1β, and TGF-β were chosen for EC activation. Although, we did not prove the inhibitory effect of OA-NO2 on IFN-γ production in macrophages, this cytokine was also used as an exemplary mediator. Subsequently, we investigated the effect of stimuli and OA-NO2 on EC-mediated production of chemokines and pro-inflammatory cytokines, including RANTES, IL-6, MCP-5, and GM-CSF. Importantly, the levels of all these mediators were influenced by IFN-γ, IL-1β, and TNF-α in different ways. Our results confirm that RANTES and IL-6 are produced in murine ECs in response to IL-1β, TNF-α, and IFN-γ [4]. OA-NO2 was able to inhibit the production of RANTES and IL-6 in all active treatments, highlighting the broad impact of OA-NO2 in the modulation of chemokine production in vascular cells. Moreover, we detected MCP-5 production in ECs. We observed significant enhancement of its production after IFN-γ administration. This effect was inhibited by OA-NO2, affirming the role of this fatty acid derivative in regulating monocyte/macrophage attraction. This finding was also supported by functional assays demonstrating that OA-NO2 reduce IFN-γ-induced chemotaxis of macrophages in vitro. In agreement with the study by Maruyama (1993), IL-1β was the only pro-inflammatory mediator used which stimulated the production of GM-CSF [6]. Importantly, in our study, OA-NO2 significantly inhibited its production. GM-CSF is a crucial cytokine responsible for macrophage differentiation into the pro-inflammatory (M1) phenotype [28, 29]. We suggest that during vascular inflammation, OA-NO2 modulates macrophage-EC intercellular communication by inhibiting GM-CSF production in ECs and IL-1β production in macrophages, which contributes to reciprocal regulation of these two cell types. In contrast to other EC activators, TGF-β did not have a stimulatory effect on pro-inflammatory cytokine production in ECs.
Activated ECs enhance cell surface adhesion molecule expression, enabling the recruitment of immune cells to the site of inflammation. In agreement with literature describing changes in the expression and distribution of adhesive molecules [6, 30] we detected enhanced ICAM-1 expression in MS-1 cells and HUVECs after administration of IFN-γ or TNF-α, respectively. Similar to previous in vitro responses [21, 31], we showed here that OA-NO2 is able to reverse ICAM-1 over-expression. Generally, changes in adhesion molecule expression are accompanied by alterations in functional properties of endothelium [32]. We demonstrated herein that IFN-γ- and OA-NO2-induced changes in ICAM-1 expression corresponded with a decreased ability of macrophages to adhere to ECs. Moreover, even lower concentrations of OA-NO2 significantly downregulated ICAM-1 expression in murine ECs. While both upstream and downstream signaling pathways of ICAM-1 are linked with STATs, MAPKs, and NF-κB regulation (in combination with phospholipase-A2), these signaling molecules represent one of the possible targets and modulators of OA-NO2 action in inflammatory responses [2, 5]. Therefore, the effect of OA-NO2 on these signaling pathways was tested. STAT1 and STAT3, induced only in ECs exposed to IFN-γ [33, 34], were significantly downregulated by OA-NO2. OA-NO2 also decreased the TNF-α- and IL-1β-mediated phosphorylation of NF-κB as well as IL-1β-mediated phosphorylation of all MAPKs detected. Cytokine-derived activation of all these pathways was described previously [4, 33, 35, 36]. It was also confirmed that TNF-α and IFN-γ induced the phosphorylation of ERK [36, 37], which was sensitive to OA-NO2 inhibition. These results indicate that OA-NO2 decreases the phosphorylation of signaling molecules that are strongly induced by pro-inflammatory mediators and thus modifies EC function during immune responses. These results are in accordance with the effect of OA-NO2 on individual signaling pathway responses (e.g. STATs, MAPKs, and NF-κB) [2, 4, 33, 34, 36, 37].
