Abstract
Therapeutic targeting of late-stage breast cancer is limited by an inadequate understanding of how tumor cell signaling evolves during metastatic progression and by the currently available small molecule inhibitors capable of targeting these processes. Herein, we demonstrate that both β3 integrin and fibroblast growth factor receptor-1 (FGFR1) are part of an epithelial-mesenchymal transition (EMT) program that is required to facilitate metastatic outgrowth in response to fibroblast growth factor-2 (FGF2). Mechanistically, β3 integrin physically disrupts an interaction between FGFR1 and E-cadherin, leading to a dramatic redistribution of FGFR1 subcellular localization, enhanced FGF2 signaling and increased three-dimensional (3D) outgrowth of metastatic breast cancer cells. This ability of β3 integrin to drive FGFR signaling requires the enzymatic activity of focal adhesion kinase (FAK). Consistent with these mechanistic data, we demonstrate that FGFR, β3 integrin and FAK constitute a molecular signature capable of predicting decreased survival of patients with the basal-like subtype of breast cancer. Importantly, covalent targeting of a conserved cysteine in the P-loop of FGFR1-4 with our newly developed small molecule, FIIN-4, more effectively blocks 3D metastatic outgrowth as compared to currently available FGFR inhibitors. In vivo application of FIIN-4 potently inhibited the growth of metastatic, patient-derived breast cancer xenografts and murine-derived metastases growing within the pulmonary microenvironment. Overall, the current studies demonstrate that FGFR1 works in concert with other EMT effector molecules to drive aberrant downstream signaling, and that these events can be effectively targeted using our novel therapeutics for the treatment of the most aggressive forms of breast cancer.
Keywords: breast cancer, metastasis, FGFR, integrin, EMT, covalent kinase inhibitor
Introduction
Fibroblast growth factor receptor (FGFR) is an emerging target for the treatment of triple negative breast cancer (TNBC), Herceptin-resistant Her2+ BC, and tamoxifen-resistant ER+ BCs (1–4). The FGFR family is composed of 4 tyrosine kinase receptors, FGFR1-4. FGFR1-3 undergo significant alternative splicing resulting in two primary isoforms with different ligand binding sites (-iiib or -iiic) and either full-length (α) or truncated (β) forms of the receptor (5). FGFR1 also undergoes gene amplification and translocation, particularly in the luminal B subtype of BC (6). In addition to gene amplification, transcriptional expression of FGFR1-iiic is dramatically increased by epithelial-mesenchymal transition (EMT) (1,7,8). We recently demonstrated that FGFR1 is a unique marker of EMT as its expression remains elevated through the oncogenic reversion of this process, or mesenchymal-epithelial transition (MET) (1). This is a key finding as highly aggressive BC cells can readily transition between epithelial and mesenchymal states (9,10). Therefore, the maintained upregulation of FGFR1 during epithelial-mesenchymal plasticity contributes to the potential of this growth factor signaling pathway as a therapeutic target for the treatment of metastatic BC. However, it remained to be determined whether enhanced FGFR expression alone is sufficient to drive FGF-mediated metastatic tumor growth or if other aspects of EMT are also required for this process.
In addition to modulated expression of growth factor receptors, another critical aspect of EMT is the production of extracellular matrix proteins and their cognate sensing molecules (11). Along these lines, EMT-mediated upregulation of αvβ3 integrin is well established, as is its ability to interact with TGF-β receptors and epidermal growth factor receptor (EGFR) (12,13). Furthermore, biochemical studies and results from endothelial cells suggest that β3 integrin also can bind to FGFs and may form a ternary complex with FGF and FGFR1 (14,15,16). Given these previous findings, we hypothesized that β3 integrin and FGFR1 work in concert during EMT to promote the metastatic progression of BC.
To date, nearly all clinically used kinase inhibitors are ATP-competitive, non-covalent compounds whose pharmacodynamics are limited by competition with the large intracellular pool of ATP (17). The recent FDA approval and clinical success of the covalent EGFR inhibitor Osimertinib for the treatment of lung cancer and the BTK inhibitor Ibrutinib for the treatment of B-cell-derived tumors serves as a proof-of-principle for the continued development of covalent kinase inhibitors as anti-cancer therapeutics (18). We recently developed FIIN-2, a potent and selective covalent pan-FGFR targeting agent that inhibits FGFR1 in biochemical and cellular assays at nanomolar concentrations (19). Despite being very potent in cellular assays, FIIN-2 proved not to be a suitable in vivo drug candidate. Therefore, a major objective of the current study was to develop and conduct preclinical, in vivo validation of FINN-4, our newly formulated covalent inhibitor of FGFR. Taken together, our studies present mechanistic insight into how EMT as a process contributes to FGFR signaling in metastatic BC. Moreover, our findings suggest that β3 integrin, FAK and FGFR can serve as useful biomarkers for the application of our next-generation, covalent inhibitor of FGFR for the treatment of metastatic BC.
Materials and Methods
Cell lines and Reagents
Murine D2.A1 were obtained from Dr. Fred Miller in 2010 (Wayne State University, Detroit, MI), while murine 4T1 and NMuMG were purchased from the ATCC in 2008. Bioluminescent 4T1 and D2.A1, cells were engineered to stably express firefly luciferase under the selection of Zeocin as previously described (20). All cell lines were tested and verified via the IMPACT III analysis at IDEXX Bioresearch on April 2, 2014. NMuMG cells expressing Twist and β3 integrin were constructed via stable transduction using pBabe and pMSCV viral particles, respectively. Plasmids encoding the FGFR1-eGFP fusion protein were a kind gift from Michael Stachowiak (Department of Chemistry, State University, Buffalo, NY). Cellular depletion of β1 and β3 integrin expression was achieved by lentiviral transduction of previously verified pLKO.1 shRNA vectors (Thermo Scientific, Table S1) as described (21). Ectopic expression of FGFR1-α-IIIc was accomplished as previously described and selected for using Neomycin (1). The covalent FGFR inhibitor FIIN-2 was synthesized as described previously (19). FIIN4 was synthesized with the same synthetic route as FIIN-2, with 3-nitrobenzylamine instead of 4-nitrobenzylamine as the starting material. PF-562,271 was provided by Pfizer and Defactinib was purchased from Selleckchem.
