Abstract
Objective
To determine the expression and function of the microRNA-29 family (miR-29a, 29b, 29c) in human leiomyoma and myometrium.
Study Design
Basic Science Experimental Design
Setting
Academic medical center
Patients
Women undergoing surgery for symptomatic uterine fibroids.
Interventions
Overexpression and knockdown of miR-29a, 29b, and 29c in primary leiomyoma and myometrial cells.
Main outcome measures
(1) Expression of the miRNA-29 family members in vivo in leiomyoma versus myometrium; (2) Major fibrillar collagen (I, II, III) expression in leiomyoma and myometrial cells with manipulation of miR-29 species.
Results
Members of the miR-29 family (29a, 29b, 29c) are all down-regulated in leiomyoma versus myometrium in vivo. The expression of the miR-29 family can be successfully modulated in primary leiomyoma and myometrial cells. Overexpression of the miR-29 family in leiomyoma cells results in down-regulation of the major fibrillar collagens. Down-regulation of the miR-29 species in myometrium results in an increase in collagen type 3 deposition.
Conclusions
The miR-29 family is consistently down-regulated in leiomyoma compared to matched myometrial tissue. This down-regulation contributes to the increased collagen seen in leiomyomas versus myometrium. When miR-29 members are overexpressed in leiomyoma cells, protein levels of all of the major fibrillar collagens decrease. Mir-29 members are potential therapeutic targets in this highly prevalent condition.
Keywords: leiomyoma, fibroids, microRNA, collagen, extracellular matrix
Introduction
Leiomyomas, or fibroids as they are more commonly known, are benign uterine smooth muscle tumors that represent the most common tumor in reproductive aged women. By the age of 50, these tumors have a prevalence of 60 to 65% in Caucasian women and greater than 80% in African-American women in the United States. While they are clinically symptomatic in only 20 to 40% of women who have them, most women who have symptoms from fibroids have multiple symptoms which include heavy uterine bleeding, pelvic pain, infertility, and recurrent pregnancy loss (1). Due to the prevalence and the sequelae of these tumors, they continue to be the leading cause of hysterectomy in the United States and annually represent up to $34B dollars in cost nationally (2). Despite the public health impact of these tumors, we are still early in our understanding of their pathogenesis.
The primary distinguishing factor between these tumors and their adjacent normal myometrial tissue is the abundance of extracellular matrix. Multiple gene and tissue microarrays have demonstrated that the matrix consists largely of the major fibrillar collagens (types I, II, and III) (3). While advances have been made in understanding the pathophysiology of the growth of these tumors there is still not a good understanding of the molecular basis of the extracellular matrix dysregulation seen in leiomyomas (4).
MicroRNAs (miRNAs) have been found to be novel regulators of fibrosis in a number of disease processes including liver fibrosis, lung fibrosis, and cardiac fibrosis. MicroRNAs are 20 to 25 nucleotide long noncoding RNAs that are involved in regulation of gene expression via translational repression (5). This repression is the result of either destruction of mRNA or destabilization and prevention of translation of mRNA. Over 3,000 miRNAs have been identified (6) and bioinformatic estimates place the number of miRNA target sites in the human genome at greater than 45,000 (7). Further, it is now speculated that more than 60% of human protein coding genes are regulated by miRNAs (7). We and others have demonstrated that in addition to differential gene expression between leiomyoma and myometrium, there is differential expression of miRNAs as well (8-10), suggesting that miRNAs play a role in gene regulation in these tumors. While several studies have demonstrated that hormonal and growth factor regulation of miRNAs in leiomyomas alters cell proliferation (11), few have demonstrated a functional role for them in terms of extracellular matrix overproduction (12).
In previously published microarray analysis, we identified 81 differentially expressed microRNAs between leiomyomas and myometrial tissue; among them, miRNA-29b and 29c were both identified as being among the most significantly down-regulated in leiomyoma versus myometrium (8). The down-regulation of these particular microRNAs is especially as the miR-29 family of miRNAs have been implicated in fibrosis in other disease processes including fibrosis after myocardial infarction (13), pulmonary fibrosis (14) and systemic sclerosis (15). Based on recent studies in other fibrotic diseases (16-20), we believe that miRNAs may play a functional role in the aberrant extracellular matrix components found in leiomyomas. While a previous study has investigated miR-29b, to our knowledge, the entire miR-29 family has not been considered (21).
