Abstract
The cytokine IL-1β plays a central role in inflammatory responses that are initiated by microbial challenges, as well as in those that are due to endogenous processes (often called “sterile” inflammation). IL-1β secretion that occurs independently of microbial stimulation is typically associated with the presence of endogenous alarmins, such as extracellular ATP (an indicator of cytopathic damage). Here we show that IL-2 activated human iNKT cells stimulate the secretion of IL-1β protein by human peripheral blood monocytes in a manner that requires neither the presence of microbial compounds nor signaling through the extracellular ATP receptor P2X7. Monocyte IL-1β production was specifically induced by iNKT cells, since similarly activated polyclonal autologous T cells did not have this effect. Secretion of IL-1β protein occurred rapidly (within 3-4 hours), and required cell contact between the iNKT cells and monocytes. Similar to IL-1β production induced by TLR stimulation, the iNKT-induced pathway appeared to entail a two-step process involving NFκB signaling and IL1B gene transcription, as well as assembly of the NLRP3 inflammasome and activation of caspase 1. However, in contrast to the classical inflammasome-mediated pathway of IL-1β production, activation of monocytes via P2X7 was dispensable for iNKT-induced IL-1β secretion and potassium efflux was not required. Moreover, the iNKT-induced effect involved caspase 8 activity, yet induced little monocyte death. These results suggest that IL-2 activated human iNKT cells induce monocytes to produce IL-1β through a distinctive pathway that does not require the presence of microbial danger signals or alarmins associated with cytopathic damage.
Introduction
Invariant natural killer T (iNKT) cells are a subset of T lymphocytes that are characterized by their use of a semi-invariant TCR that recognizes lipids as antigens presented by CD1d, a conserved non-classical antigen presenting molecule that is constitutively expressed by B lymphocytes and a number of myelo-monocytic cell types including monocytes, macrophages, and myeloid DCs (1). Lipids recognized as antigen by iNKT cells include endogenous species produced by mammalian cells (i.e. ‘self’ lipids) (2-5), and thus, iNKT cells are not dependent on microbial infection for their activation. Therefore, interactions of iNKT cells with CD1d+ APCs that occur in the absence of microbial infections have important relevance for understanding their physiological functions.
In the absence of microbial challenge, a fraction of the iNKT cells appear to reside within the vasculature where intravital microscopy studies have visualized them randomly crawling along endothelial surfaces, occasionally detaching but rapidly re-attaching a short way downstream (6). Vascular endothelial surfaces may thus be an important location for iNKT cell interactions with CD1d+ APCs. Monocytes are the most abundant CD1d+ cell type in human venous blood, and are also abundant in murine liver vascular sinusoids where there is a high frequency of resident iNKT cells (7-9), and therefore monocytes may be of particular relevance as APCs for iNKT cells in non-infected situations. However, while prior studies have established that iNKT cells interact with monocytic cell types during microbial infections (10), little is known about the outcome of iNKT-monocyte interactions in the absence of microbial signals.
iNKT cells and monocytes likely meet up in blood vessels at sites where vascular endothelial cells have up-regulated their cell surface ICAM-1, an effect that can occur as a result of exposure to cytokines, oxidized lipids, or vascular damage in the absence of microbial products. Peripheral iNKT cell populations typically express high levels of the PLZF transcription factor, which not only bestows innate-like functional properties (11, 12), but also confers elevated cell surface expression of LFA-1, which is the adhesion ligand of ICAM-1 (9). As a result, iNKT cells and monocytes (which also express both LFA-1 and ICAM-1), may both accumulate at endothelial sites where ICAM-1 has become up-regulated. Consistent with this, iNKT cells have been found to be enriched in vascular endothelial plaques, and CD1d+ APCs are also found at these sites (13-17). It is also clear that iNKT cells can play an important role in atherogenesis and other vascular pathologies as a result of their production of IFN-γ (18, 19). However, it is not clear whether iNKT cells also contribute to vascular pathology by inducing monocytes to secrete additional key cytokines.
Here we have investigated the ability of IL-2 activated human iNKT cells to activate the release of pro-inflammatory cytokines by resting human peripheral blood monocytes in the absence of microbial ligands. In particular, we have focused on iNKT-mediated induction of IL-1β secretion, since this cytokine plays a critical role in the pathophysiology of sterile inflammation (20, 21). This area of study is of interest from both a basic immunological and a clinical perspective, since the cellular and molecular mechanisms leading to IL-1β release remain a very active area of investigation and understanding the role of iNKT cells in the induction of IL-1β release by human blood monocytes may reveal new insights into their contributions to both protective and pathogenic inflammatory responses, and may be particularly relevant for their role in vascular disease.
Methods
Isolation of primary monocytes
50 mL of blood was collected from self-identified healthy donors via venipuncture and mixed with heparin (clinical grade, 10 units/mL blood). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation (Ficoll-Paque PLUS). Following purification, PBMCs were incubated with magnetic beads (whole blood CD14 beads from Miltenyi Biotech), and monocytes were purified by magnetic sorting of positively selected cells.
iNKT cells and polyclonal T cells
iNKT cells were initially produced by sorting single cells from blood of healthy subjects using α-GalCer loaded CD1d-Fc tetramers, as described (22). iNKT cells were maintained in IL-2 containing medium (RPMI 1640, 15% heat-inactivated bovine calf serum from Hyclone, 3% human AB serum from Gemini, 2 mM L-glutamine, 100 μg/ml penicillin and streptomycin), and were periodically stimulated to proliferate by the addition of PHA and irradiated PBMCs. Polyclonal T cells were isolated from PBMCs of healthy subjects by magnetic sorting using negative selection (pan T cell isolation beads, Miltenyi Biotech), then expanded in culture using PHA and irradiated PBMC in IL-2 containing medium. Prior to use in experiments, iNKT cells and polyclonal T cells were cultured for at least two weeks without addition of PHA or irradiated PBMCs, but in medium supplemented with 200 IU/mL IL-2 (Peprotech).
Flow cytometric analysis of monocyte CD69 expression
Freshly isolated PBMCs alone or combined with iNKT cells at ratios ranging from 200:1 to 10:1 PBMC:iNKTs were suspended in culture medium (RPMI 1640, 10% fetal bovine serum, 2 mM L-glutamine, 100 μg/ml penicillin and streptomycin, 1 mM sodium pyruvate, and 0.1% 2-mercaptoethanol) and placed in a humidified incubator at 37 °C and 5% CO2 for 4 hours. The cells were then transferred into blocking buffer (PBS containing 2% FBS, 2% human AB serum, 0.3 mg/mL ovalbumin, and 1 mg/mL bovine serum albumin) and on ice for 30 minutes. The cells were then stained with fluorescently labeled anti-CD69 and anti-CD14 antibodies, or isotype-matched negative controls (Biolegend), and analyzed on a BD LSRII flow cytometer. Samples were gated by forward and side scatter to exclude debris and aggregated material, and then on cells showing positive staining for CD14 compared to the isotype control to identify monocytes. The percent of cells in the monocyte gate that show positive staining for CD69 compared to the isotype control was then determined for each incubation condition.
