Abstract
AIM
This cross-sectional investigation aimed to assess the value of non-invasive measures of temporal respiratory–swallow coupling in individuals with ataxic swallowing.
METHOD
Twenty participants with ataxia telangiectasia were presented with water and pudding boluses. Their 193 swallows were compared with 2200 swallows from 82 age-matched healthy controls. The two components of airway protection during swallowing that were analyzed were: direction of peri-deglutitive airflow and duration of deglutitive inhibition of respiratory airflow (DIORA).
RESULTS
Safe expiratory patterns of peri-deglutitive airflow occurred significantly less often in participants with ataxia telangiectasia than in age-matched control participants (younger p<0.015 and older p<0.001). The frequency of an expiratory pattern of peri-deglutitive airflow increased with age in participants in the comparison group (p=0.006), but not in those with ataxia telangiectasia (p=0.234). With age, mean duration of DIORA decreased in controls (p<0.001) but was unchanged in participants with ataxia telangiectasia (p=0.164).
INTERPRETATION
Non-invasive quantitative measures of respiratory–swallow coupling capture temporal relationships that plausibly contribute to airway compromise from dysphagia. Changes in respiratory–swallow coupling observed with advancing age in control participants were not seen in participants with ataxia telangiectasia. Measures of perturbations may herald swallowing problems prior to development of pulmonary and nutritional sequelae.
Ataxia telangiectasia is a genetic disease associated with sino-pulmonary infections, neurological deterioration with concomitant bulbar dysfunction, increased sensitivity to ionizing radiation, immunodeficiency, a decline in lung function, and early death.1–6 Pulmonary complications become more frequent by the second decade of life and are a leading cause of death in individuals with ataxia telangiectasia.2,3 Oropharyngeal dysphagia is common, progressive, and a risk factor for frequent respiratory infections.2,3,6 Early detection and treatment of swallowing dysfunction appears to decrease morbidity associated with dysphagia, and improve quality of life in those affected individuals we have followed over the last two decades at the Ataxia-Telangiectasia Clinical Center.2,3,6 Standard means of swallowing assessment with videofluoroscopic swallow study are limited by concerns about radiation exposure, particularly in patients with progressive conditions warranting serial evaluations.6 Regrettably, development of non-invasive standardized approaches for the detection of dysphagia have not evolved substantially, despite advancing appreciation of the high impact that swallowing dysfunction has on health.
A non-invasive means of swallowing assessment is especially appealing for those individuals (e.g. children) who have enhanced sensitivity to the ill effects of ionizing radiation. Moreover, a technique that permits repeated assessment of the temporal relationships over multiple swallows, particularly under more familiar conditions than that achievable in the radiology suite, is of special importance given the effects of attention and examination conditions upon swallowing dynamics. Because the defining feature of ataxia is ‘lack of order’, assessment of the variation in swallowing coordination is key. Disruptions in the temporal relationships between breathing and swallowing have been linked to oropharyngeal dysphagia and are common in other diseases and disorders.7–10 Clinical tools that provide valid and reliable methods for the serial detection of perturbations in respiratory–swallow coupling are thus sorely needed, and may have substantial impact on therapy. Early detection and treatment may well prevent the sequelae of chronic swallowing dysfunction.
Two components of respiratory–swallow coordination that contribute to airway protection during swallowing are the direction of peri-deglutitive airflow and the duration of deglutitive inhibition of respiratory airflow (DIORA) – i.e. the interval of breathing cessation while a bolus passes through the pharynx. While the relationships of these two components to swallowing safety are not yet completely worked out, post-swallow expiration is considered to be a facilitator of airway clearance that in turn limits aspiration from residue matter in the pharynx or supraglottic region.8,11 That these variables are potentially meaningful is suggested by observations that individuals with neurogenic dysphagia and chronic lung disease more frequently manifest both inspiratory peri-deglutitive air flow and longer durations of DIORA.8–10
This study was conducted to evaluate the ability to assess these non-invasive measures of respiratory–swallow coupling in a cohort of individuals at high risk for advancing dysphagia and the potential value of identified changes relative to healthy controls. We hypothesized that ataxia telangiectasia participants would exhibit more frequent disruptions in the temporal relationships between respiration and deglutition with advancing age given the progression of their neurodegenerative disease. Specifically, we hypothesized that peri-deglutitive expiratory patterns would occur less frequently, thereby causing an increase in the frequency of inspiratory airflow patterns, and DIORA durations would become longer and more variable in older participants with ataxia telangiectasia.