The goal of this project is to validate the differential expression of the entire miR-29 family (miR-29a, miR-29b, and miR-29c) in leiomyoma versus myometrium and to determine whether these miRNAs have a functional role in leiomyoma ECM pathogenesis. Based on miRNA microarray studies done by this lab and others, as well as studies done in other fibrotic diseases, we hypothesize that the all members of the miR-29 family will be down-regulated in leiomyoma versus myometrium. We further hypothesize that this down-regulation contributes to the increased collagen production in these tumors and that overproduction of the miR-29 species will lead to decreased collagen production in leiomyoma cells.
MATERIALS AND METHODS
Study Subjects
Uterine leiomyoma and matched myometrial tissue were collected from subjects (n=20) undergoing hysterectomy for symptomatic uterine leiomyoma, and leiomyoma alone was collected from an additional 10 subjects. The subjects were all premenopausal women 27 to 49 years old, who were on no hormonal medications within 3 months of surgery and who were non-smokers. All of the subjects gave written informed consent for participation in the study. The study protocol was approved by the IRB of Northwestern University and all surgeries were performed at Northwestern Memorial Hospital.
Tissue Specimens
The resected tissue was collected in the operating room and taken directly to the pathology department where samples were provided to the research team within one hour of being removed from the subject. Leiomyoma ranged from 4cm to 12cm in greatest dimension and samples were routinely obtained at 1-2cm from the outer capsule of the leiomyoma to avoid variation of findings due to location within the tumor. All of the fibroids were either subserosal or intramural. No submucosal fibroids were used in this study. Myometrium was collected from within 2cm of the excised leiomyoma. The tissues were rinsed in cold phosphate buffered saline (PBS) three times and were either flash frozen and stored at −80°C, cut into 2-3mm cubed pieces and placed in vials containing RNALater® (Ambion, Austin, TX) for nucleic acid preservation, or were immediately digested for primary cell isolation.
Nucleic acid isolation
RNA was isolated from either tissue or cells using the protocol previously described (8). Briefly, flash frozen tissue specimens were homogenized using a mortar and pestle and liquid nitrogen. The crushed tissue was allowed to incubate in Tri-Reagent (Sigma) for 5 minutes then mixed with one-fifth volume of chloroform. This mixture was then kept on ice for 15 minutes and then centrifuged at 14,000rpm for 20 minutes. The resultant clear aqueous layer was transferred and mixed with an equal volume of isopropanol. The mixture was then incubated at 4°C for 10 minutes and then centrifuged at 14,000rpm for 20 minutes. The liquid was removed from the vial, and the formed pellet was resuspended and 1ml of 75% ethanol and centrifuged at 8,000rpm for 10 minutes. The liquid was again removed and the pellet was allowed to air dry at room temperature for 5 minutes at which time it was resuspended in diethylpyrocarbonate-treated (DEPC) sterile water. The RNA purity and concentration were determined by spectrophotometry using the NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE).
Cell Culture
Surgical tissue specimens were rinsed in PBS and placed in a mixture of Hanks Balanced Salt Solution (HBSS) containing DNase at a concentration of 150 mg/mL and collagenase at a concentration of 1.5mg/mL. The tissue in the HBSS mixture was placed in an agitating incubator for 5 to 8 hours at 37°C. The digested tissue solution was then filtered with a 100 micron cell filter to remove any undigested debris and the resultant solution was centrifuged to collect isolated cells. The digestion mixture was removed from the pelleted cells and the cells were rinsed in fresh cell media (DMEM/F12 with 10% FBS and 1% antibiotic-antimycotic solution) and then plated in 15cm cell culture plates.
MiRNA Expression PCR
MicroRNA expression was determined by real time reverse transcriptase polymerase chain reaction (real time RT-PCR). Complementary DNA (cDNA) was made from 10ng of total RNA from each sample using the TaqMan micro RNA reverse transcription kit (Applied Biosystems, Foster City, CA) and microRNA specific primers for miR-29a, miR-29b, and miR-29c (Applied Biosystems). These cDNA samples, along with RNU48 as a loading control, were then amplified using the ABI TaqMan MiRNA PCR Kit (Applied Biosystems) and the ABI Prism 7900HT sequence detection system (Applied Biosystems). The real time PCR was performed in triplicate in a minimum of three subjects per experiment. Values for each microRNA were normalized to the expression levels of RNU 48 using 2−ΔΔCT methodology (22).