Stimulation of monocytes
Freshly isolated monocytes (2.5×104 cells) were combined with iNKT cells or autologous polyclonal T cells (5×104 cells) per well in a total volume of 250 μL of culture medium in a round-bottom 96-well tissue culture plate. The cells were placed in a humidified incubator at 37 °C and 5% CO2 for the indicated amounts of time prior to withdrawal of supernatants for analysis. Alternatively, monocytes (2.5×104 cells/well) were incubated in culture medium containing 250 ng/mL lipopolysaccharide (LPS) purified from Salmonella enterica (Sigma-Aldrich). Where indicated, the following inhibitors were included in the culture medium: 10 μM MCC950 (Cayman Chemical); 30 μM KN-62 (Sigma-Aldrich); 50 μM Z-YVAD-FMK (Invivogen); 10 μM U0126 (Sigma-Aldrich); 10 μM Bay 11-7082 (Adipogen); 50 μM IKKγ NEMO Binding Domain (NBD) Inhibitory Peptide (Eurogentec), 5-20 μM caspase 8 inhibitor peptide Z-IED-FMK (BD Biosciences); 3 μM glycogen synthase kinase-3β inhibitor GSK (Aobious). Alternatively, the following blocking reagents were included during iNKT-monocyte co-culture: anti-CD40L mAb (clone 24-31); anti-LTβR mAb (clone 31G4D8); anti-BAFF mAb (clone 148725); osteoprotegerin (to prevent activation of RANK by RANKL) (GIBCO). For inhibitor or pathway blockade analyses, 1×105 monocytes and 1×105 iNKT cells or 1 μg/ml LPS were used, and the cells were co-incubated for only 3-4 hours prior to harvesting culture supernatants for analysis.
Determination of secreted cytokines
Culture supernatants were tested in triplicate by sandwich ELISA for IL-10, IL-6, IL-1β, or IL-12p70 (Biolegend). ELISA plates were read at two different wavelengths, 450 nm and 57nm, and for each well the 570 nm value is subtracted from the 450 nm value to reduce non-specific background signal. Cytokine protein concentrations were estimated by comparison to a standard curve of serially diluted recombinant cytokine tested in parallel (Biolegend).
Transwell assays
To examine whether soluble factors were sufficient to induce monocyte IL-1β production, 1×106 freshly isolated monocytes were placed in the lower well of a transwell plate, and 5×105 iNKT cells were placed in a transwell insert containing a membrane with 0.22 μm pores (Corning). Where indicated, 1 μg/mL anti-CD3 mAb (OKT3) and 5 μg/mL CD28 (clone CD28.2) were included in the culture medium, or iNKT cells were used that had been exposed for the preceding 4-5 hours to plates coated with 5 μg/mL anti-CD3 mAb (OKT3). Samples of culture supernatants were withdrawn after 24 hours and analyzed by ELISA for IL-1β.
Supernatant transfer assays
To assess whether contact between monocytes and iNKT cells resulted in the generation of a soluble factor capable of inducing monocytic IL-1β production, iNKT cells and monocytes were co-incubated (5×104 iNKT cells and 2.5×104 monocytes) for 2-3 hours at 37 °C and 5% CO2. Supernatants were removed and filtered through a 0.2 μm membrane, then added to wells containing 2.5×104 monocytes and the cells were incubated for 24 hours at 37 °C and 5% CO2 before supernatants were harvested for analysis.
qRT-PCR
Monocytes and iNKT cells or LPS (1 mg/mL) with or without inhibitors (concentrations listed above) were co-cultured for 2-3 hours at 37°C. After 2 hours, cells were collected and RNA was isolated via ethanol precipitation (Autogen). RNA samples were DNAse treated (Turbo DNAse kit, Invivogen) and converted to cDNA (iScript Select cDNA, BioRad). qRT-PCR was set up using Kapa Syber and the following primers: GAPDH Forward 5′-GGAGCGAGATCCCTCCAAAAT-3′; GAPDH Reverse 5′-GGCTGTTGTCATACTTCTCATGG-3′; IL-1β Forward 5′-CAGGCTGCTCTGGGATTCTC-3′ IL-1β Reverse 5′-CCTGGAAGGAGCACTTCATCT-3′.
YoPro1 uptake assay
Freshly-isolated monocytes were incubated in HBS (130 mM NaCL, 5 mM KCl, 20 mM HEPES, 0.1% BSA, 10 mM glucose, pH to 7.4 with NaOH) and stained with allophycocyanin labeled anti-CD14 mAb (Biolegend). The cells were washed and resuspended in K-glutamate buffer (130 mM K-glutamate, 5 mM KCl, 2 mM HEPES, .1% BSA, 10 mM glucose, pH to 7.4 with KOH). To these samples were added 1 μM YoPro1 (Molecular Probes) and either 250 μM BzATP (Sigma, in 250 mM HEPES) or a 1:1 ratio of iNKT cells. Samples containing BzATP were protected from light and incubated at room temperature for 20 minutes. To facilitate cell-cell contact, the iNKT cell-monocyte co-cultures were spun briefly (3 minutes at 400 RCF), protected from light, and incubated at 37 °C. At the end of the incubation period, the reactions were stopped by the addition of 100 mM MgCl2. The cells were resuspended in HBS and assessed by flow cytometry using a BD LSR II cytometer. Gating for monocytes performed based on CD14 expression and forward and side-scatter characteristics, and YoPro1 fluoresence intensity of the gated cells was determine using the FL1 channel.
Analysis of monocyte death
Release of cytoplasmic contents was assessed using the lactose dehydrogenase release assay (Pierce LDH cytotoxicity assay kit): Monocytes (30,000/well) were cultured alone, with LPS (250 ng/mL), with LPS and nigericin (250 ng/mL LPS, 6.5 uM nigericin), or with iNKT cells (30,000/well) for a total of 16 hours (nigericin added in the last two hours of culture). % LDH release was determined by colorimetric analysis according to the manufacturer's protocol. Monocytes were also assessed for apoptotic phenotype and loss of membrane integrity as follows: Monocytes (100,000/well) were cultured for 3 hours alone, with LPS (250 ng/mL), with LPS and nigericin (6.5 uM added for the last 20 minutes of incubation), or with iNKT cells (100,000 per well). After incubation, cells were washed in PBS containing 2% FBS, 2% human AB serum, 0.3 mg/mL ovalbumin, and 1 mg/mL bovine serum albumin, then stained with antibodies against CD14 (63D3) and CD3 (OKT3). Cells were then washed in binding buffer provided by the Biolegend Annexin V apoptosis detection kit, stained with propidium iodide (10 uL/sample) and APC-labeled annexin V (5 uL/sample), and analyzed by flow cytometry.
Statistical analyses
Data were analyzed using GraphPad Prism 5 Software (GraphPad Software, La Jolla, CA). To make comparisons between two unpaired groups, the student's two-sided unpaired T test was used. When appropriate, a two-sided paired T test was used. To make comparisons between >2 groups, a one-way ANOVA with a Tukey post hoc test was used to determine statistical differences.
Results
iNKT cells induce rapid activation of peripheral blood monocytes
We investigated the ability of IL-2-exposed clonal human iNKT cells to activate human peripheral blood monocytes. Freshly isolated PBMCs were incubated for 4 hours with titrated doses (0-10%) of human iNKT cells that had been cultured in medium containing IL-2. The samples were stained for CD14 to identify monocytes, and cell surface expression levels of CD69 on the monocytes were assessed, since this marker has been shown to be rapidly up-regulated by human peripheral blood monocytes after activation (23). The addition of iNKT cells resulted in a subset of the monocytes showing up-regulated CD69 (Figure 1A). The fraction of the monocytes that showed elevated CD69 increased as the percent of iNKT cells added was titrated from 0-10% (Figure 1B). These results suggested that IL-2-stimulated iNKT cells activate human peripheral blood monocytes rapidly (within 4 hours) and in a dose-dependent manner.
