Abstract
Diabetes is a major stroke risk factor and is associated with poor functional recovery after stroke. Accumulating evidence indicates that the worsened outcomes may be due to hyperglycemia-induced cerebral vascular complications, especially disruption of the blood-brain barrier (BBB). The present study tested a hypothesis that the activation of hypoxia inducible factor-1 (HIF-1) was involved in hyperglycemia-aggravated BBB disruption in an ischemic stroke model. Non-diabetic control and Streptozotocin-induced type I diabetic mice were subjected to 90 min transient middle cerebral artery occlusion (MCAO) followed by reperfusion. Our results demonstrated that hyperglycemia induced higher expression of HIF-1α and vascular endothelial growth factor (VEGF) in brain microvessels after MCAO/reperfusion. Diabetic mice showed exacerbated BBB damage and tight junction disruption, increased infarct volume as well as worsened neurological deficits. Furthermore, suppressing HIF-1 activity by specific knock-out endothelial HIF-1α ameliorated BBB leakage and brain infarction in diabetic animals. Moreover, glycemic control by insulin abolished HIF-1α up-regulation in diabetic animals and reduced BBB permeability and brain infarction. These findings strongly indicate that HIF-1 plays an important role in hyperglycemia-induced exacerbation of BBB disruption in ischemic stroke. Endothelial HIF-1 inhibition warrants further investigation as a therapeutic target for the treatment of stroke patients with diabetes.
Keywords: HIF-1, diabetic stroke, hyperglycemia, BBB, insulin
Introduction
Ischemic stroke is a leading cause of death and long-term disability in the United States and worldwide, and diabetes is the most rapidly increasing risk factor for stroke. Approximately 30-40% of ischemic stroke patients present with admission hyperglycemia, either due to diabetes or due to a generalized stress reaction (Gray et al., 2007). The relative risk of cerebrovascular disease or stroke is 2 to 6-fold higher in diabetes (Rosamond et al., 2007). In addition to the increased stroke incidence, diabetes and hyperglycemia are associated with worsened stroke outcomes, leading to increased mortality and poor functional recovery (Capes et al., 2001; Kruyt et al., 2010; Martini and Kent, 2007). Since diabetic patients are at a higher risk of stroke and have poorer prognosis compared to the non-diabetic population, a better understanding on how diabetes affects ischemic stroke outcome is pivotal for developing better prevention and treatment strategies before and after an ischemic insult.
During ischemic stroke, cerebral ischemia and subsequent reperfusion result in harmful consequences, including the breakdown of the blood-brain barrier (BBB), which leads to severe neurologic deficits through aggravation of edema formation and brain hemorrhage (Borlongan et al., 2012; Jung et al., 2010; Latour et al., 2004). Acute hyperglycemia increases infarct volume, brain swelling and hemorrhagic transformation in rat models (McBride et al., 2016). The incidence and severity of BBB damage is markedly higher in stroke patients with diabetes or stress hyperglycemia than those without, implying a prominent role for high blood glucose in the development and exacerbation of BBB disruption (Baird et al., 2003; Capes et al., 2001; Rosso et al., 2015). Moreover, in acute stroke patients who received tPA treatments, hyperglycemia was associated with increased mortality and worse clinical outcomes (Masrur et al., 2015). However, the mechanisms underlying diabetes- and hyperglycemia-mediated exacerbation in ischemic brain injury remain largely unexplored and are of great importance in identifying novel therapeutic targets for cerebrovascular protection.
Hypoxia-inducible factor 1 (HIF-1) is a key transcription factor in response to hypoxia/ischemia. It is a heterodimer of two subunits, the regulatable HIF-1α and constitutively expressed and stable HIF-1β. The activity of HIF-1 is primarily determined by the level of the α subunit. HIF-1 is implicated in cerebral vascular disorders in various pathological conditions, such as ischemic stroke, sabarachnoid hemorrhage, and traumatic brain injury. Inhibition of HIF-1 ameliorates hypoxia-induced BBB disruption and the subsequent brain damage in both adult (Chen et al., 2007) and neonatal rodent ischemic stroke models (Chen et al., 2008). Previous studies have shown that HIF-1 disrupts BBB integrity by enhancing the expression of its target gene vascular endothelial growth factor (VEGF) (Yeh et al., 2007), a potent vascular permeability enhancing factor. Furthermore, we have reported that high glucose treatment upregulates HIF-1α expression in in vitro endothelial cell culture (Yan et al., 2012). In addition, increased HIF-1α expression was found in the retina (Pouliot et al., 2012) and renal tubular epithelial cells (Tang et al., 2012) of diabetic animal models. In light of the above, we determined the effect of hyperglycemia on the expression of HIF-1α and its down-stream factor VEGF using an ischemic stroke model of mouse. We also examined whether BBB dysfunction and brain damage could be prevented by inhibition of endothelial HIF-1α. Finally, we determined if normalization of blood glucose levels in diabetic animals could prevent enhanced BBB breakdown through modulation of HIF-1α pathway.
Materials and Methods
Animals
All procedures using animals were approved by the Institutional Animal Care and Use Committees of University of Kansas and conformed to the National Institutes of Health Guidelines for use of animals in research. Animals were maintained in a climate-controlled vivarium with a 12 h light-dark cycle with free access to food and water. Male wide type (WT) C57BL/6 mice were from Charles River Laboratory (Wilmington, MA). Mice (B6.129-hif-1αtm3Rsjo/J) carrying homozygous HIF-1α floxed alleles (HIF-1αF/F) were generated by engineering loxP sites flanking exon 2 of the HIF-1α gene as described previously (Ryan et al., 2000) and bought from the Jackson Laboratory (stock number: 007561, Bar Harbor, ME).Tie2-Cre transgenic mice (B6.Cg-Tg (Tek-cre)1Ywa/J) expressing cre recombinase under the control of the receptor tyrosine kinase Tek (Tie2)promoter were generated as described previously (Kisanuki et al., 2001) and also bought form the Jackson Laboratory (stock number: 004128). All mice strains were maintained on a C57BL/6J background. All animals were acclimated to the environment for 7 days before the experiments. The mouse strain B6.Cg-Tg (Tek-cre) was crossed with homozygote HIF-1αF/F mice to generate Cre+/−: HIF-1αF/Wt, which were crossed with homozygote HIF-1αF/F mice to generate HIF-1α mutants Cre+/−: HIF-1αF/F, designated as endothelial specific HIF-1α knock-out HIF-1αΔ/Δ as described previously (Diebold et al., 2012). Littermates with the Cre−/−: HIF-1αF/F genotypes were used as controls for each group of experiments. For genotyping, genomic DNA was isolated from tail biopsies collected at 21 d of age using the DNeasy genomic DNA isolation kit (Qiagen, Valencia, CA). HIF-1αF and wild-type alleles were detected using the following primers: 5′-CGT GTG AGA AAA CTT CTG GAT G- 3′ and 5′-AAA AGT ATT GTG TTG GGG CAG T-3′. Transgenic mice expressing Cre recombinase were identified using primers: 5′-GCG GTC TGG CAG TAA AAA CTA TC-3′ and 5′-GTG AAA CAG CAT TGC TGT CAC TT-3′. The PCR reactions were performed with the Omni Clenttaq polymerase (DNA Polymerase Company, St. Louis, MO). The products were run on a 3% agarose gel for HIF-1α or Cre.
Middle cerebral artery occlusion (MCAO)
Transient focal cerebral ischemia was induced by surgical occlusion of the middle cerebral artery. The procedure of MCAO followed by reperfusion was conducted using an intraluminal model as previously described (Liu et al., 2004). For the anesthesia, 2.0% isoflurane in medical O2 was used for induction, and 1.0% for maintenance. Duration of anesthetic exposure was kept the same for each animal. Following a midline neck incision, external carotid artery (ECA), internal carotid artery (ICA), and pterygopalatine artery of ICA were exposed. A silicone rubber-coated monofilament nylon suture (Doccol Corporation, Sharon, MA) with a diameter of 0.23 mm was inserted into the ICA via a slit on the ECA. The suture was advanced along the ICA to the extent of 9 to 10 mm from the bifurcation of mice. Reperfusion was produced by gently withdrawing the suture until the suture tip reached the bifurcation and the incision closed 90 min after the onset of ischemia. After surgery, the animals were allowed to recover from anesthesia while being given food and water ad libitum. Buprenorphine was administrated at 0.1mg/kg subcutaneously as post-operative analgesia. For all animals used in this study, successful MCAO was confirmed by laser Doppler flowmetry (LDF) (Moor Instruments, Wilmington, DE) as described in the literature (Takagi et al., 1994). During ischemia, LDF regional cerebral blood flow dropped to 17.2 ± 4.3% of the preischemic level; and after reperfusion the blood flow was restored to 88.4 ± 4.6% of pre-ischemic level. During the experiment, the mice body temperature was maintained within the range of 37.0 ± 0.5°C by the heating pad. Sham-operated animals underwent the same anesthesia and surgical procedures without the occlusion of MCA. Animals that did not show any neurological deficits or died within 24 h after MCAO were excluded.