Oligonucleotide Transfection
To modify the miR-29 expression levels in leiomyoma and myometrial cells, we used the Life Technologies PremiRs to overexpress the target miRNAs in leiomyoma cells, and AntimiRs to down-regulate the targeted miRNAs in myometrial cells (Life Technologies, Austin, TX). Pre-miR and Anti-miR transfection controls were utilized for the respective experiments. For the Pre-miR transfections of leiomyoma cells, the passage one primary cells were plated in 6cm dishes and grown to 50% confluency in standard media (DMEM/F12 with 10% FBS and 1% antimycotic/antibiotic solution). The cells were then incubated in OptiMEM Reduced Serum Medium (Life Technologies) and transfected with Pre-miRs using FuGENE HD (Promega, Madison, WI) as the transfection agent, or Anti-miRs using RNAiMAX (Life Technologies) as the transfection agent.
Protein Isolation
After 48 hours of transfection, cell protein lysates were collected with M-PER (ThermoFisher, Waltham, MA) using the manufacturers protoco and protein was isolated. The lysates were transferred to fresh tubes and the protein was quantified with the Pierce BCA Colorometric kit (ThermoFisher). The proteins were stored at −80°C until ready for immunoblotting.
Immunoblotting
Isolated proteins were loaded onto 3-12% Novus Bis-Tri gels (Life Technologies) at 30mg per lane. The gels were run on the Power Ease 500 per protocol. The separated proteins were transferred to a 20um nitrocellulose membrane. Transfer was confirmed with Ponceau S staining of the membrane which was rinsed off with 10% acetic acid. The membranes were blocked with 1% milk in 0.1% TBS-Tween (TBS-T) for 2 hours at room temperature and then incubated overnight at 4° in either COL1A1, COL2A1, or COL3A1 primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The following morning, the membranes were rinsed in fresh TBS-T and incubated in horseradish peroxidase conjugated secondary antibody (Sigma-Aldrich, St. Louis, MO) for 2 hours at room temperature. The secondary antibody was removed and the membranes were rinsed with fresh TBS-T. Chemiluminescent detection was done with ECL Prime (GE Life Sciences, Marlboro, MA). After collagen detection, the membranes were stripped with Restore Western Blot Stripping Buffer (ThermoFisher) and incubated in anti-human beta actin (Sigma Aldrich) as a loading control according the manufacturers protocol. To better quantify the differences seen after anti-miR and pre-miR treatments, Image Studio Lite Western Blot Analysis Software (LI-CORE Biosciences, Lincoln, NE) was used per the developer’s guidelines. Semi-quantitative protein data are presented as a western blot and quantitative protein data are presented on a mean-fold difference of treated cells relative to their respective controls, corrected for the beta actin internal control.
Statistical Analysis
Real time RT-PCR data was assessed using 2−ΔΔCT calculations normalized to RNU48 (22). The data are reported as mean± standard error of the mean (SEM). Means of paired samples were compared using Student’s paired two-tailed t-test. A P<.05 was considered statistically significant.
RESULTS
Differential miR-29 expression in leiomyoma versus myometrium in vivo
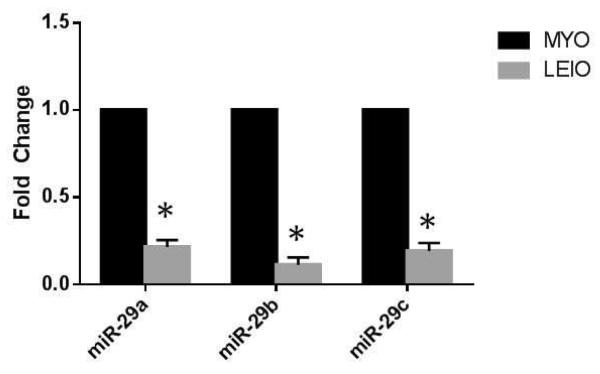
MicroRNA-29 expression was assessed in matched leiomyoma and myometrial tissue from 20 subjects who had undergone hysterectomy (Figure 1). Using qRT-PCR, we found that all of the miR-29 species (29a, 29b, and 29c) were significantly down-regulated in leiomyoma versus myometrium in vivo (n=20, P<.01).
Figure 1.
Real time PCR of miR-29 family species in matched myometrium (MYO) and leiomyoma (LEIO) tissue pair (n=20). MiR-29a, 29b, and 29c all show significantly decreased expression in leiomyoma relative to myometrium. *P<.001 relative to matched control.