Figure 1. iNKT cells activate human blood monocytes in a dose-dependent manner.
A) Freshly isolated human PBMCs were incubated alone or with IL-2 activated human iNKT cells for 4 hours, then stained with antibodies against CD14 and CD69 or isotype-matched negative controls. Samples were gated by forward and side scatter and on cells expressing CD14 (as determined by comparison to isotype). Plots show monocyte CD69 staining (filled histograms) compared to its isotype control (dashed line) for one representative experiment. Similar results were observed in two additional experiments using unrelated PBMCs. B) Plot showing the percent of the CD14+ population with anti-CD69 staining above isotype as a function of the percent iNKT cells added. Symbols represent the means from 3 independent analyses, with error bars showing the standard deviations. C) Freshly isolated monocytes were incubated alone, with a 2:1 ratio of iNKT cells, or with 250 ng/mL LPS. After 24 hours culture supernatants were tested for cytokines using a standardized ELISA. The plot shows the amount of the indicated cytokines in the monocyte alone or monocyte+iNKT cell conditions as a percentage of the LPS response. Each symbol shows the results from an independent analysis.
To investigate the functional outcome of this activation pathway, monocytes were purified from PBMCs using anti-CD14 magnetic beads and co-incubated with iNKT cells or with purified LPS. Supernatants were collected from the cultures and tested for IL-6, IL-10, IL-12p70, and IL-1β using standardized ELISAs. Exposure to LPS induced robust IL-6, IL-10, and IL-1β production (typically in the range of 0.5-1 ng/ml for IL-10 and IL-1β, and about 10 ng/ml for IL-6), but little or no detectable IL-12p70. Monocyte-iNKT cell co-cultures produced similar amounts of secreted IL-6 and IL-1β as LPS stimulated monocytes, whereas IL-10 was consistently less efficiently produced in the monocyte-iNKT co-cultures (Figure 1C). IL-12p70 was not detected in iNKT-monocyte co-culture supernatants (data not shown). These results indicated that interactions between activated iNKT cells and peripheral blood monocytes can lead to the release of substantial quantities of both IL-6 and IL-1β. However, since the secretion of IL-6 can be induced by a wide variety of stimuli (including exposure to IL-1β), whereas IL-1β secretion typically requires the coordination of multiple activation pathways, we focused our subsequent analyses on the induction of this cytokine.
We first confirmed the reproducibility of IL-1β secretion in monocyte-iNKT co-cultures. Six different human iNKT clonal lines were each tested against monocytes isolated from at least three different subjects. We consistently observed that exposing monocytes to iNKT cells resulted in significantly greater IL-1β secretion than that detected from monocytes cultured alone (Figure 2A). However, we noted that the quantity of IL-1β detected in the co-cultures varied over about a 100-fold concentration range (Figure 2A). The variation in IL-1β amounts did not seem to depend on the specific iNKT clone used for the co-culture, and instead most of the variation seemed to stem from the use of monocytes isolated from different human subjects, suggesting that the differences in IL-1β levels were largely due to the monocytes. This was supported by our observation that there were substantial inter-subject variations in the amount of IL-1β produced by monocytes in response to LPS, and the amount of IL-1β from iNKT-monocyte co-cultures tracked closely with the respective monocyte responses to LPS (Figure 2B). Moreover, we were not able to detect IL-1β in culture supernatants from anti-CD3 stimulated iNKT cells (data not shown). Based on these results, we concluded that exposure to activated iNKT cells stimulates monocytes to secrete IL-1β.
Figure 2. Induction of monocyte IL-1β secretion by iNKT cells.
A) Monocytes were cultured alone or with the indicated iNKT cell clones, and after 24 hours the concentration of IL-1β protein in the culture supernatants was determined using a standardized ELISA. Each symbol represents the mean concentration of IL-1β detected from an independent analysis using the indicated iNKT cell clones. B) Monocytes from 3 unrelated human subjects were co-cultured with the same iNKT clone (PP1.3) or stimulated with 250 ng/mL LPS, and after 24 hours secreted IL-1β protein concentrations were assessed by ELISA. The plot shows the means and standard deviations from 3 replicates. C) Freshly isolated monocytes were incubated alone, with a 2:1 ratio of iNKT cells, or with 250 ng/mL LPS, and culture supernatants collected at the indicated time points were tested for secreted IL-1β by ELISA. The plot shows the results from one representative experiment; similar results were obtained in 4 additional experiments. Symbols show the means from 3 replicate samples, with error bars indicating the standard deviations (not always visible on the scale shown). D) Monocytes were incubated alone or with a 2:1 ratio of IL-2 activated polyclonal autologous T cells (cultured in the same way as the iNKT cells shown in the other panels), and culture supernatants were assessed at the indicated time points for secreted IL-1β by ELISA. E) Freshly isolated monocytes (1x105 cells) were stimulated with 1 μg/mL LPS or co-cultured with 1×105 iNKT cells for 2-3 hours. Total RNA was harvested and IL1B mRNA was measured by qRT-PCR. The plot shows the mean IL1B signal normalized by the signal from iNKT cells alone. Each symbol shows the results from an independent analysis.
We next investigated the kinetics of IL-1β secretion in iNKT-monocyte co-cultures compared to monocytes stimulated by LPS. The time course of IL-1β accumulation in the culture supernatant appeared similar in the two conditions, with detectable amounts of IL-1β first appearing after 3-4 hours, and IL-1β levels approaching a plateau by about 8-12 hours (Figure 2C). To confirm that the accumulation of IL-1β was not simply due to some sort of stress produced by co-culturing monocytes with activated T cells, we performed control experiments in which freshly isolated monocytes were co-incubated with autologous polyclonal T cells that had been cultured in an identical manner to the conditions used for the iNKT cell clones. At no time point was any IL-1β detectable in cultures of monocytes with polyclonal T cells (Figure 2D), illustrating the selectivity of the monocyte-iNKT IL-1β effect.
Finally, we used qRT-PCR to determine whether the secreted IL-1β protein we observed in the supernatants of iNKT-monocyte co-cultures was associated with transcription of IL1B mRNA. Monocytes were exposed to LPS or iNKT cells for a period of 2-3 hours, total RNA was isolated, and qRT-PCR was performed. RNA isolated from iNKT cells alone did not show any evidence of IL1B transcript. Monocytes alone typically showed very little signal for IL1B transcript, and RNA from iNKT-monocyte co-cultures showed significantly elevated signal for IL1B message (Figure 2E). Thus, exposing freshly isolated human monocytes to IL-2 activated iNKT cells results in the induction of IL1B gene transcription.
