Abstract
During acute infections, naïve antigen-specific CD8 T cells are activated and differentiate into effector T cells, the majority of which undergo contraction after pathogen clearance. A small population of CD8 T cells persists as memory to protect against future infections. We investigated the role of adhesion and degranulation promoting adapter protein (ADAP) in promoting CD8 T cell responses to a systemic infection. Naïve antigen-specific CD8 T cells lacking ADAP exhibited a modest expansion defect early after Listeria monocytogenes or vesicular stomatitis virus infection but comparable cytolytic function at the peak of response. However, reduced numbers of ADAP-deficient CD8 T cells were present in the spleen after the peak of the response. ADAP deficiency resulted in a greater frequency of CD127+ CD8 memory precursors in secondary lymphoid organs during the contraction phase. Reduced numbers of ADAP-deficient KLRG1− resident memory CD8 T cell (TRM) precursors were present in a variety of non-lymphoid tissues at the peak of the immune response, and consequently the total numbers of ADAP-deficient TRM were reduced at memory time points. TRM cells that did form in the absence of ADAP were defective in effector molecule expression. ADAP-deficient TRM cells exhibited impaired effector function after Ag re-challenge, correlating with defects in their ability to form T:APC conjugates. However, ADAP-deficient TRM cells responded to TGFβ signals and recruited circulating memory CD8 T cells. Thus, ADAP regulates CD8 T cell differentiation events following acute pathogen challenge that are critical for the formation and select functions of TRM cells in non-lymphoid tissues.
INTRODUCTION
In response to pathogenic infection, naive antigen-specific CD8 T cells differentiate into effectors, expand and migrate to non-lymphoid tissues (NLTs)2 (1–3). As the infection is resolved, these effector CD8 T cells die en masse and a population of long-lived memory CD8 T cells are produced and stably maintained (1). Effector CD8 T cells expressing the receptor for IL-7 (IL-7Rα, CD127) and negative for killer cell lectin-like receptor G1 (KLRG1) are more likely to survive contraction and are present in the circulation and in secondary lymphoid organs (SLOs) during memory (4, 5). Memory T cells with the cell surface phenotype KLRG1hi CD127int CD27lo CD62Llo preferentially localize to the red pulp of the spleen and NLTs to provide robust protective immunity, in spite of suboptimal recall proliferation (6). Moreover KLRG1lo CD8 T cells are the predominant population that gives rise to resident memory CD8 T (TRM) cells, a newly defined lineage of CD8 memory T cells that are seeded into NLTs following infection but do not recirculate (7). As a whole, CD8 memory T cells protect the host from secondary infection by being present in SLOs, blood and NLTs and responding to infection by proliferating and eliciting effector functions (1).
The initial interaction between the naïve T cell and antigen presenting cell (APC) can dictate the formation and function of memory CD8 T cells. Naïve T cell contact with cognate antigen initiates TCR-signaling events that culminate in increased adhesion of the T cell to the APC and initiation of transcriptional pathways (8, 9). Reduced TCR signaling (10, 11), or reduced T-APC interactions (12) prevents effective priming and results in reduced cytotoxic T lymphocyte (CTL) functions and altered memory generation. These studies have suggested that molecules that regulate positive signaling from the TCR to transcriptional pathways and T-APC adhesion are vital for balancing the production of terminally-differentiated effectors and memory cells.
Adhesion and degranulation promoting adapter protein (ADAP) is a cytosolic adapter protein that coordinates the formation of signaling complexes after TCR signaling. ADAP positively regulates both T-APC interactions and NF-κB and JNK transcriptional pathways (9, 13–17). A fraction of ADAP is constitutively associated with Src kinase-associated phosphoprotein of 55 kDa (SKAP55) (18). The ADAP-SKAP55 signaling module promotes optimal T-APC interactions by facilitating TCR inside-out signaling to integrins (18, 19). ADAP not associated with SKAP55 positively regulates the activation of NF-κB and JNK in a TCR-inducible manner (15–18). The downstream effects of TCR-inducible interactions of ADAP with caspase recruitment domain (CARD) membrane-associated guanylate kinase (MAGUK) protein 1 (CARMA-1) and, transforming growth factor-β (TGF-β)-activated protein kinase (TAK-1) promote T cell entry into the cell cycle (9, 15–17). The ADAP-CARMA-1-TAK-1 signalosome is also required for cytokine and chemokine production by NK cells after NKG2D or CD137 stimulation (20).
Although we and others have demonstrated defects in T-APC contacts, entry of T cells into the cell cycle, proliferation, differentiation and survival in the absence of ADAP (13–17, 19, 21), several recent reports have documented a negative regulatory function for ADAP in T cells (22, 23). We have recently shown that ADAP-deficient T cells have an enhanced response to weak agonist peptide ligands in naïve CD8 T cells (22). In addition, uninfected ADAP-deficient mice have an enhanced frequency and number of CD8 T cells with a memory phenotype that is associated with enhanced responsiveness of ADAP-deficient CD8 T cells to the homeostatic cytokine IL-15 (22). Additional recent reports indicate that ADAP−/ − CTLs show enhanced cytolytic function both in vitro and in vivo (23), although CD8 CTLs lacking ADAP exhibit normal cytotoxicity in response to an allogeneic graft (24, 25). In this study, we examined the response of ADAP-deficient CD8 T cells to a systemic infection. We show that while lack of ADAP modestly impairs clonal expansion, it does not impinge on effector function, and that ADAP deficiency results in strikingly enhanced contraction, changes in the frequency of memory CD8 T cell precursors, and a reduced number of CD8 memory T cells in both secondary lymphoid organs and NLTs.
MATERIALS AND METHODS
Mice
C57BL/6 (B6) wild-type and ADAP−/− mice were generated as previously described (13). OT-I ADAP−/− mice were generated by crossing ADAP−/− mice with OT-I mice (The Jackson Laboratory, Bar Harbor, ME). Thy1.1+ P14 transgenic mice were fully backcrossed to C57BL/6J and maintained in our colony (26). B6 CD45.1 recipient mice were purchased from The Jackson Laboratory. Mice were housed in specific pathogen-free facilities at the University of Minnesota. All experimental protocols involving the use of mice were approved by the Institutional Animal Care and Use Committee at the University of Minnesota.
Flow cytometry and reagents
Cell surface staining for flow cytometry was performed with ice-cold HBSS supplemented with 2% bovine serum (FACS buffer). Single cell suspensions were washed with FACS buffer, stained with surface marker antibodies, then washed twice before multi-parameter flow cytometric detection on a BD LSRFortessa (Becton Dickinson, San Jose, CA, USA). For IFN-γ staining on day 7 post infection, splenocytes were harvested and single cell suspensions were incubated in complete T cell media ± 10 ng/ml SIINFEKL peptide for 5 hours at 37°C in the presence of Golgi Plug (BD Biosciences) at a 1:1000 final dilution, then washed twice with FACS buffer. Intracellular staining for IFN-γ (1:100 dilution) or Granzyme B (1:50 dilution) was performed using the BD Cytofix/Cytoperm kit (BD Biosciences). Briefly, cells were stained with surface antibodies, washed with FACS buffer, fixed with 300 μl BD Cytofix/Cytoperm, and incubated at 4°C for 30 min. Cells were washed twice with 1X BD Perm/Wash buffer then incubated with intracellular staining antibodies for 30 min. Cells were washed twice with 1X BD Perm/Wash buffer, and resuspended in FACS buffer for flow cytometry. Directly conjugated fluorescent antibodies used include: CD4 (clone GK1.5), CD8α (clone 53–6.7), CD45.1 (clone A20), CD45.2 (clone 104), B220 (clone RA3-6B2) and KLRG1 (clone 2F1) (Tonbo); CD8-β (clone YTS156.7.7); CD16/32 (clone 93), CD69 (clone H1.2F3); CD127 (clone SB/199), I-Ab (clone 25-9-17), Ter-119 (clone TER-119), Vα2 (clone B20.1), CD29 (clone HMB1-1), LPAM (clone DATK32) (Biolegend); CD43 (clone 1B11); CD44 (clone IM7) and CD103 (clone M290), Thy1.1 (Clone OX-7) (BD Biosciences); CD27 (clone LG.7F9); F4/80 (clone BM8), CD11a (clone M17/4), and IFN-γ (clone XMG1.2) (eBioscience); Granzyme B (clone GB11) (Invitrogen). Complete T cell media (TCPM) consisted of RPMI 1640 supplemented with 10% FCS, 4 mM L-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U/ml penicillin and streptomycin, 10 mM HEPES, and 5 mM 2-ME.
