Abstract
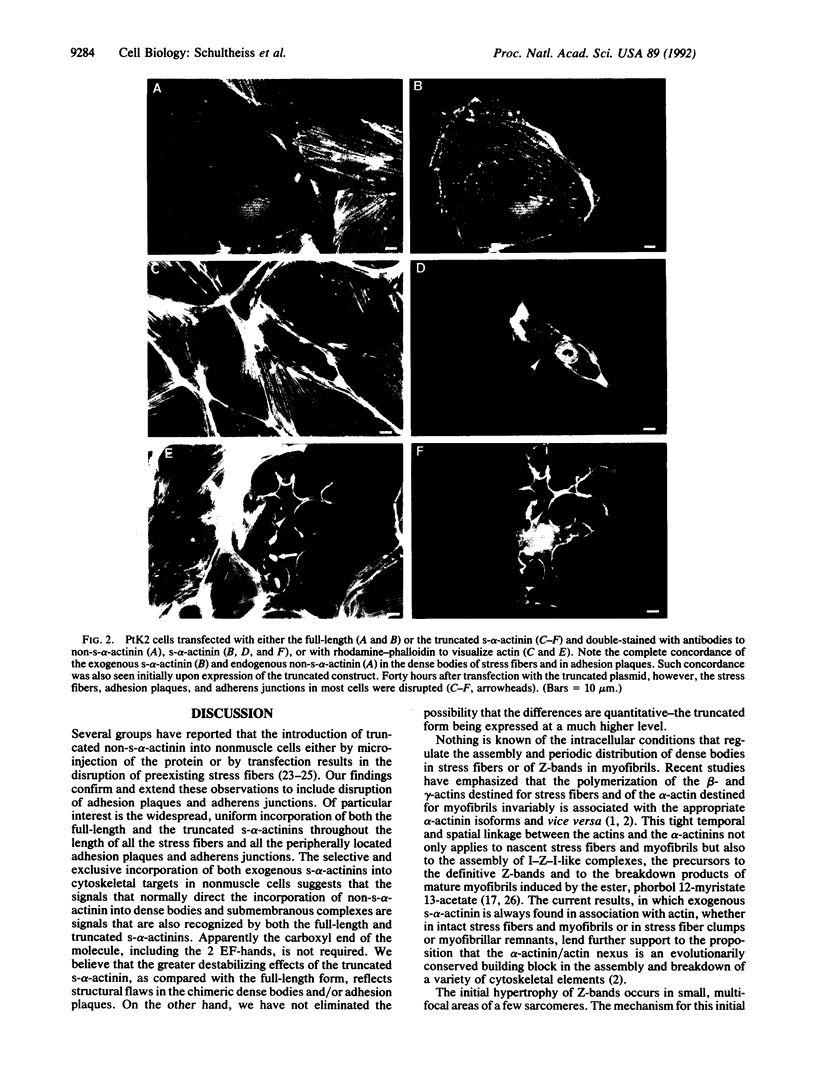
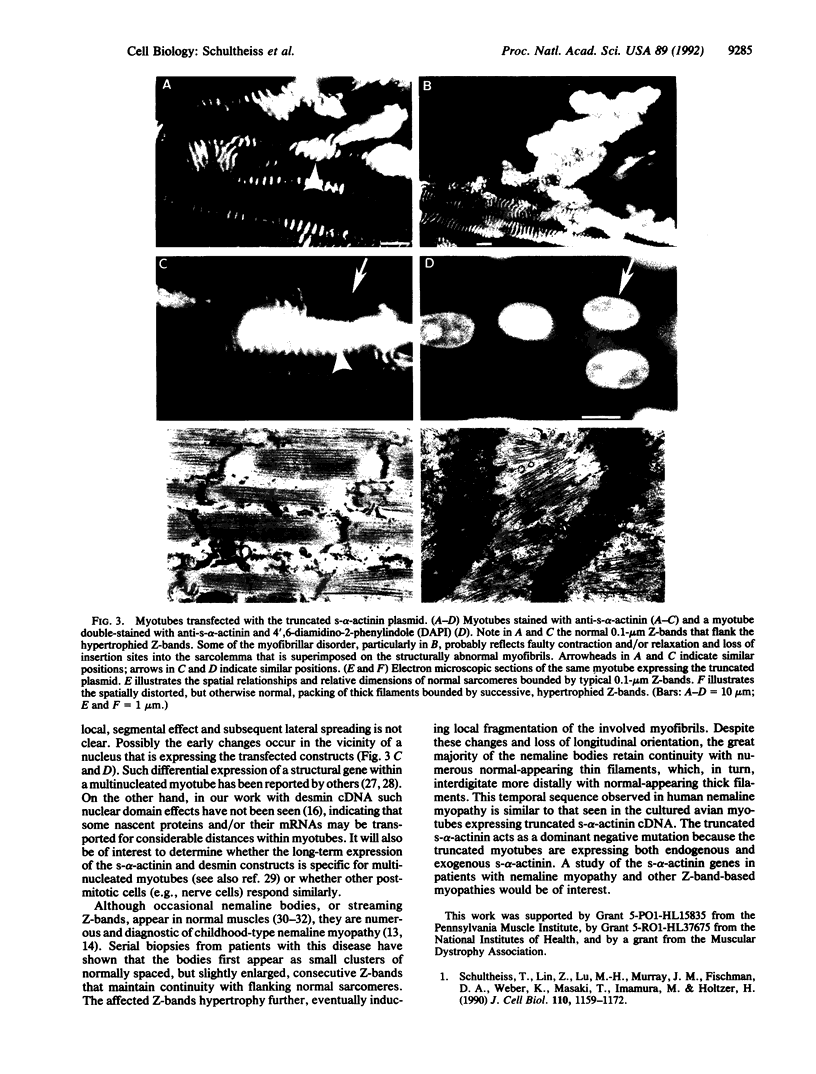
In many nonmuscle cells, nonsarcomeric alpha-actinin is distributed in the dense bodies of stress fibers, adhesion plaques, and adherens junctions. In striated muscle, a sarcomeric isoform of alpha-actinin (s-alpha-actinin) is found in the Z-bands of myofibrils and subsarcolemmal adhesion plaques. To understand the role(s) of the alpha-actinin isoforms in the assembly and maintenance of such cytoskeletal structures, full-length or truncated s-alpha-actinin cDNAs were expressed in PtK2 cells and in primary skeletal myogenic cells. We found the following. (i) In transfected PtK2 cells the truncated s-alpha-actinin was rapidly incorporated into preexisting dense bodies, adhesion plaques, and adherens junctions. With time these structures collapsed, and the affected cells detached from the substrate. (ii) In myotubes the truncated s-alpha-actinin was incorporated into nascent Z-bands. Many of these progressively hypertrophied, forming nemaline-like bodies. With time the affected myofibrils fragmented, and the myotubes detached from the substrate. (iii) In both cell types the truncated s-alpha-actinin was significantly more disruptive of the cytoskeletal structures than the full-length molecule. (iv) Pools of "over-expressed" full-length or truncated protein did not self-aggregate into homogeneous, amorphous complexes; rather the exogenous proteins selectively colocalized with the same cohort of cytoskeletal proteins with which the endogenous alpha-actinin normally associates. The similarity among the hypertrophied Z-bands in transfected myotubes, the nemaline bodies in patients with nemaline myopathies, and the streaming Z-bands seen in various muscle pathologies raises the possibility that the genetically determined nemaline bodies and the pathologically induced Z-band alterations may reflect primary and/or post-translational modifications of s-alpha-actinin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimura C., Suzuki T., Yanagisawa M., Imamura M., Hamada Y., Masaki T. Primary structure of chicken skeletal muscle and fibroblast alpha-actinins deduced from cDNA sequences. Eur J Biochem. 1988 Nov 15;177(3):649–655. doi: 10.1111/j.1432-1033.1988.tb14419.x. [DOI] [PubMed] [Google Scholar]
- Baron M. D., Davison M. D., Jones P., Critchley D. R. The sequence of chick alpha-actinin reveals homologies to spectrin and calmodulin. J Biol Chem. 1987 Dec 25;262(36):17623–17629. [PubMed] [Google Scholar]