Induction of diabetes and administration of insulin
Six-week-old mice were rendered diabetic with intraperitoneal injections of freshly prepared streptozotocin (STZ). Immediately before injection, STZ was dissolved in 0.2 ml sodium citrate buffer (pH 4.5). Mice received one round of STZ injection each day for three consecutive days (day 1: 85 mg/kg, day 2: 70 mg/kg, and day 3: 55 mg/kg). We chose the dose of STZ based on a number of previous publications (Kennedy and Zochodne, 2005; Murray et al., 2005; Neitzel et al., 2003; Panagia et al., 2005; Urban et al., 2010). Three days after the last injection, mice with fasting blood glucose > 290mg/dl were deemed diabetic (One-Touch Ultra glucometer). Because it has been reported that increasing BBB permeability in STZ-induced diabetic animals requires at least 4 weeks, the mice were subjected to MCAO 4 weeks after the STZ injection (Huber et al., 2006).
For the long-term insulin treatment, insulin (0.7 U/mouse/d, subcutaneously) was administrated daily from third day after the last STZ injection until the mice were sacrificed (Thomas et al., 2013). For the mice treated with insulin only at the onset of reperfusion, continuous administration of intermediate-acting insulin was performed during the 24 h reperfusion. Mice were treated with three times of insulin injection at 0, 8, and 16 h of reperfusion at a total dose of 0.7 U. Another group of STZ mice were treated with a low dose of insulin which was not able to correct the mice blood glucose level. Mice were injected with insulin at the onset of reperfusion at 2 U/kg (about 0.04-0.05 U/mouse) (Fanne et al., 2011; Rizk et al., 2006). The level of blood glucose in each group before and after ischemia was summarized in Table 1. For the insulin receptor signaling activation experiments (Supplementary Fig. 1), non-diabetic control mice received one dose of insulin injection at 2 U/kg. For acute hyperglycemia induced by glucose injection (Supplementary Fig. 3), mice received continuous intraperitoneal injection of 25% glucose solution during reperfusion period (Akamatsu et al., 2015). The blood glucose level in the induced-hyperglycemic mice was maintained above 300 mg/dL throughout the reperfusion.
Table 1.
Mice body weight and blood glucose levels.
| Weight (g) | Blood Glucose (mg/dl) | |||
|---|---|---|---|---|
| Baseline | Final | Before MCAO | After MCAO (admission) | |
| Control | 22.1±1.8 | 27.8±2.3 | 116±15 | 127±28 |
| Diabetic | 21.9±1.6 | 22.4±2.1 | 473±32* | 465±38* |
| Long-term insulin | 21.6±1.9 | 26.9±2.5 | 129±32 | 167±35 |
| Insulin at reperfusion | 22.5±1.5 | 23.1±1.7 | 489±43* | 158±39 |
| Low dose insulin | 22.3±1.5 | 22.9±1.2 | 467±28* | 423±37* |
Data are expressed as means ± SD.
p< 0.05 vs. control animals.
Isolation of cerebral microvessels
Since the BBB is formed at the level of cerebral microvessels, the microvessels were isolated from mouse brains to analyze protein expressions of HIF-1α, VEGF, and tight junction (TJ) proteins. At the indicated time points of reperfusion, mice were anesthetized and euthanized by decapitation. The brain microvessels were isolated from freshly removed brains as described previously (Seelbach et al., 2007). Briefly, brains were removed from the skull and immediately immersed in ice-cold PBS. Choroid plexus, meninges, cerebellum, and brain stem were removed, and ipsilateral and contralateral hemispheres were separated and homogenized in isolation buffer separately. Then 26% dextran was added to the homogenates, and samples were centrifuged (5800g, 4°C) for 10 min. The supernatants were discarded; pellets were re-suspended and filtered through a 100 μm mesh filter. The filtered homogenates were re-pelleted by centrifuge (1500 g, 10 min), and either smeared on microscope slides for immunostaining by fluorescence microscopy or re-suspended in lysis buffer for Western blotting analysis.
Western blotting
Cerebral microvessels isolated from contralateral and ipsilateral hemispheres were homogenized in an ice-cold RIPA buffer with 1μg/ml of a protease inhibitor cocktail (Thermo scientific, Rockford, IL), and sonicated for 10 s. The homogenates were centrifuged at 14,000 rpm for 15 min at 4°C, and the supernatants were collected. For the experiments in Supplementary Fig. 1, the whole brains were isolated and homogenized. Standard Western blotting procedures were followed. The primary antibodies were rabbit anti-HIF-1α (Millipore, Billerica, MA), ZO-1 (40-2200, Invitrogen, Carlsbad, CA), occludin (33-1500, Invitrogen), claudin-5 (34-1600, Invitrogen), VEGF (sc-507, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-phospho-Akt (Thr308) (#9275, Cell Signaling Technology, MA, USA), rabbit anti-Akt (#9272, Cell Signaling Technology), rabbit anti-phospho-p70 S6K (Thr389) (#9205, Cell Signaling Technology), rabbit anti-p70S6K (#9202, Cell Signaling Technology), and β-actin (sc-1616, Santa Cruz). The secondary antibody was goat anti-rabbit IgG-HRP (sc-2030, Santa Cruz Biotechnology, Santa Cruz, CA). β-actin was used as an internal control.
Immunohistochemical staining
Freshly isolated microvessels were spread onto microscope slides and heat-fixed for 10 min at 95°C followed by treatment with 4% paraformaldehyde for 10 min. The microvessels were then washed with PBS and permeabilized in PBS containing 0.1% Triton-X100 for 10 min. The nonspecific binding sites were blocked with PBS containing 0.05 % triton-X100 and 0.25 % BSA for 1 h. Primary ZO-1 antibody was diluted (1:100) in blocking buffer and incubated with sections overnight at 4°C. Secondary antibody was donkey anti-rabbit Alexa 488 (Molecular Probes, Carlsbad, CA). Images were routinely captured with a Leica DMI 4000B fluorescent microscope. All immunohistochemical staining data were obtained in a blinded manner.
Evaluation of neurological deficits, infarct size and brain edema volume
At 24 h reperfusion, mice were evaluated for neurological deficits in a blinded fashion based on a modified scoring standard of Rogers et al.(Rogers et al., 1997) with: 0=no deficit; 1=failure to extend right forepaw fully; 2=decreased grip of the right forelimb while tail gently pulled; 3=spontaneous movement in all directions, contralateral circling only if pulled by the tail; 4=circling or walking to the right; 5=walks only when stimulated; 6=unresponsive to stimulation with a depressed level of consciousness. In addition, mortality was calculated at 24 h after MCAO/reperfusion.
Mice were anesthetized and euthanized by decapitation immediately after scoring. The brains were removed and sectioned into 2 mm slices. The slices were incubated in a 2% solution of TTC in PBS (pH 7.4) at 37°C for 30 min and fixed in 10% formalin. TTC staining has been widely used to reflect accurately the extent of irreversible ischemic damage in cerebral tissues in rodents (Bederson et al., 1986). TTC-stained brain sections were photographed using a digital camera (Powershot 400 digital camera, Canon) and analyzed using Image J for determination of infarct area and brain edema. To compensate for the effect of brain edema, the corrected infarct volume was calculated as previously described. The brain edema percentage was determined as (ipsilateral volume–contralateral volume)/contralateral volume.
Determination of BBB permeability
BBB permeability was assessed by measuring extravasation of Evans blue (EB) dye. EB (2% in saline, 6 ml/kg body weight) was injected through tail vein right after reperfusion according to a previous report (Liu et al., 2011). At the end of reperfusion, mice were transcardially perfused with saline under anesthesia until colorless perfusion fluid was obtained from the right atrium. After decapitation, the brain was removed and sectioned into 2 mm slices. The whole brain and brain sections were photographed using a digital camera (Powershot 400 digital camera, Canon). Then, the tissue from contralateral and ipsilateral hemispheres was separately weighed and soaked in 1 ml of 50% trichloroacetic acid solution. After homogenization and centrifugation, the extracted EB was diluted with ethanol (1:3); and fluorescence intensity was measured at 620 nm and 680 nm for excitation and emission, respectively, using a fluorescence reader. The tissue content of EB dye was quantified from a linear standard curve derived from known amounts of the dye and was expressed as micrograms per gram of tissues.
