Abstract
The clinical use of anthracyclines to treat various canine cancers is limited by the development of cardiotoxicity. The intra-cardiac synthesis of anthracycline C-13 alcohol metabolites (e.g. daunorubicinol) contributes to the development of cardiotoxicity. Canine carbonyl reductase 1 (cbr1) catalyzes the reduction of daunorubicin into daunorubicinol. Recent mapping of the cbr1 locus by sequencing DNA samples from dogs from various breeds revealed a cluster of conserved motifs for the transcription factor Sp1 in the putative promoter region of cbr1. We hypothesized that the variable number of Sp1 motifs could impact the transcription of canine cbr1. In this study, we report the functional characterization of the canine cbr1 promoter. Experiments with reporter constructs and chromatin immunoprecipitation show that cbr1 transcription depends on the binding of Sp1 to the proximal promoter. Site-directed mutagenesis experiments suggest that the variable number of Sp1 motifs impacts the transcription of canine cbr1. Inhibition of Sp1-DNA binding decreased canine cbr1 mRNA levels by 54% in comparison to controls, and also decreased enzymatic carbonyl reductase activity for the substrates daunorubicin (16%) and menadione (23%). The transactivation of Sp1 increased the expression of cbr1 mRNA (67%), and increased carbonyl reductase activity for daunorubicin (35%) and menadione (27%). These data suggest that the variable number of Sp1 motifs in the canine cbr1 promoter may impact the pharmacodynamics of anthracyclines in canine cancer patients.
Keywords: Anthracyclines, Canine carbonyl reductases, Gene regulation, Specificity protein 1
Introduction
The clinical use of the anthracyclines doxorubicin and daunorubicin for the treatment of cancers in dogs is hampered by the development of cardiotoxicity in some patients (Astra et al., 2003). In the veterinary clinic, the incidence of this serious complication is around 18% (Gillings et al., 2009). Anthracycline-related cardiotoxicity may induce sudden death by arrhythmias, and approximately 2 to 9% of the cases culminate in overt congestive heart failure (CHF) (FitzPatrick et al., 2010; Ratterree et al., 2012).
Olson et al. proposed that the synthesis of anthracycline C-13 alcohol metabolites (i.e. daunorubicinol and doxorubicinol) in the myocardium contributes to cardiac damage (Olson et al., 1988). In different species (e.g. humans, mice, and rabbits), the synthesis of anthracycline C-13 alcohol metabolites is catalyzed by carbonyl reductases (CBRs) and aldo-keto reductases (AKRs) (Mordente et al., 2001; Mordente et al., 2003; Quinones-Lombrana et al., 2014; Schaupp et al., 2015). In the domestic dog, two genes located in chromosome 31 encode for carbonyl reductase 1 (cbr1) and carbonyl reductase 3 (cbr3). Recently, we reported the catalytic properties of canine cbr1 for the prototypical quinone substrate menadione, the anthracycline daunorubicin, and the flavonoid inhibitor rutin. Enzyme kinetics studies with recombinant canine cbr1 showed that “wild-type” cbr1 (i.e. cbr1 D218) and the variant isoform cbr1 V218 are capable of catalyzing the NADPH-dependent reduction of daunorubicin into its corresponding C-13 alcohol metabolite (Ferguson et al., 2015).There is a paucity of data describing the catalytic properties of the homologous canine cbr3.
Variability in the expression of the cbr1 gene may contribute to the erratic pharmacology of anthracyclines in canines. A recent mapping of the cbr1 locus by sequencing 97 genomic DNA samples from dogs from various breeds revealed that the putative proximal promoter region of cbr1 contains a cluster of conserved motifs for the transcription factor Sp1 (Cheng et al., 2012). The number of Sp1 motifs in samples from individual dogs varied from 6 to 8 in comparison to the reference DNA sequence from a Boxer dog (GenBank, http://www.ncbi.nlm.nih.gov/genome/guide/dog). It is known that polymorphic promoter variants that alter the number of Sp1 sites modulate the transcription of pharmacogenetically relevant genes. For example, variability in the number of Sp1 sites impacts the promoter activity of human ALOX 5 (Arachidonate 5-lipoxygenase) and the individual’s response to ALOX 5 inhibitors (Drazen et al., 1999; Kim et al., 2005). The factors that govern the transcription of canine cbr1 remain largely unexplored. Thus, the first aim of this study was to investigate the potential promoter activity of a DNA construct encompassing up to 729 base pairs (bp) of genomic sequence 5′ upstream the translation start site of canine cbr1. Second, studies were conducted to determine whether Sp1 regulates the transcription of canine cbr1. These studies provide insights into the regulation of canine cbr1 and constitute a platform for future analyses aimed to test whether interindividual variability in the number of Sp1 motifs impacts the pharmacology of anthracyclines in dogs with cancer.