METHOD
Participants
Respiratory–swallow patterns were recorded in children and young adults with ataxia telangiectasia during routine visits to the Johns Hopkins Ataxia-Telangiectasia Clinical Center (2006–2007) and Neurology Workshop for Ataxia-Telangiectasia (Chicago, 2007). Criteria for diagnosis of ataxia telangiectasia included the presence of characteristic neurological features (gait ataxia, oculomotor dysfunction, dysarthria, and a movement disorder) and at least one of the following: oculocutaneous telangiectasia, elevated levels of alpha-fetoprotein in serum, or spontaneous or x-irradiation-induced chromosomal breakage.5 All participants fed orally. Body mass index (BMI) scores within 2 years of respirodeglutometer instrumentation (RDG) recordings were obtained from the medical records, when available. Dysphagia was determined by clinical presentations and videofluoroscopic swallow study findings as previously described.6 Although radiological swallowing assessments were not part of the protocol for this study, three participants partook in videofluoroscopic swallow studies within 1 year of respiratory–swallow recordings because of caregiver concerns about worsening swallowing function (Table I).
Table 1.
Characteristics and dysphagic concerns for younger and older participants with ataxia telangiectasia
| Age (y:mo) |
Sex | Dysphagic concerns | Mealtime concerns |
Respiratory concerns |
BMI z- score |
|
|---|---|---|---|---|---|---|
| Younger (pre-pubertal) | ||||||
| 1 | 9:0 | M | Occasional and more frequent cough with very thin liquids Trouble with hard-to-chew foods Drooling when tired |
None | Frequent URIs |
1.54 |
| 2 | 7:7 | F | Occasional drooling | Long mealsa Picky eating |
None | −0.02 |
| 3 | 9:9 | M | Occasional mealtime cough | Long mealsa | None | −1.00 |
| 4 | 10:7 | M | Cough with very thin liquids | None | None | 1.68 |
| 5 | 11:0 | F | Drooling when concentrating | Long mealsa | None | −1.68 |
| 6 | 13:5 | M | Occasional cough with very thin liquids G-Tube placed: 9y 7mo of age |
None | None | ^ |
| 7 | 13:9 | F | Occasional cough with liquids | None | None | −1.30 |
| Older (post-pubertal) | ||||||
| 1 | 12:6 | F | Intermittent cough with liquids and solids Persistent drooling Dysphagia on VFSSb |
Long mealsa | Sinopulmona ry infections |
−0.27 |
| 2 | 12:9 | F | None | None | None | 0.84 |
| 3 | 14:8 | M | None | None | None | −2.69 |
| 4 | 15:4 | F | Cough/choke with liquids and solids Dysphagia on VFSSb |
None | Yes, increasing |
−0.37 |
| 5 | 16:0 | F | Occasional cough and nasopharyngeal reflux with very thin liquids Sensation of food sticking in throat |
None | None | −0.32 |
| 6 | 16:0 | F | Occasional cough with liquids | None | None | −1.38 |
| 7 | 18:2 | M | None | None | None | −2.49 |
| 8 | 18:8 | F | Drooling when tired | None | None | ^ |
| 9 | 18:9 | M | Cough with liquids Trouble with hard-to-chew foods Dysphagia on VFSSb G-Tube placed: 12y 7mo of age |
None | None | ^ |
| 10 | 18:11 | M | Cough/choke with liquid Fatigue interferes with chewing |
None | None | ^ |
| 11 | 19:6 | M | None | None | None | −1.28 |
| 12 | 20:2 | M | Occasional cough with very thin liquids Sensation of food sticking in throat |
None | None | −2.30 |
| 13 | 20:7 | M | Occasional cough and sensation of nasopharyngeal reflux with very thin liquids |
None | None | −1.23 |
Meals of 45 minutes or longer.
VFSS completed within 1 year of RDG recordings. All were positive for supraglottic penetration while drinking thin liquids by straw.
Gastrostomy placed for nutritional intake or BMI measures >2 years before or after acquiring respiratory–swallow coordination measures.
BMI, body mass index; URI, upper respiratory infection; VFSS, videofluoroscopic swallow study.