Statistical analysis
The results were presented as mean with a standard deviation of mean. Comparisons of Western blotting, EB leakage, edema formation, and infarct volumes were carried out by ANOVA test, followed by Tukey's correction (R 3.0.1). Neurological scores were compared using Kruskal-Wallis analysis followed by Bonferroni correction. A p<0.05 was considered statistically significant.
Results
Diabetic mice demonstrated increased BBB permeability and worsened stroke outcomes after ischemia/reperfusion
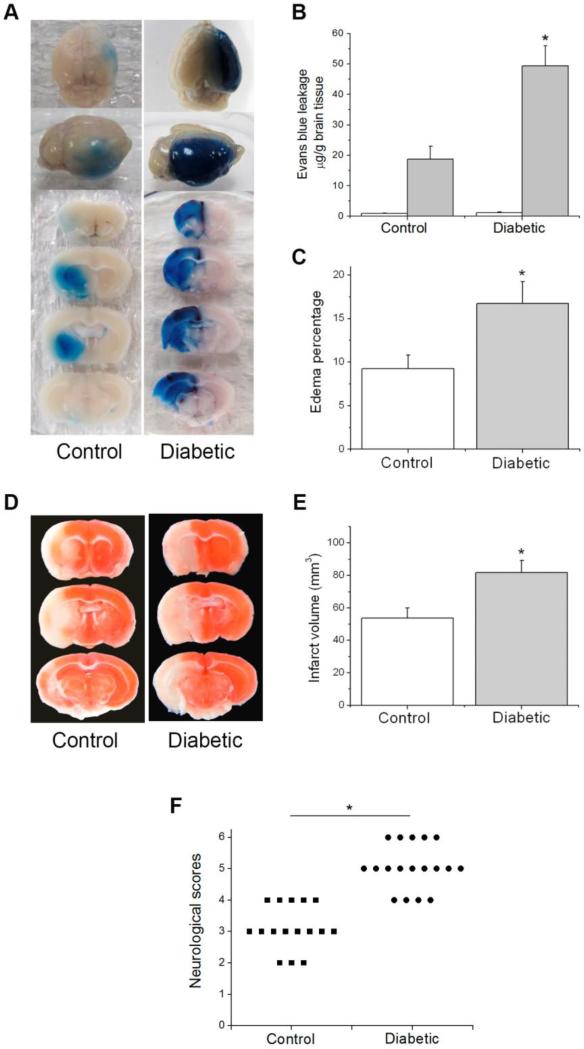
We utilized STZ-induced diabetic mice as a model of chronic hyperglycemia that occurs in diabetes mellitus. At 4 weeks after STZ injection, the diabetic mice exhibited significantly higher level of blood glucose than the non-diabetic control mice (Table 1). Both diabetic and control mice were subjected to 90 min MCAO and 24 h reperfusion. BBB permeability was assessed at the end of reperfusion. As shown in Fig. 1, EB leaked mainly into the ipsilateral hemisphere with only insignificant, low background level in the contralateral hemisphere of both control and diabetic mice, suggesting that the 4-week diabetes didn't elevate BBB permeability to macromolecular proteins such as albumin under basal condition. Diabetic mice showed remarkable increase in EB leakage in the ipsilateral hemisphere, which indicated that hyperglycemia aggravated stroke-induced BBB breakdown. Consistent with the increased EB leakage, more pronounced brain edema was observed in the ipsilateral brains of diabetic mice (Fig. 1C). As BBB dysfunction after stroke might affect infarct expansion and stroke outcome, we evaluated the infarct volume and functional recovery in control and diabetic mice. Diabetic mice demonstrated significantly increased lesion volume (82 mm3, compared to 54 mm3 in the control group) and more severe neurological impairments (Fig. 1E and F). In summary, diabetic mice exhibited increased BBB leakage, edema formation, infarct volume and neurological deficits after transient focal ischemia. These results suggest that the STZ-induced diabetic mouse is a good model for investigating the molecular mechanism underlying hyperglycemia-enhanced BBB disruption in stroke.
Fig. 1.
Effect of diabetes on ischemia/reperfusion-induced brain injury. Control and diabetic mice were subjected to 90 min MCAO followed by 24 h reperfusion. (A) Representative images of EB extravasation in a whole brain and coronal sections. (B) Quantification of EB leakage in contralateral and ipsilateral hemispheres (n=5). White bars, contralateral hemisphere; dark bars, ipsilateral hemisphere. (C) Quantification of brain edema percentage (n=6 (control), 8 (diabetic)). (D) Representative TTC staining images of brain sections. (E) Quantification of infarct volume estimated by TTC stained sections (n=6 (control), 8 (diabetic)). (F) Quantification of neurological deficit scores rate (n= 16 (control), 18 (diabetic)). Values are means ± SD, *p< 0.05 vs. control animals.
Hyperglycemia enhanced endothelial HIF-1α and VEGF expression in ischemic brains during reperfusion
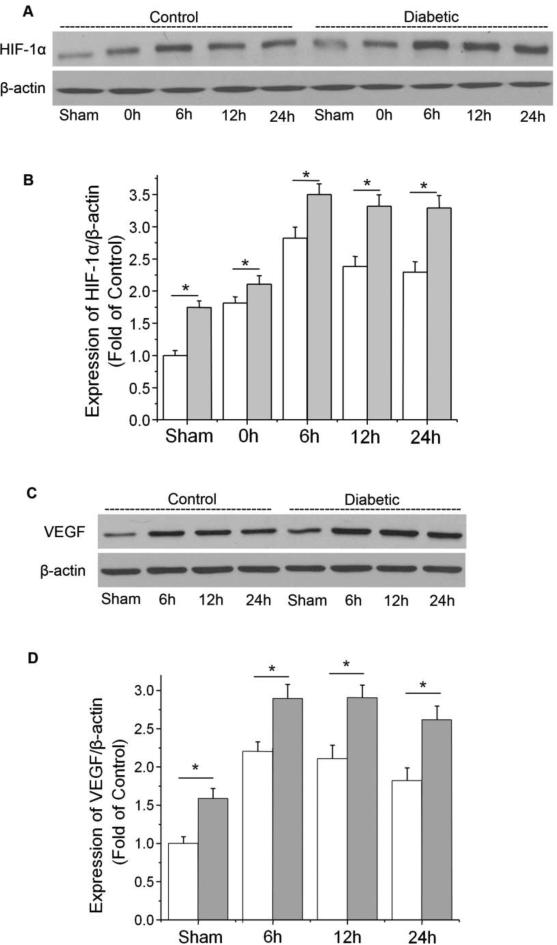
To study whether endothelial HIF-1α was induced by hyperglycemia in ischemic brains, we isolated the brain microvessels from ipsilateral brains at various time points after ischemia and determined the expression of HIF-1α in microvessel homogenates. As shown in Fig. 2A, ischemia increased the endothelial HIF-1α protein level in control (non-diabetic) mice. Reperfusion did not decrease but further elevated the HIF-1α level, which peaked at 6 h of reperfusion and remained elevated for at least 24 h. In diabetic mice, the protein level of HIF-1α was significantly increased at all time points following MCAO, suggesting that hyperglycemia further enhanced cerebral endothelial HIF-1α expression throughout the post-ischemic period. It is noteworthy that diabetic mice showed higher HIF-1α basal level than control mice.
Fig. 2.
Effect of hyperglycemia on the expression of HIF-1α and VEGF in ischemic brain microvessels. Control and diabetic mice were subjected to 90 min MCAO followed by reperfusion (0 h to 24 h). HIF-1α was analyzed by Western blotting in cerebral microvessel lysates from ipsilateral hemispheres of mice at 0, 6, 12, and 24 h of post-ischemia. VEGF was analyzed at 6, 12, and 24 h post-ischemia. (A) Representative Western blots of HIF-1α. (B) Quantification of the HIF-1α protein level. (C) Representative Western blots of VEGF. (D) Quantification of the VEGF protein level. White bars, control ischemic brains; dark bars, diabetic ischemic brains. Values were normalized to β-actin and sham-operated control. Values are means ± SD, n=5. *p< 0.05 vs. control animals.