Negative selection of naïve OT-I T cells, adoptive transfer, pathogen challenge and local peptide challenge
Lymph nodes were harvested from wild-type (CD45.1/2) and ADAP−/− (CD45.2) mice and CD8 naïve mature T cells were isolated by negative magnetic bead enrichment similar to previously described methods (27). Briefly, single-cell suspensions were incubated with the following FITC-conjugated antibodies: CD4, B220, F4/80, CD16/32, I-Ab, Ter119, and CD44. After washing, cells were incubated with anti-FITC microbeads and passed over LS columns on magnets according to manufacturer’s instructions (Miltentyi Biotec, Auburn, CA) to capture non-CD8 cells. Purity of the flow-through fraction was > 95% CD8+ CD44lo CD122lo. Purified cells were cotransferred into CD45.1 recipients at a 1:1 ratio, and unless noted otherwise, either 3 x 103 or 1 x 104 cells of each genotype were transferred. Mice were challenged with 500 CFU of Listeria monocytogenes-OVA (LM-OVA) (retro-orbital) (28) or 1 x 106 PFU of vesicular stomatitis virus (VSV-OVA) (tail vein) (28, 29). For secondary challenge of LM-OVA mice, animals were given 1 x 106 PFU of VSV-OVA. Local SIINFEKL peptide stimulation was given transcervically (t.c.) with 50 μg of peptide, as described (29), and FRT was harvested 12 hours post challenge, as described below. For circulating memory P14 recruitment, 9 x 104 naïve P14 Thy1.1+ T cells were transferred to Thy 1.2+ hosts and challenged with 2 x 105 PFU of LCMV. After 30+ days memory P14 cells were enriched from the peripheral lymph nodes and spleen by negative selection using the Easy Sep™ Mouse CD8+ T cell enrichment kit (Stem Cell Technologies) according to the manufacturer’s instructions, and 5 x 105 cells were transferred into hosts containing WT or ADAP−/− OT-I T cells on D30+ challenge with VSV-OVA.
BrdU labeling and detection
BrdU labeling and detection was performed as described (30). Briefly, to label actively dividing OT-I T cells on days 6–8 post infection, mice were injected with 1 mg of BrdU (Sigma-Aldrich) i.p. and spleens were harvested 5 hours post injection. Cells were processed and surface staining was performed as described above. Cells were fixed and permeabilized with the BD Cytofix/Cytomerm kit as per the manufacturer’s instructions, incubated with 1 mg/ml DNase I (Sigma-Aldrich) for 60 min at 37°C, washed and stained with anti-BrdU (Invitrogen).
In vivo killing assay
An in vivo killing assay was performed similarly to a previously described study (31). Bulk splenocytes (CD45.1/2) were used as targets. Cells were labeled with an intravital dye, CFSE (Invitrogen). Briefly, cells were resuspended in PBS with 5% FBS at 2 x 107 cells/ml. CFSE diluted in PBS was added 1:1 to the cells [5 μM (hi) or 0.5 μM (lo) final concentrations] and incubated at 25°C for 5 min. Cells were quenched by washing twice with PBS with 2% FBS. An aliquot of CFSElo labeled cells were pulsed with 10 nM of SIINFEKL peptide for 30 min at 37°C. Cells were washed twice before mixing 1:1 with CFSEhi labeled cells. Mixed populations of peptide pulsed or unpulsed cells were transferred into mice (CD45.1) that had received WT (CD45.2) or ADAP−/− (CD45.2) OT-I T cells and challenged with LM-OVA 7 days prior. After 1–5 hours, splenocytes were harvested and the ratio of CFSElo:CFSEhi was determined by flow cytometry. Specific killing is calculated by: (1− (control ratio/experimental ratio)) x 100 (31).
In vivo intravenous injection of anti-CD8 and lymphocyte isolation from NLTs
To discriminate blood-borne cells from parenchymal cells, mice were given an intravenous injection of anti-CD8α (5 μg) for 3 min, as described (32). Mice were euthanized and organs were harvested. Single cell suspensions of lymph nodes and spleen were processed as described above. Salivary glands were processed as described previously (3). Briefly, salivary glands were minced and incubated at 37°C in a solution of RPMI 1640/10%FCS/2 mM MgCl2/2mM CaCl2/HEPES/L-glutamine medium containing 100U/ml collagenase type I (Worthington Biochemical) with mixing for 45min. Female reproductive tract (FRT), including the uterine horns, cervix and vaginal tissue, were processed as described (26). Briefly, the FRT was removed and minced into small pieces, then incubated in a solution of RPMI 1640/10%FCS/2 mM MgCl2/2mM CaCl2/HEPES/L-glutamine medium containing 50 mg/100 ml collagenase type IV (Sigma) for 1 hour at 37°C. Lymphocytes from all NLTs were purified on a 44/67% Percoll gradient (800 x g at 20°C for 20 min).
Ex vivo functional analysis of memory cells isolated from NLTs
To evaluate the ability of memory CD8 T cells to form conjugates with APCs, naïve WT (Thy1.1 or CD45.1) and ADAP−/− (CD45.1) OT-I T cells were transferred into CD45.2 mice and the mice infected with VSV-OVA as described above. After 120–160 days, spleen, SG, and FRT were removed and processed as described above to generate single cell suspensions that were then re-suspended in 10 ml (spleen) or 1.1 ml (SG and FRT) of TCPM.
Ex vivo conjugate assays were performed by modifying our previously described conjugate assay (14, 15, 18, 21, 22), using splenic dendritic cells enriched from mice treated for 14 days with a B16 tumor expressing FLT3 ligand (33). Briefly, single cell suspensions obtained from spleens of FLT3 conditioned mice, which contained 25–35% CD11c+MHCII+ dendritic cells, were labeled with Cell Tracker Orange (CTO, Thermo Fisher) as previously described (14, 15, 18, 21, 22) and then pulsed with the indicated doses of SIINFEKL peptide for 30 mins at 37C in warm TCPM, washed, and resuspended at 5 x 106 cells/ml in pre-warmed TCPM. 100 μl (0.5 x 106) of peptide-pulsed splenocytes were added to triplicate wells of a 96-well round-bottom plate, followed by the addition of 100 μl of pre-warmed unlabeled lymphocytes isolated from the spleens, SG, or FRT of VSV-OVA infected mice isolated as described above. The plate was immediately spun down for 30s to pellet the cells together, incubated for exactly 10 min at 37C, centrifuged briefly again, supernatant dumped, and finally the pellets were vortexed vigorously for 30s on a plate shaker. Wells were then immediately fixed by the addition of 2% paraformaldehyde for 20 min, washed, and then stained for CD8α, CD45.1, Thy1.1, CD11c, B220, and MHCII. Conjugates were defined as memory OT-I T cells (CD8α+ and CD45.1+ or Thy1.1+) that were also CTO+. The majority of conjugates within this CTO+ population were formed with CD11c+ or B220+ and MHCIIhi cells.
To analyze the capacity of memory OT-I cells to respond to TGFβ signals, single cell suspensions isolated from memory spleen, SG and FRT tissues as described above were resuspended in T cell media as described above and 100 μl aliquoted into FACS tubes and pre-warmed to 37C for 30–60 mins. Samples were then simulated by adding 100 μl of control TPCM or TCPM containing 40 ng/ml TGFβ (RnD Systems, Minneapolis, MN) to yield a final concentration of 20 ng/ml TGFβ. After 20 mins at 37C, signaling was stopped by the addition of 16% PFA to 1% final concentration and the samples were incubated an additional 10 min at 37C to fix cells, followed by the addition of 2 ml of ice-cold PBS. Cells were then spun down and resuspended in 500 μl of 95% ice-cold methanol in water and permeabilized for 30 min on ice, washed 1x with PBS, washed 1x FACS buffer, and then stained for CD8α, CD8β, CD45.1 and/or Thy1.1, and phospho-SMAD2/3-PE (pSMAD2 Ser 465/467/pSMAD3 423/425; Cell Signaling, clone D27F4) at a dilution of 1:100 for 30 min on ice. Cells were washed with FACS buffer and immediately analyzed by flow cytometry.
Statistics
Graphpad Prism (version 5.03, Graphpad Software, La Jolla, CA) was used to determine statistical significance using Student’s unpaired two-tailed t tests. The p value cutoffs and notation were used as follows: *, p < 0.05; **, p < 0.01; ***, p < 0.001. For ADAP−/− numbers as a percent of WT analysis, statistics were performed using a normalized value = 100.