Patient derived xenografts and in vivo drug treatments
Patient tumor tissues (HCI-015) were obtained from the Hunstman Cancer Institute (HCI) Preclinical Research Resource (PRR). Transfer of tumor tissues was conducted under IRB approval from both HCI and Purdue University. Approximately 1mm3 pieces of tumor tissue were engrafted onto the mammary fat pads of NSG mice according to published procedures (22). Cohorts of these tumor-bearing mice were treated with FIIN-4 (25 mg/kg) every 48 hours. Mammary tumor sizes were measured using digital calipers and the following equation was used to approximate tumor volume (V=(length^2)*(width)*(0.5)). In separate experiments mice were inoculated via the tail vein with ex-vivo 4T1-L4 pulmonary metastases. These pulmonary tumor-bearing mice were similarly treated with FIIN-4. In both cases FIIN-4 was originally suspended in DMSO and then further diluted in a solution 0.5% carboxymethyl cellulose and 0.25% Tween-80 to a final concentration of 10% DMSO for administration to animals via oral gavage. All animal experiments were conducted under IACUC approval from Purdue University.
Immuno assays
For signaling assays cells were serum deprived for 6 hours (NMuMG) or overnight (D2.A1) in the presence or absence of the indicated inhibitors and cells were subsequently stimulated with the indicated growth factors. For immunoblot assays, lysates generated from 2D- and 3D-cultures were prepared as described previously (23). Antibodies used herein are described in the Supplementary Table S2. For immunocytochemistry, cells were fixed in 4% formalin, blocked and stained with the indicated antibodies (Table S2). For GFP-Trap experiments (GFP-Trap_A, ChromoTek), cells were rinsed in 1% formaldehyde, lysed in 1 mL membrane co-IP buffer (50mM TRIS, 15mM EGTA, 100mM NaCl, 0.1% Triton X-100, pH 7.5, and protease inhibitor cocktail (Sigma) added just before use), and 20 μL of supernatant was saved for input control. IHC images of FGFR1 in patient tumors were gathered from the human protein atlas (24,25).
mRNA transcript analyses
For real-time PCR analysis, NMuMG cells were stimulated with TGF-β1 (5 ng/ml) for 48 hours, at which point total RNA was isolated using RNeasy Plus Kit (Qiagen). Alternatively, RNA was similarly isolated from 4T1 in vitro cultured cells or ex vivo lung metastases. Afterward, total RNA was reverse transcribed using the iScript cDNA Synthesis System (BioRad), and semi-quantitative real-time PCR was conducted using iQ SYBR Green (BioRad) as described previously (23). For identification of the FGFR splice variants PCR products were visualized by gel electrophoresis. The oligonucleotide primer pairs used have been previously described (1).
Enzymatic assays
The enzymatic activities against FGFR1-4 were tested in Z′-Lyte assays with ATP concentrations of Km for each kinases; all the protocols are available from Life Technologies (www.invitrogen.com/drugdiscovery/). The MLM stability assays were previously reported and are commercially available from Scripps Florida (26).
In Vivo Pharmacokinetic Studies
Male Swiss albino mice were dosed via oral gavage (intravenous, 0.1% v/v Tween 80, 0.5% w/v NaCMC in water at a dose of 10 mg/kg) or via intravenous tail vein injection (suspensions in 5% NMP, 5% solutol HS in normal saline at a dose of 2 mg/kg). Blood samples were collected at 0.08, 0.25, 0.5, 1, 2, 4, 8 and 24 h (i.v.) and at Predose, 0.25, 0.5, 1, 2, 4, 6, 8 and 24 h (p.o.). Plasma samples were separated by centrifugation of whole blood and stored below −70°C until bioanalysis. All samples were processed for analysis by protein precipitation using acetonitrile and analyzed with fit-for-purpose LC/MS/MS method (LLOQ, 1.06 ng/mL). Pharmacokinetic parameters were calculated using the non-compartmental analysis tool of WinNonlin Enterprise software (version 6.3).
Cell biological assays
Cell viability assays were performed using the Cell Titer Glo assay according to the manufacture’s instructions (Promega). Visualization of the actin cytoskeleton was performed by staining fixed cells with FITC-conjugated Phalloidin according to the manufacture’s instructions (Thermo Scientific). FGFR1-4 transformed Ba/F3 cell viability assays were conducted via services from Carna Biosciences (http://www.carnabio.com).
3D-organotypic growth assays
Cells were diluted in complete media supplemented with 5% Cultrex (Trevigen, Gaithersburg, MD, USA) and seeded onto solidified Cultrex cushions (50 μl/well) contained in 96-well plates (1×104 cells/cm2). Cultrex cushions were supplemented with fibronectin or Collagen I where indicated. Longitudinal bioluminescent growth assays were performed as described (1,23,27).
Kaplan-Meier plots
The MTCI BreastMark, an on-line biomarker validation tool (http://glados.ucd.ie/BreastMark/) was employed to estimate survival probabilities for BC patients split into two groups based on ITGB3, PTK2B, and FGFR1 gene expression. This analysis performed by extracting gene expression values and overall patient survival data from 714 basal-like BCs.
Statistical analyses
Statistical values were defined using an unpaired Student’s T-test, where a P value < 0.05 was considered significant. Statistically significant differences in the overall survival of 4T1-pulmonary tumor bearing mice were analyzed using a log-rank test and a two-way ANOVA test. P values for all experiments are indicated.
Results
Twist-mediated induction of EMT enhances FGFR signaling in mammary epithelial cells
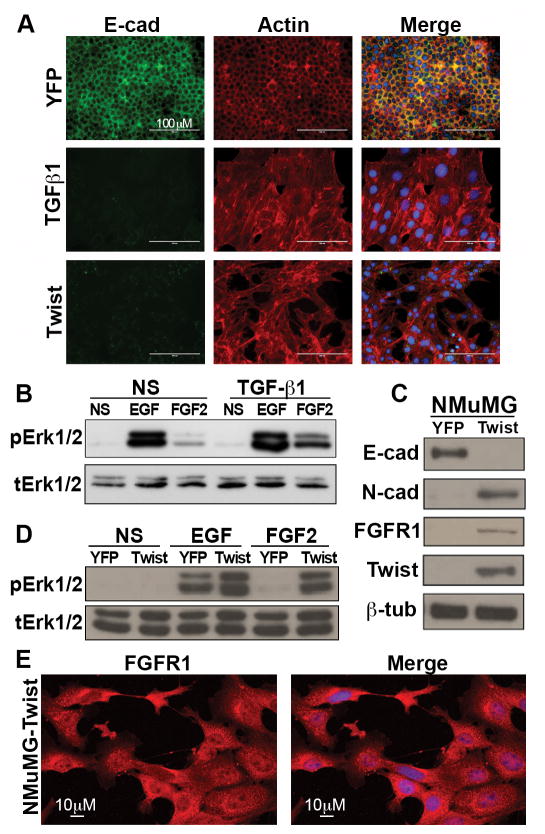
We and others have previously demonstrated that FGFR1 is upregulated during the process of TGF-β1-induced EMT (Fig 1A; 1,8). Consistent with these findings pretreatment with TGF-β1 facilitates FGF2-mediated phosphorylation of Erk1/2 (Fig. 1B). We next sought to define the downstream mechanisms responsible for increased expression of FGFR1 during induction of EMT. Similar to TGF-β1 treatment, expression of the basic helix-loop-helix transcription factor Twist also led to a robust induction of EMT in the NMuMG cell model, which included the upregulation of the –iiic splice variant of FGFR1 (Fig. 1A and 1C; Supplementary Fig. S1). Importantly, expression of Twist did not cause a corresponding decrease in FGFR2, an event that is characteristic of TGF-β-induced EMT, and expression of FGFR3 and 4 were not affected by Twist or TGF-β (Supplementary Fig. S1; (28)). However, similar to TGF-β1-induced EMT, a Twist-mediated EMT event was also sufficient to enhance FGF2-induced phosphorylation of Erk1/2 (Fig. 1D). These findings demonstrate that EMT events that include enhanced FGFR1 expression can facilitate a differential response to subsequent FGF2 ligand stimulation.