The Joint Committee for Clinical Investigation of the Johns Hopkins Hospital and the Human Participants Committee at the University of Illinois Urbana–Champaign approved this study.
Instrumentation and initial measurements
RDG instrumentation (DFI Enterprises, Inc, Morrisville, NY, USA) provides non-invasive measures of respiratory air flow during swallowing.12–14 RDG records multiple temporal measures of respiration and swallowing, including the direction of nasal air flow, onset of laryngeal displacement, onset of surface electromyographic activity from submental muscles, and swallow-associated acoustic signals from a surface microphone affixed to the neck. A ‘chatter’ channel records the examiner’s instructions and conversation, and a trigger channel is used to identify the occurrence of each event of interest. Outputs from the analogue wave forms were simultaneously inputted into CODAS (DATAQ Instruments Inc., Akron, Ohio, USA) at a per channel sampling rate of 1000 per second. Recorded data were analyzed with WINDAQ (DATAQ Instruments Inc., Akron, Ohio, USA).
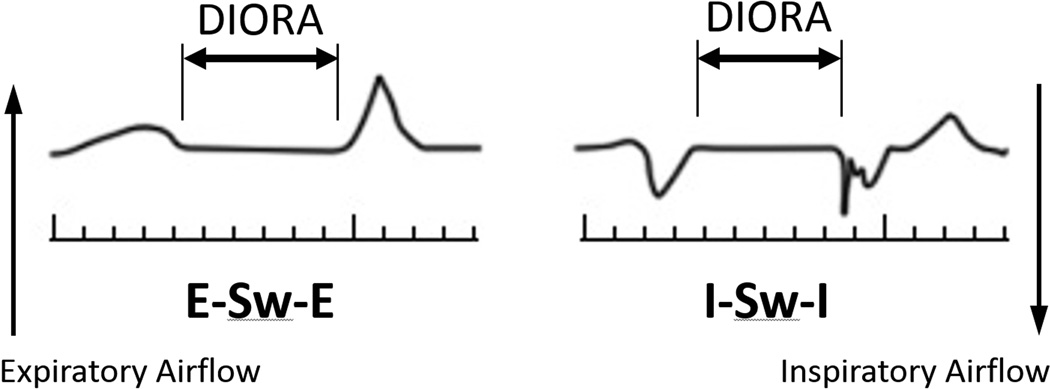
Each RDG examination was analyzed according to previously published protocols.12–14 DIORA duration was defined as the interval between nasal respiratory airflow cessation and re-onset. Pre- and post-swallow respiratory airflow patterns were determined by direction of airflow immediately before and after DIORAs (Fig. 1).
Figure 1.
Schematic of expiration–swallow–expiration (E–Sw–E) and inspiration–swallow–inspiration (I–Sw–I) respiratory airflow patterns bracketing deglutitive inhibition of respiratory airflow (DIORA).
Protocol
Participants with ataxia telangiectasia were seated, connected to the various RDG recording instruments, and partook in an evaluation protocol described by Klahn and Perlman.12 Participants were instructed to hold each water or pudding bolus in the mouth until instructed to swallow. Each participant was presented with ten randomly ordered boluses (five pre-measured 3ml boluses of water from a medicine cup and five 3ml boluses of commercially available pudding by spoon). Our experience in participants with ataxia telangiectasia suggested the necessity of decreasing the offered bolus to 3ml, because participants typically split 5ml boluses into two swallows.6 Comparisons were made to a previously acquired data set of 5ml bolus swallows in healthy age-matched control participants.
Data analysis
DIORA data were logarithmically transformed to normalize data distribution. Statistical tests were carried out to test significance of log respiratory inhibition durations by sub-groups. Because sex effects were not seen in any subgroup, sex data were pooled according to pubertal status and bolus type (water or pudding). The PROCEDURE MIXED in SAS 9.1.3 (SAS Inc., Cary, North Carolina, USA) was used to implement the tests and each ‘participant’ was treated as random so that the correlation of the swallows from the same participant was accounted for, and the variances were estimated for ataxia telangiectasia and control participants separately to account for the possible heterogeneity of variance.