Material and Methods
Cell culture
MDCK (Madin-Darby canine kidney. American Type Culture Collection, Manassas,VA) were routinely cultured in T75 flasks using DMEM (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (Sigma-Aldrich, St.Louis, MO), 100 U/mL penicillin (Thermo Fisher Scientific), and 100 μg/mL streptomycin (Thermo Fisher Scientific). Cultures were grown and maintained at low passage numbers (n < 12) using standard incubation conditions at 37 °C, 5% CO2, and 95% relative humidity.
Reagents
Mithramycin A, NADPH, monobasic potassium phosphate, dibasic potassium phosphate, phosphate-buffered saline (PBS), daunorubicin hydrochloride, and menadione sodium bisulfate were purchased from Sigma-Aldrich. Mithramycin A (Sigma-Aldrich) solutions were prepared with phosphate-buffered saline (PBS). Control treatments included equal volumes of PBS vehicle. Daunorubicin and menadione stock solutions were prepared in 0.1 M potassium phosphate buffer (pH 7.4).
Canine cbr1 reporter constructs and site-directed mutagenesis
A 729 bp DNA fragment from the canine cbr1 locus (−21 to −750 bp upstream the translation initiation codon A+1TG) was amplified by PCR from a Beagle dog genomic DNA sample (Orthopedic Foundation for Animals, OFFA) with the following primers: cbr1f, 5′-CACTAGGTTTGCTTTCAC-3′, and cbr1r, 5′-GCAAGGGCGTGTTTGCTCC-3′. A second 209 bp DNA fragment was amplified by PCR using the cbr1r reverse primer and the following forward primer: cbr1_sp1-GAGCGACAAGGGCATTGGA-3′. Both PCR products were cloned into the HindIII site of the PGL4.17 basic vector (Promega, Madison, WI).
The binding sequences for the reported Sp1 sites (consensus and non-consensus) were mutated with the QuikChange II XL-site-directed mutagenesis kit (Agilent, Santa Clara, CA)(Cheng et al., 2012). The following primers were used for site-directed mutagenesis (mutated bases are underlined): Sp1.A forward primer 5′-GCCCCTAGCCACGCCTATCCTGCGCCTGCGTAG-3′, Sp1.A reverse primer 5′-CTACGCAGGCGCAGGATAGGCGTGGCTAGGGGC-3′; Sp1.B forward primer 5′-CCTCCTCGGGAAGCACAGCCCAGGCCACGCC-3′, Sp1.B reverse primer 5′-GGCTGGCCTGGGCTGTGCTTCCCGAGGAGG; Sp1.C forward primer 5′-AGCCACGCCCAGGCACAGCCCAGACCACGCC-3′, Sp1.C reverse 5′-GGCGTGGTCTGGGCTGTGCCTGGGCGTGGCT-3′; Sp1.D forward primer 5′-GGCCACGCCCAGACACAGCCCCTAGCCACGC-3′, Sp1.D reverse primer 5′-GCGTGGCTAGGGGCTGTGTCTGGGCGTGGCC-3′; Sp1.E forward primer 5′-GACCACGCCCCTAGCACAGCCCCTAGCCACGCC-3′, Sp1.E reverse primer 5′-GGCGTGGCTAGGGGCTGTGCTAGGGGCGTGGTC-3′; Sp1.F forward primer 5′-AGCCACGCCCCTAGCACAGCCCGCCCTGCGCCT-3′, Sp1.F reverse primer 5′-AGGCGCAGGGCGGGCTGTGCTAGGGGCGTGGCT-3′; Sp1.G forward primer 5′-GGTGCCGCGGACCATACCAGGGCCCGGGA-3′, Sp1.G reverse primer 5′-TCCCGGGCCCTGGTATGGTCCGCGGCACC-3′. All constructs were verified by DNA sequencing.