The dysregulation of ·NO production is a feature of endothelial homeostasis disruption [9]. Herein, the only mediator which induced changes in ·NO production in MS-1 cells was IFN-γ, as a result of eNOS activation. iNOS expression was not elevated in cytokine-treated MS-1 cells. Moreover, OA-NO2 did not induce changes in IFN-γ-upregulated ·NO synthesis, suggesting that lower concentrations of OA-NO2 were not effective. We hypothesize that the IFN-γ-induced increase in ·NO production represents a compensatory effect associated with the activation of ECs; however, the exact mechanism of this action remains to be elucidated. At higher concentrations, NO2-FAs might be a direct source of ·NO, mediating endothelium-dependent vasorelaxation, but in vivo NO2-FAs do not acutely affect blood pressure or heart rate [20, 38, 39].
EndMT contributes significantly to the excessive deposition of the extracellular matrix resulting in fibrotic processes. We used phalloidin staining to identify changes in filamentous actin stress fibers arrangement in ECs that is characteristic for active fibroblasts [19]. Upon TGF-β treatment, MS-1 cells reorganized their actin cytoskeleton to the long stress fibers that cross the cell body, as previously described [40]. Importantly, OA-NO2 significantly inhibited this process. Subsequently, the small cytoplasmic protein FSP1 and the structural protein α-SMA were chosen as markers of fibrotic responses. The filament-associated mesenchymal marker FSP1 belongs to the superfamily of cytoplasmic calcium binding proteins and was recently identified in transdifferentiated ECs, leukocytes, and hematopoietic cells [11, 13, 14, 41-43]. It is not present in normal ECs. In our study, FSP1 expression was induced by TGF-β after 24 h; and after 48 h of treatment this effect was more pronounced. OA-NO2 significantly reduced FSP1 expression at both times. In general, the number of cells expressing α-SMA should increase during the time of incubation with TGF-β [44]. In agreement with this, we observed upregulation of α-SMA expression in TGF-β-treated MS-1 cells after 6 d, but not after 24 or 48 h. OA-NO2 significantly inhibited the effect of TGF-β after 6 d. These results affirmed that whilst FSP1 is the more consistent marker of early stage of fibrotic process, α-SMA is expressed rather in latter stages [11]. Subsequently, we observed significant induction of both Smad2 and Smad3 phosphorylation in MS-1 cells treated with TGF-β. This effect was unequivocally reduced by OA-NO2. Together with the observation that TGF-β did not activate STAT, MAPK, or NF-κB signaling, we postulate that Smad activation represents one of the key events where OA-NO2 modulates signaling events leading to EndMT. These results, confirmed also in HUVECs, are in agreement with previous observations of the involvement of Smad proteins in the regulation of fibrosis [2, 25]. Nevertheless, this does not exclude the involvement of other signaling molecules or pathways in the inception of fibrotic processes and the actions of OA-NO2.
In aggregate, we show that the pathological activation of ECs and macrophages can be modulated by NO2-FA-mediated signaling actions that impact macrophage-EC intercellular communication – specifically by decreasing the adhesive properties of activated endothelium and inhibiting pro-inflammatory cytokine production by ECs and macrophages. Importantly, OA-NO2 also prevents the transformation of ECs into the pro-fibrotic phenotype, by blocking EndMT triggered by cytokine activation of endothelium.
Supplementary Material
Highlights.
Nitro-oleic acid regulates cooperation of macrophages and endothelial cells in inflammation.
Nitro-oleic acid attenuates the inflammatory response of endothelial cells.
Nitro-oleic acid affects STAT, MAPK, and NF-κB signaling in activated endothelial cells.
Nitro-oleic acid downregulates TGF-β-induced EndMT in endothelial cells.
Smad2/3 activation is suppressed by nitro-oleic acid in TGF-β treated endothelial cells.
Acknowledgements
We thank Hana Kolarova and Jana Kudova for their expertise confocal microscopy. This work was supported by the Czech Science Foundation (no. 13-40824P). LK and MP were supported by the European Regional Development Fund, the projects NPS II no. LQ1605 and FNUSA-ICRC no. CZ.1.05/1.1.00/02.0123 from MEYS CR and BAF by NIH grants R01-HL058115, R01-HL64937, PO1-HL103455.