Comparison of waveform assessments by two graduate students experienced in RDG protocols generated inter-rater reliability measures. Kappa statistics of agreement were computed for identification of the direction of peri-deglutitive airflow and on the log transformation of the durations of DIORAs. Kappa values calculated from data generated by 10 controls for identification of direction of peri-deglutitive airflow were in the substantial agreement range (kappa=0.79) and for duration of DIORAs were in almost perfect agreement (kappa=0.97).15 For five participants with ataxia telangiectasia, agreement scores for direction of peri-deglutitive airflow patterns were in the moderate range (kappa=0.50) and duration of DIORAs were in the substantial range (kappa=0.68).15 Differences in BMI z-scores were examined using t-tests.
RESULTS
Demographics
Twenty participants with ataxia telangiectasia (11 males, nine females) ranging in age from 9 to 21 years, participated in recordings of respiration during deglutition. Characteristics of the participants, including dysphagia and mealtime concerns, are listed in Table I. All participants fed orally. At the time of this investigation, two participants had percutaneous gastrostomy tubes placed for nighttime supplemental nutrition. BMI z-scores from these two participants were excluded from calculations. Mean BMI z-scores were comparable for younger and older ataxia telangiectasia participants (−1.13 vs −1.15; p=0.486).
Interface between respiratory airflow and deglutition
Using standardized RDG respiratory–swallow protocols, 193 swallows recorded from 20 participants with ataxia telangiectasia were compared with 2200 swallows recorded from 82 matched healthy control participants with typical development. All participants with ataxia telangiectasia were able to take part in this protocol; the average number of swallows recorded per participant was 9.65 (range 5–10). Pubertal status was used to assign participants with ataxia telangiectasia to younger and older age groups and identify corresponding controls. Pre- and post-pubertal groupings ranged from 9 to 14 years and 13 to 21 years for participants with ataxia telangiectasia, and from 8 to 10 and 18 to 25 years for those in the comparison group.
Direction of peri-deglutitive airflow
Four possible patterns of peri-deglutitive airflow can be identified with RDG recordings: expiration–swallow–expiration (E–Sw–E), expiration–swallow–inspiration (E–Sw–I), inspiration-swallow-expiration (I–Sw–E), and inspiration–swallow–inspiration (I–Sw–I) (Fig. 1). Although E–Sw–E, the putative safest pattern,11,16 is observed most commonly in both control participants and those with ataxia telangiectasia, it occurred significantly less often in the younger and older ataxia telangiectasia groups than their age-matched controls (p<0.015 and p<0.001). It is notable that the frequency of E–Sw–E coupling did not increase with age/increasing neurological impairment in the ataxia telangiectasia cohort as it did in the comparison group (p=0.234 and p=0.006). The most worrisome pattern of respiratory–deglutition coupling, I–Sw–I, was equally frequent in older and younger participants of both groups (ataxia telangiectasia, p=0.206; comparison group, p=0.130), but was more common in the older group of participants with ataxia telangiectasia than their controls (p<0.001).
DIORA duration
Group mean durations of DIORA were comparable in both age groups of participants with ataxia telangiectasia (younger 0.81s [SD 0.31s] and older 0.91s [SD 0.28s], p=0.164). In contrast, group mean DIORA durations decreased with age in controls (0.91s [SD 0.65s] vs 0.72s [SD 0.19s], p<0.001). DIORA durations were similar for younger participants with ataxia telangiectasia and their controls (0.81s [SD 0.31s] vs 0.91s [SD 0.65s], p=0.212), but with the decrease of mean DIORA durations in older healthy controls, a difference was observed between the older groups (ataxia telangiectasia, 0.91s [SD 0.28s] vs control, 0.72s [SD 0.19s], p<0.005).
With respect to bolus type, younger and older participants with ataxia telangiectasia had comparable individual mean DIORA durations during liquid (0.80s [SD 0.31s] vs 0.88s [SD 0.27s], p=0.266) and pudding swallows (0.81s [SD 0.30s] vs 0.94s [SD 0.29s], p=0.235). For control participants, individual mean DIORA durations for liquid swallows decreased with age (1.09s [SD 0.81s] vs 0.71s [SD 0.18s], p<0.001) but remained stable for pudding swallows 0.73s [SD 0.32s] vs 0.73s [SD 0.20s], p=0.651).