VEGF is a well-characterized transcriptional target of HIF-1. It is a potent vascular permeability enhancing factor that increases BBB leakage in the post-ischemic brain. VEGF expression in ischemic brain microvessels was substantially increased at 6 h following reperfusion and was maintained for at least 24 h, which correlated well with the time course of HIF-1α expression (Fig. 2C and D). The protein levels of VEGF in both sham and ischemic brains of diabetic mice were significantly higher than that of control mice, suggesting that diabetes enhanced the expression of HIF-1 down-stream factor VEGF in brain microvessels.
Hyperglycemia enhanced TJ protein degradation in microvessels of ischemic brains
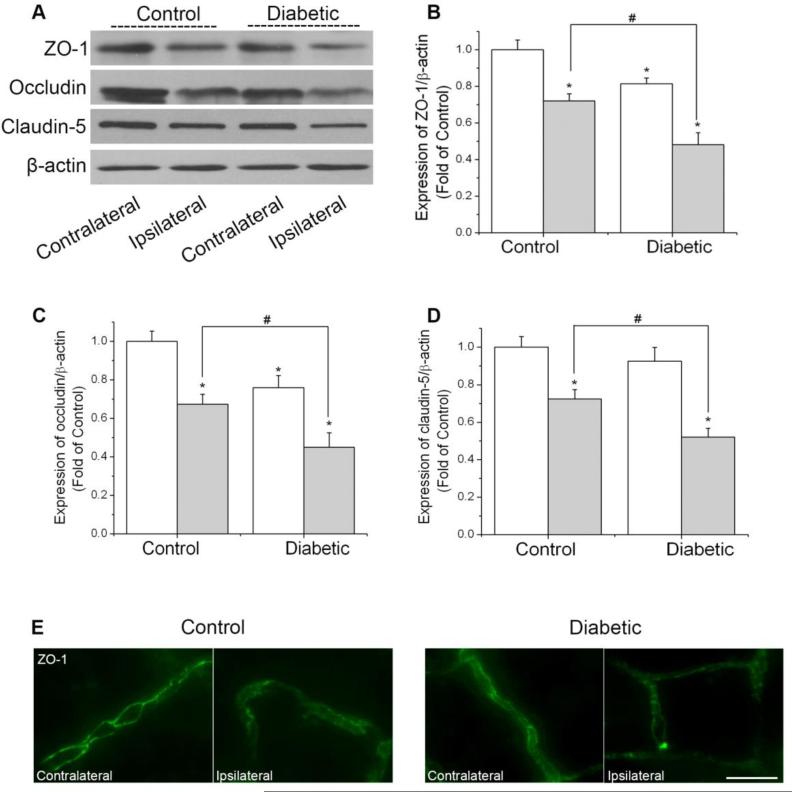
The TJs between endothelial cells of cerebral capillaries are critical in maintaining BBB integrity (Almutairi et al., 2015). Decreased TJ protein expressions or variations in subcellular TJ protein localization are associated with alterations in BBB permeability. TJs consist of the transmembrane proteins (e.g. claudins, occludin) and cytoplasmic accessory proteins (e.g. ZO-1) that connect the transmembrane proteins to the actin cytoskeleton. To determine whether exacerbated BBB disruption induced by hyperglycemia is associated with decreased levels of TJ proteins following cerebral ischemia, we determined the protein levels of ZO-1, occludin, and claudin-5 in isolated brain microvessels. As shown in Fig. 3, consistent with the BBB permeability results, the level of all three proteins in the ischemic hemisphere of diabetic mice were significantly reduced, compared with that of control mice. Diabetic mice also showed lower level of occludin and ZO-1 in contralateral hemisphere. Furthermore, we studied the arrangement pattern of ZO-1 on brain microvessels by immunostaining. The immunostaining study revealed relatively continuous and linear staining of ZO-1 in the contralateral brain microvessels. Ischemia/reperfusion diminished staining intensity and disrupted the continuity of ZO-1. Moreover, the loss of ZO-1 was even more obvious in the diabetic ipsilateral brain microvessels. The results above demonstrated that diabetes further disrupted BBB by decreasing TJ proteins in ischemic brain.
Fig. 3.
Effect of diabetes on the expression of ZO-1, occludin, and claudin-5 in ischemic brain microvessels. Mice were subjected to 90 min MCAO followed by 24 h reperfusion. The protein levels of ZO-1, occludin, and claudin-5 were analyzed in isolated brain microvessels from contralateral and ipsilateral hemispheres of mice. (A) Representative Western blots of ZO-1, occludin, and claudin-5. (B) Quantification of the ZO-1 protein level. (C) Quantification of the occludin protein level. (D) Quantification of the claudin-5 protein level. White bars, contralateral hemispheres; dark bars, ipsilateral hemispheres. Values were normalized to β-actin and contralateral hemispheres of control animals. Values are means ± SD, n = 5. *p< 0.05 vs. contralateral hemispheres from control animals. #p< 0.05 vs. ipsilateral hemispheres from control animals. (E) Immunostaining of ZO-1 on isolated brain microvessels. Scale bar, 20 μm.
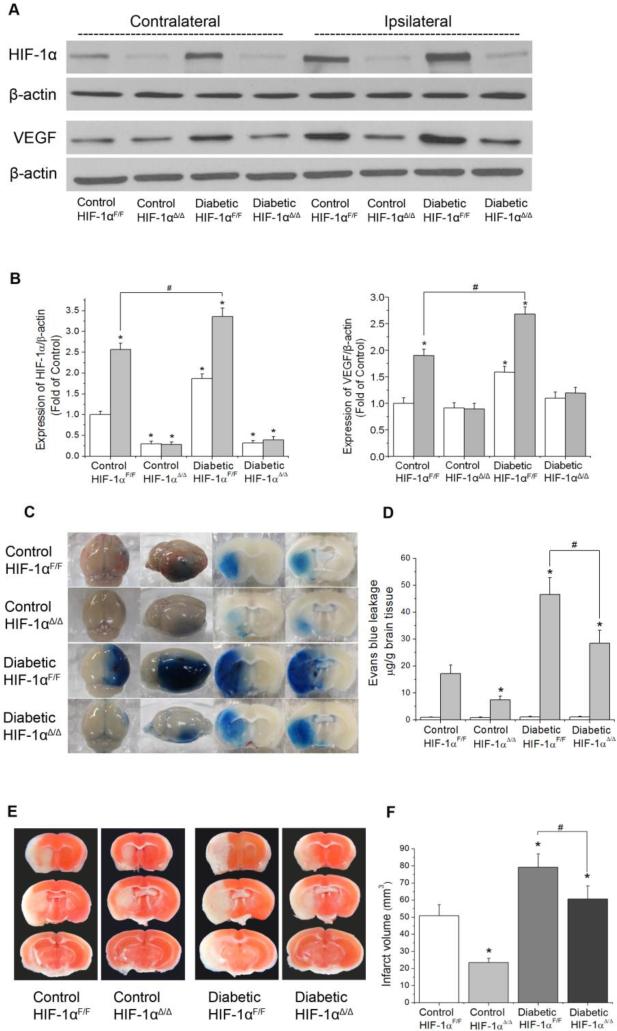
Endothelial-specific HIF-1α knock-out reduced BBB permeability and brain infarction in diabetic mice
To investigate the specific role of HIF-1 in hyperglycemia-aggravated BBB disruption, we utilized endothelial-specific HIF-1α knock-out mice (HIF-1αΔ/Δ). The deletion was achieved by crossing mice bearing loxP-floxed HIF-1α alleles with transgenic mice expressing Cre recombinase under control of Tek promoter. Both wild-type and HIF-1α knock-out mice were rendered diabetes and subjected to 90 min MCAO and 24 h reperfusion. To evaluate the efficiency of HIF-1α ablation in endothelial cells of the HIF-1αΔ/Δ mice, we detected HIF-1α expression in cerebral microvessel homogenates from contra- and ipsilateral hemisphere. As expected, post-ischemic accumulation of HIF-1α was dramatically attenuated in the both control and diabetic HIF-1αΔ/Δ mice (Fig. 4A). Correspondingly, the expression of HIF-1 target gene VEGF was also significantly decreased in the ischemic brain of HIF-1αΔ/Δ mice compared with that of wild-type mice. (Fig. 4A). We observed that the EB extravasation was significantly reduced in diabetic HIF-1αΔ/Δ mice (Fig. 4C), suggesting that inhibition of endothelial HIF-1α partially restored BBB integrity. Moreover, the brain infarct volume was reduced from 79 mm3 in diabetic wild-type to 61 mm3 in diabetic HIF-1αΔ/Δ mice. It is of interest to point out that deletion of endothelial HIF-1α also attenuated BBB leakage and brain damage in non-diabetic mice, which is in line with previous reports that accumulation of HIF-1α and its downstream target VEGF promotes BBB permeability in ischemic stroke (Yeh et al., 2007). In summary, we demonstrated that hyperglycemia-exacerbated BBB dysfunction was heavily dependent on its ability to activate HIF-1α-VEGF pathway since its detrimental effects were markedly alleviated in HIF-1αΔ/Δ mice.