Abbreviations
- α-SMA
α-smooth muscle actin
- DMEM
Dulbecco's modified Eagle's medium
- EC
endothelial cell
- EndMT
endothelial-mesenchymal transition
- eNOS
endothelial nitric oxide synthase
- FBS
fetal bovine serum
- FSP1
fibroblast-specific protein 1
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- ICAM-1
intercellular adhesion molecule-1
- IFN-γ
interferon-γ
- IL-1β
interleukin-1β
- IL-6
interleukin-6
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MCP-5
murine monocyte chemoattractant protein
- NF-κB
nuclear factor-κ B
- NO2-FA
nitro-fatty acid
- OA-NO2
nitro-oleic acid
- PPARγ
peroxisome proliferator-activated receptor γ
- RANTES
regulated on activation normal T cell expressed and secreted
- STAT
signal transducer and activator of transcription
- TGF-β
transforming growth factor-β
- TNF-α
tumor necrosis factor-α
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Bruce A. Freeman and Steven Woodcock acknowledge interest in Complexa Inc. All other authors declare no conflicts of interest with respect to the contents of this manuscript.
References
- 1.Popa C, Netea MG, van Riel PL, van der Meer JW, Stalenhoef AF. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. Journal of lipid research. 2007;48:751–762. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Ambrozova G, Martiskova H, Koudelka A, Ravekes T, Rudolph TK, Klinke A, Rudolph V, Freeman BA, Woodcock SR, Kubala L, Pekarova M. Nitro-oleic acid modulates classical and regulatory activation of macrophages and their involvement in pro-fibrotic responses. Free radical biology & medicine. 2015 doi: 10.1016/j.freeradbiomed.2015.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoo NK, Rudolph V, Cole MP, Golin-Bisello F, Schopfer FJ, Woodcock SR, Batthyany C, Freeman BA. Activation of vascular endothelial nitric oxide synthase and heme oxygenase-1 expression by electrophilic nitro-fatty acids. Free radical biology & medicine. 2010;48:230–239. doi: 10.1016/j.freeradbiomed.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mai J, Virtue A, Shen J, Wang H, Yang XF. An evolving new paradigm: endothelial cells--conditional innate immune cells. Journal of hematology & oncology. 2013;6:61. doi: 10.1186/1756-8722-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawson C, Wolf S. ICAM-1 signaling in endothelial cells. Pharmacological reports : PR. 2009;61:22–32. doi: 10.1016/s1734-1140(09)70004-0. [DOI] [PubMed] [Google Scholar]
- 6.Maruyama H, Toda K, Uno K, Miyake K, Matsushima K, Yamamoto K, Mori JK, Masuda T. Murine endothelial cell line cells, F-2: interaction with leukocytes and cytokines production. Microbiology and immunology. 1993;37:895–903. doi: 10.1111/j.1348-0421.1993.tb01721.x. [DOI] [PubMed] [Google Scholar]
- 7.Sarafi MN, Garcia-Zepeda EA, MacLean JA, Charo IF, Luster AD. Murine monocyte chemoattractant protein (MCP)-5: a novel CC chemokine that is a structural and functional homologue of human MCP-1. The Journal of experimental medicine. 1997;185:99–109. doi: 10.1084/jem.185.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Experimental cell research. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- 9.Huang PL. Endothelial nitric oxide synthase and endothelial dysfunction. Current hypertension reports. 2003;5:473–480. doi: 10.1007/s11906-003-0055-4. [DOI] [PubMed] [Google Scholar]
- 10.Kolosova I, Nethery D, Kern JA. Role of Smad2/3 and p38 MAP kinase in TGF-beta1-induced epithelial-mesenchymal transition of pulmonary epithelial cells. Journal of cellular physiology. 2011;226:1248–1254. doi: 10.1002/jcp.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. The Journal of clinical investigation. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Meeteren LA, ten Dijke P. Regulation of endothelial cell plasticity by TGF-beta. Cell and tissue research. 2012;347:177–186. doi: 10.1007/s00441-011-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nature medicine. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 14.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer research. 