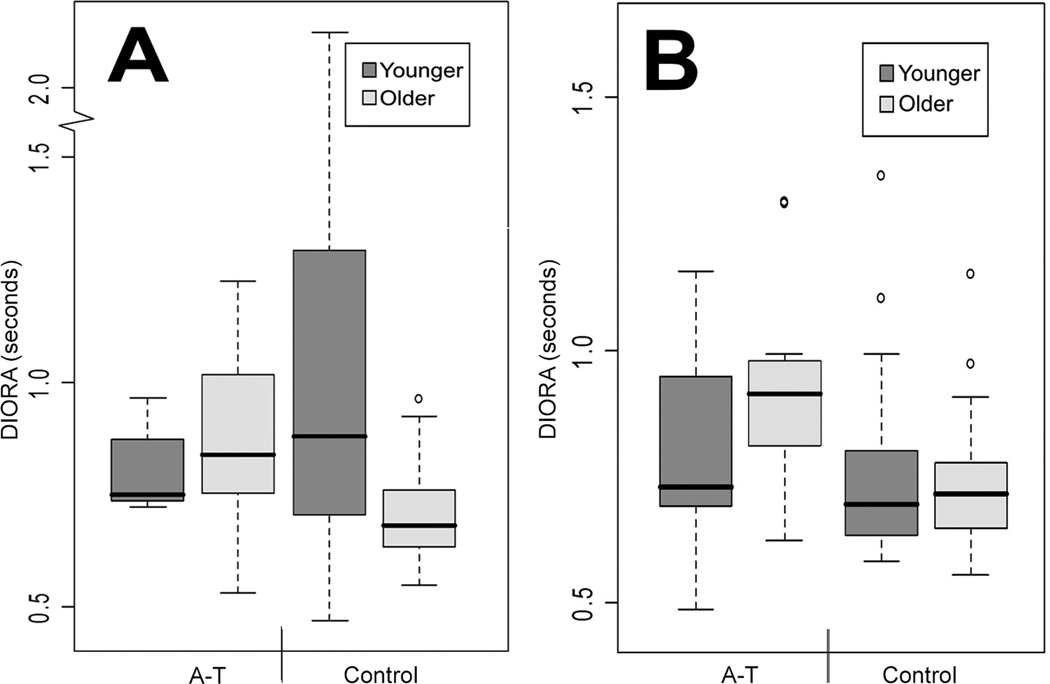
Variability of mean DIORA durations was greatest for younger controls during liquid swallows and decreased with age. In contrast, variability of DIORA durations was greater for older participants with ataxia telangiectasia than controls during liquid and pudding swallows (Fig. 2).
Figure 2.
Distribution by box plots of duration of deglutitive inhibition of respiratory airflow (DIORA) produced by participants with ataxia telangiectasia (A-T) and control subjects during (a) liquid and (b) pudding swallows.
DISCUSSION
This investigation demonstrates that non-invasive assessment measurement of respiratory–deglutitive coupling in a cohort of participants at risk for swallowing incoordination is both possible and of potential clinical value. Our participants with ataxia telangiectasia were able to take part in respiratory–swallow protocols that detected aberrant respiratory–swallow relationships.
Perturbations identified in the direction of peri-deglutitive nasal airflow and duration of DIORAs have been associated with an increased risk of aspiration.8,17,18 Our experience suggests that assessment of these temporal measures of respiratory–swallow coupling may have practical value in clinical assessment of individuals at risk for dysphagia.
Four participants (two were siblings) had no dysphagic concerns or clinical concerns associated with aspiration. This is an insufficient number for statistical analysis, but age, neurological z-score, and BMI were not predictive. These observations may reflect the high degree of variability of neurological genotype or phenotype, or the small sample size.