RESULTS
Altered immune response to Listeria monocytogenes in the absence of ADAP
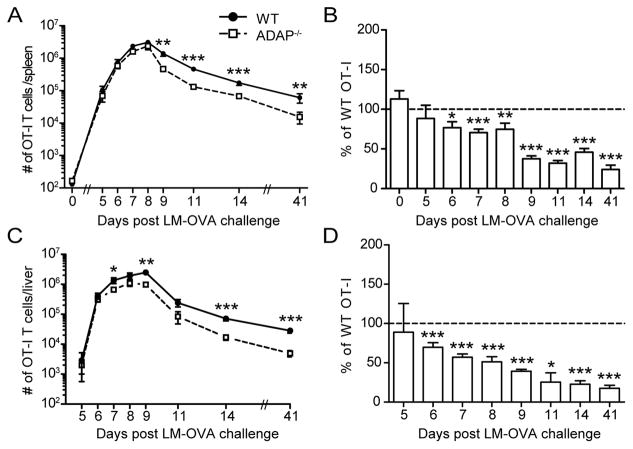
We evaluated the response of ADAP-deficient CD8 T cells in vivo following infection of mice with the model pathogen, Listeria monocytogenes (LM). Since ADAP-deficient mice contain an elevated number of memory phenotype CD8 T cells when compared with wild-type mice (22), we utilized an adoptive transfer approach. We isolated naïve OT-I CD8 T cells from wild-type and ADAP-deficient peripheral lymph nodes (pLNs) and co-transferred these cells at an equal ratio into naïve recipients. To be sure that we were analyzing the response of naïve CD8 T cells, we verified the expression of CD122 and CD44 after purification and only transferred CD8 T cells with greater than 98% of CD122lo CD44lo populations (Supplemental Fig. 1A). A genetically modified LM expressing ovalbumin (LM-OVA) was used to challenge recipient animals, generating a robust expansion of wild-type T cells (Fig. 1A) (28). Although ADAP is required for optimal high affinity T-APC interactions and proliferation by CD4 T cells in response to a peptide Ag (14), clonal expansion of ADAP-deficient OT-I CD8 T cells measured in the spleen after LM-OVA infection was robust and relatively similar to WT OT-I CD8 T cells (Fig. 1A). However, a small but statistically insignificant decrease in the total number of ADAP−/− OT-I cells was observed near the peak of the response (Fig. 1A, day 7–8). However, analysis of ADAP-deficient OT-I T cell frequency as a percentage of wild-type revealed that statistically significant reductions in ADAP-deficient CD8 T cell numbers were present on days 6–8 post challenge (Fig. 1B). Additionally, more striking differences were observed during the contraction phase of the immune response between day 8 and day 14 post challenge (Fig. 1A–B). To further investigate the reduction of ADAP-deficient OT-I T cells on days 6–8 post challenge, we assessed late state proliferation by incorporation of BrdU. We were not able to detect statistically significant differences in BrdU accumulation at days 6–8 post infection (Supplemental Fig. 1 B and C). We also assessed OT-I T cells in the liver, a site known to be infected by Listeria monocytogenes. We observed a reduced number and frequency of ADAP-deficient T cells in the liver beginning at day 6 after LM-OVA infection, and at all times after the peak of the immune response (Fig. 1C–D). Thus, ADAP is not required for initial CD8 T cell responses to LM-OVA, but appears to be required for maintenance during contraction and formation of memory cells in both the spleen and in infected tissues.
Figure 1. ADAP is not required for initial expansion, but is required for optimal survival to memory after LM-OVA challenge.
Naïve WT (CD45.1/2) and ADAP−/− (CD45.2) OT-I T cells were cotransferred into CD45.1 hosts and challenged with LM-OVA. Splenocytes (A–B) or lymphocytes from the liver (C–D) were isolated and stained for cell surface markers. (A and C) Number of WT (black circles) or ADAP−/− (open squares) OT-I T cells after LM-OVA challenge. (B and D) The frequency of ADAP−/− OT-I T cells at each time point is expressed as a percentage of WT OT-I T cells. The results are compiled from 12 independent experiments, with at least 4 mice per time point (± SEM). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Loss of ADAP does not alter effector T cell responses
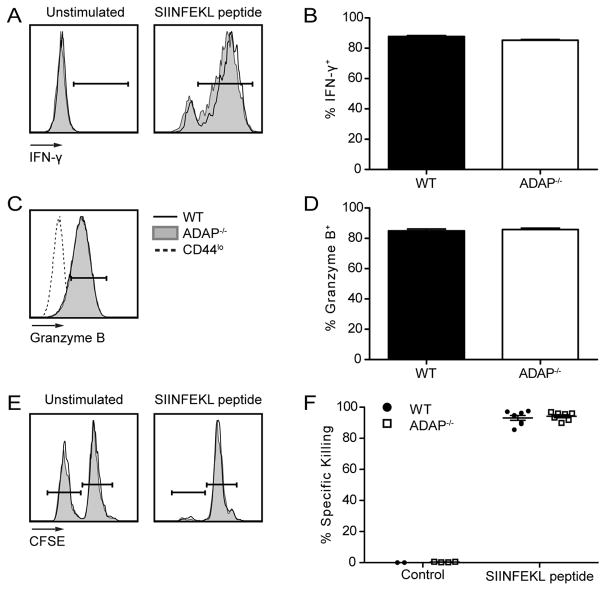
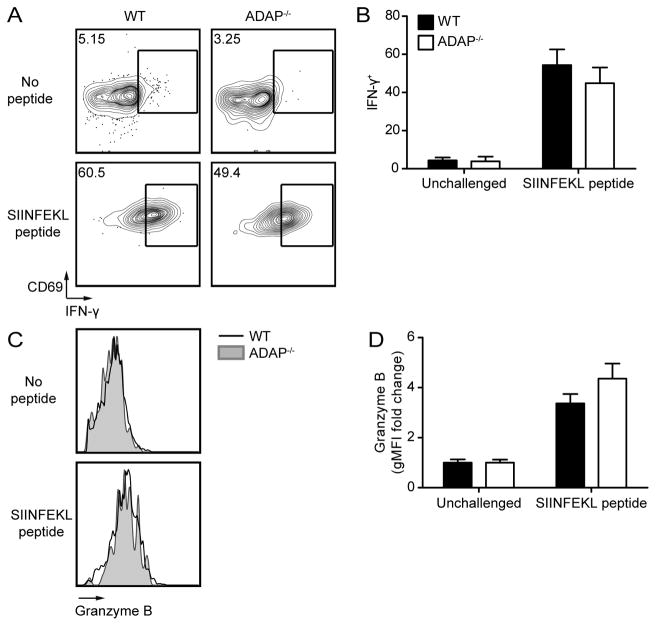
CD8 CTLs are vital for the immune response against LM (34). CD8 CTLs produce effector cytokines IFN-γ and Granzyme B, and specifically kill antigen-expressing cells (31, 35, 36). To test the requirement for ADAP in CTL functions, we analyzed effector functions on day 7 post LM-OVA challenge. The production of IFN-γ was similar between wild-type and ADAP-deficient CD8 T cells (Fig. 2A and B). Similarly, Granzyme B expression remained unaltered in the absence of ADAP (Fig. 2C and D). To more specifically test CTL function, we performed an in vivo cytotoxicity assay (31). We challenged mice with LM-OVA after single transfer of wild-type or ADAP-deficient OT-I T cells. On day 7 post challenge, SIINFEKL peptide pulsed target cells were transferred and specific killing was assessed 1–5 hours later. Both wild-type and ADAP-deficient OT-I T cells efficiently killed peptide-pulsed cells at all time points analyzed (Fig. 2E–F and Supplemental Fig. 1F). Consistent with previous findings (24, 25), we conclude that ADAP is not required for CD8 CTL functions.
Figure 2. Development of effector functions in CD8 T cells is independent of ADAP.
LM-OVA challenged mice were generated as in Figure 1. Splenocytes were isolated and stained for cell surface markers and intracellular proteins on day 7 post LM-OVA challenge. (A–B) Splenocytes were stimulated ex vivo with SIINFEKL peptide for 5 hours. (A) IFN-γ staining of unstimulated (left) and SIINFEKL stimulated (right) WT (black) and ADAP−/− (grey shaded) OT-I T cells. Gate indicates cells that are positive for IFN-γ. (B) Percentage of IFN-γ+ WT (black bar) or ADAP−/− (white bar) OT-I T cells in the spleen, based on the gate shown in A. (C) Granzyme B staining of WT or ADAP−/− OT-I T cells, or CD44lo host cells (dashed line). (D) Percentage of Granzyme B+ WT or ADAP−/− OT-I T cells in the spleen, based on the gate shown in C. (E–F) WT (CD45.2) or ADAP−/− (CD45.2) OT-I T cells were transferred into separate CD45.1 hosts, and challenged with LM-OVA. On day 7 post challenge, an in vivo killing assay was performed as described in Materials and Methods. Spleens were harvested 5 hours after transfer of targets and specific killing was determined. (E) Representative target frequencies from hosts containing WT or ADAP−/− OT-I T cells. (F) Percentage specific killing. The results (B, D and F) are compiled from at least 2 independent experiments with 4 mice per experiment (± SEM).