Figure 1. Twist-mediated induction of EMT enhances FGF2 signaling in mammary epithelial cells.
(A) NMuMG cells expressing YFP were either not stimulated or stimulated for 48 hours with TGF-β1 (5ng/ml). These cells were stained for expression and localization of E-cadherin (E-cad) or the actin cytoskeleton. Similarly, NMuMG cells were stably transduced with Twist to drive a morphologically-similar EMT event. (B) NMuMG cells were pretreated with TGF-β1 as in panel A and subsequently stimulated with either EGF (50 ng/ml) or FGF2 (20 ng/ml) for 30 minutes. These cells were then analyzed by immunoblot for downstream phosphorylation of Erk1/2. Expression of total Erk1/2 was analyzed as a loading control. (C) Expression of the EMT markers N-cadherin (N-cad) and E-cadherin (E-cad) as well as FGFR1 were analyzed by immunoblot in control (YFP) and Twist expressing NMuMG cells. Twist and β-tubulin (β-tub) served as loading controls. (D) NMuMG cells expressing YFP or Twist as shown in panel C were stimulated with either EGF or FGF2 and downstream phosphorylation of Erk1/2 was assessed by immunoblot. Expression of total Erk1/2 was analyzed as a loading control. (E) Twist expressing NMuMG cells were stained for expression and localization of FGFR1 and DAPI to visualize the nuclei. All data in panels A–E are representative of at least three independent experiments yielding similar results. Images in panel A were collected on an EVOS FL fluorescence microscope, while images in panel E were collected on a Nikon A1R confocal microscope.
β3 integrin redistributes FGFR1 and promotes FGF2 signaling
We next sought to elucidate the impact of EMT on FGFR1 subcellular localization and signaling capacity. Immunofluorescent staining in combination with confocal microscopy revealed that FGFR1 was localized throughout Twist-expressing NMuMG cells (Fig. 1E). To delineate the influence of the overall EMT event on this localization profile of FGFR1, NMuMG cells were stably transfected with an FGFR1-eGFP fusion construct and appropriate FGFR1 expression levels were isolated by FACS (Fig. 2A and Supplementary Fig. S2). In contrast to the localization of endogenous FGFR1 that is induced upon Twist-mediated EMT, ectopic expression of FGFR1 predominantly localized at the cell-cell junctions in NMuMG cells (Fig. 1E and 2A). However, upon recombinant expression of β3 integrin FGFR1-eGFP displayed more diffuse localization throughout the cell that was very similar to what was observed for endogenous FGFR1 upon expression of Twist (Fig. 1E and 2A). To further characterize this pronounced change in subcellular localization, we conducted GFP-precipitation experiments using cells expressing the FGFR-eGFP fusion construct (Fig. 2B). Consistent with our fluorescence images, we observed FGFR1 to be in complex with E-cadherin (E-cad) under control conditions, but upon coexpression with β3 integrin, FGFR moved out of this complex and was observed to interact with β3 integrin itself (Fig. 2B). These cell culture data are supported by our analysis of the Human Protein Atlas database, which revealed similarly distinct subcellular localizations of endogenous FGFR1 in a primary breast tumor (Patient 4229) as compared to metastatic patient tumor (Patient 1874) isolated from the lymph node (Fig. 2C). The functional consequence of this change in complex localization is evidenced by the requirement of both β3 integrin and FGFR1 to facilitate enhanced pErk1/2 phosphorylation in response to FGF2 (Fig. 2D and Supplementary Fig S3A). These data suggest that through the process of EMT, upregulation of both FGFR1 and β3 integrin are required for BC cells to aberrantly sense FGF2 as a proliferative factor.
Figure 2. β3 integrin redistributes FGFR1 and promotes FGF2 signaling.
(A) NMuMG cells were stably transfected with an FGFR1-eGFP fusion protein. These cells were subsequently transduced with control or β3 integrin (β3-Int) encoding viral particles. Differential localization of FGFR1-eGFP was observed upon recombinant expression of β3 integrin. (B) NMuMG cells constructed to express FGFR1-eGFP and/or β3 Integrin were lysed and FGFR1-eGFP was precipitated using GFP-TRAP as detailed in the methods. FGFR1-eGFP protein complexes were analyzed by immunoblot for the presence of FGFR1-eGFP(GFP), E-cadherin (E-cad), and β3 integrin (β3-Int). Presence of these proteins and β-tubulin (β-tub) in the input lysate served as loading controls. (C) Patient tumor samples isolated from a primary tumor (P4229) and a lymph node metastasis (P1874) demonstrate plasma membrane versus diffuse cellular localization of FGFR1, respectively. (D) NMuMG cells were constructed to express FGFR1 and/or β3 Integrin (β3-Int) and these cells were stimulated with FGF2 (2ng/ml) for 5 minutes. Subsequent to stimulation cell lysates were analyzed by immunoblot for the phosphorylation of Erk1/2. Presence of β3 integrin, total Erk1/2 and β-tubulin (β-tub) served as loading controls. Data in panels A, B and D are representative of at least three independent experiments yielding similar results. Images in panels A were collected on an EVOS FL fluorescence microscope.