Transfections
Twenty four hours prior to transfections, MDCK cells were plated in 24-well plates. Cells were transfected with the specific canine cbr1 luciferase reporter construct or the backbone vector (150 ng) plus the internal control plasmid pRL-TK (15 ng) using the Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific). In co-transfection assays, MDCK cells were transfected with 100 ng of the cbr1 reporter constructs, 15 ng of pRL-TK, and 150 ng of Sp1 expression vector or empty pCMV6-XL6 plasmid (OriGene, Rockville, MD). Twenty four hours post-transfection, cultures were washed once with PBS; cells were lysed in freshly diluted passive lysis buffer (100 μl/well, Promega) by incubating the plates at room temperature on a shaker at 200 rpm for 60 min. Luciferase reporter gene activities were determined with the Dual-Luciferase Reporter Assay System (Promega) per the manufacturer's instructions. Light intensity was measured in a Synergy HT luminometer equipped with proprietary software for data analysis (BioTek, Winooski, VT). Corrected firefly luciferase activities were normalized to renilla luciferase activities and expressed relative to the averaged activity of the −230/−21cbr1 construct which was assigned an arbitrary value of 100.
Chromatin Immunoprecipitation
Assays were performed using the ChIP-Enzymatic Express kit (Active Motif, Carlsbad, CA) according to the manufacturer’s instructions. Briefly, MDCK cells were cross-linked with 1% formaldehyde at room temperature for 5 minutes, repeatedly washed with ice-cold PBS, and lysed using a Dounce homogenizer followed by centrifugation. The nuclear pellet was suspended in enzymatic shearing mixture and digested at 37 °C for 12 min to shear the DNA. A fraction of the mixture of protein-DNA complex was used as “input DNA”. Sheared chromatin (15 μg) was then incubated overnight at 4°C with protein G magnetic beads and 5 μg of anti-Sp1 antibody (sc-14027-X, Santa Cruz Biotechnology, Dallas, TX) or normal rabbit IgG. Immuno-precipitated DNA was eluted from protein G beads, then the cross-linking was reversed and the DNA was purified. A 209-bp fragment of the canine cbr1 proximal promoter region was amplified by PCR with the cbr1_sp1 and cbr1r primers. PCR products were electrophoresed on a 2% agarose gel stained with ethidium bromide for visualization.
Quantification of canine cbr1 mRNA expression
Total RNA was isolated from cultured cells with Trizol reagent (Thermo Fisher Scientific). Canine cbr1 mRNA expression was analyzed by qRT-PCR with specific primers (cbr1 forward: 5′-GAAACCCCAAGGCAGAGTGG-3′; cbr1 reverse: 5′-CTGTGCACGCCTTTCTTTGTG-3′) following the MIQE guidelines (Bustin et al., 2009). Briefly, total RNA (25 ng) was reverse transcribed and amplified with iTaq Universal SYBR Green One-Step Kit (Bio Rad). Canine cbr1 and canine actb (reference gene, actb forward: 5′-GATCAAGATCATCGCACCCC-3′, and actb reverse: 5′-CGGTTTCTGCGCAAGTTAGG-3′) were amplified in parallel in an CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA) with the following cycling parameters: 50 °C for 10 min (reverse transcription), 95 °C for 1 min, followed by 40 cycles of 95 °C for 15 s and 56 °C for 30 s. Calibration curves were prepared to analyze linearity (r2 > 0.99) and PCR efficiency for the amplification of canine cbr1 (efficiency: 97%) and actb (efficiency: 99%). For each sample, the averaged Ct values for cbr1 were normalized against the averaged Ct values for actb using the dCt method (Schmittgen and Livak, 2008).
Cell viability
The viability of mithramycin A treated MDCK cells was assessed by recording the reduction of 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT. Sigma-Aldrich). Briefly, cells were plated in 96 well plates and treated with mithramycin A as described. Then, 20 μL of MTT solution (5 mg/mL) were added to each well followed by incubation at 37 °C for 4 h. After incubation, the medium was removed and 100 μL of DMSO were added into each well. The plate was gently rotated on an orbital shaker for 10 min to dissolve the MTT precipitate. The absorbance at 570 nm was recorded with a Synergy HT microplate reader (BioTek). Cellular viability was expressed as percentages relative to control incubations.