Altered memory generation in the absence of ADAP
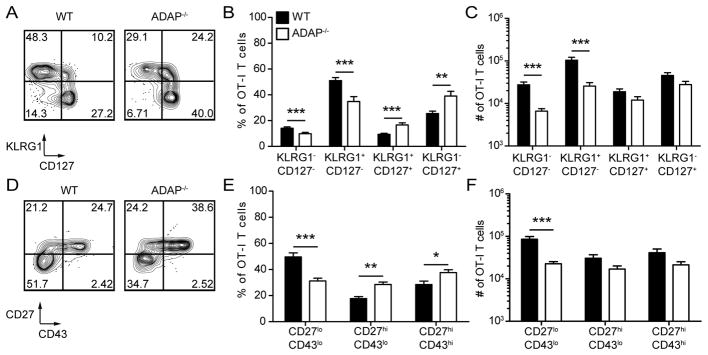
During contraction, CD8 T cells expressing higher levels of cytokine receptors are more likely to survive and be maintained as memory T cells (37). Although reduced numbers of ADAP-deficient T cells were present at memory time points (Fig. 1), a greater frequency of ADAP-deficient OT-I T cells expressed CD127 on day 14 post challenge (Fig. 3A and B). In addition, the reduced numbers of ADAP-deficient OT-I T cells can be traced to a lower frequency of CD127− populations (Fig. 3C). Of note, greater frequencies of KLRG1+ CD127+ cells and reduced frequencies of KLRG1− CD127− were also observed (Fig. 3A and B). This trend was maintained into memory time points, where frequencies and numbers of KLRG1+ memory cells were still reduced in the absence of ADAP (data not shown).
Figure 3. CD8 T cell effector differentiation is dependent on ADAP.
LM-OVA challenged mice were generated as in Figure 1. Splenocytes were isolated and stained for cell surface markers on day 14 (A–C) or day 30+ (D–E) post challenge. (A) CD127 and KLRG1 staining. Numbers represent the percentage of cells in each quadrant. (B) Percentage or (C) number of WT (black bars) or ADAP−/− (white bars) OT-I T cells from each quadrant. (D) CD43 and CD27 staining. Numbers represent the percentage of cells in each quadrant. (E) Percentage and (F) number of CD43loCD27lo, CD43loCD27hi and CD43hiCD27hi WT (black bars) or ADAP−/− (white bars) OT-I T cells. The results (B and C) are compiled from 4 independent experiments, with 4 mice per experiment (± SEM), and the results shown in (E and F) are compiled from 2 independent experiments with at least 3 mice per experiment (± SEM). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
At memory time points, the markers CD27 and CD43 can be used to identify memory CD8 T cells with recall capabilities against secondary infections (6, 38). Three main populations are observed after LM challenge: CD27hi CD43lo, CD27hi CD43hi, and CD27lo CD43lo, with the latter population mediating rapid protective immunity upon pathogen re-encounter (6). At one month post challenge, all three populations were observed in wild-type OT-I T cells at similar frequencies as previously published data (Fig. 3D and E) (6). Reduced frequencies and numbers of the CD27lo CD43lo population were observed in the absence of ADAP (Fig. 3D–F). In contrast, an increase in the frequency of both CD27hi CD43lo and CD27hi CD43hi populations does not alter the relative numbers of these populations in the absence of ADAP (Fig. 3D–F). In summary, using two distinct functional subsetting systems of memory CD8 T cells, we have found altered frequencies of several memory CD8 T cell subsets in the absence of ADAP.
Loss of ADAP does not alter memory CD8 T cell expansion after systemic secondary challenge
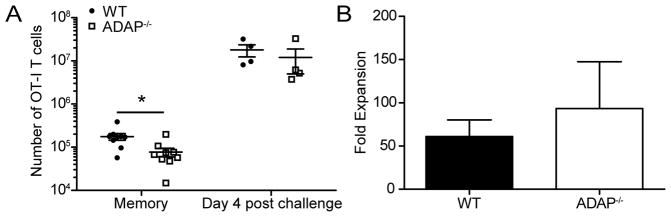
To test the memory recall potential of ADAP-deficient OT-I T cells, we transferred naïve wild-type and ADAP-deficient OT-I T cells into naïve hosts and then challenged with LM-OVA. After 45 days, these mice were then challenged with vesicular stomatitis virus expressing OVA (VSV-OVA) through an intravenous route. After 4 days, we assessed the fold expansion of wild-type and ADAP-deficient OT-I T cells. Both wild-type and ADAP-deficient OT-I T cells responded robustly to VSV-OVA secondary challenge (Fig. 4A and B). The fold expansion was not statistically different between ADAP-deficient and wild type OT-I T cells, demonstrating that there is not a defect in the proliferative response of ADAP-deficient CD8 memory T cells to a systemic secondary challenge.
Figure 4. CD8 T cell secondary proliferation is independent of ADAP.
LM-OVA challenged mice were generated as in Figure 1. At day 45+ animals were given a systemic challenge with 1 x 106 PFU of VSV-OVA. After 4 days, spleens were harvested and stained for surface molecules. (A) Number of WT (black circles) or ADAP−/− (open squares) OT-I T cells in the spleen. (B) Fold expansion after VSV-OVA challenge. Data are representative of two experiments with 4 mice per experiment. *, p < 0.05.
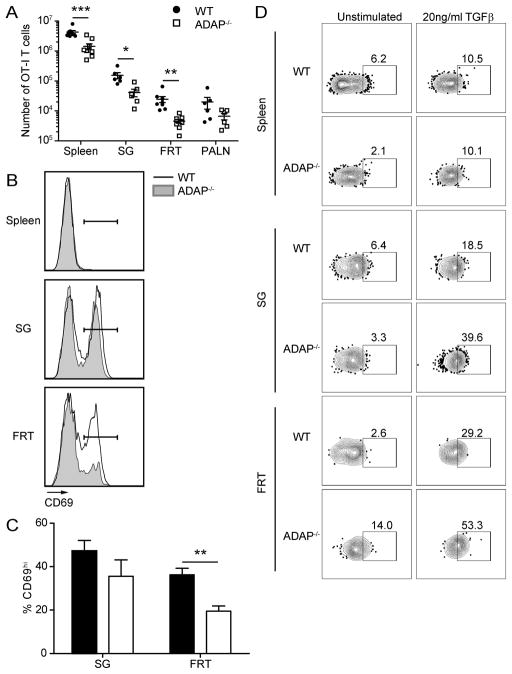
Reduced TRM cell populations at memory time points in the absence of ADAP
As we observed altered memory populations in the absence of ADAP in SLOs, we next assessed the number and phenotype of CD8 memory T cells in NLTs. Reduced numbers of ADAP-deficient OT-I T cells were observed in the small intestine (SI) intraepithelial layer (IEL) and lamina propia (LP), and salivary gland (SG) at memory time points, but not at day 7 post infection (Supplemental Figure 2B and F). We were unable to detect sufficient numbers of OT-I memory T cells in the female reproductive tract (FRT) to perform functional assays after infection with LM-OVA. Therefore, we instead performed the primary challenge with VSV-OVA, which also generates a systemic infection that results in the localization of wild-type memory CD8 T cells to NLTs. As with LM-OVA, when we examined the spleens of VSV-OVA infected animals we observed equivalent expansion of wild-type and ADAP−/− OT-I T cells, and increased contraction of ADAP-deficient T cells (data not shown). We also analyzed the blood of animals infected with VSV-OVA and observed reduced frequencies of ADAP−/− cells when co-transferred with WT cells but not when transferred alone (Supplemental Fig. 3A–B). No differences in T cell integrin (CD29/β1, α4β7/LPAM, or CD11a) expression were observed between circulating WT and ADAP−/− T cells following infection (Supplemental Fig. 3 F–K), so it is unlikely that subsequent differences in memory populations are due to these canonical tissue homing receptors (39). Examination of the circulating effector memory (TEM /CD44+CD62L−) central memory (TCM /CD44+CD62L+) populations revealed similar frequencies at day 7 and 14 but increased TEM and reduced TCM cells in the circulation 45 days post VSV-OVA infection (Supplemental Fig. 3C–E and data not shown). At memory time points ranging from 45 to 160 days post-infection, reduced numbers of ADAP-deficient OT-I T cells were found in the SG and in particular in the FRT (Fig. 5A and data not shown). Previous studies have shown that the vast majority of memory CD8 T cells found in both of these tissues are TRM (40). We next assessed the expression of CD69 on T cells founds in the SG and FRT, since CD69 expression has been used as a surrogate marker of TRM in these tissues (3). A large percentage of wild-type OT-I cells expressed CD69 in both the SG and FRT. While a comparable frequency of CD8 memory OT-I T cells lacking ADAP expressed CD69 in the SG, the percentage of ADAP-deficient T cells that expressed CD69 in the FRT was reduced by 50% when compared to wild-type OT-I memory T cells in the FRT (Fig. 5B and C). The tissue signals that regulate conversion into TRM phenotype cells are not fully understood but responsiveness to TGFβ is one mechanism that is thought to control TRM generation (26, 41). To evaluate whether the defect in TRM populations traces to an intrinsic inability of ADAP−/− T cells to respond to TGFβ, we assessed the capacity of WT or ADAP−/− memory T cells to induce phosphorylation of SMAD2 and SMAD3 following TGFβ stimulation ex vivo (42). Surprisingly, despite our consistent observation of defective TRM phenotype cells in SG and especially the FRT, we did not observe defects in pSMAD2/3 formation following TGFβ stimulation (Fig. 5D); indeed, ADAP−/− cells showed a trend towards slightly increased pSMAD2/3 activation in our assay.