Modification of microRNA in leiomyoma and myometrial Cells
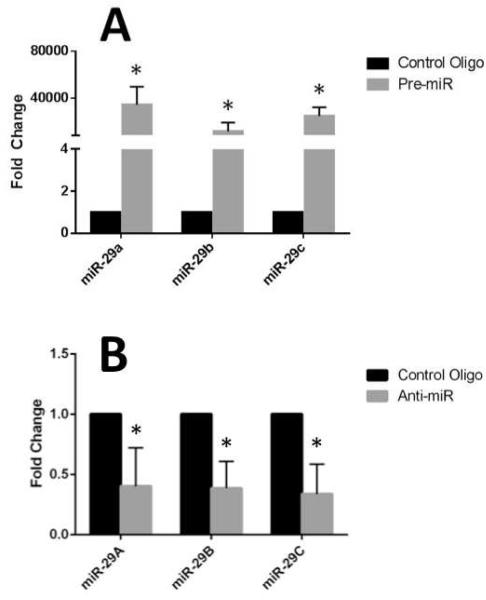
To assess the function of miR-29 in leiomyoma and myometrial cells, we had to first confirm that their expression could be manipulated. Using the Ambion miRNA precursors (Pre-miRs) we were able to successfully overexpress all three miR-29 members in leiomyoma cell culture as determined by qRT-PCR (n=5) (Figure 2A). Also using Anti-miR miRNA inhibitors, we were able to successfully inhibit expression of the miR-29 species in cultured myometrial cells (n=5) as determined by qRT-PCR (Figure 2B).
Figure 2.
(A) Transfection of leiomyoma smooth muscle cells with miR-29 Pre-miRs resulting in overexpression versus control; *P<.001. (B) Transfection of myometrial smooth muscle cells with miR-29 Anti-miRs resulting in decreased expression versus control. *P<.05.
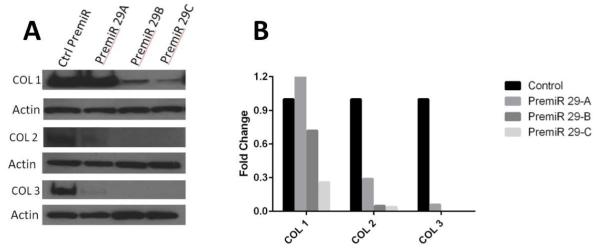
Overexpression of miR-29 leads to decreased collagen production in leiomyoma cells
The functional significance of miR-29 differential expression in relation to collagen production was determined by overexpressing miR-29 a, b, and c in leiomyoma cells, using the aforementioned miR-29 precursors. The over expression of the miR-29 family members lead to a significant reduction in the major collagens (I, II, and III) relative to the control Pre-miR. The experiment was performed in cells from four subjects. A representative experiment is shown in Figure 3. Overexpression of miR-29b and 29c showed a more robust suppression of the major collagens than miR-29a.
Figure 3.
(A) Western blot of leiomyoma cells transfected with Pre-miRs to induce overexpression of miR-29 family results in down-regulation of the major fibrillar collagens. (B) Quantitative Pre-miR data presented as a mean-fold difference of treated cells relative to their respective controls, corrected for the beta actin internal control.
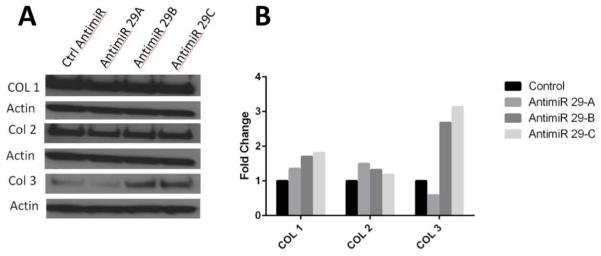
Inhibition of miR-29 in myometrial cells leads to increased collagen production
The knockdown of miR-29 family members in myometrial cells was assessed in cells from four subjects. A representative experiment is shown in Figure 4. The knockdown of miR-29 had no impact on collagen type 1 or collagen type 2, but resulted in increased collagen type 3 relative to the control Anti-miR.
Figure 4.
Western blot of myometrial cells transfected with Anti-miRs to down-regulate miR-29 expression results in increased expression of collagen type 3. (B) Quantitative Anti-miR data presented as a mean-fold difference of treated cells relative to their respective controls, corrected for the beta actin internal control.