Mechanism of monocyte-iNKT interaction leading to IL-1β production
To investigate the mechanism underlying iNKT cell induction of monocyte IL-1β production, we first addressed the possibility that soluble factors generated by iNKT cells were responsible. Monocytes were exposed to transwell inserts containing either iNKT cells alone, iNKT cells in the presence of anti-CD3 and anti-CD28 mAbs, or iNKT cells that were pre-stimulated by exposure to plate-bound anti-CD3 mAb. In contrast to co-cultures where monocytes and iNKT cells were able to make contact, exposing monocytes to soluble factors released by iNKT cells contained within transwell inserts did not result in any detectable IL-1β secretion, regardless of whether the iNKT cells were given a TCR stimulus or not (Figure 3A). These results indicated that contact between iNKT cells and monocytes was required for induction of IL-1β production, however, it remained a possibility that an initial contact between iNKT cells and monocytes results in the generation of a soluble factor that might provide the signaling needed to induce IL-1β secretion. To test this possibility, monocytes and iNKT cells were co-cultured for 2-3 hours. At this point, culture supernatants were removed, filtered to remove any cells or large debris, then added to freshly isolated monocytes. Consistent with our prior time course analyses, supernatants from these 2-3 hr iNKT-monocyte co-cultures did not contain detectable IL-1β. Moreover, there was no secreted IL-1β detected from monocytes that were cultured for 24 hours with the filtered iNKT-monocyte supernatants (Figure 3B), suggesting the supernatants also did not contain soluble factors that were sufficient to induce IL-1β secretion. Based on these results we concluded that iNKT cells stimulate monocyte IL-1β secretion through a contact dependent mechanism.
Figure 3. Contact between monocytes and iNKT cells is required to induce IL-1β secretion.
A) Monocytes (1×106 cells) were placed in the lower wells of transwell plates and 5×105 iNKT cells were placed in the transwell inserts; after 24 hours culture supernatants were harvested and tested for IL-1β protein by ELISA. In the “no stim” condition IL-2 activated iNKT cells were placed into the transwell inserts, in the “anti-CD3 + anti-CD28 mAb” condition these antibodies were included in the culture medium for the duration of the experiment, and in the “pre-activated iNKT” condition iNKT cells that had been exposed to plate-bound anti-CD3 mAb were placed into the transwell insert. In parallel, monocytes were cultured alone or co-cultured with a 2:1 ratio of IL-2 activated iNKT cells (“in contact”). Each symbol represents the mean cytokine detected from an independent experiment. B) Monocytes and iNKT cells were co-cultured for 2-3 hours and supernatants were removed and filtered through a 0.2 μm membrane. This supernatant was then added to resting monocytes, and after 24 hours the culture supernatant was assayed for IL-1β protein by ELISA. In parallel, monocytes were cultured alone or co-cultured with a 2:1 ratio of IL-2 activated iNKT cells (“co-culture”). Each symbol represents the mean cytokine detected from an independent experiment.
NF-κB signaling
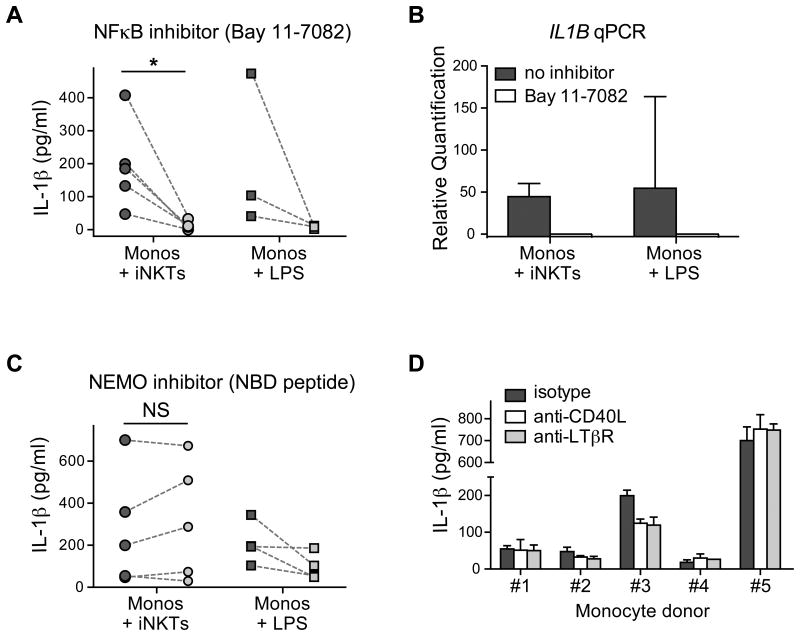
Earlier studies of IL-1β production by myeloid cell types have delineated two critical steps prior to secretion of the mature cytokine: i) NF-κB signaling that leads to IL1B gene transcription, and ii) inflammasome activation that leads to post-translational processing of the IL-1β polypeptide chain (24). Therefore, to further investigate the mechanism of IL-1β production during monocyte interactions with iNKT cells, we first investigated NF-κB signaling pathways. Monocytes were exposed to iNKT cells for 10-30 minutes, then the cells were lysed, and Western blotting was performed using antibodies against phospho-p65 to detect activation of the canonical NF-κB pathway, or p100/p52 to evaluate the non-canonical NFκB pathway. Both pathways appeared to be activated within 30 minutes of iNKT-monocyte contact (data not shown). We next tested the impact of Bay 11-7082, a chemical compound that has broad inhibitory effects on NF-κB activation (25). We focused these experiments on the earliest time points at which we were able to detect secreted IL-1β protein (3-4 hours of stimulation) so as to avoid potential contributions from autocrine signaling pathways that can induce IL-1β, such as TNFα or IL-1β itself. Inclusion of the Bay 11-7082 inhibitor during monocyte-iNKT cell interactions led to a profound reduction of the amount of IL-1β protein secretion compared to co-cultures that were treated with vehicle alone (Figure 4A). Additionally, inclusion of the Bay 11-7082 inhibitor abrogated the signal for IL1B transcript detected by qRT-PCR (Figure 4B). Together, these results indicated that iNKT-mediated induction of monocyte IL-1β protein secretion is dependent on NF-κB activation of IL1B gene transcription.
Figure 4. Involvement of NFκB signaling in iNKT-induced monocyte IL-1β secretion.
A) Monocytes were incubated for 3-4 hours with iNKT cells, or in medium containing 1 μg/mL LPS, in the presence (light symbols) or absence (dark symbols) of the broad spectrum NFκB inhibitor Bay 11-7082, and secreted IL-1β protein was determined by ELISA. Each symbol represents the mean cytokine detected from an independent analysis with dashed lines connecting the paired inhibitor and control treatments. B) Monocyteswere stimulated with 1 μg/mL LPS or co-cultured with iNKT cells in the presence or absence of the Bay 11-7082 inhibitor for 2-3 hours. Total RNA was harvested and IL1B mRNA was measured by qRT-PCR. The plot shows mean relative quantitation of the indicated samples in relation to the IL1B mRNA signal from unstimulated monocytes (which was set at a value of 1), with error bars representing the 95% confidence interval of the means. C) Monocytes were incubated for 3-4 hours with iNKT cells, or in medium containing 1 μg/mL LPS, in the presence (light symbols) or absence (dark symbols) of a selective IKK-γ (NEMO) inhibitor peptide, and secreted IL-1β protein was determined by ELISA. Each symbol represents the mean cytokine detected from an independent analysis with dashed lines connecting the paired inhibitor and control treatments. D) Monocytes isolated from five different subjects were incubated with iNKT cells for 3-4 hours in the presence of anti-CD40L or anti-LTbR blocking mAbs, and secreted IL-1β was determined by ELISA. Bars represent the means and standard deviations from 3 replicate analyses.