Figure 5. ADAP is required for optimal CD8 TRM cell presence at memory time points.
Naïve WT (CD45.1/2) and ADAP−/− (CD45.2) OT-I T cells were cotransferred into CD45.1 hosts and challenged with VSV-OVA. Tissues were harvested and single cell suspensions were stained for cell surface markers at D33+ challenge. (A) Number of WT (black circles) or ADAP−/− (open squares) OT-I T cells in the spleen, SG, FRT or para-aortic lymph node (PALN) after VSV-OVA challenge. (B) CD69 staining from WT (black line) and ADAP−/− (grey shaded) OT-I T cells from spleen, SG and FRT. Gate represents CD69hi population. (C) Percentage of wild-type or ADAP−/− OT-I cells with CD69hi staining. The results in (A) are compiled from 3 independent experiments, with at least 4 mice per experiment (± SEM). The results in (C) are compiled from 2 independent experiments, with at least 3 mice per experiment (± SEM). (D) Single cell suspensions from the indicated tissues were prepared from mice 140 days post VSV-OVA infection and treated with control media or media containing 10 ng/ml TGFβ for 20 min and then stained for phospho-SMAD2/3. **, p < 0.01.
Reduced numbers of ADAP-deficient CD8 TRM cells alter cytokine responses to local reactivation
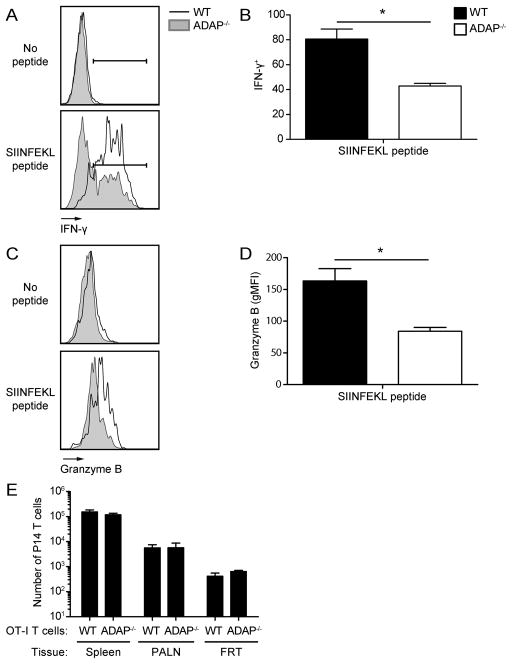
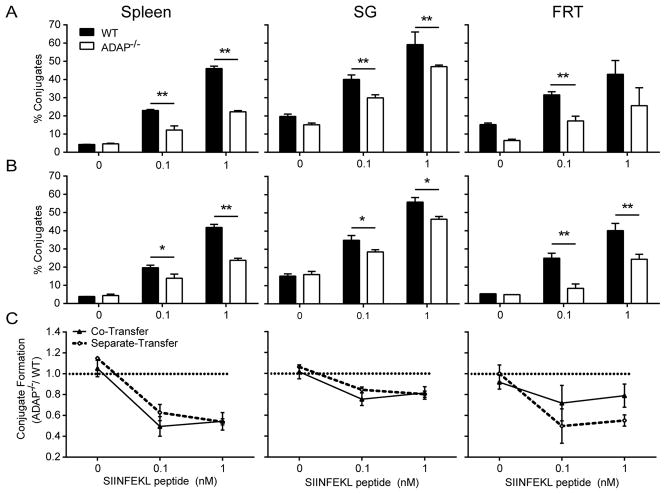
We next assessed the functionality of ADAP-deficient TRM cells in a reactivation assay using local peptide challenge in the FRT (29). After stimulation, ADAP-deficient TRM cells produced IFN-γ at the same levels as wild-type (Fig. 6A and B). Granzyme B production was also similar between wild-type and ADAP-deficient TRM cells (Fig. 6C and D). These experiments were performed in mice that had also received wild-type OT-I T cells. To verify that ADAP-deficient TRM cells are functional in the absence of cotransferred wild-type OT-I T cells, we repeated these experiments using mice that received only wild-type OT-I T cells or ADAP-deficient OT-I T cells. In the absence of the generation of a population of wild-type OT-I TRM cells, ADAP-deficient OT-I TRM produced significantly less IFN-γ and Granzyme B after local peptide challenge (Fig. 7A–D). This result suggests that the presence of wild-type OT-I T cells may aid ADAP-deficient OT-I T cells in the ability to detect antigen. To determine if this defect in in vivo peptide restimulation is associated with an intrinsic defect in the ability of ADAP−/− memory TRM cells to recognize SIINFEKL on APCs, as has been previously described for naïve ADAP−/− CD4 (14) and naïve and memory phenotype CD8 (22) T cells, we performed ex vivo T:APC conjugate assays using memory OT-I cells isolated from mice 120–160 days following infection with VSV-OVA infection. At all peptide doses tested, T:APC conjugate formation of ADAP−/− memory cells was defective in cells isolated from spleen, SG, and FRT (Fig. 8 and Supplementary Fig. 4A). While overall conjugate formation was somewhat enhanced in the NLTs we analyzed (SG and FRT) compared to spleen, ADAP−/− memory cells in these tissues retained defects in T:APC conjugate formation both when transferred together with WT (Fig. 8A) or in separate mice (Fig. 8B). However, consistent with our observation that defects in IFN-γ production by ADAP−/− memory cells following local peptide challenge were diminished in the presence of WT memory cells, careful analysis of conjugate formation from the FRT tissues that were co-transferred with WT and ADAP−/− T cells revealed that the observed defect in conjugate formation by ADAP−/− memory T cells was partially diminished (FRT panels in Fig. 8A and 8C) compared to mice that were separately transferred with either WT or ADAP−/− T cells and infected. However, this difference did not quite reach statistical significance in our repeated assays (Fig. 8C, FRT). One possible explanation for this trend is that the local tissue positioning of ADAP−/− memory T cells may differ in the presence or absence of WT memory T cells, altering the access of ADAP−/− memory T cells to APCs in vivo. However, we did not observe striking differences in the distribution or local clustering of ADAP−/− memory T cells in the FRT when transferred alone or together with WT cells (Supplemental Fig. 4B–D).
Figure 6. CD8 TRM cell effector functions are not dependent on ADAP.
VSV-OVA challenged mice were generated as in Figure 5. On day 30+ post infection, animals were t.c. challenged with SIINFEKL peptide. At 12 hours post challenge, spleen, para-aortic lymph node (PALN) and FRT were harvested and stained for cell surface markers and intracellular cytokines. (A) Representative IFN-γ and CD69 staining from WT or ADAP−/− OT-I T cells from FRT. (B) Percentage of IFN-γ+ WT (black bars) or ADAP−/− (white bars) OT-I T cells in the FRT. (C) Representative Granzyme B staining from WT or ADAP−/− OT-I T cells. (D) Fold change in gMFI of Granzyme B+ WT or ADAP−/− OT-I T cells over unstimulated in the FRT. The results (B and D) are compiled from 3 independent experiments, with at least 3 mice per experiment (± SEM).
Figure 7. Reduced numbers of CD8 TRM cells in the absence of ADAP blunts the response to local antigen challenge, but not recruitment of circulating memory CD8 T cells.