2007;67:10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 15.Kryczka J, Boncela J. Leukocytes: The Double-Edged Sword in Fibrosis. Mediators of inflammation. 2015;2015:652035. doi: 10.1155/2015/652035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Laboratory investigation; a journal of technical methods and pathology. 2013;93:844–854. doi: 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- 17.Piera-Velazquez S, Li Z, Jimenez SA. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. The American journal of pathology. 2011;179:1074–1080. doi: 10.1016/j.ajpath.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Techasen A, Loilome W, Namwat N, Dokduang H, Jongthawin J, Yongvanit P. Cytokines released from activated human macrophages induce epithelial mesenchymal transition markers of cholangiocarcinoma cells. Asian Pacific journal of cancer prevention : APJCP. 2012;13(Suppl):115–118. [PubMed] [Google Scholar]
- 19.Stramer BM, Austin JS, Roberts AB, Fini ME. Selective reduction of fibrotic markers in repairing corneas of mice deficient in Smad3. Journal of cellular physiology. 2005;203:226–232. doi: 10.1002/jcp.20215. [DOI] [PubMed] [Google Scholar]
- 20.Freeman BA, Baker PR, Schopfer FJ, Woodcock SR, Napolitano A, d'Ischia M. Nitro-fatty acid formation and signaling. The Journal of biological chemistry. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang J, Lee KE, Lim JY, Park SI. Nitrated fatty acids prevent TNFalpha-stimulated inflammatory and atherogenic responses in endothelial cells. Biochemical and biophysical research communications. 2009;387:633–640. doi: 10.1016/j.bbrc.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Shin E, Yeo E, Lim J, Chang YH, Park H, Shim E, Chung H, Hwang HJ, Chun J, Hwang J. Nitrooleate mediates nitric oxide synthase activation in endothelial cells. Lipids. 2014;49:457–466. doi: 10.1007/s11745-014-3893-8. [DOI] [PubMed] [Google Scholar]
- 23.Klinke A, Moller A, Pekarova M, Ravekes T, Friedrichs K, Berlin M, Scheu KM, Kubala L, Kolarova H, Ambrozova G, Schermuly RT, Woodcock SR, Freeman BA, Rosenkranz S, Baldus S, Rudolph V, Rudolph TK. Protective effects of 10-nitro-oleic acid in a hypoxia-induced murine model of pulmonary hypertension. American journal of respiratory cell and molecular biology. 2014;51:155–162. doi: 10.1165/rcmb.2013-0063OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoo NK, Freeman BA. Electrophilic nitro-fatty acids: anti-inflammatory mediators in the vascular compartment. Current opinion in pharmacology. 2010;10:179–184. doi: 10.1016/j.coph.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rudolph TK, Ravekes T, Klinke A, Friedrichs K, Mollenhauer M, Pekarova M, Ambrozova G, Martiskova H, Kaur JJ, Matthes B, Schwoerer A, Woodcock SR, Kubala L, Freeman BA, Baldus S, Rudolph V. Nitrated fatty acids suppress angiotensin II-mediated fibrotic remodelling and atrial fibrillation. Cardiovascular research. 2016;109:174–184. doi: 10.1093/cvr/cvv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rostamzadeh A, Zumbrunn T, Jongen LM, Nederkoorn PJ, Macdonald S, Lyrer PA, Kappelle LJ, Mali WP, Brown MM, van der Worp HB, Engelter ST, Bonati LH, I.-M.S. Investigators, M.R.I.S. following centers enrolled patients in the International Carotid Stenting Study, U.T.N. University Medical Centre, S. University Hospital Basel, R.T.N. Erasmus Medical Centre, N.u.T.U.K. Newcastle Acute Hospitals Nhs Foundation Trust, A.T.N. Academic Medical Centre, L.U.K. University College London Hospitals Nhs Foundation Trust, S.U.K. Sheffield Teaching Hospitals Nhs Foundation Trust Predictors of acute and persisting ischemic brain lesions in patients randomized to carotid stenting or endarterectomy. Stroke; a journal of cerebral circulation. 2014;45:591–594. doi: 10.1161/STROKEAHA.113.003605. [DOI] [PubMed] [Google Scholar]