To investigate the roles of the canonical and non-canonical NF-κB pathways, we tested the effect of a peptide that has been shown to inhibit NEMO (also known as IKK-γ), a component that plays a critical role in activation of the canonical NF-κB pathway but is not required for the non-canonical pathway. Addition of the NEMO Binding Domain (NBD) inhibitory peptide completely failed to reduce monocyte IL-1β secretion induced by iNKT cells, although it was associated with up to a 50% reduction in monocyte IL-1β secreted in response to LPS (Figure 4C). This observation suggested that the iNKT-induced pathway might act completely or in part through non-canonical NF-κB activation. We therefore tested whether IL-1β secretion was inhibited by antibody blockade of receptors that are known to induce non-canonical NF-κB activation, including CD40, LTβR, RANK, and BAFF. However, we were not able to observe reproducible inhibitory effects on iNKT-induced IL-1β secretion from blocking monocyte stimulation through any of these pathways (Figure 4D, and data not shown). Thus, iNKT cells may engage an NF-κB-activating receptor on monocytes that is not yet well-characterized.
Inflammasome activation
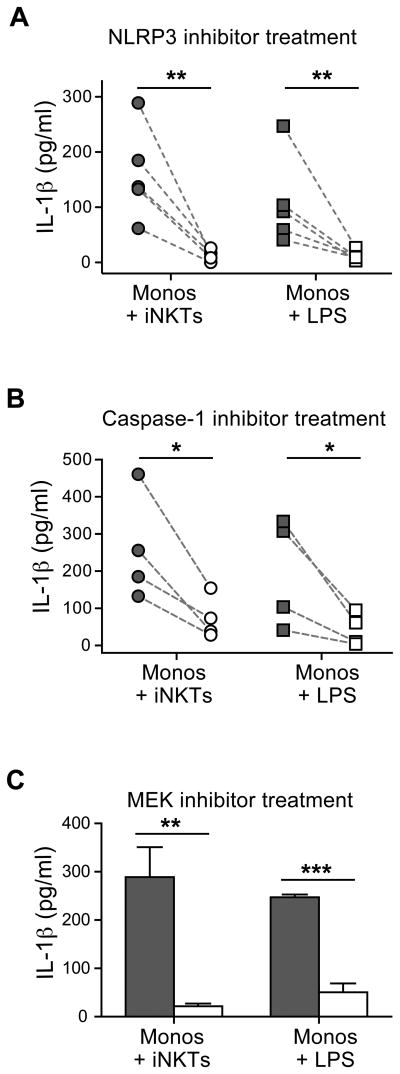
Unlike most cytokines, which are secreted immediately following translation of the polypeptide chain, translation of the mRNA transcript for IL-1β yields a pro-form of the cytokine that must be processed by proteolytic cleavage to produce the biologically active mature cytokine that is then released from the cell. Since this proteolytic processing step has previously been shown to involve activation of caspases by the NLRP3 inflammasome, we tested the impact of a small molecule inhibitor (MCC950) that selectively targets the NLRP3 inflammasome (26). Inclusion of this inhibitor in iNKT-monocyte co-cultures significantly inhibited IL-1β protein detected in the culture supernatant after 3-4 hours (Figure 5A). Addition of a peptide inhibitor of caspase-1 (Z-YVAD-FMK) also led to a significant reduction of secreted IL-1β protein (Figure 5B). Finally, we noted that inclusion of a chemical inhibitor (U0126) that prevents phosphorylation of ERK1 led to significant reductions in secreted IL-1β protein, which is consistent with recent results showing that ERK1 is required for priming of the NLRP3 inflammasome to activate caspase-1 in human monocytes (27). These results establish that iNKT-mediated induction of IL-1β secretion by human monocytes requires the participation of the NLRP3 inflammasome and involves activated caspase-1.
Figure 5. Requirement for inflammasome activation for iNKT-induced monocyte IL-1β secretion.
Monocytes were incubated for 3-4 hours with iNKT cells, or in medium containing 1 μg/mL LPS, in the presence of inhibitor (light symbols) or of vehicle alone (dark symbols), and secreted IL-1β protein was determined by ELISA. Each symbol represents the mean cytokine detected from an independent analysis, with dashed lines connecting the paired inhibitor and vehicle control treatments. Panel (A) shows results using the selective NLRP3 inflammasome inhibitor MCC950; panel (B) shows results using the specific caspase-1 inhibitor peptide Z-YVAD-FMK. C) Effect of the specific MEK inhibitor U0126. Bars represent means and standard deviations from one representative experiment out of a total of three.
No requirement for P2X7 signaling
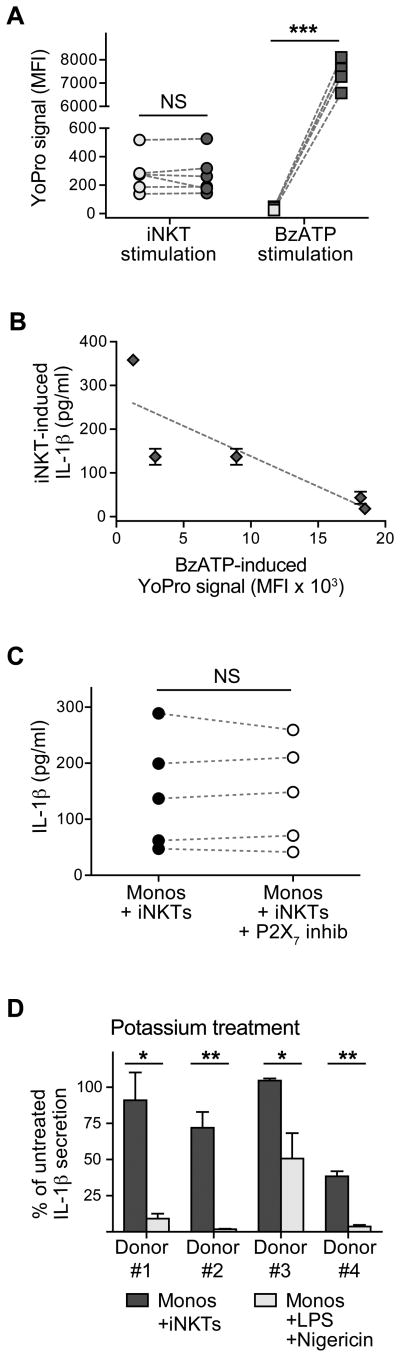
Prior studies of IL-1β production by macrophages have indicated that the NLRP3 inflammasome typically becomes engaged as a result of signaling through the P2X7 receptor (28). P2X7 is a ligand-gated ion channel embedded in the plasma membrane that becomes activated by binding extracellular purines (e.g. ATP) (29). Prolonged P2X7 stimulation leads to the activation of pannexin-1, an accessory channel that allows the entry of large molecules into the cytoplasm (30). To investigate the ability of iNKT cells to stimulate monocytes via P2X7, we tested monocyte uptake of a fluorescent dye (YoPro1) during exposure to iNKT cells or the potent P2X7 agonist BzATP. Whereas exposure to BzATP reproducibly led to a more than 100-fold increase in monocyte fluorescent signal, there was no increased dye fluorescence in monocytes that were exposed to iNKT cells (Figure 6A). Thus, exposure to iNKT cells does not result in P2X7 signaling that is sufficient to open monocyte pannexin-1 channels.
Figure 6. Signaling through P2X7 is dispensable for iNKT cell-induced monocyte IL-1β production.