Naïve WT (CD45.2) or ADAP−/− (CD45.2) OT-I T cells were single transferred into CD45.1 hosts, and challenged with VSV-OVA. (A–D) On day 30+ post infection, animals were t.c. challenged with SIINFEKL peptide. At 12 hours post challenge, FRTs were harvested and stained for cell surface markers and intracellular cytokines. (A) Representative IFN-γ staining from WT or ADAP−/− OT-I T cells. (B) Percentage of IFN-γ+ WT (black bars) or ADAP−/− (white bars) OT-I T cells. (C) Representative Granzyme B staining from WT or ADAP−/− OT-I T cells. (D) Fold change in gMFI of Granzyme B+ WT or ADAP−/− OT-I T cells over unstimulated. (E) On day 30+ post infection animals received P14 memory cells, followed by t.c. challenge with SIINFEKL peptide. At 48 hours post challenge FRTs were harvested and stained for cell surface markers. (E) Number of circulating memory P14 cells recruited into the FRT. The results (B, D and E) are representative of 2 independent experiments, with 3 mice per group (± SEM). *, p < 0.05.
Figure 8. Impaired T:APC conjugate formation by ADAP−/− TRM cells isolated from NLTs.
Naïve WT (CD45.1 for single-transfer and Thy1.1 for co-transfer) or ADAP−/− (CD45.1) OT-I T cells were co-transferred (A) or separately transferred (B) into CD45.2 hosts, and challenged with VSV-OVA. On Day 120–160 following infection, the indicated tissues were isolated and processed to generate single cell suspensions that were mixed with DC-enriched splenocytes that were pre-pulsed with the indicated concentrations of SIINFEKL peptide. The formation of T:APC conjugates was determined by flow cytometry as described in Materials and Methods. (A) and (B) depict T:APC conjugate formation efficiency in a representative experiment from co-transferred (A) and separately transferred mice (B). Compiled results from 4 independent experiments, normalized to the conjugate efficiency of WT memory cells in each assay, are shown in (C).
Local resident CD8 T cells secrete IFN-γ to induce VCAM-1 expression on local vascular endothelium after cognate antigen stimulation to recruit circulating memory CD8 T cells in a non-specific manner (43). As ADAP-deficient OT-I TRM cells, in the absence of wild-type OT-I TRM cells, produce less IFN-γ than their wild-type counterparts, we next assessed the ability of ADAP-deficient CD8 TRM to recruit circulating memory P14 T cells after local antigen challenge. Memory P14 T cells were generated by infection of naïve recipient mice with LCMV after transfer of donor naïve P14 T cells. After at least 30 days post LCMV infection, memory P14 T cells were enriched from spleen and pLNs and transferred into VSV-OVA memory mice containing wild-type or ADAP−/− OT-I T cells. The following day, mice were locally challenged with SIINFEKL peptide and the ability of memory P14 T cells to migrate to the FRT was assessed 48 hours later. We observed comparable recruitment of memory P14 T cells to the FRT in mice containing either wild-type or ADAP-deficient OT-I TRM cells (Fig. 7E). This suggests that the reduced amount of IFN-γ produced by ADAP-deficient OT-I TRM cells is sufficient to draw in non-antigen specific circulating memory T cells in response to a local challenge.
DISCUSSION
In this study, we analyzed the role of ADAP in regulating naïve CD8 T cell responses to systemic pathogens. We utilized an adoptive transfer approach to assess clonal expansion, effector CTL functions, contraction, memory generation, and the functionality of memory CD8 T cells in the absence of ADAP. Sustained TCR signaling is required for optimal proliferation and clonal expansion of naïve CD8 T cells (44), while upregulation of numerous transcription factors facilitates differentiation into effector CD8 CTLs (4, 45–47). Initial T-APC interactions during priming must be stable and long-lived for survival of activated CD8 T cells during contraction (12). Alterations in the quality of TCR signaling can change the balance between effector and memory CD8 T cell generation (11). Our findings support a function for ADAP as a positive regulator of CD8 T cell contraction and memory generation, including the formation of memory in NLTs.
Previous studies of both CD8 and CD4 T cells demonstrated a reduced interaction of ADAP-deficient T cells with APCs expressing strong agonist ligands in vitro (9, 14, 19, 21, 22, 24, 25). In addition, ADAP-deficient CD4 T cells expressing the DO11.10 transgenic TCR exhibited defects in clonal expansion following peptide Ag challenge in the presence of IFA (19). We observed a modest defect in clonal expansion of ADAP-deficient OT-I T cells up to the peak of the immune response following LM infection, and larger defects in the ability of ADAP-deficient T cells to survive the contraction phase. This may reflect intrinsic differences between CD4 and CD8 T cells. Alternatively, differences in TCR affinity for Ag between the DO11.10 TCR and the OT-I TCR may result in differences in the requirement for ADAP during clonal expansion. It is also important to note that previous in vitro assays testing the role of ADAP in CD8 T cell proliferation were performed with either Ab-mediated or allogeneic DC-mediated TCR stimulation (24, 25). Our studies evaluated the response of ADAP-deficient CD8 T cells in vivo in response to a pathogen, where the CD8 T cells receive TCR stimulation, costimulation and inflammatory cytokine signals. Our results are consistent with other work showing that CD8 T cells activated by ICAM-1-deficient DCs undergo robust clonal expansion, but exhibit increased contraction (12). This suggests that CD8 T cells that have sub-optimal interactions with APCs, either via loss of ADAP expression in T cells or loss of ICAM-1 expression on DCs, do not exhibit large proliferation and clonal expansion defects, but instead generate activated CD8 T cells with decreased survival potential during the contraction phase.
The differentiation of naïve CD8 T cells into effector CTLs is partially dependent on the cytokine milleu during priming (4, 48–50). IL-12 is the major inflammatory cytokine produced in response to LM (34, 51). IL-12 drives expression of the transcription factor T-bet, and the effector cytokine IFN-γ (4, 51). Additionally, T-bet promotes the expression of IFN-γ and Granzyme B (4, 47). At the peak of the immune response following LM infection, we observed equal production of both IFN-γ and Granzyme B between wild-type and ADAP-deficient CD8 T cells. This suggests that ADAP does not regulate IL-12 signaling in CD8 T cells. This is in contrast to the negative regulatory role that ADAP has recently been shown to play in naïve CD8 T cell responses to the homeostatic cytokine IL-15 (22). IL-12 signaling occurs via the cell surface receptors IL-12Rβ1 and IL-12Rβ2, the former of which binds Jak2, and triggers STAT4 phosphorylation (51). In contrast, IL-15 signaling is initiated by binding of IL-15 to the IL-15Rβ and γC molecules on the cell surface, which activates JAK1 and 3, followed by activation of STAT5 (52).
Integrins are used by NK cells for target recognition (53) and strong adhesion mediated by the LFA-1 integrin is required for target cell lysis by CD8 CTLs and NK cells (54). Although ADAP is important for optimal interactions between naïve T cells and Ag-laden APCs both in vitro and in vivo (14, 19, 21), there have been conflicting reports on the role of ADAP in killing of target cells by effector CD8 T cells (24, 25) or NK cells (20, 55). One study reported enhanced cytolytic function of ADAP−/− tumor-specific CTLs in vitro and in vivo (23), while another study reported that CD8 CTLs lacking ADAP exhibited normal cytotoxicity in response to an allogenic graft (25). CD8 T cells primed by ICAM-1-deficient DCs also exhibit normal targeted lysis (12). We observed that ADAP-deficient Ag-specific CTLs generated following LM infection had a comparable ability to kill target cells as wild-type CTLs. This suggests that activated CD8 T cells likely do not require ADAP to mediate physical contact with target cells sufficient to initiate cell lysis. This is likely due to increased integrin function and functional activity on CTLs compared with naïve CD8 T cells. We note that the adoptive transfer approach utilized in this study allowed us to directly compare the differentiation of CD122lo CD44lo naïve wild-type and ADAP-deficient CD8 T cells into CTLs following LM infection. Analysis of CTL function in intact ADAP-deficient mice is complicated by our recent finding that ADAP-deficient mice have an elevated number of memory phenotype CD8 T cells (22), which have been shown to enhance targeted lysis (56, 57).
After clearance of the pathogen, the majority of antigen-specific CD8 T cells die while a small percentage of cells are maintained into the memory phase (1). At the peak of infection, activated CD8 T cells that have upregulated CD127 and downregulated KLRG1 are more likely to survive the contraction phase (4, 5). We observed that a greater percentage of ADAP-deficient CD8 T cells upregulated CD127 and downregulated KLRG1, as compared to wild-type, during the contraction phase. Our findings are similar to the response of T cells expressing a form of Src homology 2 domain-containing leukocyte phosphoprotein of 76 kD (SLP-76) where the tyrosine residues that mediate SLP-76 binding to inducible T cell kinase (Itk), (Y145) and non-catalytic region of tyrosine kinase (Nck) and Vav1 (Y112/128) after TCR stimulation were mutated to phenylalanine (11). This mutant form of SLP-76 impaired proximal TCR signaling, but did not alter antigen-specific CD8 T cell expansion or contraction. However, activation of T cells expressing this SLP-76 mutant resulted in a greater percentage of CD127hi cells and reduced percentage of KLRG1hi cells during contraction (11). Since ADAP also inducibly binds to SLP-76 after TCR stimulation, our results suggest that the impact of ADAP deficiency on the expression of these markers on activated CD8 T cells may be occurring through similar mechanisms.