- Belkin A. M., Koteliansky V. E. Interaction of iodinated vinculin, metavinculin and alpha-actinin with cytoskeletal proteins. FEBS Lett. 1987 Aug 17;220(2):291–294. doi: 10.1016/0014-5793(87)80832-3. [DOI] [PubMed] [Google Scholar]
- Blanchard A., Ohanian V., Critchley D. The structure and function of alpha-actinin. J Muscle Res Cell Motil. 1989 Aug;10(4):280–289. doi: 10.1007/BF01758424. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Changeux J. P. Compartmentalized transcription of acetylcholine receptor genes during motor endplate epigenesis. New Biol. 1991 May;3(5):413–429. [PubMed] [Google Scholar]
- Endo T., Masaki T. Differential expression and distribution of chicken skeletal- and smooth-muscle-type alpha-actinins during myogenesis in culture. J Cell Biol. 1984 Dec;99(6):2322–2332. doi: 10.1083/jcb.99.6.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett D. W. The sporadic occurrence in cardiac muscle of anomalous Z bands exhibiting a periodic structure suggestive of tropomyosin. J Cell Biol. 1968 Jan;36(1):266–270. doi: 10.1083/jcb.36.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Volk T., Volberg T., Bendori R. Molecular interactions in adherens-type contacts. J Cell Sci Suppl. 1987;8:251–272. doi: 10.1242/jcs.1987.supplement_8.14. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Bischoff R., Holtzer H. Mitosis and intermediate-sized filaments in developing skeletal muscle. J Cell Biol. 1968 Sep;38(3):538–555. doi: 10.1083/jcb.38.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P., Smith G., Critchley D. R. Expression of a muscle-type alpha-actinin cDNA clone in non-muscle cells. Eur J Cell Biol. 1989 Oct;50(1):162–169. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Actin, alpha-actinin, and tropomyosin interaction in the structural organization of actin filaments in nonmuscle cells. J Cell Biol. 1976 Feb;68(2):202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. X., Eshleman J., Grund C., Fischman D. A., Masaki T., Franke W. W., Holtzer H. Differential response of myofibrillar and cytoskeletal proteins in cells treated with phorbol myristate acetate. J Cell Biol. 1989 Mar;108(3):1079–1091. doi: 10.1083/jcb.108.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. X., Holtzer S., Schultheiss T., Murray J., Masaki T., Fischman D. A., Holtzer H. Polygons and adhesion plaques and the disassembly and assembly of myofibrils in cardiac myocytes. J Cell Biol. 1989 Jun;108(6):2355–2367. doi: 10.1083/jcb.108.6.