A) CD14-stained monocytes were incubated for 20 minutes with the fluorescent dye YoPro1 alone (light symbols), or in the presence of a 1:1 ratio of iNKT cells or in medium containing 250 μM of the selective P2X7 agonist BzATP (dark symbols), and dye uptake was assessed by flow cytometry. Each symbol represents the mean fluorescence intensity of the YoPro1 dye in the CD14-positive cells from one independent analysis, with lines connecting unstimulated monocytes with their paired treatments. B) Plot showing the relationship between YoPro1 analysis of monocyte P2X7 function and iNKT-induced IL-1β release for monocytes from five unrelated human subjects. C) Monocytes were incubated for 3-4 hours with iNKT cells, or in medium containing 1 μg/mL LPS, in the presence of the KN-62 P2X7 inhibitor (light symbols) or of vehicle alone (dark symbols), and secreted IL-1β protein was determined by ELISA. Each symbol represents the mean cytokine detected from an independent analysis, with dashed lines connecting the paired inhibitor and vehicle control treatments. D) Monocytes were incubated for 3-4 hours with iNKT cells, or exposed to 1 μg/mL LPS and 6.5 μM nigericin, in an RPMI-based culture medium in the presence or absence of 40 μM K+ supplementation, and secreted IL-1β protein was determined by ELISA. The plot shows the IL-1β secreted in the presence of K+ supplementation as a percentage of that produced in the absence of added K+.
To further investigate the role of P2X7 functionality in the iNKT-mediated pathway of monocyte IL-1β secretion, we took advantage of the observation that there is a tremendous amount of allelic polymorphism in the human P2X7 locus that results in functional differences in the ability of the P2X7 receptor to promote pannexin-mediated dye uptake (31). Comparing the BzATP-induced dye uptake levels of monocytes from 5 unrelated subjects with the amount of IL-1β released by their monocytes in response to iNKT cells, we observed no evidence that high P2X7 functionality was associated with greater iNKT-induced IL-1β secretion, and instead subjects with high P2X7 activity may actually tend towards reduced monocyte responsiveness to iNKT cells (Figure 6B).
To directly address the requirement for P2X7 function during induction of monocyte IL-1β secretion by iNKT cells, we tested the impact of a chemical inhibitor of P2X7 (KN-62). As shown in Figure 6C, there was no reduction in secreted IL-1β protein levels after 3-4 hours of monocyte-iNKT co-culture in the presence of the KN-62 inhibitor, despite our confirmation that similar KN-62 treatment was sufficient to inhibit stimulation of P2X7 by BzATP (data not shown). One of the contributions of the P2X7 ion channel to secretion of mature IL-1β may be that it allows the efflux of cytoplasmic K+ ions from the cell, creating a cytoplasmic environment that promotes activation of the NLRP3 inflammasome complex (32, 33). To test whether the iNKT-induced pathway requires K+ efflux, we assessed the impact of raising the concentration of extracellular K+. The amount of IL-1β secreted in response to iNKT-mediated activation showed little or no decrease at elevated extracellular K+ concentrations, whereas this treatment typically almost completely abrogated monocyte IL-1β secretion induced by LPS and nigericin (Figure 6D).
A recent study revealed the existence of a novel pathway in human monocytes in which mature IL-1β is released following an activation process that does not require P2X7 signaling and is not blocked by the inhibition of K+ efflux (34). This novel pathway was found to involve caspase-8, and did not induce pyroptosis (34). We therefore first investigated the impact of caspase-8 inhibition on the iNKT-induced pathway. Addition of a peptide that specifically inhibits caspase-8 (Z-IETD-FMK) led to significantly reduced IL-1β secretion by iNKT-stimulated monocytes, but had little or no impact on monocyte IL-1β secretion in response to LPS and nigericin (Figure 7A). Analysis of cell death using an LDH release assay suggested that there was a modest, but statistically significant, increase in cell death in monocyte-iNKT cell co-cultures compared to monocytes alone (Figure 7B). The amount of LDH release in monocyte-iNKT cell co-cultures was similar to that observed for monocytes exposed to LPS, and significantly less than that observed in the presence of nigericin (Figure 7B). Since the LDH assay did not allow us to distinguish monocyte from iNKT death in the co-cultures, we used flow cytometric analysis of propidium iodide and annexin V staining to specifically assess monocyte membrane integrity and expression of apoptotic markers. This analysis also revealed slightly elevated levels of monocytes showing loss of membrane integrity in the iNKT co-culture compared to monocytes cultured alone, but also showed significantly less monocyte death than the nigericin condition (Figure 7C). Together, these results suggest that iNKT cells induce secretion of IL-1β by human peripheral blood monocytes via a mechanism that is distinct from the classical pathway of inflammasome activation.
Figure 7. Dependence on caspase-8 but little induction of monocyte death.
A) Monocytes were incubated for 14 hours with iNKT cells, or with 250 ng/mL LPS with 6.5 μM nigericin added for the last 2 hours. Experiments were performed in the presence of 5 μM caspase-8 inhibitor peptide Z-IETD-FMK and 3 μM glycogen synthase kinase-3β inhibitor (light grey) or of vehicle alone (dark grey), and secreted IL-1β protein was determined by ELISA. The left plot shows results from one representative analysis; the right plot shows compiled results from analyses of 4 different monocyte donors. B) Cell death associated with release of cytoplasmic contents was assessed by analysis of percent lactase dehydrogenase (LDH) release after 14 hours of monocyte culture alone, or with iNKT cells, or with 250 ng/mL LPS with 6.5 μM nigericin added for the last 2 hours. Each symbol represents the mean % LDH release detected from an independent analysis. C) Flow cytometric analysis of monocyte expression of markers associated with apoptosis and loss of membrane integrity. Monocytes were incubated for 3-4 hours alone, or with iNKT cells, or in medium containing 1 μg/mL LPS, or 1 μg/mL LPS and 6.5 μM nigericin. The zebra-contour plots show propidium iodide and Annexin staining of CD14+CD3- cells from one representative analysis (lower gate represents cells with early apoptotic changes; upper gate represents intact cells showing late apoptotic changes). The plot on the right shows compiled results from four independent analyses of the percent of the monocytes showing high propidium iodide staining indicating loss of membrane integrity.
Discussion
Our results outline a novel pathway of IL-1β production by human monocytes. This pathway does not require exposure to microbial compounds, and instead is activated by contact with IL-2 exposed iNKT cells. While involving both NF-κB signaling and activation of the NLRP3 inflammasome, P2X7 signaling is not required for iNKT-mediated induction of monocyte IL-1β secretion. This is an intriguing observation, since P2X7 activation appears to be a critical component of the cell death that is often associated with IL-1β release. Prior studies have established that activation through the P2X7 receptor results in potassium ion efflux from the cytoplasm of the cell, which leads to activation of the NLRP3 inflammasome and ensuing activation of caspases that generate the active form of gasdermin D, an executor of pyroptosis (35, 36). We show here that P2X7 activation is not required for iNKT-mediated induction of IL-1β release by monocytes, and that the iNKT-induced pathway is associated with comparatively modest levels of cell death.
Since this iNKT-induced monocyte IL-1β production pathway appears not to be highly pyroptotic, its inflammatory effects may ordinarily be comparatively limited and not necessarily associated with a spiraling inflammatory response or pathology. However, in the context of other pro-inflammatory signals such as microbial ligands or damage-associated alarmins, the iNKT-induced pathway may further amplify monocyte IL-1β production. Moreover, although our data indicate that activation by exogenous lipid antigens (e.g. α-GalCer) is not required for iNKT cells to induce monocyte IL-1β secretion, our prior studies suggest that presentation of such antigens stabilizes the cell-cell conjugation of iNKT cells with monocytic APCs (37), which may increase the duration of the iNKT-induced response. Furthermore, the production of certain self lipids recognized by human iNKT cells, such as lyso-phosphatidylcholine (LPC), is dramatically upregulated during inflammatory responses (2, 38-41). Thus, increased CD1d-mediated presentation of antigenic self lipids during inflammation may also lead to enhanced iNKT interactions with monocytic APCs that in turn increases the amount or duration of IL-1β output.