Fig. 4.
Effect of endothelial-specific HIF-1α deficiency on the BBB permeability and brain infarction in diabetic mice. Mice were subjected to 90 min MCAO followed by 24 h reperfusion. The protein levels of HIF-1α and VEGF were analyzed by Western blotting in cerebral microvessel lysates from contralateral and ipsilateral hemispheres of mice. (A) Representative Western blots of HIF-1α and VEGF. (B) Left: quantification of the HIF-1α protein level; right: quantification of the VEGF protein level (n=5). Values were normalized to β-actin and contralateral hemispheres of control animals. *p< 0.05 vs. contralateral hemispheres from control animals. #p< 0.05 vs. ipsilateral hemispheres from control animals. (C) Representative images of EB extravasation in a whole brain and coronal sections. (D) Quantification of EB leakage in contralateral and ipsilateral hemispheres (n=5). White bars, contralateral hemisphere; dark bars, ipsilateral hemisphere. (E) Representative TTC staining images of brain sections. (F) Quantification of infarct volume estimated by TTC stained sections (n=4). Values are means ± SD, *p<0.05 vs. control animals.
Normalizing blood glucose by insulin abolished HIF-1α upregulation in diabetic mice
Blood glucose of diabetic mice was normalized by long-term insulin administration to verify that the upregulation of HIF-1α and the exacerbated BBB disruption observed in diabetic mice were due to hyperglycemia. As expected, the long-term insulin-treated diabetic mice showed similar HIF-1α protein level in both contra-and ipsilateral hemispheres, compared with the non-diabetic control. In addition, a group of diabetic mice only received insulin treatment at the time of reperfusion was included to further explore the effects of hyperglycemia on HIF-1α expression and BBB disruption at different stages of ischemia. We found normalizing the blood glucose during post-ischemic reperfusion significantly decreased the HIF-1α expression in the ipsilateral hemisphere, compared with the non-treated diabetic mice (Fig. 5A), suggesting that reperfusion significantly elevates HIF-1α expression in ischemic brain exposed to high glucose. Both the long-term or reperfusion insulin administrations attenuated EB extravasation and brain infarction in diabetic mice (Fig. 5 D-G), which indicates that the adverse effect of hyperglycemia in stroke largely results from high blood glucose in reperfusion.
Fig. 5.
Effect of normalizing blood glucose on HIF-1α expression, BBB permeability, and brain infarction in diabetic mice. Mice were subjected to 90 min MCAO followed by 24 h reperfusion. Long term insulin treatment: mice were treated with insulin (0.7 U) daily from the 3rd day after diabetic induction; Acute insulin treatment: mice were administrated with continuous insulin (0.7 U) during the 24 h reperfusion. The protein level of HIF-1α was analyzed by Western blotting in cerebral microvessel lysates from contralateral and ipsilateral hemispheres of mice.(A) Representative Western blots of HIF-1α. (B, C) Quantification of the HIF-1α protein level in contralateral hemispheres (white bars) and ipsilateral hemispheres (dark bars) (n=5). Values were normalized to β-actin and contralateral hemispheres of control animals. *p< 0.05 vs. control animals. #p< 0.05 vs. diabetic animals. (D) Representative images of EB extravasation in a whole brain and coronal sections. (E) Quantification of EB leakage in contralateral and ipsilateral hemispheres (n=5). White bars, contralateral hemisphere; dark bars, ipsilateral hemisphere. (F) Representative TTC staining images of brain sections. (G) Quantification of infarct volume estimated by TTC stained sections (n=8 (diabetic), 6 (long-term insulin), 5 (insulin at reperfusion)). Values are means ± SD, *p< 0.05 vs. diabetic animals.
It was unclear from the above results whether insulin protected ischemic brain by blood glucose correction or by its activation of signaling pathways. To differentiate the protective mechanism of insulin, a group of diabetic mice were treated with a low dose of insulin which was not able to correct the blood glucose level. The low dose insulin increased the phosphorylation of two key kinases in the insulin receptor signaling pathway: Akt and 70 kDa ribosomal S6 kinase (p70S6K) in the mouse brain (Supplementary Fig. 1). In the meantime, the low dose insulin did not affect HIF-1α expression, BBB permeability or brain injury at all, compared with the non-treated diabetic mice (Supplementary Fig. 2). These results suggest that the effects of insulin were predominantly via correcting the level of blood glucose rather than activating insulin receptors signaling pathway.
In addition, to specifically validate the effect of high glucose in reperfusion on the level of HIF-1α, we induced acute hyperglycemia (above 300 mg/dL) during reperfusion period by consecutive glucose injections in non-diabetic mice (Akamatsu et al., 2015). As shown in supplementary Fig. 3, glucose administration significantly increased HIF-1α protein level and EB extravasation in the ischemic brain. These results further support the concept that high blood glucose in reperfusion causes HIF-1α upregulation and BBB damage in ischemic brain.
Discussion
This study provides several major findings on the effect of diabetes on neurovascular and functional outcomes of stroke. First, we demonstrated that STZ-induced diabetes enhanced the expression of HIF-1α and VEGF in ischemic brain microvessels, which was accompanied by increased BBB disruption, elevated infarct volumes, severe edema formation, and worsened neurological deficits. Second, the detrimental effect of diabetes on cerebral vascular damage was partially reversed by specific inhibition of endothelial HIF-1α, suggesting that activation of HIF-1α is an important underlying mechanism responsible for the BBB hyperpermeability. Lastly, both chronic and acute glycemic control abolished HIF-1α upregulation in the ischemic brain of diabetic mice and lessened neurovascular injury in diabetes. In addition, glucose administration during reperfusion enhanced HIF-1α expression and BBB breakdown. These results together indicate that hyperglycemia-induced HIF-1α increase is a major mediator for BBB damage in diabetic stroke.
HIF-1's role in cerebral ischemia injury is still arguable. On the one hand, HIF-1 regulates a broad range of genes that facilitate cellular adaption to low oxygen conditions. Its targets include the genes that code for molecules participating in angiogenesis, erythropoiesis, energy metabolism, and cell proliferation (Semenza, 2003; Sharp and Bernaudin, 2004). Each of these functions potentially contributes to neuronal survival under ischemic conditions. Indeed, HIF-1 has been reported to protect neurons against cerebral ischemic damage (Jones and Bergeron, 2001; Ogle et al., 2012). Furthermore, neuron-specific knockdown of HIF-1α was demonstrated to aggravate tissue damage and reduce survival rate of mice subjected to MCAO (Baranova et al., 2007) and in vitro neuronal death (Guo et al., 2009). On the other hand, several groups have reported opposite effects of HIF-1 in cerebral ischemia, showing that HIF-1 is a likely mediator of BBB disruption. For example, Yeh et al. demonstrated that HIF-1α inhibitor YC-1was able to prevent ischemia/reperfusion-induced BBB hyperpermeability in both rat brain endothelial cell culture and in in vivo model (Yeh et al., 2007). Results by Chen et al. showed that early inhibition of HIF-1α by 2-methoxyestradiol provided neuroprotection after neonatal hypoxia-ischemia by preserving BBB integrity and attenuating brain edema. Moreover, HIF-1α upregulation by dimethyloxaylglycine increased the permeability of BBB and brain edema (Chen et al., 2008). The discrepancy of these observations may be partly explained by distinctive effects of HIF-1 down-stream targets in different cell types. For example, the angiogenic factor VEGF is the best defined HIF-1 target protein in vascular biology (Forsythe et al., 1996). It has been reported that VEGF mediates neuroprotection in cerebral ischemia by its angiogenic and neurotropic effects (Sun et al., 2003). However, VEGF is a strong inducer of vascular permeability and plays a critical role in causing BBB disruption and cerebral edema (Zhang et al., 2000). Experimental evidence has shown that VEGF down-regulates the expression of TJ proteins such as claudin-5 (Argaw et al., 2009), occludin (Argaw et al., 2009) and ZO-1 (Fischer et al., 2002) in brain microvasculature. In this study, we observed significant increase in both HIF-1α and VEGF expression in diabetic brain microvessels, which correlates with aggravated BBB leakage after stroke. Our results support the concept that enhanced HIF-1α activity in diabetic mice brain promotes BBB damage after ischemia, possibly through upregulating VEGF expression. To further evaluating the contribution of endothelial HIF-1α signaling in BBB disruption, we utilized an endothelial specific HIF-1α knock-out mouse model. We found that inhibition of HIF-1α and its down-stream VEGF expression attenuated BBB breakdown and brain infarction in both normoglycemic and hyperglycemic mice after MCAO/reperfusion, indicating that endothelial HIF-1α is an important mediator of BBB disruption in ischemic stroke.