Kinetic Analysis
Cytosolic protein extraction was performed using the NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer's instructions. Daunorubicin reductase activity and menadione reductase activity were measured in cytosols of MDCK cells by monitoring the rate of oxidation of the NADPH cofactor (molar absorption coefficient: 6220 M−1cm−1) essentially as reported (Gonzalez-Covarrubias et al., 2007). Briefly, reaction mixtures (200 μl) were assembled in a clear flat-bottom 96-well Costar plate (Sigma-Aldrich) with 0.1 M potassium phosphate buffer (pH 7.4), cytosolic protein (10 μg), and daunorubicin (400 μM) or menadione (200 μM). The mixtures were then equilibrated to 37°C for 3 minutes, followed by the addition of NADPH to a final concentration of 200 μM. Immediately following the addition of NADPH, the absorbance at 340 nm was monitored every 30 seconds for 2 hours using a Synergy HT plate reader (Bio-Tek). Initial enzymatic velocity (V0) was determined by linear regression (ΔAbs340/minute), followed by conversion to nanomoles NADPH/min using the Gen5 software (BioTek).
Bioinformatics
The presence of transcription factor binding motifs in canine cbr1 was examined with the PhysBinder Web-based algorithm (http://bioit.dmbr.ugent.be/physbinder/index.php)(Broos et al., 2013). Protein sequence alignment was performed with MultAlin (Corpet, 1988).
Statistical Analysis
Statistics were computed with Excel 2013 (Microsoft Office; Microsoft, Redmond, WA) and GraphPad Prism version 4.03 (GraphPad Software Inc., La Jolla, CA). The Kolmogorov–Smirnov test was used to analyze the normality of datasets. Data are expressed as mean ± standard deviation (SD). P values < 0.05 were considered significant.
Results
Analysis of the canine cbr1 promoter
The putative canine cbr1 promoter was amplified by PCR from a Beagle dog genomic DNA sample. This DNA sample showed a sequence variant in the polymorphic “hot spot” region that differs slightly from the GenBank reference sequence from a Boxer dog (Fig. 1A) (Cheng et al., 2012). Computer-assisted analysis of the amplified product (~800 bp upstream the translation initiation codon A+1TG) pinpointed a cluster of binding motifs for the transcription factor Sp1 (Fig. 1A)(Cheng et al., 2012). In this region, there were two motifs (A and G) containing the canonical binding sequence for Sp1 (GGGCGG), and five motifs with the alternative Sp1 binding sequence (GCCACGCC) (Fig. 1A) (Raiber et al., 2012; Tamaki et al., 1995). To test for gene promoter activity, a 729-bp fragment was cloned into a pGL4.17 luciferase reporter vector, and the resulting construct was transiently transfected into cultures of MDCK cells. The 729 bp cbr1 construct exerted significant gene promoter activity (Fig. 1B). Next, the 729 bp cbr1 construct was used as a template to generate a shorter cbr1 promoter construct (209 bp) containing the cluster of seven Sp1 binding sites. Gene reporter assays showed that the “short” cbr1 promoter construct exhibited significant promoter activity in MDCK cells. In fact, both “short” and “long” cbr1 constructs exhibited similar promoter activities (−750/−21cbr1: 94.1 ± 19.7 RLUs vs. −230/−21cbr1: 100.0 ± 12.4; Student’s t test P = 0.199; Fig. 1B). Thus, it appears that the −230 to −21 bp region containing the cluster of Sp1 sites is important for basal canine cbr1 expression.
Fig. 1.
Analysis of the canine cbr1 promoter. (A) Schematic representation of canine cbr1 promoter constructs showing seven potential binding sites for Sp1. Canonical Sp1 binding sites (GGGCGG) are represented with white boxes. Non-canonical Sp1 binding sites (GCCACGCC) are represented by grey boxes. DNA sequence comparison (Beagle vs Boxer GenBank reference sequence) corresponding to variant D(B) Luciferase activities of the canine cbr1 constructs −750/−21 and −230/−21 in MDCK cells. Normalized luciferase activities were expressed relative to the values from −230/−21cbr1 construct that was assigned an arbitrary value of 100. Data represent the mean ± standard deviation of three independent experiments.