Altered differentiation of activated CD8 T cells could prevent the seeding of NLTs and generation of CD8 TRM cells, which do not recirculate and thus play a critical role in protective host immunity to pathogen rechallenge. We observed a reduced number of KLRG1− ADAP-deficient TRM precursors in the FRT on day 7 after challenge (Supplemental Fig. 2C and D) that was associated with a reduced number of ADAP-deficient CD8 TRM in this NLT at memory time points. The percentage of ADAP-deficient TRM in the FRT expressing CD69 was also reduced when compared to wild-type TRM in the FRT. However, no differences in CD69 expression were observed on ADAP-deficient TRM in the SG, even though there was a lower number of ADAP-deficient TRM in this NLT as well. This suggests that there may be tissue-specific differences in the ADAP-dependent regulation of CD8 TRM generation. Another recent study reported that infection of ADAP−/− mice with influenza results in enhanced mortality that was associated with increased numbers of CD8 T cells in the lung but reduced levels of CD103 expression by these CD8 T cells (58). In addition to the analysis of different pathogens, we note two important technical differences between this study and our current work. First, we analyzed the response of ADAP-deficient CD8 T cells to LM infection by adoptively transferring a small population of naïve ADAP-deficient CD8 T cells into wild-type mice, rather than analyzing the response of ADAP-deficient mice. This was important because of increased numbers of CD8 memory phenotype T cells in intact ADAP-deficient mice (22) that would likely have enhanced proliferative potential and reduced responses to TGFβ signals compared to wild-type mice, thereby masking direct comparison of the activation of naïve CD8 T cells. Second, in order to clearly identify CD8 TRM in NLTs, we utilized an in vivo labeling approach that allowed us to discriminate circulating, blood-borne CD8 T cells found in tissues from tissue-resident T cells (32). In our LM and VSV infection models, we did not observe decreased CD103 upregulation on ADAP-deficient CD8 TRM cells as was previously described following influenza infection (58). We also directly examined the ability of tissue memory T cells to respond to TGFβ ex vivo, which is known to regulate CD103 expression and is associated with the formation of TRM cells in NLTs (7, 41, 59). However, we did not observe defects in the ability of ADAP−/− memory T cells to phosphorylate SMAD2/3, compared to WT memory T cells. Together with our observations of the altered contraction of ADAP−/− T cells following clonal expansion, this suggests that both the timing of and access to tissue signals, and the differentiation state of memory precursors, may be a critical factor in TRM conversion that is regulated by ADAP. These processes remain largely uncharacterized, and future work will be needed to further define the mechanism(s) by which ADAP regulates the generation of TRM cells.
CD8 TRM cells provide rapid and near-sterilizing protection at the sites of pathogen entry (7, 29, 43). Upon Ag recognition, CD8 TRM cells can specifically kill target cells, as well as recruit and activate both innate and adaptive immune cells (29, 43). The secretion of IFN-γ by CD8 TRM cells induces local inflammation, recruiting immune cells from the blood (29). We found that ADAP-deficient CD8 TRM cells at memory time points produced lower levels of IFN-γ and Granzyme B when compared to wild-type CD8 TRM cells. This was consistent with our observation that as with naïve ADAP−/− T cells, T:APC conjugate formation of ADAP−/− TRM cells isolated from these tissues was defective compared to wild-type memory cells. This result suggests that the signals that regulate TCR mediated LFA-1 activation are similar in both naïve and TRM populations. Additionally, as with naïve cells isolated from secondary lymphoid tissues, local NLT responses to antigen are critically dependent on T:APC conjugate formation downstream of ADAP. We also observed that the defect in effector molecule production by ADAP-deficient CD8 TRM cells was not present when both wild-type and ADAP-deficient CD8 TRM cells were responding to Ag in the same host, and likewise that defects in T:APC conjugate formation by ADAP−/− TRM cells were somewhat negated in the presence of wild-type TRM cells. The ability of wild-type CD8 TRM cells to “rescue” the deficit in IFN-γ and Granzyme B production by ADAP-deficient CD8 TRM cells is intriguing and may reflect the increased number of CD8 TRM cells responding to Ag in mice that received both wild-type and ADAP-deficient CD8 T cells prior to VSV infection. The dampened role for ADAP in ex vivo conjugate assays performed in the presence of wild-type memory T cells (in co-transferred animals) is consistent with this result but additional studies will be required to fully explain this observation. Other studies have reported that T cell-T cell interactions may influence CD8 T cell function (27, 60), suggesting that CD8 TRM function in NLTs may be facilitated by a certain threshold of CD8 TRM cells required for optimal effector molecule expression upon Ag stimulation. In mice that received only adoptively transferred naïve ADAP-deficient CD8 T cells, the total number of CD8 TRM cells and the percentage of those cells expressing IFN-γ after Ag re-challenge were reduced compared to recipient mice that received only wild-type CD8 T cells. However, despite these deficits in CD8 TRM cell number and IFN-γ production, we did not observe a defect in the ability of ADAP-deficient CD8 TRM cells to recruit circulating memory CD8 T cells. This result suggests that the amount of IFN-γ being produced by ADAP-deficient CD8 TRM cells is sufficient to mediate this important CD8 TRM cell function, and thus that some but not all aspects of TRM development and function are regulated by ADAP.
In summary, our studies have defined a role for ADAP in the differentiation of memory CD8 T cells and the presence of CD8 TRM in NLTs. ADAP is required for optimal balance in the production of both effector and memory precursor CD8 T cells after pathogen challenge. Our results suggest that alterations in the balance between effector and memory CD8 T cells prevent optimal seeding of NLTs. These findings reinforce the importance of future studies to identify how many and what type of CD8 T cells are generated after vaccination to promote immunity both in SLOs and at the front lines of pathogen entry.
Supplementary Material
Acknowledgments
We thank F. Shoyama, T. Rivard, S. Jin, S. O’Flanagan, K. Benson, P. O’Hare, and K. Young for mouse genotyping and colony maintenance. Dr. K. Anderson and E. Thompson provided assistance with injections and animal harvests. We thank Drs. S. Jameson, D. Masopust and E. Peterson for helpful discussion and critical review of the manuscript. We thank the University of Minnesota Flow Cytometry Resource for technical assistance.
Footnotes
This work was supported by National Institutes of Health grant R01 AI038474 (to Y.S.). Y.S. is also supported by the Harry Kay Chair in Biomedical Research at the University of Minnesota.
Abbreviations: ADAP, adhesion and degranulation promoting adapter protein; CARMA-1, caspase recruitment domain (CARD) membrane-associated guanylate kinase (MAGUK) protein; FRT, female reproductive tract; gMFI, geometric mean fluorescence intensity; IL-7Rα, interleukin-7 receptor α; Itk, inducible T cell kinase; KLRG1, killer cell lectin-like receptor G1 (KLRG1); Listeria monocytogenes, LM; Nck, non-catalytic region of tyrosine kinase; NLTs, non-lymphoid tissues; PALN, para-aortic lymph node; pLN, peripheral lymph node; SG, salivary gland; SKAP55, Src kinase-associated phosphoprotein of 55 kDa; SLO, secondary lymphoid organ; SLP-76, Src homology 2 domain-containing leukocyte phosphoprotein of 76 kD; TAK-1, transforming growth factor-β (TGF-β)-activated protein kinase; t.c., transcervically; TRM cells, resident memory T cells; VSV, vesicular stomatitis virus.