Although genetic depletion of endothelial HIF-1α reduced BBB leakage and brain infarct volume in diabetic mice, the BBB permeability in diabetic HIF-1αΔ/Δ mice was still significantly higher than the non-diabetic mice even though HIF-1α accumulation was largely diminished. These results indicate that there might be other pathways that contribute independently of HIF-1α to the BBB disruption. For example, the activation of protein kinase C (PKC) is implicated in cerebral microvascular dysfunction in hyperglycemic stroke (Shao and Bayraktutan, 2013). PKC activity is rapidly increased in endothelium in response to hyperglycemia due to de novo synthesis of diacylglycerol, the primary activator of PKC (Kouroedov et al., 2004). PKC activation can directly affect BBB permeability through its ability to phosphorylate ZO-1, disrupt TJs, and promote excessive superoxide anion radical production from NADPH oxidase, which can induce endothelial barrier dysfunction. Cipolla et al. has demonstrated that the inhibition of PKC-β reversed the enhanced BBB permeability and prevented edema formation in STZ-induce diabetic rats subjected to MCAO (Cipolla et al., 2011). Another important contributor to vascular damage is inflammation. Hyperglycemia is known to be associated with increased expression of several pro-inflammatory transcription factors, such as NF-κB. These factors regulate the inflammatory responses by increasing pro-inflammatory cytokines and promoting the adhesion of inflammatory cells to the vascular endothelium, which leads to BBB breakdown (Kruyt et al., 2010; Martini and Kent, 2007). Moreover, our experiments only inhibited the HIF-1 signaling in endothelial cells of HIF-1αΔ/Δ mice. VEGF could also be expressed by reactive astrocytes and astrocytic end-feet lie in close proximity to microvascular endothelium (Argaw et al., 2009). VEGF secreted from astrocytes may interact with VEGF receptors on the ischemic vessels and induce BBB leakage in a paracrine manner (Zhang et al., 2002).
Many studies have reported increased brain injury in hyperglycemic animals after reperfusion. However, in ischemic animals without reperfusion, hyperglycemia seemed to have no adverse effect and might even be beneficial (Helgason, 1988; Kagansky et al., 2001). Indeed, a previous publication by Suh et al. has shown that glucose plays a detrimental role in the reperfusion injury as removing glucose from the medium during reoxygenation completely prevents post-ischemic neuron death (Suh et al., 2008). The question of when hyperglycemia has its most deleterious effects is of great clinical importance since it provides guidelines for optimal glycemic control in acute ischemic stroke. We tested the hypothesis that the adverse effect of hyperglycemia may become most evident during reperfusion. The current study showed that both chronic insulin treatment and acute insulin treatment during reperfusion suppressed HIF-1α expression in ischemic brain microvessels of diabetic mice, indicating that hyperglycemia-induced HIF-1α accumulation was largely due to the high blood glucose during reperfusion. Acute glycemic control at post-ischemic reperfusion in previously untreated diabetic subjects inhibited hyperglycemia-induced BBB permeability and reduced brain injury. Moreover, hyperglycemia that initiated at the time of reperfusion by glucose injection induced significant increase in HIF-1α accumulation and BBB disruption in non-diabetic mice. These results together suggest that the high glucose during reperfusion is a major contributor to HIF-1α upregulation and BBB disruption. Insulin is the most commonly used agent to regulate blood glucose and has been shown to reduce ischemic brain damage in animal models (Hamilton et al., 1995; Harada et al., 2009; Wass et al., 1996) and confer better survival outcomes in stroke patients (Gentile et al., 2006). It is unclear whether its protective effects are due to blood glucose correction or due to its direct interaction with brain tissues. To differentiate the protective mechanism of insulin, we administrated STZ mice with a low dose of insulin (2U/kg) which was not able to correct the mouse blood glucose levels but activated insulin receptor signaling pathway in the mouse brain. The low dose of insulin did not affect HIF-1α expression, BBB permeability, or brain injury, indicating that the effects of insulin are predominantly via alteration in the level of blood glucose rather than activation of insulin receptor signaling pathways. A previous study showed that most of insulin's protective effect was abolished by concomitant glucose infusion in a MCAO rat model (Hamilton et al., 1995), which is in line with our observations that insulin benefits ischemic stroke by reducing blood glucose levels. Although it is still debatable whether glucose-lowering treatment improves clinical outcome in patients with ischemic stroke, our study suggest a favorable outcome of glycemic control given at the onset of reperfusion. Consistent with our research, previous studies have reported that continuous glycemic control in the post stroke period alleviates neuronal damage (Harada et al., 2009) and improves the functional outcome after stroke (Prakash et al., 2013).
Our present report is the first to demonstrate that HIF-1α is up-regulated in the brain microvessels of diabetic stroke mice. This is in line with many recent findings in other tissues such as hearts (Marfella et al., 2002), peripheral nerves (Chavez et al., 2005), pancreas (Haligur et al., 2012), retina (Ly et al., 2011), and kidney glomeruli (Isoe et al., 2010). Studies based on samples from human diabetic patients have also shown increased HIF-1α levels in pre-retinal membranes (Lim et al., 2010) and vitreous fluid (Wang et al., 2009). Although the mechanism of high glucose-induced HIF-1α expression is not clear, the following factors may contribute to its stabilization. First, high glucose may stabilize HIF-1α by inhibiting HIF-specific prolyl hydroxylases (PHDs) activity through increasing the two tricarboxylic acid (TCA) cycle metabolites, succinate and fumarate. Numerous studies have reported that intracellular succinate or fumarate can inhibit PHDs to stabilize HIF-1α in both in vitro and in vivo models (Briere et al., 2005; Hewitson et al., 2007; Isaacs et al., 2005; Koivunen et al., 2007; MacKenzie et al., 2007; Pan et al., 2007; Pollard et al., 2005; Selak et al., 2005; Tannahill et al., 2013; Zhang et al., 2011). It is well established that hydroxylation of HIF-1α by PHDs at its proline residues targets it for proteasomal degradation (Semenza, 2010). Succinate and fumarate have been found to inhibit PHDs by competing with enzymes co-substrate α-ketoglutarate for binding to the catalytic center, thereby leading to HIF-1α stabilization and activation (MacKenzie et al., 2007; Semenza, 2010). Elevated succinate concentrations have been detected in the kidney and plasma of diabetic mouse models as well as high glucose-treated endothelial cell culture (Supplementary Fig. 4). In addition, a previous study has reported that post-ischemic increase of fumarate/α-ketoglutarate ratio stabilizes HIF-1α at reoxygenation by decreasing its hydroxylation (Serra-Perez et al., 2010). Therefore, we speculate that increased level of succinate and/or fumarate may mediate the high glucose-induced HIF-1α expression in our diabetic stroke model. Second, hyperglycemia-induced reactive oxygen species (ROS) overproduction may mediate HIF-1α accumulation. Increased ROS has been reported to stabilize HIF-1α in certain cell types (Nanduri et al., 2015). Under normoxic conditions, high glucose has been reported to induce mitochondrial superoxide overproduction in the endothelial cells (Makino et al., 2010; Piconi et al., 2006). Moreover, during ischemia/reperfusion, glucose itself is a requisite electron donor for reperfusion-induced superoxide production (Suh et al., 2008). Hyperglycemia further accelerates ROS generation following cerebral ischemic reperfusion (Kruyt et al., 2010; Tsuruta et al., 2010). Increased level of intracellular ROS may inhibit PHDs activity by oxidizing their co-factor Fe2+ to Fe3+, thereby promoting HIF-1α stabilization. Although most evidence indicates that glucose-induced HIF-1α accumulation is due to protein stabilization, we do not completely exclude the possibility that high glucose may enhance HIF-1α expression at transcription level. Indeed, a previous study reported that high glucose enhances carbohydrate response element binding protein (ChREBP) binding to HIF-1α promoter and increases HIF-1α mRNA level. (Isoe et al., 2010). Future investigation is needed to elucidate the molecular mechanism responsible for the upregulation of HIF-1α in brain endothelial cells.