DISCUSSION
In the present study we demonstrate that the entire miR-29 family is down-regulated in leiomyoma versus myometrium. Using a primary cell culture model we further demonstrated that the members of the miR-29 family are able to regulate the expression of the major fibrillar collagens in both leiomyoma and myometrial cells. To our knowledge this is one of the first studies to report a functional role of microRNA in the extracellular matrix production in leiomyomas.
Though the etiologies of fibrotic disease can vary significantly, several features are shared by all fibrotic disorders – including activation of myofibroblast cells and excess extracellular matrix production (23). Through their post-transcriptional effect on gene expression, microRNAs have been demonstrated to regulate this process of collagen production in a number of diseases. Cardiac fibrosis was one of the first fibrotic diseases in which miRNAs were implicated as having a contributory role. Since that time, miRNAs have been implicated in a number of fibrotic disease states including systemic sclerosis (24), renal fibrosis (25), pulmonary fibrosis (26), and liver fibrosis (27). For example, Duisters et al. found that miR-30 targets connective tissue growth factor (CTGF) (28), while Thum and colleagues demonstrated that miR-21 targets sprout1 and regulated fibroblast survival (29).
The miR-29 family, which is composed of miR-29a, miR-29b, and miR-29c, has specifically been heavily implicated in fibrotic disease (8). This family of microRNAs, differing only in two or three bases, are encoded and transcribed in tandem by two genes located on chromosome 7 and chromosome 1, respectively (30). MiR-29b, in particular, has been suggested to play a especially significant role in fibrotic disease (31). It was first implicated in cardiac fibrosis by van Rooij et al. (32). Maurer et al. found that it was down-regulated and contributed to the progression of systemic sclerosis (31). Other studies have demonstrated that down-regulation of miR-29 contributes to the progression of liver fibrosis (33, 34) and renal fibrosis (25, 35), however the role of the miR-29 family in leiomyoma is largely unexplored.
In 2008, our first generation microarray analysis (8) suggested that miR-29b and miR-29c are down-regulated in leiomyoma; a finding supported by this study. Adding to the findings of Qiang and colleagues, which identified a relationship between ECM production and down-regulation of miR-29b (21), in this study we considered the entire miR-29 family. Beyond reporting the observational finding that the miR-29 family was down-regulated in leiomyoma, as it is in other fibrotic diseases, we were able to establish the functional significance of miR-29 in relation to collagen production by demonstrating an increase in the major fibrillar collagen expression following down-regulation of all members of the miR-29 family. Conversely, we also found that over expression of the miR-29 family members lead to a significant reduction in the major collagens – further supporting the functional significance of miR-29 differential expression.
While our primary finding that down regulation of the miR-29 family is associated with an increase in major fibrillar collagen secretion in leiomyoma is novel, the miR-29 family, miR-29b in particular, has been associated with the fibrotic process in other organs (13, 15, 36). MiR-29 has been found to directly bind to the 3′UTR of collagen type 1 suggesting in part a direct effect of miR-29 on the suppression of collagen translation (37). In addition, the TGFβ/smad pathway has been implicated in miR-29 regulation in renal, pulmonary, and cardiac fibrosis (38). TGFβ is known to be dysregulated i.e. increased, in leiomyoma relative to myometrium (39, 40). This increase, in part, could also explain the downregulation of miR-29 seen in leiomyoma.
The findings of this paper are consistent with previous studies by our lab and others, adding further support to the growing body of research suggesting the miR-29 family plays an important role in fibroid pathogenesis. While this study may be limited by the inherent potential for inter-subject variability when using human samples, the use of human tissue is also a strength as these results are extremely relevant to the clinical disease process at hand. Though only 4-5 samples were utilized to perform miR over- and under- expression studies, miR-29 expression was consistently down-regulated in all samples used and the results of the siRNA and over-expression studies were highly consistent. While the results were highly consistent across all subjects, we did not have menstrual cycle phase data and therefore were not able to comment on possible impact of hormonal status. While these data indicate a role of miR-29 in major fibrillar collagen production, they do not confirm a direct effect of miR-29 the collagen genes. Additional studies are needed to identify the pathway(s) through which the miR-29 family exerts its effect on collagen.