- 27.Ambrozova G, Pekarova M, Lojek A. Effect of polyunsaturated fatty acids on the reactive oxygen and nitrogen species production by raw 264.7 macrophages. European journal of nutrition. 2010;49:133–139. doi: 10.1007/s00394-009-0057-3. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nature reviews. Immunology. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 29.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1992;6:2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- 31.Cui T, Schopfer FJ, Zhang J, Chen K, Ichikawa T, Baker PR, Batthyany C, Chacko BK, Feng X, Patel RP, Agarwal A, Freeman BA, Chen YE. Nitrated fatty acids: Endogenous anti-inflammatory signaling mediators. The Journal of biological chemistry. 2006;281:35686–35698. doi: 10.1074/jbc.M603357200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aird WC. Endothelial cell heterogeneity. Cold Spring Harbor perspectives in medicine. 2012;2:a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Caterina R, Bourcier T, Laufs U, La Fata V, Lazzerini G, Neish AS, Libby P, Liao JK. Induction of endothelial-leukocyte interaction by interferon-gamma requires coactivation of nuclear factor-kappaB. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:227–232. doi: 10.1161/01.atv.21.2.227. [DOI] [PubMed] [Google Scholar]
- 34.Kano A, Wolfgang MJ, Gao Q, Jacoby J, Chai GX, Hansen W, Iwamoto Y, Pober JS, Flavell RA, Fu XY. Endothelial cells require STAT3 for protection against endotoxin-induced inflammation. The Journal of experimental medicine. 2003;198:1517–1525. doi: 10.1084/jem.20030077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadad N, Tuval L, Elgazar-Carmom V, Levy R. Endothelial ICAM-1 protein induction is regulated by cytosolic phospholipase A2alpha via both NF-kappaB and CREB transcription factors. J Immunol. 2011;186:1816–1827. doi: 10.4049/jimmunol.1000193. [DOI] [PubMed] [Google Scholar]
- 36.Lin FS, Lin CC, Chien CS, Luo SF, Yang CM. Involvement of p42/p44 MAPK, JNK, and NF-kappaB in IL-1beta-induced ICAM-1 expression in human pulmonary epithelial cells. Journal of cellular physiology. 2005;202:464–473. doi: 10.1002/jcp.20142. [DOI] [PubMed] [Google Scholar]
- 37.Sano H, Nakagawa N, Chiba R, Kurasawa K, Saito Y, Iwamoto I. Cross-linking of intercellular adhesion molecule-1 induces interleukin-8 and RANTES production through the activation of MAP kinases in human vascular endothelial cells. Biochemical and biophysical research communications. 1998;250:694–698. doi: 10.1006/bbrc.1998.9385. [DOI] [PubMed] [Google Scholar]
- 38.Schopfer FJ, Baker PR, Giles G, Chumley P, Batthyany C, Crawford J, Patel RP, Hogg N, Branchaud BP, Lancaster JR, Jr., Freeman BA. Fatty acid transduction of nitric oxide signaling. Nitrolinoleic acid is a hydrophobically stabilized nitric oxide donor. The Journal of biological chemistry. 2005;280:19289–19297. doi: 10.1074/jbc.M414689200. [DOI] [PubMed] [Google Scholar]
- 39.Trostchansky A, Rubbo H. Nitrated fatty acids: mechanisms of formation, chemical characterization, and biological properties. Free radical biology & medicine. 2008;44:1887–1896. doi: 10.1016/j.freeradbiomed.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 40.Moustakas A, Stournaras C. Regulation of actin organisation by TGF-beta in H-ras-transformed fibroblasts. Journal of cell science. 1999;112(Pt 8):1169–1179. doi: 10.1242/jcs.112.8.1169. [DOI] [PubMed] [Google Scholar]
- 41.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. American journal of physiology. Heart and circulatory physiology. 2013;305:H1363–1372. doi: 10.1152/ajpheart.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, Sasik R, Hardiman G, Karin M, Brenner DA. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:308–313. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: FSP1. The Journal of cell biology. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arciniegas E, Sutton AB, Allen TD, Schor AM. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. Journal of cell science. 1992;103(Pt 2):521–529. doi: 10.1242/jcs.103.2.521. [DOI] [PubMed] [Google Scholar]
- 45.Silber HA, Bluemke DA, Ouyang P, Du YP, Post WS, Lima JA. The relationship between vascular wall shear stress and flow-mediated dilation: endothelial function assessed by phase-contrast magnetic resonance angiography. J.Am.Coll.Cardiol. 2001;38:1859–1865. doi: 10.1016/s0735-1097(01)01649-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.