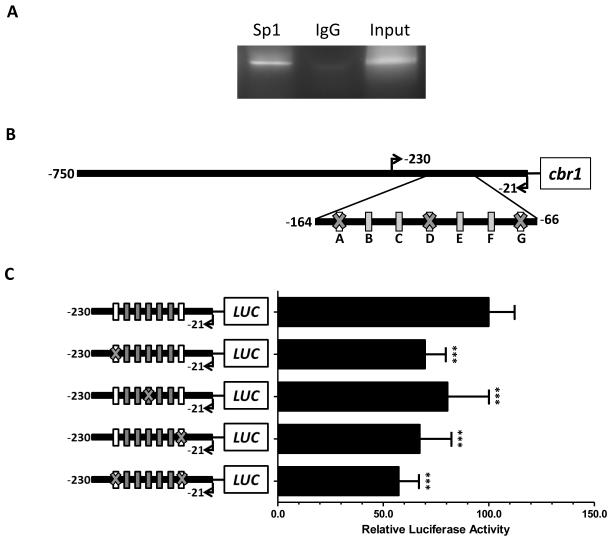
Next, the presence of interactions between Sp1 and the proximal promoter region of canine cbr1 was examined with chromatin immunoprecipitation assays. Immunoprecipitation of DNA with an anti-Sp1 antibody allowed the PCR amplification of the canine cbr1 promoter fragment containing the seven Sp1 sites (Fig. 2A).
Fig. 2.
Characterization of Sp1 interactions with canine cbr1 promoter. (A) Chromatin immunoprecipitation (ChIP) evaluation of Sp1 binding to canine cbr1 promoter. (B) Schematic representation of the potential binding sequences in the canine cbr1 promoter modified by site-directed mutagenesis. (C) Luciferase activities of mutated cbr1 constructs. Inset: luciferase activity values for constructs mutated in the non-consensus Sp1 sites B, C, E, and F. For each mutated construct, normalized luciferase activities were expressed relative to the values from −230/−21cbr1 construct that was assigned an arbitrary value of 100. Data represent the mean ± standard deviation of three independent experiments. ***P < 0.001, (Student's t-test).
Site directed mutagenesis studies were performed by mutating the “short” cbr1 promoter construct to further examine the potential contribution of Sp1 sites to gene expression. The binding cores of the two canonical Sp1 binding sequences (i.e. sites A and G) were mutated (Fig. 2B). Gene reporter assays showed that mutation of the binding cores in sites A and G led to significant decreases (site A: 30% decrease, Student’s t test P < 0.001; site G: 33% decrease, P < 0.001) in promoter activity in comparison to the activity of the non-mutated cbr1 construct (Fig. 2C). Site-directed mutagenesis of the Sp1 binding cores in both sites A and G led to a 43% decrease in promoter activity (Student’s t test P < 0.001, Fig. 2C). To assess the transcriptional impact of the additional non-consensus Sp1 binding motifs, the binding core sequences of sites B, C, D, E and F were mutated. For example, gene reporter assays showed that mutation of the binding core in site D induced a significant decrease in promoter activity (20%, Student’s t test P < 0.001. Fig. 2C). Mutations in the binding core sequences of the non-consensus Sp1 sites B, C, E, and F also modified promoter activity (Fig. 2C inset).
Involvement of Sp1 in canine cbr1 expression and activity
Mithramycin A binds to DNA GC boxes and inhibits the binding of Sp1 to its corresponding transcriptional motifs (Blume et al., 1991). We examined mithramycin A blockade of the interactions between Sp1 and the proximal promoter region of canine cbr1 with ChIP assays. PCR amplification of the canine cbr1 promoter was not observed when immunoprecipitated DNA extracted from mithramycin A treated MDCK cells was incubated with a specific anti-Sp1 antibody (Fig. 3A).
Fig. 3.