In summary, the miR-29 family of miRNAs is significantly down-regulated in leiomyoma versus myometrium. This differential expression contributes to the excess extracellular matrix seen in leiomyomas versus myometrium. The disease burden of leiomyomas remains disturbingly high given that they are benign tumors without direct multisystem sequelae. With limited medical treatment options currently FDA approved for leiomyomas, and no long-term medical options available, the miR-29 family potentially represents a novel therapeutic target in the treatment and perhaps prevention of these prevalent morbid tumors. The findings demonstrated in our study suggest that the miR-29 family is a potential therapeutic target for uterine leiomyomas. A miR-29 mimic that could be delivered locally, could treat and potentially prevent the development of these collagen laden tumors.
Capsule.
The microRNA-29 family is down-regulated in leiomyoma versus myometrium. This down-regulation leads to collagen overexpression in leiomyoma and can be reversed by overexpressing microRNA-29 in leiomyoma cells.
Acknowledgements
The authors would like to thank Jazzmyne Dickens for her technical assistance.
Financial Support: National Institutes of Health – NICHD Grants R21HD077479 (EEM) and K12HD050121 Northwestern University Women’s Reproductive Health Research (WRHR) Scholar Award (EEM) and P01057877 (SEB); Harold Amos Medical Faculty Development Award, Robert Wood Johnson Foundation (EEM); Friends of Prentice Women’s Health Research Award (EEM); The Woman’s Board of Northwestern Memorial Hospital Award (EEM); and Northwestern University.
Footnotes
Disclosure: None of the authors have a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–92. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 2.Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211–e1. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertility and sterility. 2004;82(Suppl 3):1182–7. doi: 10.1016/j.fertnstert.2004.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Q, Mas A, Diamond MP, Al-Hendy A. The Mechanism and Function of Epigenetics in Uterine Leiomyoma Development. Reprod Sci. 2015 doi: 10.1177/1933719115584449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen K, Rajewsky N. The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet. 2007;8:93–103. doi: 10.1038/nrg1990. [DOI] [PubMed] [Google Scholar]
- 6.Londin E, Loher P, Telonis AG, Quann K, Clark P, Jing Y, et al. Analysis of 13 cell types reveals evidence for the expression of numerous novel primate- and tissue-specific microRNAs. Proc Natl Acad Sci U S A. 2015;112:E1106–15. doi: 10.1073/pnas.1420955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marsh EE, Lin Z, Yin P, Milad M, Chakravarti D, Bulun SE. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertility and sterility. 2008;89:1771–6. doi: 10.1016/j.fertnstert.2007.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, et al. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosomes Cancer. 2007;46:336–47. doi: 10.1002/gcc.20415. [DOI] [PubMed] [Google Scholar]
- 10.Georgieva B, Milev I, Minkov I, Dimitrova I, Bradford AP, Baev V. Characterization of the uterine leiomyoma microRNAome by deep sequencing. Genomics. 2012;99:275–81. doi: 10.1016/j.ygeno.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Pan Q, Luo X, Chegini N. Differential expression of microRNAs in myometrium and leiomyomas and regulation by ovarian steroids. J Cell Mol Med. 2008;12:227–40. doi: 10.1111/j.1582-4934.2007.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Karmon AE, Cardozo ER, Rueda BR, Styer AK. MicroRNAs in the development and pathobiology of uterine leiomyomata: does evidence support future strategies for clinical intervention? Hum Reprod Update. 2014;20:670–87. doi: 10.1093/humupd/dmu017. [DOI] [PubMed] [Google Scholar]
- 13.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–32. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cushing L, Kuang PP, Qian J, Shao F, Wu J, Little F, et al. miR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–94. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–43. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 16.Adam O, Lohfelm B, Thum T, Gupta SK, Puhl SL, Schafers HJ, et al. Role of miR-21 in the pathogenesis of atrial fibrosis. Basic Res Cardiol. 2012;107:278. doi: 10.1007/s00395-012-0278-0. [DOI] [PubMed] [Google Scholar]