Pre- and post-expiratory airflow patterns
Pre- and post-deglutitive expiratory airflow coupling is the predominant respiratory pattern in healthy adults, and is considered the safest pattern of coupling for swallowing.11,16 Regardless of age, E–Sw–E was the most common pattern for both ataxia telangiectasia and healthy participants. Those with ataxia telangiectasia, however, generated proportionately fewer E–Sw–E airflow patterns than their age-matched healthy controls. Participants with ataxia telangiectasia generated more frequent inspiratory airflow patterns either before deglutition, after deglutition, or both; the least safe pattern, I–Sw–I, occurred significantly more often in older participants with ataxia telangiectasia than in their controls.8,17
To consider pre- or post-swallowing inspiratory patterns individually, variability in the direction of respiratory airflow before swallowing is well-known and appears to be less worrisome of the non-E–Sw–E patterns. Expiratory airflow precedes swallowing (E–Sw) in 76% to 90% of swallows in individuals without dysphagia, and 70% to 83% of swallows in those with dysphagia or conditions associated with dysphagia.8,10,14,19 Direction of airflow preceding swallows is influenced by multiple factors including the swallowing task (e.g. self-feeding vs being fed), the presence of oral phase problems, and potentially delays in the initiation of complex volitional tasks – as has been observed in individuals with ataxia telangiectasia.4,8,9
In contrast, post-swallow expiratory airflow (Sw–E) appears to be the better marker of swallowing safety, characterizing 91% to 100% of the swallows produced by adults without dysphagia.14,18 In this investigation, the frequency of Sw–E occurred in 91% to 93% of the swallows in healthy controls and 78% to 80% of the swallows in participants with ataxia telangiectasia. Data extrapolated from other investigations using similar protocols showed the prevalence of post-swallow inspiratory airflow (Sw–I) coupling in the normal population is approximately 11% for children between 2 and 11 years of age and ranges from 0% to 8% in older children and adults.14,16,20 In this investigation, the prevalence of Sw–I coupling in healthy controls (9% younger and 7% older) was within the reported normal ranges. In contrast, Sw–I patterns were more than twofold higher for participants with ataxia telangiectasia than controls, occurring in 22% of swallows of younger and 20% of swallows of older participants with ataxia telangiectasia. These findings demonstrate that aberrant respiratory–swallow coordination is present in participants with ataxia telangiectasia and may play a role in their dysphagia.
Duration of DIORA
Our findings of DIORA durations and their variability in older ataxia telangiectasia participants were consistent with previous reports of longer and more variable DIORA durations in children and adults with neurological dysphagia.7,9 Older ataxia telangiectasia participants had the longest DIORA durations and they were significantly longer than their age-matched controls. Nonetheless, DIORA durations for our older ataxia telangiectasia participants were within ranges (0.6–2.00s; median: 1s) for healthy adults.12,21 We suspect that the overlap of these findings is related to differences in instrumentation and procedural protocols,12,17 age of participants,22 and bolus size.14
To our knowledge, this is the first study to characterize perturbations in respiratory–swallow coupling as possible arrested development. Many features of neurological impairment in individuals with ataxia telangiectasia, including the onset of oropharyngeal dysphagia, become more apparent in the second decade of life.4,6 We anticipated that measure of dysphagia would follow a similar course of increasing manifestation with advancing age. As expected respiratory–swallow patterns for younger participants with ataxia telangiectasia and their age-matched controls were similar. Contrary to our expectations, the frequency of perturbations in respiratory–swallow coupling did not increase significantly with age in participants with ataxia telangiectasia. Instead, measures of respiratory–swallow coordination remained unchanged while those of controls changed with normal development. These findings fit either a model of impaired development or one of degeneration, a quandary that has been apparent in other neurological dimensions of ataxia telangiectasia.4,23 Selley et al. demonstrated that respiratory–swallow interactions mature through the second decade of life and remain stable thereafter.20 Taken together, it is plausible that the expression of the swallowing dysfunction is related to interactions between the type and severity of the neurodegenerative insults and their timing relative to other developmental, growth, and compensatory processes.24,25 We speculate that compensations, which were successfully used by younger participants with ataxia telangiectasia, became less advantageous with disease progression or increased demands at older ages. Longitudinal studies that identify and track disease-specific patterns of respiratory–swallow coupling can increase our understanding of the natural history of the dysfunction, elucidate the interface between neurodegenerative and developmental processes, and identify optimal timing for the introduction of treatments before the emergence of symptomatic lung injury or nutrition compromise.
Study limitations and future directions
We acknowledge several methodological limitations. We used a cross-sectional design to model how changes in respiratory–swallow coordination apply to individuals with ataxia telangiectasia over time. Another limitation is that we decreased the bolus size from 5ml used in the control to 3ml for ataxia telangiectasia participants; this was because our previous observations showed that a 3ml bolus size is tolerated as a single bolus swallow in those with ataxia telangiectasia.6 Nonetheless, this difference in bolus size is unlikely to influence our findings, given that respiratory–swallow coupling is relatively invariant for boluses between 3ml and 20ml swallows of thin liquids through syrup consistencies.17
Non-invasive measures of respiratory–swallow coupling hold promise of capturing the development of aberrant temporal relationships that potentially compromise swallowing safety and may provide information that increases our understanding of the interplay between the timing of neurodegenerative and developmental processes. Although the RDG has provided valuable insight into the aberrant physiological basis of respiratory–swallow coordination and potentially useful clinical measures, analysis of data is costly and very labor intensive. Consequently, this technique is not part of routine clinical care, and at present is used primarily in research, including clinical trials.