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M. H., DiLullo C., Schultheiss T., Holtzer S., Murray J. M., Choi J., Fischman D. A., Holtzer H. The vinculin/sarcomeric-alpha-actinin/alpha-actin nexus in cultured cardiac myocytes. J Cell Biol. 1992 Jun;117(5):1007–1022. doi: 10.1083/jcb.117.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron B. J., Ferrans V. J., Roberts W. C. Ultrastructural features of degenerated cardiac muscle cells in patients with cardiac hypertrophy. Am J Pathol. 1975 Jun;79(3):387–434. [PMC free article] [PubMed] [Google Scholar]
- Masaki T., Endo M., Ebashi S. Localization of 6S component of a alpha-actinin at Z-band. J Biochem. 1967 Nov;62(5):630–632. doi: 10.1093/oxfordjournals.jbchem.a128717. [DOI] [PubMed] [Google Scholar]
- Mimura N., Asano A. Further characterization of a conserved actin-binding 27-kDa fragment of actinogelin and alpha-actinins and mapping of their binding sites on the actin molecule by chemical cross-linking. J Biol Chem. 1987 Apr 5;262(10):4717–4723. [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko F. M., Burridge K. Disruption of the actin cytoskeleton after microinjection of proteolytic fragments of alpha-actinin. J Cell Biol. 1991 Aug;114(3):481–491. doi: 10.1083/jcb.114.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlath G. K., Rich K., Webster S. G., Blau H. M. Localization of muscle gene products in nuclear domains. Nature. 1989 Feb 9;337(6207):570–573. doi: 10.1038/337570a0. [DOI] [PubMed] [Google Scholar]
- Schultheiss T., Lin Z. X., Ishikawa H., Zamir I., Stoeckert C. J., Holtzer H. Desmin/vimentin intermediate filaments are dispensable for many aspects of myogenesis. J Cell Biol. 1991 Sep;114(5):953–966. doi: 10.1083/jcb.114.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss T., Lin Z. X., Lu M. H., Murray J., Fischman D. A., Weber K., Masaki T., Imamura M., Holtzer H. Differential distribution of subsets of myofibrillar proteins in cardiac nonstriated and striated myofibrils. J Cell Biol. 1990 Apr;110(4):1159–1172. doi: 10.1083/jcb.110.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuue Y., Goto S., Imamura M., Obinata T., Masaki T., Endo T. Transfection of chicken skeletal muscle alpha-actinin cDNA into nonmuscle and myogenic cells: dimerization is not essential for alpha-actinin to bind to microfilaments. Exp Cell Res. 1991 Dec;197(2):158–167. doi: 10.1016/0014-4827(91)90418-t. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M., Robson R. M., Stromer M. H., Dahl D. S., Oda T. Nemaline myopathy rod bodies. Structure and composition. J Neurol Sci. 1982 Oct;56(1):35–56. doi: 10.1016/0022-510x(82)90059-4. [DOI] [PubMed] [Google Scholar]