Three dimensional cell culture increases expression of β3 integrin and enhances FGF2 signaling
To better recapitulate the in vivo condition we next sought to examine FGF2 signaling under 3D culture conditions. When the NMuMG cells were cultured under 3D growth conditions containing basement membrane extract (BME) FGFR1 overexpression alone, irrespective of β3 integrin expression, was sufficient to facilitate acinar filling in response to exogenous FGF2 (Fig. 3A). We were able to quantify this FGF2-mediated growth response using bioluminescence (Fig. 3B). To extend these studies beyond our overexpression systems we utilized the metastatic D2.A1 cells, a system we have recently shown responds poorly to FGF2 when cultured on 2D plastic, but is sensitive to FGFR inhibition in 3D culture and in vivo (23). Growth of these cells on 2D plastic yields drastically different growth morphologies as compared to 3D culture of the same cells on BME, Fibronectin, or Collagen I (Fig. 3C). Immunoblot analysis of these differential cultures demonstrated a robust upregulation of β3 integrin in 3D culture conditions as compared to 2D (Fig. 3D). Consistent with the notion that FGFR1 and β3 integrin are required for aberrant FGF2 signaling, we observed a dramatic increase in FGF2-induced phosphorylation of Erk1/2 when these cells were cultured under 3D conditions as compared to traditional 2D culture conditions (Fig. 3E). Together with our data from the previous figures these findings suggest that EMT in combination with structural changes in the in vivo growth environment contribute to upregulation of β3 integrin and the creation of aberrant FGF2 signaling complexes in metastatic BC cells.
Figure 3. Three dimensional cell culture increases expression of β3 integrin and enhances FGF2 signaling.
(A) Control (CNTRL) and FGFR1 expressing NMuMG cells were grown under 3D culture conditions in the presence of exogenous FGF2 (20 ng/ml) for 10 days. Brightfield images showing the resulting hollow acinar structures (top) and filled structures (bottom) were collected using an EVOS FL microscope. (B) Control NMuMG cells or cells expressing FGFR1 and/or β3 integrin (β3-Int) were cultured in the presence or absence of exogenous FGF2 as in panel A and overall cell growth was quantified by bioluminescence. Data in panel B are the mean ± SD of triplicate wells. Data in panels A and B are representative of at least three independent experiments yielding similar results. (C) Metastatic D2.A1 cells were grown on tissue culture plastic or in 3D culture conditions containing basement membrane extract (BME) alone or supplemented with additional Fibronectin or Collagen I as described in the materials and methods. Photomicrographs represent typical growth morphologies of these cells when grown in these various conditions. These brightfield images were collected using a TS100 Nikon microscope at 100x magnification. (D) Following 10 days of culture in the conditions shown in panel C (Fibronectin = FN; Col I = Collagen I) cells were lysed and analyzed by immunoblot for expression of β3 integrin (β3-Int). Actin served as a loading control. (E) D2.A1 cells were cultured on tissue culture plastic or in 3D culture conditions as shown in panel C. These cells were stimulated with FGF2 (20 ng/ml) for 30 minutes and analyzed for downstream phosphorylation of Erk1/2. All data in panels C–E are representative of at least three independent experiments yielding similar results.
β3 integrin is required for FGF2-mediated signaling and 3D cellular outgrowth
Our findings in the NMuMG cell model demonstrated that FGFR1 and β3 integrin were both required for robust FGF2-mediated activation of Erk1/2. To further explore the role of integrins in facilitating FGF2 signaling in metastatic cells we depleted the expression of either β1 or β3 integrin in the D2.A1 cells using previously established and verified shRNAs (Fig. 4A; (21)). As previously observed in other models, depletion of β1 integrin led to a robust compensatory increase in the expression of β3 integrin in D2.A1 cells, but this event did not affect the ability of FGF2 to activate Erk1/2 phosphorylation (Fig. 4A; (21)). More importantly, directed depletion of β3 integrin in the D2.A1 cells inhibited the ability of FGF2 to stimulate phosphorylation of Erk1/2 (Fig. 4A; Supplementary Fig. S3B). To better establish the biological significance of these events, we cultured these cells under 3D growth conditions and quantified cellular outgrowth using bioluminescence (Fig. 4B). Addition of exogenous FGF2 enhanced the outgrowth of control D2.A1 cells (Fig. 4B and 4C). As reported previously, depletion of β1 integrin did lead to a robust inhibition of D2.A1 outgrowth (29). However, this inhibition of basal outgrowth could be completely rescued by the addition of exogenous FGF2, findings that are consistent with the enhanced levels of β3 integrin in these cells (Fig. 4B and 4C). Finally, directed depletion of β3 integrin similarly lead to a significant inhibition of basal 3D outgrowth by the D2.A1 cells, but unlike depletion of β1 integrin this event completely prevented the ability of exogenous FGF2 to stimulate the outgrowth of the D2.A1 cells (Fig. 4B and 4C). These data clearly indicate that β3 integrin is necessary for FGF2-mediated 3D outgrowth of metastatic BC cells.
Figure 4. β3 integrin is required for FGF2-mediated signaling and 3D cellular outgrowth.
(A) D2.A1 cells were depleted for expression of either β1 integrin or β3 integrin using gene-specific shRNAs and these cells were not stimulated (NS) or were stimulated with either EGF (50ng/ml) or FGF2 (20ng/ml) for 30 minutes and subsequently analyzed by immunoblot for phosphorylation of Erk1/2. Expression of total Erk1/2, β3 and β1 integrin served as a loading control. Data are representative of at least three independent experiments. (B) D2.A1 cells depleted for β1 or β3 integrin as shown in panel A were cultured under 3D conditions in the presence or absence of exogenous FGF2 (20ng/ml) for 8 days. Cellular outgrowth under these conditions was quantified by bioluminescence. Changes in cellular outgrowth are shown as the percentage increase relative to the plated bioluminescent value (T0). Data are the mean values of three independent experiments completed in triplicate resulting in the indicated P values. (C) Representative brightfield photomicrographs of the 3D culture conditions described and quantified in panel B. These images were collected using a TS100 Nikon microscope at 100x magnification.