In ataxia telangiectasia, aberrant patterns may represent arrested development, neurodegeneration, or a combination of both. Detection of prolongation of DIORAs and decreased frequency of expiratory–deglutitive coupling may facilitate identification of swallowing problems in asymptomatic individuals, and lead to prompt initiation of treatment before development of sequelae in persons at increased risk for the development of dysphagia.
What this paper adds.
Non-invasive technologies can quantify respiratory–swallow coordination associated with progressive ataxia.
Ataxic dysphagia is characterized by aberrant respiratory–swallow coupling.
This aberrant coupling is associated with increased risk of aspiration.
Perturbations in respiratory–swallow coupling may signify impaired development and/or neurodegeneration.
Acknowledgments
The authors wish to thank the participants and their families for contributing to this project. We also are grateful for contributions of Karen Rosquist, RN, and Jennifer Wright, RN, nurse coordinators for the Ataxia-Telangiectasia Clinical Center; Georgia Malandraki, Yihe Zu, and Christina Bronson-Lowe for their assistance with data analysis and reliability measures; and Will Crawford and Renee Allard for their assistance with illustrations. This work was supported by the National Institutes of Health grant NIDCD R24 DC008646-02 and the Johns Hopkins Institute for Clinical and Translational Research (ICTR), which is funded in part by grant number UL1 TR 000424-06 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH, the ICTR, or NCATS. Additional support was received from Ataxia Telangiectasia Children’s Project, Coconut Creek, FL, USA.
ABBREVIATIONS
- BMI
Body mass index
- DIORA
Deglutitive inhibition of respiratory airflow
- RDG
Respirodeglutometer
Footnotes
The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
REFERENCES
- 1.Swift M. Genetics and epidemiology of ataxia-telangiectasia. Kroc Found Ser. 1985;19:133–146. [PubMed] [Google Scholar]
- 2.McGrath-Morrow SA, Gower WA, Rothblum-Oviatt C, et al. Evaluation and management of pulmonary disease in ataxia-telangiectasia. Pediatr Pulmonol. 2010;45:847–859. doi: 10.1002/ppul.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford TO, Skolasky RL, Fernandez R, Rosquist KJ, Lederman HM. Survival probability in ataxia telangiectasia. Arch Dis Child. 2006;91:610–611. doi: 10.1136/adc.2006.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crawford TO. Ataxia telangiectasia. Semin Pediatr Neurol. 1998;5:287–294. doi: 10.1016/s1071-9091(98)80007-7. [DOI] [PubMed] [Google Scholar]
- 5.Crawford TO, Mandir AS, Lefton-Greif MA, et al. Quantitative neurologic assessment of ataxia-telangiectasia. Neurol. 2000;54:1505–1509. doi: 10.1212/wnl.54.7.1505. [DOI] [PubMed] [Google Scholar]
- 6.Lefton-Greif MA, Crawford TO, Winkelstein JA, et al. Oropharyngeal dysphagia and aspiration in patients with ataxia telangiectasia. J Pediatr. 2000;136:225–231. doi: 10.1016/s0022-3476(00)70106-5. [DOI] [PubMed] [Google Scholar]
- 7.Rempel G, Moussavi Z. The effect of viscosity on the breath–swallow pattern of young people with cerebral palsy. Dysphagia. 2005;20:108–112. doi: 10.1007/s00455-005-0006-0. [DOI] [PubMed] [Google Scholar]
- 8.Troche MS, Huebner I, Rosenbek JC, Okun MS, Sapienza CM. Respiratory–swallowing coordination and swallowing safety in patients with Parkinson's disease. Dysphagia. 2011;26:218–224. doi: 10.1007/s00455-010-9289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selley WG, Parrot LC, Lethbridge PC, et al. Non-invasive technique for assessment and management planning of oral-pharyngeal dysphagia in children with cerebral palsy. Dev Med Child Neurol. 2000;42:617–623. doi: 10.1017/s0012162200001158. [DOI] [PubMed] [Google Scholar]