Interestingly, the iNKT-induced monocyte activation pathway we have characterized here appears similar to that revealed by a recent analysis, which showed that exposing human peripheral blood monocytes to purified LPS alone drove IL-1β production in a caspase-8- and NLRP3-dependent manner that bypassed P2X7 signaling and could not be blocked by inhibiting potassium efflux (34). This alternative pathway of NLRP3 inflammasome activation did not induce cell death. There was a key role in the alternative pathway of NLRP3 activation for toll-like receptor adaptor molecule 1 (TRIF), which is specifically associated with MyD88-independent activation through TLR4. Thus, while purified LPS induced this non-pyroptotic pathway, other TLR-ligands activated the classical NLRP3 inflammasome pathway in a way that did induce cell death (34). Our results indicate that iNKT cells activate monocyte IL-1β secretion through a cell contact-dependent process that may involve non-canonical NFκB signaling. However, it is not clear what cell surface receptor is triggered by iNKT cells to induce NFκB signaling in the monocytes or whether this receptor activates TRIF. We also observed that iNKT-induced IL-1β secretion was inhibited by preventing ERK phosphorylation, and thus it is possible that MAPK signaling induced in monocytes by contact with iNKT cells is able to activate the “alternative” NLRP3 inflammasome pathway in this system.
The results presented here may have important implications for the role of iNKT cells in human vascular pathology, such as atherosclerosis. Atherosclerosis is a disease characterized by the growth of fatty plaques in the arterial intima. Inflammatory conditions such as hyperlipidemia induce the upregulation of adhesion molecules (including ICAM-1) on endothelial cells, which leads to the accumulation of leukocytes at the site of endothelial activation. iNKT cells have been shown to aggravate atherosclerosis (18, 42-49), but the precise mechanism by which they induce their pro-atherosclerotic effects—plaque growth, induction of apoptosis, promotion of angiogenesis—have not been fully elucidated. It is clear that arterial lesions typically include both CD1d+ APCs and iNKT cells (13-17). iNKT cells isolated from human atherosclerotic plaques appear to induce apoptosis of smooth muscle cells, potentially through IFNγ-induced upregulation of FAS or through the secretion of the lytic proteins granzyme and perforin B (15, 49). Additionally, iNKT cells have been shown to secrete IL-8 when activated, which has been shown to promote angiogenesis, a feature of unstable atherosclerotic plaques (17). To these pro-atherogenic effects may now be added iNKT-mediated induction of IL-1β release. IL-1 is known to promote angiogenesis and induce apoptosis (50), and in humans it appears to enhance plaque development in a manner that is independent of cholesterol metabolism (51). Thus, exploring the connection between iNKT cells and IL-1β production by monocytic cells during atherogenesis will be an important area of future study.
Acknowledgments
Financial Support: Funding provided by NIH R01AI074940 and R21AI116007 (JEG, AS), NIH T32 AI055397 and T32 GM008692 and NIH UL1TR000427 (LEF), NIH R01CA188034 (J-DS), and an AAI Careers in Immunology Fellowship (ET).
References
- 1.Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions. Nature Reviews Immunology. 2013;13:101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- 2.Fox LM, Cox DG, Lockridge JL, Wang X, Chen X, Scharf L, Trott DL, Ndonye RM, Veerapen N, Besra GS, Howell AR, Cook ME, Adams EJ, Hildebrand WH, Gumperz JE. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- 4.Facciotti F, Ramanjaneyulu GS, Lepore M, Sansano S, Cavallari M, Kistowska M, Forss-Petter S, Ni G, Colone A, Singhal A, Berger J, Xia C, Mori L, De Libero G. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nature Immunology. 2012;13:474–480. doi: 10.1038/ni.2245. [DOI] [PubMed] [Google Scholar]
- 5.Kain L, Webb B, Anderson BL, Deng S, Holt M, Costanzo A, Zhao M, Self K, Teyton A, Everett C, Kronenberg M, Zajonc DM, Bendelac A, Savage PB, Teyton L. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian alpha-linked glycosylceramides. Immunity. 2014;41:543–554. doi: 10.1016/j.immuni.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liew PX, Kubes P. Intravital imaging - dynamic insights into natural killer T cell biology. Frontiers in Immunology. 2015;6:240. doi: 10.3389/fimmu.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geissmann F, Cameron TO, Sidobre S, Manlongat N, Kronenberg M, Briskin MJ, Dustin ML, Littman DR. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velazquez P, Cameron TO, Kinjo Y, Nagarajan N, Kronenberg M, Dustin ML. Cutting edge: activation by innate cytokines or microbial antigens can cause arrest of natural killer T cell patrolling of liver sinusoids. J Immunol. 2008;180:2024–2028. doi: 10.4049/jimmunol.180.4.2024. [DOI] [PubMed] [Google Scholar]
- 9.Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, Meng F, Luster AD, Bendelac A. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med. 2011;208:1179–1188. doi: 10.1084/jem.20102630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegde S, Fox L, Wang X, Gumperz JE. Autoreactive natural killer T cells: promoting immune protection and immune tolerance through varied interactions with myeloid antigen-presenting cells. Immunology. 2010;130:471–483. doi: 10.1111/j.1365-2567.2010.03293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, Pandolfi PP, Sant'Angelo DB. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nature Immunology. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobryshev YV, Lord RS. Expression of heat shock protein-70 by dendritic cells in the arterial intima and its potential significance in atherogenesis. J Vasc Surg. 2002;35:368–375. doi: 10.1067/mva.2002.121067. [DOI] [PubMed] [Google Scholar]
- 14.Bobryshev YV, Lord RS. Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. J Histochem Cytochem. 2005;53:781–785. doi: 10.1369/jhc.4B6570.2005. [DOI] [PubMed] [Google Scholar]
- 15.Chan WL, Pejnovic N, Hamilton H, Liew TV, Popadic D, Poggi A, Khan SM. Atherosclerotic abdominal aortic aneurysm and the interaction between autologous human plaque-derived vascular smooth muscle cells, type 1 NKT, and helper T cells. Circ Res. 2005;96:675–683. doi: 10.1161/01.RES.0000160543.84254.f1. [DOI] [PubMed] [Google Scholar]
- 16.Melian A, Geng YJ, Sukhova GK, Libby P, Porcelli SA. CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am J Pathol. 1999;155:775–786. doi: 10.1016/S0002-9440(10)65176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kyriakakis E, Cavallari M, Andert J, Philippova M, Koella C, Bochkov V, Erne P, Wilson SB, Mori L, Biedermann BC, Resink TJ, De Libero G. Invariant natural killer T cells: Linking inflammation and neovascularization in human atherosclerosis. Eur J Immunol. 2010;40:3268–3279. doi: 10.1002/eji.201040619. [DOI] [PubMed] [Google Scholar]