Pharmacological inhibition of integrin:FGFR signaling blocks three dimensional metastatic cell growth
Given the critical nature of EMT in facilitating aberrant FGF2-mediated signaling and cellular outgrowth we next sought to establish a pharmacological means to inhibit this process. Addition of three different FGFR inhibitors for 18 hours prior to stimulation with FGF2 demonstrated the enhanced durability of our recently described covalent inhibitor of FGFR (FIIN-2) to block the function of this receptor as compared to a reversible FGFR inhibitor (BGJ-398) in metastatic D2.A1 cells (Fig. 5A; Supplementary Fig. S3C). However, FIIN-2 only showed moderate mouse liver microsomal (MLM) stability (T1/2 = 4.0 min), precluding its further development as a drug candidate. Further optimization of FIIN-2 lead to formulation of FIIN-4, which showed rather improved MLM stability (T1/2 = 16.6 min), and maintained good enzymatic and cellular IC50s against FGFR1-4 as well as a similar kinase selectivity profile as FIIN-2 (Supplementary Fig. S4A and S4B, Table S3). FIIN-4 also demonstrated good overall pharmacokinetic properties, with T1/2 of 2.4 h, an area under the curve (AUC) value of 935 ng*hr/mL following a 10 mg/Kg oral dose and moderate oral bioavailability of 31% (Supplementary Fig. S4C). Similar to FIIN-2, FIIN-4 also demonstrated robust long-term inhibition of FGFR signaling in the D2.A1 cells (Fig. 5A). Treatment with FIIN-4 did not affect the ability of EGF to induce Erk1/2 phosphorylation or increase the 3D growth of NMuMG cells, demonstrating the specificity of this compound for FGFR (Supplementary Fig. 5). In addition to directly targeting FGFR we also investigated the potential downstream mechanisms of FGFR:β3 integrin signaling by utilizing two different inhibitors of FAK, PF-562,271/VS-6062 (PF-271) and VS-6063 (Defactinib). Long-term inhibition of FAK using both compounds inhibited FGF2-mediated phosphorylation of Erk1/2 in a dose dependent fashion (Fig. 5B and 5C). Moreover, a short-term pretreatment (10 minutes) with Defactinib was also highly effective at blocking FGF2-mediated phosphorylation of Erk1/2 (Fig. 5D). The importance of robust long-term inhibition of FGFR signaling is emphasized by the fact that covalent inhibition of FGFR led to decreased 3D outgrowth following a single dose of inhibitor, results that were not observed with the competitive inhibitor, BGJ-398 (Fig. 5E). Defactinib was similarly capable of blocking overall 3D outgrowth after only a single dose of this compound (Fig. 5E and 5F). Finally, treatment with Trametinib, an FDA approved allosteric inhibitor of MEK1/2, was similarly capable of inhibiting the 3D outgrowth of the D2.A1 cells and completely blocked the ability of FGF2 to induce outgrowth of our FGFR1 overexpressing NMuMG cells (Fig. 5E; Supplementary Fig. 5). Taken together with our previous findings these data strongly suggest that FAK is functioning downstream of β3 integrin and FGFR1 to facilitate an FGF2:Erk1/2 signaling axis and outgrowth of metastatic BC cells.
Figure 5. Pharmacological inhibition of FAK blocks FGF2:Erk1/2 signaling.
(A) D2.A1 cells were pretreated for 18 hours with the covalent FGFR inhibitors FIIN-4 or FIIN-2, or the Type I FGFR inhibitor BGJ-398 at the indicated concentrations. (B and C) D2.A1 cells were pretreated for 18 hours with the indicated concentration of the FAK inhibitors PF-271 or Defactinib. (D) D2.A1 cells were pretreated for 10 minutes with indicated concentrations of Defactinib. In panels A–D cells were serum starved for 18 hours with or without inhibitor pretreatment and cells were subsequently stimulated with FGF2 (20ng/ml) for 30 minutes and analyzed by immunoblot for downstream phosphorylation of Erk1/2. Expression of total Erk1/2 was analyzed as a loading control. (E) D2.A1 cells were grown in 3D culture for 4 days at which point a single treatment with the indicated inhibitors of FGFR (BGJ, FIIN-2, FIIN-4), FAK (Defact) or MEK1/2 (Tram) were added for 4 days. Following this treatment period cells were allowed to grow for an additional 4 days and luminescence was quantified. Changes in cellular outgrowth are shown as a percentage relative to the untreated control (CNTRL) luminescence value. Data are the mean values ±SE of at least six replicates completed over at least 2 independent experiments resulting in the indicated P values. (F) Brightfield photomicrographs of final 3D cell clusters formed after the total 12 days of growth. These images were collected using a TS100 Nikon microscope at 100x magnification.
Covalent targeting of FGFR blocks metastatic tumor growth
We next sought to specifically evaluate the role of FGFR:β3 signaling in the metastatic tumor microenvironment. We have recently demonstrated that the 4T1 model of advanced stage BC undergoes a spontaneous EMT during in vivo metastasis as compared to the epithelial phenotype of these cells when cultured in vitro (23,12). Consistent with these findings, ex vivo RT-PCR analyzes clearly demonstrate that a clonal, luciferase-expressing 4T1 cell line (4T1-L4) displayed robust upregulation of FGFR1 following pulmonary metastasis from the mammary fat pad (Fig. 6A and Supplementary Fig. S6). Further expression analyses between in vitro and ex-vivo 4T1-L4 lung metastases demonstrated enhanced expression of β3 integrin, and indicated the upregulation of all the major isoforms of FGFR1 (Fig. 6A). Along these lines, FIIN-2 and FIIN-4 potently inhibited cellular viability of ex vivo 4T1-L4 pulmonary metastases (Fig. 6B). In contrast, similar experiments showed no affect of FIIN molecules on cell viability of normal murine mammary gland cells (data not shown). Consistent with these viability data, FIIN-2 and FIIN-4 abrogated the constitutive phosphorylation of Erk1/2 in ex vivo 4T1-L4 pulmonary metastases, even at low nanomolar concentrations (Fig. 6C). We also specifically monitored organotypic, 3D tumor cell growth within these heterogeneous ex vivo cultures via their stable expression of firefly luciferase, in which treatment with FIIN-2 or FIIN-4 led to a dramatic eradication of tumor cells (Fig. 6D–6E). To evaluate the in vivo efficacy of FIIN-4, ex-vivo 4T1-L4 pulmonary metastases were re-inoculated into the pulmonary microenvironment of fresh cohorts of mice via a tail vein injection, and pulmonary tumor growth was quantified by bioluminesence (Fig. 6F). Forty-eight hours after metastatic inoculation, we initiated FIIN-4 therapy via oral gavage. Consistent with our 3D culture data, in vivo FIIN-4 therapy led to a potent inhibition of pulmonary tumor growth (Fig. 6, F and G). Fifteen days after tail vein inoculation the first control mouse was sacrificed due to pulmonary tumor burden, at which point FIIN-4 treatments were stopped. Even with this discontinuation of therapy, FIIN-4 still resulted in a highly significant prolongation in survival (Supplementary Fig. S7). Taken together, these findings clearly demonstrate that FGFR is a major driver of EMT-associated metastatic outgrowth and its function can be successfully antagonized within the metastatic microenvironment through the systemic administration of FIIN-4.