In conclusion, in this study we found that hyperglycemia significantly increased HIF-1α and its downstream factor VEGF expression in brain microvessels after MCAO. The enhanced HIF-1 expression might represent an important mechanism for aggravated ischemic damage, particularly BBB disruption, during hyperglycemic stroke. Our study suggests that targeting HIF-1α may provide a novel therapeutic option for BBB protection in ischemic stroke patients with admission hyperglycemia.
Supplementary Material
Highlights.
Hyperglycemia enhanced HIF-1α and VEGF expression in ischemic brain microvessels.
Suppressing endothelial HIF-1α activity ameliorated BBB leakage and brain injury.
Correcting blood glucose abolished HIF-1α upregulation in brain microvessels and reduced BBB permeability.
ACKNOWLEDGEMENTS
This work was supported in part by National Institutes of Health grants NS058807 from NINDS and a startup fund from the University of Kansas Center for Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: Z.Z. and H.S. were responsible for study concept and design. Z. Z. was responsible for data acquisition and analysis. J. Y. was responsible for knock out animal model. Z. Z. and H.S. were responsible for drafting of the manuscript and figures and editing.
Potential Conflicts of Interest
Nothing to report.
References
- Akamatsu Y, et al. Impaired leptomeningeal collateral flow contributes to the poor outcome following experimental stroke in the Type 2 diabetic mice. J Neurosci. 2015;35:3851–64. doi: 10.1523/JNEUROSCI.3838-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almutairi MM, et al. Factors controlling permeability of the blood-brain barrier. Cell Mol Life Sci. 2016;73:57–77. doi: 10.1007/s00018-015-2050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argaw AT, et al. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci U S A. 2009;106:1977–82. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird TA, et al. Persistent poststroke hyperglycemia is independently associated with infarct expansion and worse clinical outcome. Stroke. 2003;34:2208–14. doi: 10.1161/01.STR.0000085087.41330.FF. [DOI] [PubMed] [Google Scholar]
- Baranova O, et al. Neuron-specific inactivation of the hypoxia inducible factor 1 alpha increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007;27:6320–32. doi: 10.1523/JNEUROSCI.0449-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bederson JB, et al. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–8. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, et al. Breaking the barrier in stroke: what should we know? A mini-review. Curr Pharm Des. 2012;18:3615–23. doi: 10.2174/138161212802002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briere JJ, et al. Mitochondrial succinate is instrumental for HIF1alpha nuclear translocation in SDHA-mutant fibroblasts under normoxic conditions. Hum Mol Genet. 2005;14:3263–9. doi: 10.1093/hmg/ddi359. [DOI] [PubMed] [Google Scholar]
- Capes SE, et al. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001;32:2426–32. doi: 10.1161/hs1001.096194. [DOI] [PubMed] [Google Scholar]
- Chavez JC, et al. Transient expression of hypoxia-inducible factor-1 alpha and target genes in peripheral nerves from diabetic rats. Neurosci Lett. 2005;374:179–82. doi: 10.1016/j.neulet.2004.10.050. [DOI] [PubMed] [Google Scholar]
- Chen C, et al. Multiple effects of 2ME2 and D609 on the cortical expression of HIF-1alpha and apoptotic genes in a middle cerebral artery occlusion-induced focal ischemia rat model. J Neurochem. 2007;102:1831–41. doi: 10.1111/j.1471-4159.2007.04652.x. [DOI] [PubMed] [Google Scholar]
- Chen W, et al. HIF-1alpha inhibition ameliorates neonatal brain injury in a rat pup hypoxicischemic model. Neurobiol Dis. 2008;31:433–41. doi: 10.1016/j.nbd.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolla MJ, et al. Inhibition of protein kinase Cbeta reverses increased blood-brain barrier permeability during hyperglycemic stroke and prevents edema formation in vivo. Stroke. 2011;42:3252–7. doi: 10.1161/STROKEAHA.111.623991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold I, et al. The HIF1 target gene NOX2 promotes angiogenesis through urotensin-II. J Cell Sci. 2012;125:956–64. doi: 10.1242/jcs.094060. [DOI] [PubMed] [Google Scholar]
- Fanne RA, et al. Insulin and glucagon share the same mechanism of neuroprotection in diabetic rats: role of glutamate. Am J Physiol Regul Integr Comp Physiol. 2011;301:R668–73. doi: 10.1152/ajpregu.00058.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, et al. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile NT, et al. Decreased mortality by normalizing blood glucose after acute ischemic stroke. Acad Emerg Med. 2006;13:174–80. doi: 10.1197/j.aem.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Gray CS, et al. Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol. 2007;6:397–406. doi: 10.1016/S1474-4422(07)70080-7. [DOI] [PubMed] [Google Scholar]
- Guo S, et al. Specific inhibition of HIF-1 exaggerates cell injury induced by in vitro ischemia through deteriorating cellular redox environment. J Neurochem. 2009;108:1309–21. doi: 10.1111/j.1471-4159.2009.05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haligur M, et al. Early degenerative effects of diabetes mellitus on pancreas, liver, and kidney in rats: an immunohistochemical study. Exp Diabetes Res. 2012;2012:120645. doi: 10.1155/2012/120645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MG, et al. Insulin reduction of cerebral infarction due to transient focal ischemia. J Neurosurg. 1995;82:262–8. doi: 10.3171/jns.1995.82.2.0262. [DOI] [PubMed] [Google Scholar]
- Harada S, et al. The development of glucose intolerance after focal cerebral ischemia participates in subsequent neuronal damage. Brain Res. 2009;1279:174–81. doi: 10.1016/j.brainres.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Helgason CM. Blood glucose and stroke. Stroke. 1988;19:1049–53. doi: 10.1161/01.str.19.8.1049. [DOI] [PubMed] [Google Scholar]
- Hewitson KS, et al. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J Biol Chem. 2007;282:3293–301. doi: 10.1074/jbc.M608337200. [DOI] [PubMed] [Google Scholar]
- Huber JD, et al. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am J Physiol Heart Circ Physiol. 2006;291:H2660–8. doi: 10.1152/ajpheart.00489.2006. [DOI] [PubMed] [Google Scholar]
- Isaacs JS, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–53. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Isoe T, et al. High glucose activates HIF-1-mediated signal transduction in glomerular mesangial cells through a carbohydrate response element binding protein. Kidney Int. 2010;78:48–59. doi: 10.1038/ki.2010.99. [DOI] [PubMed] [Google Scholar]
- Jones NM, Bergeron M. Hypoxic preconditioning induces changes in HIF-1 target genes in neonatal rat brain. J Cereb Blood Flow Metab. 2001;21:1105–14. doi: 10.1097/00004647-200109000-00008. [DOI] [PubMed] [Google Scholar]
- Jung JE, et al. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41:172–9. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagansky N, et al. The role of hyperglycemia in acute stroke. Arch Neurol. 2001;58:1209–12. doi: 10.1001/archneur.58.8.1209. [DOI] [PubMed] [Google Scholar]
- Kennedy JM, Zochodne DW. Experimental diabetic neuropathy with spontaneous recovery: is there irreparable damage? Diabetes. 2005;54:830–7. doi: 10.2337/diabetes.54.3.830. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, et al. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–42. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Koivunen P, et al. Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem. 2007;282:4524–32. doi: 10.1074/jbc.M610415200. [DOI] [PubMed] [Google Scholar]
- Kouroedov A, et al. Selective inhibition of protein kinase Cbeta2 prevents acute effects of high glucose on vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 2004;110:91–6. doi: 10.1161/01.CIR.0000133384.38551.A8. [DOI] [PubMed] [Google Scholar]
- Kruyt ND, et al. Hyperglycemia in acute ischemic stroke: pathophysiology and clinical management. Nat Rev Neurol. 2010;6:145–55. doi: 10.1038/nrneurol.2009.231. [DOI] [PubMed] [Google Scholar]
- Latour LL, et al. Early blood-brain barrier disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–77. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- Lim JI, et al. A comparison of hypoxia-inducible factor-alpha in surgically excised neovascular membranes of patients with diabetes compared with idiopathic epiretinal membranes in nondiabetic patients. Retina. 2010;30:1472–8. doi: 10.1097/IAE.0b013e3181d6df09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, et al. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cerebr. Blood Flow Metab. 2004;24:343–9. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- Liu W, et al. Normobaric hyperoxia protects the blood brain barrier through inhibiting Nox2 containing NADPH oxidase in ischemic stroke. Med Gas Res. 2011;1:22. doi: 10.1186/2045-9912-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly A, et al. Early inner retinal astrocyte dysfunction during diabetes and development of hypoxia, retinal stress, and neuronal functional loss. Invest Ophthalmol Vis Sci. 2011;52:9316–26. doi: 10.1167/iovs.11-7879. [DOI] [PubMed] [Google Scholar]