- 18.Rogers L, Burchat S, Gage J, Hasu M, Thabet M, Willcox L, Ramsamy TA, Whitman SC. Deficiency of invariant V alpha 14 natural killer T cells decreases atherosclerosis in LDL receptor null mice. Cardiovasc Res. 2008;78:167–174. doi: 10.1093/cvr/cvn005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace KL, Marshall MA, Ramos SI, Lannigan JA, Field JJ, Strieter RM, Linden J. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood. 2009;114:667–676. doi: 10.1182/blood-2009-02-205492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dinarello CA. A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol. 2011;41:1203–1217. doi: 10.1002/eji.201141550. [DOI] [PubMed] [Google Scholar]
- 21.Zheng Y, Gardner SE, Clarke MC. Cell death, damage-associated molecular patterns, and sterile inflammation in cardiovascular disease. Arterioscler Thromb Vasc Biol. 2011;31:2781–2786. doi: 10.1161/ATVBAHA.111.224907. [DOI] [PubMed] [Google Scholar]
- 22.Brigl M, van den Elzen P, Chen X, Meyers JH, Wu D, Wong CH, Reddington F, Illarianov PA, Besra GS, Brenner MB, Gumperz JE. Conserved and heterogeneous lipid antigen specificities of CD1d-restricted NKT cell receptors. J Immunol. 2006;176:3625–3634. doi: 10.4049/jimmunol.176.6.3625. [DOI] [PubMed] [Google Scholar]
- 23.Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cellular Immunology. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 24.Choi AJ, Ryter SW. Inflammasomes: molecular regulation and implications for metabolic and cognitive diseases. Molecules and Cells. 2014;37:441–448. doi: 10.14348/molcells.2014.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee J, Rhee MH, Kim E, Cho JY. BAY 11-7082 is a broad-spectrum inhibitor with anti-inflammatory activity against multiple targets. Mediators of Inflammation. 2012;2012:416036. doi: 10.1155/2012/416036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O'Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nature Medicine. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghonime MG, Shamaa OR, Das S, Eldomany RA, Fernandes-Alnemri T, Alnemri ES, Gavrilin MA, Wewers MD. Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J Immunol. 2014;192:3881–3888. doi: 10.4049/jimmunol.1301974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubyak GR. P2X7 receptor regulation of non-classical secretion from immune effector cells. Cellular Microbiology. 2012;14:1697–1706. doi: 10.1111/cmi.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 30.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. The EMBO Journal. 2006;25:5071–5082. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manthei DM, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Gern JE, Lemanske RF, Jr, Denlinger LC. Protection from asthma in a high-risk birth cohort by attenuated P2X(7) function. The Journal of Allergy and Clinical Immunology. 2012;130:496–502. doi: 10.1016/j.jaci.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Munoz-Planillo R, Kuffa P, Martinez-Colon G, Smith BL, Rajendiran TM, Nunez G. K(+) efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 34.Gaidt MM, Ebert TS, Chauhan D, Schmidt T, Schmid-Burgk JL, Rapino F, Robertson AA, Cooper MA, Graf T, Hornung V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 36.Kayagaki N, I, Stowe B, Lee BL, O'Rourke K, Anderson K, Warming S, Cuellar T, Haley B, Roose-Girma M, Phung QT, Liu PS, Lill JR, Li H, Wu J, Kummerfeld S, Zhang J, Lee WP, Snipas SJ, Salvesen GS, Morris LX, Fitzgerald L, Zhang Y, Bertram EM, Goodnow CC, Dixit VM. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Chen X, Rodenkirch L, Simonson W, Wernimont S, Ndonye RM, Veerapen N, Gibson D, Howell AR, Besra GS, Painter GF, Huttenlocher A, Gumperz JE. Natural killer T-cell autoreactivity leads to a specialized activation state. Blood. 2008;112:4128–4138. doi: 10.1182/blood-2008-05-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan W, Kang SJ, Evans JE, Cresswell P. Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J Immunol. 2009;182:4784–4791. doi: 10.4049/jimmunol.0803981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cox D, Fox L, Tian R, Bardet W, Skaley M, Mojsilovic D, Gumperz J, Hildebrand W. Determination of cellular lipids bound to human CD1d molecules. PLoS One. 2009;4:e5325. doi: 10.1371/journal.pone.0005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Sagaseta J, Sibener LV, Kung JE, Gumperz J, Adams EJ. Lysophospholipid presentation by CD1d and recognition by a human Natural Killer T-cell receptor. The EMBO Journal. 2012;31:2047–2059. doi: 10.1038/emboj.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salio M, Cerundolo V. Linking inflammation to natural killer T cell activation. PLoS Biol. 2009;7:e1000226. doi: 10.1371/journal.pbio.1000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Major AS, Wilson MT, McCaleb JL, Ru Su Y, Stanic AK, Joyce S, Van Kaer L, Fazio S, Linton MF. Quantitative and qualitative differences in proatherogenic NKT cells in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:2351–2357. doi: 10.1161/01.ATV.0000147112.84168.87. [DOI] [PubMed] [Google Scholar]
- 43.Nakai Y, Iwabuchi K, Fujii S, Ishimori N, Dashtsoodol N, Watano K, Mishima T, Iwabuchi C, Tanaka S, Bezbradica JS, Nakayama T, Taniguchi M, Miyake S, Yamamura T, Kitabatake A, Joyce S, Van Kaer L, Onoe K. Natural killer T cells accelerate atherogenesis in mice. Blood. 2004;104:2051–2059. doi: 10.1182/blood-2003-10-3485. [DOI] [PubMed] [Google Scholar]
- 44.Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, Berne GP. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med. 2004;199:417–422. doi: 10.1084/jem.20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aslanian AM, Chapman HA, Charo IF. Transient role for CD1d-restricted natural killer T cells in the formation of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2005;25:628–632. doi: 10.1161/01.ATV.0000153046.59370.13. [DOI] [PubMed] [Google Scholar]
- 46.VanderLaan PA, Reardon CA, Sagiv Y, Blachowicz L, Lukens J, Nissenbaum M, Wang CR, Getz GS. Characterization of the natural killer T-cell response in an adoptive transfer model of atherosclerosis. Am J Pathol. 2007;170:1100–1107. doi: 10.2353/ajpath.2007.060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.To K, Agrotis A, Besra G, Bobik A, Toh BH. NKT cell subsets mediate differential proatherogenic effects in ApoE-/- mice. Arterioscler Thromb Vasc Biol. 2009;29:671–677. doi: 10.1161/ATVBAHA.108.182592. [DOI] [PubMed] [Google Scholar]
- 48.Subramanian S, Turner MS, Ding Y, Goodspeed L, Wang S, Buckner JH, O'Brien K, Getz GS, Reardon CA, Chait A. Increased levels of invariant natural killer T lymphocytes worsen metabolic abnormalities and atherosclerosis in obese mice. Journal of Lipid Research. 2013;54:2831–2841. doi: 10.1194/jlr.M041020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, To K, Kanellakis P, Hosseini H, Deswaerte V, Tipping P, Smyth MJ, Toh BH, Bobik A, Kyaw T. CD4+ natural killer T cells potently augment aortic root atherosclerosis by perforin- and granzyme B-dependent cytotoxicity. Circ Res. 2015;116:245–254. doi: 10.1161/CIRCRESAHA.116.304734. [DOI] [PubMed] [Google Scholar]
- 50.Bujak M, Frangogiannis NG. The role of IL-1 in the pathogenesis of heart disease. Archivum Immunologiae et Therapiae Experimentalis. 2009;57:165–176. doi: 10.1007/s00005-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Francis SE, Camp NJ, Dewberry RM, Gunn J, Syrris P, Carter ND, Jeffery S, Kaski JC, Cumberland DC, Duff GW, Crossman DC. Interleukin-1 receptor antagonist gene polymorphism and coronary artery disease. Circulation. 1999;99:861–866. doi: 10.1161/01.cir.99.7.861. [DOI] [PubMed] [Google Scholar]