Figure 6. FIIN-4 potently inhibits pulmonary metastases.
(A) Clonal 4T1-L4 cells were used to form orthotopic mammary tumors and the resultant lung metastases (LM) were analyzed ex-vivo by RT-PCR for expression of β3 integrin (ITGB3) and various isoforms of FGFR1 and FGFR2 as compared to their in vitro parental counterparts. Analysis of GAPDH served as a loading control. (B) Ex-vivo 4T1-L4 lung metastases were cultured on plastic in the presence of the indicated concentrations for the covalent FGFR inhibitors FIIN-2 or FIIN-4 for 48 hours. Data are the mean ± SE of three independent experiments normalized to no inhibitor, control values. (C) Ex-vivo 4T1-L4 lung metastases were treated with the indicated concentrations of FGFR inhibitors for 6 hours. These cultures were subsequently analyzed by immunoblot for downstream phosphorylation of Erk1/2. Expression of total Erk1/2 served as a loading control. (D) Ex-vivo 4T1-L4 lung metastases were grown under 3D culture conditions for 3 days at which point FIIN-2 or FIIN-4 were added to the cultures (arrow). Tumor cell specific 3D outgrowth was quantified by firefly luminescence. Data are normalized to the plated values and are the mean of three independent experiments completed in triplicate. (E) Photomicrographs showing inhibition of 3D growth of the ex-vivo 4T1-L4 metastases under control conditions (DMSO) and in the presence of the indicated concentrations of FGFR inhibitors. These brightfield images were collected using a TS100 Nikon microscope at 40x magnification. (F) Ex-vivo 4T1-L4 lung metastases were re-inoculated into the tail vein of female Balb/C mice. 48 hours post injection cohorts of mice were left untreated (No Drug) or were treated with FIIN-4 (25mg/kg/48hr) via oral gavage. Shown are bioluminescent images of representative mice from each group immediately following tail vein inoculation (D0) and 15 days post inoculation (D15). (G) Data are the mean ± SE of the bioluminescence (Radiance) values of 5 mice per treatment group as described in panel F, resulting in the indicated P values between the untreated and FIIN-4 therapy groups.
FIIN-4 effectively targets chemotherapy resistant, metastatic TNBC
To verify the clinical significance of an FGFR:β3 integrin complex in BC progression, we analyzed patient survival rates upon cohort bifurcation based on the mean expression value of FGFR, FAK (PTKB), and β3 Integrin (ITGB3). Analysis of a cohort of basal-like BC patients revealed that β3 integrin expression alone was associated with a significant decrease in patient survival rates, but FAK and FGFR1 alone were not (Fig. 7A). However, combination of these three genes into a signature led to a more robust separation between high and low expressing tumors (Fig. 7A; right panel). FGFR3 or FGFR4 also led to more robust bifurcation in patient survival when combined with β3 integrin and FAK (Supplementary Fig. S8). Consistent with the depletion of FGFR2 through EMT and metastasis, addition of FGFR2 to this signature nullified the ability of β3 integrin to predict for patient survival (Supplementary Fig. S8). To further support these data, TNBC brain metastases were harvested from a patient whose disease had progressed while receiving adramycin, taxol, cyclophosphamide (ATC) chemotherapy. These PDX tissues were expanded to 10 individual mice and randomized into two groups 40 days after engraftment. Those mice that received FIIN-4 demonstrated a significant delay in tumor growth as compared to the control group (Fig. 7B). Taken together with our mechanistic data these findings strongly suggest that EMT-mediated expression of FGFR1 and β3 integrin work in complex through the activity of FAK to drive metastatic tumor growth.
Figure 7. FIIN-4 effectively targets chemo-resistant metastatic TNBC.
(A) Patient cohorts bearing basal-like breast tumors were separated into two groups based on the mean expression value of the indicated single genes (FGFR1, ITGB3, or PTK2 (FAK)) or as a signature of all three genes together and patient survival was analyzed. The number of patients for each analysis is indicated, resulting in the indicated P values and hazard ratios (HR). (B) A patient-derived xenograft was established from triple-negative brain metastases that progressed on ATC chemotherapy. These tumors were expanded via surgical procedures and tumor bearing mice were split into two cohorts of 5 mice each, one of which was treated with FIIN-4 (25 mg/kg/48hr) via oral gavage (initiation of treatment is indicated by the black arrow). Tumor volumes were measured at the indicated time points. Data are presented as the mean tumor volume ± the S.E., where * indicates P≤ 0.01 between the control and FIIN-4 treatment groups.
Discussion
In the current study we have developed a first-in-class covalent inhibitor of FGFR that is capable of blocking the in vivo growth of TNBC within anatomically relevant metastatic locations. Mechanistically, we demonstrate that EMT drives the expression of β3 integrin and FGFR1, both of which are necessary for enhanced response to FGF2 (Fig. 8; (1,28)). FIIN-4 demonstrated excellent efficacy against the cell lines and tumor tissues studied herein, and our current dosing schedule (25 mg/kg/48 hr p.o.) did not result in any overt toxicity to the animals. However, a major hurdle to the clinical application of FINN-4 will be diagnostic selection of the proper TNBC patient populations. Our patient and mechanistic data begin to shed light on this problem by suggesting that expression levels of β3 integrin, FAK and FGFR1, 3, and 4 could serve as potential biomarkers for prescription of FIIN-4. Ongoing work in the lab is applying FIIN-4 to a growing set of PDX samples. Overtime we will be able to correlate several pieces of annotated data including BC subtype, previous therapy, global gene expression and protein localization data with tumor response to FIIN-4. Of particular importance may be the subcellular localization of FGFR1. Our data herein, previous studies from our lab and others, and samples contained in the Human Protein Atlas clearly demonstrate that in addition to plasma membrane signaling FGFR1 can localize to the nucleus where it can influence the expression of hundreds of genes (1,30,31). Therefore, nuclear localization of FGFR1 may serve as an important biomarker for efficacy/resistance to FIIN-4. In any event, it is encouraging that our current studies clearly demonstrate that FIIN-4 is capable of inhibiting the growth of metastatic TNBC that had progressed on chemotherapy.
Figure 8. A schematic representation of how the processes of EMT drive FGFR-dependent growth of breast cancer metastases.
In epithelial-like tumor cells FGFR1-iiic levels are low and interactions with E-cadherin limit the ability of FGF2 to stimulate Erk1/2, preventing cell growth. However, following EMT, expression levels of FGFR1-iiic and β3 integrin are increased producing multiple signaling complexes that utilize FAK to aberrantly activate Erk1/2. These molecular events promote metastatic tumor growth, but they also can be used to effectively identify those patients that will respond to FIIN4 therapy, our newly developed covalent inhibitor of FGFR.