References
- 1.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- 3.Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 6.Olson JA, McDonald-Hyman C, Jameson SC, Hamilton SE. Effector-like CD8+ T cells in the memory population mediate potent protective immunity. Immunity. 2013;38:1250–1260. doi: 10.1016/j.immuni.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- 8.Burbach BJ, Medeiros RB, Mueller KL, Shimizu Y. T-cell receptor signaling to integrins. Immunol Rev. 2007;218:65–81. doi: 10.1111/j.1600-065X.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- 9.Witte A, DJ, Baumgart K, Waldt N, Kuropka B, Freund C, Schraven B, Kliche S. Emerging Roles of ADAP, SKAP55, and SKAP-HOM for Integrin and NF-kB Signaling in T cells. Clin Cell Immunol. 2012:S12. [Google Scholar]
- 10.Palmer MJ, V, Mahajan S, Chen J, Irvine DJ, Lauffenburger DA. Signaling thresholds govern heterogeneity in IL-7-receptor-mediated responses of naive CD8+ T cells. Immunol Cell Biol. 2011;89:581–594. doi: 10.1038/icb.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith-Garvin JE, Burns JC, Gohil M, Zou T, Kim JS, Maltzman JS, Wherry EJ, Koretzky GA, Jordan MS. T-cell receptor signals direct the composition and function of the memory CD8+ T-cell pool. Blood. 2010;116:5548–5559. doi: 10.1182/blood-2010-06-292748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Peterson EJ, Woods ML, Dmowski SA, Derimanov G, Jordan MS, Wu JN, Myung PS, Liu QH, Pribila JT, Freedman BD, Shimizu Y, Koretzky GA. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science. 2001;293:2263–2265. doi: 10.1126/science.1063486. [DOI] [PubMed] [Google Scholar]
- 14.Mueller KL, Thomas MS, Burbach BJ, Peterson EJ, Shimizu Y. Adhesion and degranulation-promoting adapter protein (ADAP) positively regulates T cell sensitivity to antigen and T cell survival. J Immunol. 2007;179:3559–3569. doi: 10.4049/jimmunol.179.6.3559. [DOI] [PubMed] [Google Scholar]
- 15.Medeiros RB, Burbach BJ, Mueller KL, Srivastava R, Moon JJ, Highfill S, Peterson EJ, Shimizu Y. Regulation of NF-kB activation in T cells via association of the adapter proteins ADAP and CARMA1. Science. 2007;316:754–758. doi: 10.1126/science.1137895. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava R, Burbach BJ, Shimizu Y. NF-kappaB activation in T cells requires discrete control of IκB kinase α/β (IKKα/β) phosphorylation and IKKγ ubiquitination by the ADAP adapter protein. J Biol Chem. 2010;285:11100–11105. doi: 10.1074/jbc.M109.068999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivastava R, Burbach BJ, Mitchell JS, Pagan AJ, Shimizu Y. ADAP regulates cell cycle progression of T cells via control of cyclin E and Cdk2 expression through two distinct CARMA1-dependent signaling pathways. Mol Cell Biol. 2012;32:1908–1917. doi: 10.1128/MCB.06541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burbach BJ, Srivastava R, Ingram MA, Mitchell JS, Shimizu Y. The pleckstrin homology domain in the SKAP55 adapter protein defines the ability of the adapter protein ADAP to regulate integrin function and NF-κB activation. J Immunol. 2011;186:6227–6237. doi: 10.4049/jimmunol.1002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell JS, Burbach BJ, Srivastava R, Fife BT, Shimizu Y. Multistage T cell-dendritic cell interactions control optimal CD4 T cell activation through the ADAP-SKAP55-signaling module. J Immunol. 2013;191:2372–2383. doi: 10.4049/jimmunol.1300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rajasekaran K, Kumar P, Schuldt KM, Peterson EJ, Vanhaesebroeck B, Dixit V, Thakar MS, Malarkannan S. Signaling by Fyn-ADAP via the Carma1-Bcl-10-MAP3K7 signalosome exclusively regulates inflammatory cytokine production in NK cells. Nat Immunol. 2013;14:1127–1136. doi: 10.1038/ni.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burbach BJ, Srivastava R, Medeiros RB, O’Gorman WE, Peterson EJ, Shimizu Y. Distinct regulation of integrin-dependent T cell conjugate formation and NF-kappa B activation by the adapter protein ADAP. J Immunol. 2008;181:4840–4851. doi: 10.4049/jimmunol.181.7.4840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiege JK, Burbach BJ, Shimizu Y. Negative regulation of memory phenotype CD8 T cell conversion by adhesion and degranulation promoting adapter protein (ADAP) J Immunol. 2015;195:3119–3128. doi: 10.4049/jimmunol.1402670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li C, Li W, Xiao J, Jiao S, Teng F, Xue S, Zhang C, Sheng C, Leng Q, Rudd CE, Wei B, Wang H. ADAP and SKAP55 deficiency suppresses PD-1 expression in CD8+ cytotoxic T lymphocytes for enhanced anti-tumor immunotherapy. EMBO Mol Med. 2015;7:754–769. doi: 10.15252/emmm.201404578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian J, Pabst O, Romermann D, Skubich S, Forster R, Beckmann J, Chen JH, Hoffmann MW. Inactivation of T-cell receptor-mediated integrin activation prolongs allograft survival in ADAP-deficient mice. Transplantation. 2007;84:400–406. doi: 10.1097/01.tp.0000269724.06142.92. [DOI] [PubMed] [Google Scholar]
- 25.Tian J, Rodriguez-Barbosa JI, Pabst O, Roemermann D, Foerster R, Beckmann J, Hoffmann MW. ADAP deficiency combined with costimulation blockade synergistically protects intestinal allografts. Transpl Int. 2010;23:71–79. doi: 10.1111/j.1432-2277.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- 26.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, Masopust D. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zumwalde NA, Domae E, Mescher MF, Shimizu Y. ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation. J Immunol. 2013;191:3681–3693. doi: 10.4049/jimmunol.1201954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8+ T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hippen KL, Schram BR, Tze LE, Pape KA, Jenkins MK, Behrens TW. In vivo assessment of the relative contributions of deletion, anergy, and editing to B cell self-tolerance. J Immunol. 2005;175:909–916. doi: 10.4049/jimmunol.175.2.909. [DOI] [PubMed] [Google Scholar]
- 31.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 32.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, Masopust D. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc. 2014;9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 34.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 35.Russell JH, Ley TJ. Lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 36.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 37.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nature reviews Immunology. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 38.Hikono H, Kohlmeier JE, Takamura S, Wittmer ST, Roberts AD, Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denucci CC, Mitchell JS, Shimizu Y. Integrin function in T-cell homing to lymphoid and nonlymphoid sites: getting there and staying there. Crit Rev Immunol. 2009;29:87–109. doi: 10.1615/critrevimmunol.v29.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyarto BZ, Southern PJ, Masopust D. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell. 2015;161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rygiel KA, Robertson H, Marshall HL, Pekalski M, Zhao L, Booth TA, Jones DE, Burt AD, Kirby JA. Epithelial-mesenchymal transition contributes to portal tract fibrogenesis during human chronic liver disease. Lab Invest. 2008;88:112–123. doi: 10.1038/labinvest.3700704. [DOI] [PubMed] [Google Scholar]
- 43.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, Wu BB, Cheung KP, Ding Z, Goldrath AW. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol. 2006;7:1317–1325. doi: 10.1038/ni1403. [DOI] [PubMed] [Google Scholar]
- 46.Knell J, Best JA, Lind NA, Yang E, D’Cruz LM, Goldrath AW. Id2 influences differentiation of killer cell lectin-like receptor G1(hi) short-lived CD8+ effector T cells. J Immunol. 2013;190:1501–1509. doi: 10.4049/jimmunol.1200750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 48.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 49.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 50.Obar JJ, Lefrancois L. Early signals during CD8 T cell priming regulate the generation of central memory cells. J Immunol. 2010;185:263–272. doi: 10.4049/jimmunol.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O’Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 52.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett. 2010;127:85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Helander TS, Timonen T. Adhesion in NK Cell Function. In: Kärre K, Colonna M, editors. Specificity, Function, and Development of NK Cells. Springer; Berlin Heidelberg: 1998. pp. 89–99. [DOI] [PubMed] [Google Scholar]
- 54.Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 2004;173:3653–3659. doi: 10.4049/jimmunol.173.6.3653. [DOI] [PubMed] [Google Scholar]
- 55.Fostel LV, Dluzniewska J, Shimizu Y, Burbach BJ, Peterson EJ. ADAP is dispensable for NK cell development and function. Int Immunol. 2006;18:1305–1314. doi: 10.1093/intimm/dxl063. [DOI] [PubMed] [Google Scholar]
- 56.Akue AD, Lee JY, Jameson SC. Derivation and maintenance of virtual memory CD8 T cells. J Immunol. 2012;188:2516–2523. doi: 10.4049/jimmunol.1102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li C, Jiao S, Wang G, Gao Y, Liu C, He X, Zhang C, Xiao J, Li W, Zhang G, Wei B, Chen H, Wang H. The immune adaptor ADAP regulates reciprocal TGF-b1-integrin crosstalk to protect from influenza virus infection. PLoS Pathog. 2015;11:e1004824. doi: 10.1371/journal.ppat.1004824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang N, Bevan MJ. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cox MA, Barnum SR, Bullard DC, Zajac AJ. ICAM-1-dependent tuning of memory CD8 T-cell responses following acute infection. Proc Natl Acad Sci USA. 2013;110:1416–1421. doi: 10.1073/pnas.1213480110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.