- 10.Pinnington LL, Muhiddin KA, Ellis RE, Playford ED. Non-invasive assessment of swallowing and respiration in Parkinson's disease. J Neurol. 2000;247:773–777. doi: 10.1007/s004150070091. [DOI] [PubMed] [Google Scholar]
- 11.Paydarfar D, Gilbert RJ, Poppel CS, Nassab PF. Respiratory phase resetting and airflow changes induced by swallowing in humans. J Physiol. 1995;483:273–288. doi: 10.1113/jphysiol.1995.sp020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klahn MS, Perlman AL. Temporal and durational patterns associating respiration and swallowing. Dysphagia. 1999;14:131–138. doi: 10.1007/PL00009594. [DOI] [PubMed] [Google Scholar]
- 13.Perlman AL, He X, Barkmeier J, Van Leer E. Bolus location associated with videofluoroscopic and respirodeglutometric events. J Speech Lang Hear Res. 2005;48:21–33. doi: 10.1044/1092-4388(2005/003). [DOI] [PubMed] [Google Scholar]
- 14.Perlman AL, Ettema SL, Barkmeier J. Respiratory and acoustic signals associated with bolus passage during swallowing. Dysphagia. 2000;15:89–94. doi: 10.1007/s004550010006. [DOI] [PubMed] [Google Scholar]
- 15.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 16.Martin-Harris B, Brodsky MB, Michel Y, Ford CL, Walters B, Heffner J. Breathing and swallowing dynamics across the adult lifespan. Arch Otolaryngol Head Neck Surg. 2005;131:762–770. doi: 10.1001/archotol.131.9.762. [DOI] [PubMed] [Google Scholar]
- 17.Preiksaitis HG, Mills CA. Coordination of breathing and swallowing: effects of bolus consistency and presentation in normal adults. J Appl Physiol. 1996;81:1707–1714. doi: 10.1152/jappl.1996.81.4.1707. [DOI] [PubMed] [Google Scholar]
- 18.Hadjikoutis S, Pickersgill TP, Dawson K, Wiles CM. Abnormal patterns of breathing during swallowing in neurological disorders. Brain. 2000;123:1863–1873. doi: 10.1093/brain/123.9.1863. [DOI] [PubMed] [Google Scholar]
- 19.Preiksaitis HG, Mayrand S, Robins K, Diamant NE. Coordination of respiration and swallowing: effect of bolus volume in normal adults. Am J Physiol. 1992;263:R624–R630. doi: 10.1152/ajpregu.1992.263.3.R624. [DOI] [PubMed] [Google Scholar]
- 20.Selley WG, Flack FC, Ellis RE, Brooks WA. The Exeter Dysphagia Assessment Technique. Dysphagia. 1990;4:227–235. doi: 10.1007/BF02407270. [DOI] [PubMed] [Google Scholar]
- 21.Martin-Harris B, Brodsky MB, Price CC, Michel Y, Walters B. Temporal coordination of pharyngeal and laryngeal dynamics with breathing during swallowing: single liquid swallows. J Appl Physiol. 2003;94:1735–1743. doi: 10.1152/japplphysiol.00806.2002. [DOI] [PubMed] [Google Scholar]
- 22.Selley WG, Flack FC, Ellis RE, Brooks WA. Respiratory patterns associated with swallowing: Part 1. The normal adult pattern and changes with age. Age Ageing. 1989;18:168–172. doi: 10.1093/ageing/18.3.168. [DOI] [PubMed] [Google Scholar]
- 23.Shaikh AG, Zee DS, Mandir AS, Lederman HM, Crawford TO. Disorders of upper limb movements in ataxia-telangiectasia. PLoS One. 2013;8:e67042. doi: 10.1371/journal.pone.0067042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.La Spada AR. Neurodegeneration: a case of arrested development? Cell. 2006;127:669–671. doi: 10.1016/j.cell.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Hoche F, Seidel K, Theis M, et al. Neurodegeneration in ataxia telangiectasia: what is new? What is evident? Neuropediatrics. 2012;43:119–129. doi: 10.1055/s-0032-1313915. [DOI] [PubMed] [Google Scholar]