The connection of integrins and the extracellular matrix to downstream signaling events often involves both the scaffolding and kinase function of FAK. In the current study we demonstrate the ability of two distinct FAK inhibitors, Defactinib and PF-271, to inhibit in vitro FGF2 signaling. These data suggest that co-adminstration of FGFR and FAK inhibitors could provide an improved therapeutic response. However, the clinical utility of FAK inhibitors has been limited by their pharmacokinetics and solubility (32). Along these lines, the COMMAND trial (NCT01870609) evaluating Defactinib for the treatment of malignant pleural mesothelioma was recently terminated due to lack of efficacy. Furthermore, systemic inhibition of FAK can inhibit T-cell function, and immune cell infiltration which may detract from its tumor-cell autonomous effects (20,33). Due to these reasons the current study did not pursue combination therapies targeting both FAK and FGFR.
Recent findings point to engagement of the extracellular matrix and activation of Erk1/2 signaling as key mechanisms that are required for disseminated BC cells to overcome systemic dormancy and undergo metastatic outgrowth (34,35). Our data support and expand upon these findings by demonstrating that FGFR in complex with β3 integrin acts as a key upstream mediator of Erk1/2 activation. Indeed, our data in Figure 3 demonstrate that β3 integrin levels dramatically increase in all 3D culture conditions compared to tissue culture plastic, and this increase was somewhat inhibited by the addition of collagen I. These findings are consistent with the notion that FGFR1:β3 integrin signaling complexes are enhanced in more compliant metastatic microenvironments such as the lungs or liver. Whether the mechanisms of β3 integrin regulation by matrix compliance are related to or independent from the ability of EMT to mediate β3 integrin expression remains to be definitively determined (23).
Overall our studies herein add important mechanistic understanding to how the processes of EMT drive integrins and growth factor receptors to work in concert during the metastatic progression of BC. Going beyond these mechanistic data we utilized both patient-derived xenografts and a highly aggressive syngeneic model of metastatic BC growing within the pulmonary microenvironment to demonstrate the robust in vivo utility of FIIN-4, a first-in-class covalent inhibitor of FGFR. Finally, we identify a β3 integrin:FAK:FGFR molecular signature that is capable of indicating a poor prognosis group of basal-like BC patients that would clearly benefit from FIIN-4 therapy. These findings strongly support the clinical advancement of this exciting therapeutic strategy.
Supplementary Material
Acknowledgments
Financial Support: This research was supported in part by the National Institutes of Health (R00 CA166140) and the Showalter Trust foundation to M.K. Wendt and by Lung SPORE grant (P50 CA090578) to N.S. Gray.
Members of the Wendt Laboratory are thanked for critical reading of the manuscript. We also thank Dr. David Lum for his assistance in acquiring patient-derived tumor tissues. We also acknowledge the expertise of the personnel within the Purdue Center for Cancer Research Biological Evaluation Core (P30 CA023168).
Abbreviations list
- EMT
Epithelial-mesenchymal transition
- MET
mesenchymal-epithelial transition
- FGFR
fibroblast growth factor receptor
- TGF-β
transforming growth factor β
- FAK
focal adhesion kinase
- EGFR
epidermal growth factor receptor
- E-cad
Epithelial Cadherin
Footnotes
Conflicts of Interest: The authors have no conflicts of interest.
References
- 1.Wendt MK, Taylor MA, Schiemann BJ, Sossey-Alaoui K, Schiemann WP. Fibroblast growth factor receptor splice variants are stable markers of oncogenic transforming growth factor β1 signaling in metastatic breast cancers. Breast Cancer Res BCR. 2014;16:R24. doi: 10.1186/bcr3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azuma K, Tsurutani J, Sakai K, Kaneda H, Fujisaka Y, Takeda M, et al. Switching addictions between HER2 and FGFR2 in HER2-positive breast tumor cells: FGFR2 as a potential target for salvage after lapatinib failure. Biochem Biophys Res Commun. 2011 Apr 1;407:219–24. doi: 10.1016/j.bbrc.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Dey JH, Bianchi F, Voshol J, Bonenfant D, Oakeley EJ, Hynes NE. Targeting fibroblast growth factor receptors blocks PI3K/AKT signaling, induces apoptosis, and impairs mammary tumor outgrowth and metastasis. Cancer Res. 2010 May 15;70:4151–62. doi: 10.1158/0008-5472.CAN-09-4479. [DOI] [PubMed] [Google Scholar]
- 4.Sharpe R, Pearson A, Herrera-Abreu MT, Johnson D, Mackay A, Welti JC, et al. FGFR signaling promotes the growth of triple negative and basal-like breast cancer cell lines both in vitro and in vivo. Clin Cancer Res Off J Am Assoc Cancer Res. 2011 Aug 15;17:5275–86. doi: 10.1158/1078-0432.CCR-10-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holzmann K, Grunt T, Heinzle C, Sampl S, Steinhoff H, Reichmann N, et al. Alternative Splicing of Fibroblast Growth Factor Receptor IgIII Loops in Cancer. J Nucleic Acids. 2012;2012:950508. doi: 10.1155/2012/950508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elbauomy Elsheikh S, Green AR, Lambros MBK, Turner NC, Grainge MJ, Powe D, et al. FGFR1 amplification in breast carcinomas: a chromogenic in situ hybridisation analysis. Breast Cancer Res BCR. 2007;9:R23. doi: 10.1186/bcr1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro IM, Cheng AW, Flytzanis NC, Balsamo M, Condeelis JS, Oktay MH, et al. An EMT-driven alternative splicing program occurs in human breast cancer and modulates cellular phenotype. PLoS Genet. 2011 Aug;7:e1002218. doi: 10.1371/journal.pgen.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warzecha CC, Jiang P, Amirikian K, Dittmar KA, Lu H, Shen S, et al. An ESRP-regulated splicing programme is abrogated during the epithelial-mesenchymal transition. EMBO J. 2010 Oct 6;29:3286–300. doi: 10.1038/emboj.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooks MD, Burness ML, Wicha MS. Therapeutic Implications of Cellular Heterogeneity and Plasticity in Breast Cancer. Cell Stem Cell. 2015 Mar 9;17:260–71. doi: 10.1016/j.stem.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med. 2013 Nov;19:1438–49. doi: 10.1038/nm.