- MacKenzie ED, et al. Cell-permeating alpha-ketoglutarate derivatives alleviate pseudohypoxia in succinate dehydrogenase-deficient cells. Mol Cell Biol. 2007;27:3282–9. doi: 10.1128/MCB.01927-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, et al. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia. 2010;53:1783–94. doi: 10.1007/s00125-010-1770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marfella R, et al. Myocardial infarction in diabetic rats: role of hyperglycaemia on infarct size and early expression of hypoxia-inducible factor 1. Diabetologia. 2002;45:1172–81. doi: 10.1007/s00125-002-0882-x. [DOI] [PubMed] [Google Scholar]
- Martini SR, Kent TA. Hyperglycemia in acute ischemic stroke: a vascular perspective. J Cereb Blood Flow Metab. 2007;27:435–51. doi: 10.1038/sj.jcbfm.9600355. [DOI] [PubMed] [Google Scholar]
- Masrur S, et al. Association of Acute and Chronic Hyperglycemia With Acute Ischemic Stroke Outcomes Post-Thrombolysis: Findings From Get With The Guidelines-Stroke. J Am Heart Assoc. 2015;4:e002193. doi: 10.1161/JAHA.115.002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride DW, et al. Acute Hyperglycemia Is Associated with Immediate Brain Swelling and Hemorrhagic Transformation After Middle Cerebral Artery Occlusion in Rats. Acta neurochirurgica. Supplement. 2016;121:237–41. doi: 10.1007/978-3-319-18497-5_42. [DOI] [PubMed] [Google Scholar]
- Murray AJ, et al. Plasma free fatty acids and peroxisome proliferator-activated receptor alpha in the control of myocardial uncoupling protein levels. Diabetes. 2005;54:3496–502. doi: 10.2337/diabetes.54.12.3496. [DOI] [PubMed] [Google Scholar]
- Nanduri J, et al. HIF-1alpha activation by intermittent hypoxia requires NADPH oxidase stimulation by xanthine oxidase. PLoS One. 2015;10:e0119762. doi: 10.1371/journal.pone.0119762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neitzel AS, et al. Chylomicron and palmitate metabolism by perfused hearts from diabetic mice. Am J Physiol Endocrinol Metab. 2003;284:E357–65. doi: 10.1152/ajpendo.00380.2002. [DOI] [PubMed] [Google Scholar]
- Ogle ME, et al. Inhibition of prolyl hydroxylases by dimethyloxaloylglycine after stroke reduces ischemic brain injury and requires hypoxia inducible factor-1alpha. Neurobiol Dis. 2012;45:733–42. doi: 10.1016/j.nbd.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, et al. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol Cell Biol. 2007;27:912–25. doi: 10.1128/MCB.01223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagia M, et al. PPAR-alpha activation required for decreased glucose uptake and increased susceptibility to injury during ischemia. Am J Physiol Heart Circ Physiol. 2005;288:H2677–83. doi: 10.1152/ajpheart.00200.2004. [DOI] [PubMed] [Google Scholar]
- Piconi L, et al. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab Res Rev. 2006;22:198–203. doi: 10.1002/dmrr.613. [DOI] [PubMed] [Google Scholar]
- Pollard PJ, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–9. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- Pouliot M, et al. Ocular application of the kinin B1 receptor antagonist LF22-0542 inhibits retinal inflammation and oxidative stress in streptozotocin-diabetic rats. PLoS One. 2012;7:e33864. doi: 10.1371/journal.pone.0033864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, et al. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: relevance to stroke recovery. Stroke. 2013;44:2875–82. doi: 10.1161/STROKEAHA.113.001660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizk NN, et al. Cerebral ischemia-induced apoptosis and necrosis in normal and diabetic rats: effects of insulin and C-peptide. Brain Res. 2006;1096:204–12. doi: 10.1016/j.brainres.2006.04.060. [DOI] [PubMed] [Google Scholar]
- Rogers DC, et al. Correlation between motor impairment and infarct volume after permanent and transient middle cerebral artery occlusion in the rat. Stroke. 1997;28:2060–5. doi: 10.1161/01.str.28.10.2060. [DOI] [PubMed] [Google Scholar]
- Rosamond W, et al. Heart disease and stroke statistics--2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- Rosso C, et al. Hyperglycaemia, insulin therapy and critical penumbral regions for prognosis in acute stroke: further insights from the INSULINFARCT trial. PloS one. 2015;10:e0120230. doi: 10.1371/journal.pone.0120230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan HE, et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–5. [PubMed] [Google Scholar]
- Seelbach MJ, et al. Peripheral inflammatory hyperalgesia modulates morphine delivery to the brain: a role for P-glycoprotein. J Neurochem. 2007;102:1677–90. doi: 10.1111/j.1471-4159.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- Selak MA, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–6. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra-Perez A, et al. Extended ischemia prevents HIF1alpha degradation at reoxygenation by impairing prolyl-hydroxylation: role of Krebs cycle metabolites. J Biol Chem. 2010;285:18217–24. doi: 10.1074/jbc.M110.101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao B, Bayraktutan U. Hyperglycaemia promotes cerebral barrier dysfunction through activation of protein kinase C-beta. Diabetes Obes Metab. 2013;15:993–9. doi: 10.1111/dom.12120. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5:437–48. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- Suh SW, et al. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–63. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, et al. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–51. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi K, et al. The effect of ritanserin, a 5-HT2 receptor antagonist, on ischemic cerebral blood flow and infarct volume in rat middle cerebral artery occlusion. Stroke. 1994;25:481–5. doi: 10.1161/01.str.25.2.481. [DOI] [PubMed] [Google Scholar]
- Tang L, et al. Valsartan inhibited HIF-1alpha pathway and attenuated renal interstitial fibrosis in streptozotocin-diabetic rats. Diabetes Res Clin Pract. 2012;97:125–31. doi: 10.1016/j.diabres.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Tannahill GM, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–42. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CM, et al. Direct renin inhibition prevents cardiac dysfunction in a diabetic mouse model: comparison with an angiotensin receptor antagonist and angiotensin-converting enzyme inhibitor. Clin Sci (Lond) 2013;124:529–41. doi: 10.1042/CS20120448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruta R, et al. Hyperglycemia enhances excessive superoxide anion radical generation, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Brain Res. 2010;1309:155–63. doi: 10.1016/j.brainres.2009.10.065. [DOI] [PubMed] [Google Scholar]
- Urban MJ, et al. Inhibiting heat-shock protein 90 reverses sensory hypoalgesia in diabetic mice. ASN Neuro. 2010;2:e00040. doi: 10.1042/AN20100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, et al. Intravitreous vascular endothelial growth factor and hypoxia-inducible factor 1a in patients with proliferative diabetic retinopathy. Am J Ophthalmol. 2009;148:883–9. doi: 10.1016/j.ajo.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Wass CT, et al. Insulin treatment of corticosteroid-associated hyperglycemia and its effect on outcome after forebrain ischemia in rats. Anesthesiology. 1996;84:644–51. doi: 10.1097/00000542-199603000-00020. [DOI] [PubMed] [Google Scholar]
- Yan J, et al. HIF-1 is involved in high glucose-induced paracellular permeability of brain endothelial cells. Cell Mol Life Sci. 2012;69:115–28. doi: 10.1007/s00018-011-0731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WL, et al. Inhibition of hypoxia-induced increase of blood-brain barrier permeability by YC-1 through the antagonism of HIF-1alpha accumulation and VEGF expression. Mol Pharmacol. 2007;72:440–9. doi: 10.1124/mol.107.036418. [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. Hypoxia-inducible factor directs POMC gene to mediate hypothalamic glucose sensing and energy balance regulation. PLoS Biol. 2011;9:e1001112. doi: 10.1371/journal.pbio.1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, et al. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000;106:829–38. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, et al. Correlation of VEGF and angiopoietin expression with disruption of blood-brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:379–92. doi: 10.1097/00004647-200204000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.