- 17.He Y, Huang C, Zhang SP, Sun X, Long XR, Li J. The potential of microRNAs in liver fibrosis. Cell Signal. 2012;24:2268–72. doi: 10.1016/j.cellsig.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Iizuka M, Ogawa T, Enomoto M, Motoyama H, Yoshizato K, Ikeda K, et al. Induction of microRNA-214-5p in human and rodent liver fibrosis. Fibrogenesis Tissue Repair. 2012;5:12. doi: 10.1186/1755-1536-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macconi D, Tomasoni S, Romagnani P, Trionfini P, Sangalli F, Mazzinghi B, et al. MicroRNA-324-3p Promotes Renal Fibrosis and Is a Target of ACE Inhibition. J Am Soc Nephrol. 2012;23:1496–505. doi: 10.1681/ASN.2011121144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vettori S, Gay S, Distler O. Role of MicroRNAs in Fibrosis. Open Rheumatol J. 2012;6:130–9. doi: 10.2174/1874312901206010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiang W, Liu Z, Serna VA, Druschitz SA, Liu Y, Espona-Fiedler M, et al. Down-regulation of miR-29b is essential for pathogenesis of uterine leiomyoma. Endocrinology. 2014;155:663–9. doi: 10.1210/en.2013-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bustin SA. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J Mol Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- 23.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–40. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makino K, Jinnin M, Kajihara I, Honda N, Sakai K, Masuguchi S, et al. Circulating miR-142-3p levels in patients with systemic sclerosis. Clin Exp Dermatol. 2012;37:34–9. doi: 10.1111/j.1365-2230.2011.04158.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Komers R, Carew R, Winbanks CE, Xu B, Herman-Edelstein M, et al. Suppression of microRNA-29 expression by TGF-beta1 promotes collagen expression and renal fibrosis. J Am Soc Nephrol. 2012;23:252–65. doi: 10.1681/ASN.2011010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang S, Xie N, Cui H, Banerjee S, Abraham E, Thannickal VJ, et al. miR-31 is a negative regulator of fibrogenesis and pulmonary fibrosis. Faseb J. 2012;26:3790–9. doi: 10.1096/fj.11-202366. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Ogawa T, Enomoto M, Fujii H, Sekiya Y, Yoshizato K, Ikeda K, et al. MicroRNA-221/222 upregulation indicates the activation of stellate cells and the progression of liver fibrosis. Gut. 2012;61:1600–9. doi: 10.1136/gutjnl-2011-300717. [DOI] [PubMed] [Google Scholar]
- 28.Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, et al. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–8. doi: 10.1161/CIRCRESAHA.108.182535. 6p following 8. [DOI] [PubMed] [Google Scholar]
- 29.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–4. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 30.He Y, Huang C, Lin X, Li J. MicroRNA-29 family, a crucial therapeutic target for fibrosis diseases. Biochimie. 2013;95:1355–9. doi: 10.1016/j.biochi.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Maurer B, Stanczyk J, Jungel A, Akhmetshina A, Trenkmann M, Brock M, et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–43. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 32.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–60. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekiya Y, Ogawa T, Yoshizato K, Ikeda K, Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem Biophys Res Commun. 2011;412:74–9. doi: 10.1016/j.bbrc.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 34.Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–18. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 35.Qin W, Chung AC, Huang XR, Meng XM, Hui DS, Yu CM, et al. TGF-beta/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol. 2011;22:1462–74. doi: 10.1681/ASN.2010121308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cushing L, Kuang P, Lu J. The role of miR-29 in pulmonary fibrosis. Biochem Cell Biol. 2015;93:109–18. doi: 10.1139/bcb-2014-0095. [DOI] [PubMed] [Google Scholar]
- 37.Wang J, Chu ES, Chen HY, Man K, Go MY, Huang XR, et al. microRNA-29b prevents liver fibrosis by attenuating hepatic stellate cell activation and inducing apoptosis through targeting PI3K/AKT pathway. Oncotarget. 2015;6:7325–38. doi: 10.18632/oncotarget.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu GX, Li YQ, Huang XR, Wei L, Chen HY, Shi YJ, et al. Disruption of Smad7 promotes ANG II-mediated renal inflammation and fibrosis via Sp1-TGF-beta/Smad3-NF.kappaB-dependent mechanisms in mice. PLoS One. 2013;8:e53573. doi: 10.1371/journal.pone.0053573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffman PJ, Milliken DB, Gregg LC, Davis RR, Gregg JP. Molecular characterization of uterine fibroids and its implication for underlying mechanisms of pathogenesis. Fertility and sterility. 2004;82:639–49. doi: 10.1016/j.fertnstert.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa H, Shozu M, Okada M, Inukai M, Zhang B, Kato K, et al. Early growth response gene-1 plays a pivotal role in down-regulation of a cohort of genes in uterine leiomyoma. J Mol Endocrinol. 2007;39:333–41. doi: 10.1677/JME-06-0069. [DOI] [PubMed] [Google Scholar]