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wendt MK, Tian M, Schiemann WP. Deconstructing the mechanisms and consequences of TGF-β-induced EMT during cancer progression. Cell Tissue Res. 2012 Jan;347:85–101. doi: 10.1007/s00441-011-1199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wendt MK, Smith JA, Schiemann WP. Transforming growth factor-β-induced epithelial-mesenchymal transition facilitates epidermal growth factor-dependent breast cancer progression. Oncogene. 2010 Dec 9;29:6485–98. doi: 10.1038/onc.2010.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galliher AJ, Schiemann WP. Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res BCR. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori S, Wu C-Y, Yamaji S, Saegusa J, Shi B, Ma Z, et al. Direct binding of integrin alphavbeta3 to FGF1 plays a role in FGF1 signaling. J Biol Chem. 2008 Jun 27;283:18066–75. doi: 10.1074/jbc.M801213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rusnati M, Tanghetti E, Dell’Era P, Gualandris A, Presta M. αvβ3 Integrin Mediates the Cell-adhesive Capacity and Biological Activity of Basic Fibroblast Growth Factor (FGF-2) in Cultured Endothelial Cells. Mol Biol Cell. 1997 Dec;8:2449–61. doi: 10.1091/mbc.8.12.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mori S, Takada Y. Crosstalk between Fibroblast Growth Factor (FGF) Receptor and Integrin through Direct Integrin Binding to FGF and Resulting Integrin-FGF-FGFR Ternary Complex Formation. Med Sci. 2013 Aug 13;1:20–36. [Google Scholar]
- 17.Liu Q, Sabnis Y, Zhao Z, Zhang T, Buhrlage SJ, Jones LH, et al. Developing Irreversible Inhibitors of the Protein Kinase Cysteinome. Chem Biol. 2013 Feb 21;20:146–59. doi: 10.1016/j.chembiol.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsh V. Next-Generation Covalent Irreversible Kinase Inhibitors in NSCLC: Focus on Afatinib. Biodrugs. 2015;29:167–83. doi: 10.1007/s40259-015-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan L, Wang J, Tanizaki J, Huang Z, Aref AR, Rusan M, et al. Development of covalent inhibitors that can overcome resistance to first-generation FGFR kinase inhibitors. Proc Natl Acad Sci U S A. 2014 Nov 11;111:E4869–4877. doi: 10.1073/pnas.1403438111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wendt MK, Schiemann WP. Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-beta signaling and metastasis. Breast Cancer Res BCR. 2009;11:R68. doi: 10.1186/bcr2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parvani JG, Galliher-Beckley AJ, Schiemann BJ, Schiemann WP. Targeted inactivation of β1 integrin induces β3 integrin switching, which drives breast cancer metastasis by TGF-β. Mol Biol Cell. 2013 Nov;24:3449–59. doi: 10.1091/mbc.E12-10-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeRose YS, Gligorich KM, Wang G, Georgelas A, Bowman P, Courdy SJ, et al. Patient-derived Models of Human Breast Cancer: Protocols for In vitro and In vivo Applications in Tumor Biology and Translational Medicine. Curr Protoc Pharmacol Editor Board SJ Enna Ed--Chief Al. 2013 Mar;14(Unit14.23) doi: 10.1002/0471141755.ph1423s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP. Down-regulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell. 2011 Jul 15;22:2423–35. doi: 10.1091/mbc.E11-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berglund L, Björling E, Oksvold P, Fagerberg L, Asplund A, Szigyarto CA-K, et al. A genecentric Human Protein Atlas for expression profiles based on antibodies. Mol Cell Proteomics MCP. 2008 Oct;7:2019–27. doi: 10.1074/mcp.R800013-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, Forsberg M, et al. Towards a knowledge-based Human Protein Atlas. Nat Biotechnol. 2010 Dec;28:1248–50. doi: 10.1038/nbt1210-1248. [DOI] [PubMed] [Google Scholar]
- 26.Li X, He Y, Ruiz CH, Koenig M, Cameron MD, Vojkovsky T. Characterization of dasatinib and its structural analogs as CYP3A4 mechanism-based inactivators and the proposed bioactivation pathways. Drug Metab Dispos Biol Fate Chem. 2009 Jun;37:1242–50. doi: 10.1124/dmd.108.025932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wendt MK, Schiemann BJ, Parvani JG, Lee Y-H, Kang Y, Schiemann WP. TGF-β stimulates Pyk2 expression as part of an epithelial-mesenchymal transition program required for metastatic outgrowth of breast cancer. Oncogene. 2013 Apr 18;32:2005–15. doi: 10.1038/onc.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirakihara T, Horiguchi K, Miyazawa K, Ehata S, Shibata T, Morita I, et al. TGF-β regulates isoform switching of FGF receptors and epithelial–mesenchymal transition. EMBO J. 2011 Feb 16;30:783–95. doi: 10.1038/emboj.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci U S A. 2009 Jun 23;106:10290–5. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stachowiak MK, Stachowiak EK. Evidence-Based Theory for Integrated Genome Regulation of Ontogeny—An Unprecedented Role of Nuclear FGFR1 Signaling. J Cell Physiol. 2016 Jun 1;231:1199–218. doi: 10.1002/jcp.25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terranova C, Narla ST, Lee Y-W, Bard J, Parikh A, Stachowiak EK, et al. Global Developmental Gene Programing Involves a Nuclear Form of Fibroblast Growth Factor Receptor-1 (FGFR1) PLOS ONE. 2015 Apr 29;10:e0123380. doi: 10.1371/journal.pone.0123380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Infante JR, Camidge DR, Mileshkin LR, Chen EX, Hicks RJ, Rischin D, et al. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J Clin Oncol Off J Am Soc Clin Oncol. 2012 May 1;30:1527–33. doi: 10.1200/JCO.2011.38.9346. [DOI] [PubMed] [Google Scholar]
- 33.Wiemer AJ, Wernimont SA, Cung T-D, Bennin DA, Beggs HE, Huttenlocher A. The focal adhesion kinase inhibitor PF-562,271 impairs primary CD4+ T cell activation. Biochem Pharmacol. 2013 Sep 15;86:770–81. doi: 10.1016/j.bcp.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El Touny LH, Vieira A, Mendoza A, Khanna C, Hoenerhoff MJ, Green JE. Combined SFK/MEK inhibition prevents metastatic outgrowth of dormant tumor cells. J Clin Invest. 2014 Jan 2;124:156–68. doi: 10.1172/JCI70259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barkan D, Green JE, Chambers AF. Extracellular Matrix: A Gatekeeper in the Transition from Dormancy to Metastatic Growth. Eur J Cancer Oxf Engl 1990. 2010 May;46:1181–8. doi: 10.1016/j.ejca.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.