Regulation of canine cbr1 expression by Sp1. (A) Chromatin immunoprecipitation (ChIP) evaluation of Sp1 binding to canine cbr1 promoter in cells treated with mithramycin A (B) Luciferase activities of the canine −230/−21cbr1 construct in MDCK cells treated with 50 nM mithramycin A or co-transfected with the Sp1 expression vector for 24 hours. Normalized luciferase activities were expressed relative to the values from the control treatment that was assigned an arbitrary value of 100. (C) Effect of mithramycin A treatment on canine cbr1 mRNA levels. (D) Effect of the transfection of Sp1 expression vector on canine cbr1 mRNA levels. Data represent the mean ± standard deviation of three independent experiments. ***P < 0.001, (Student's t-test).
MDCK cells were transfected with the 209 bp canine cbr1 reporter construct and simultaneously treated with a non-cytotoxic concentration of mithramycin A for 24 hours (Table 1). The treatment with mithramycin A led to a 42% decrease in cbr1 promoter activity in comparison to control incubations (Student’s t test P < 0.001. Fig. 3B).
Table 1.
Effect of mithramycin A concentration on the viability of MDCK cells after 24 hours treatment.
| Concentration | Cell viability (%) | |
|---|---|---|
| Control | 100.0 ± 12.2 | |
| 12.5 nM | 90.7 ± 9.6 | p = 0.059 |
| 25 nM | 91.6 ± 13.3 | p = 0.135 |
| 50 nM | 98.1 ± 14.9 | p = 0.741 |
| 100 nM | 90.4 ± 10.5 | p = 0.058 |
| 200 nM | 98.7 ± 9.2 | p = 0.770 |
The human and canine Sp1 protein sequences are 98% homologous. Further analysis revealed that the Sp1 DNA binding domain is 100% identical in both species (Supplemental Fig. 1). Thus, a human Sp1 expression construct was used to perform co-transfection experiments in MDCK cells. Co-transfection of the Sp1 expression construct led to a 40% increase in the promoter activity of the cbr1 reporter construct (Student’s t test P < 0.001; Fig. 3B).
Mithramycin A treatment reduced the expression of endogenous canine cbr1 mRNA by 54% in comparison to control incubations (Student’s t test P < 0.001. Fig. 3C). Co-transfection of the Sp1 expression construct also increased the transcription of the endogenous cbr1 gene in MDCK cells as indicated by a 67% increase in cbr1 mRNA levels (Student’s t test P < 0.001; Fig. 3D).
Incubation of MDCK cells for twenty four hours with mithramycin A (50 nM) led to a 16% reduction in cytosolic daunorubicin reductase activity (Student’s t test P<0.001, Fig. 4A). In contrast, transient transfection of the cells with the Sp1 expression vector led to a 35% increase in daunorubicin reductase activity (Student’s t test P< 0.001. Fig. 4B). Similar trends were found when the enzymatic activity for the prototypical cbr substrate menadione was assessed. Mithramycin A incubation reduced cytosolic menadione reductase activity by 23% in comparison to control incubations (Student’s t test P < 0.001. Fig. 4C). Transfection of the Sp1 expression vector increased menadione reductase activity by 27% (Student’s t test P < 0.001. Fig. 4D).
Fig. 4.
Effect of Sp1 repression/activation on cytosolic cbr activity for the substrates daunorubicin and menadione. Daunorubicin and menadione reductase activity in MDCK cells treated with 50 nM mithramycin A (A and C). Daunorubicin and menadione reductase activity in MDCK cells transfected with the Sp1 expression vector for 24 hours (B and D). Data represent the mean ± SD of four independent experiments. ***P < 0.001, (Student's t-test).
Discussion
Sequencing of the canine cbr1 locus in DNA samples from dogs from various breeds identified a polymorphic “hot spot” in the putative gene promoter region containing a variable number of binding motifs (6 to 8) for the transcription factor Sp1 (Cheng et al., 2012). The putative canine cbr1 promoter does not have a TATA-box binding sequence motif (consensus: TATAAAA). This is of particular interest because Sp1 drives the transcription of genes without canonical TATA boxes (Latchman, 1990; Smale, 1997). In the present study, we identified the promoter of canine cbr1 and examined whether the variable number of Sp1 motifs impacts cbr1 gene transcription. The two 5′ DNA fragments (−729 bp and −209 bp) displayed significant gene promoter activities in canine MDCK cells. It appears that the cluster of seven Sp1 motifs is part of the “minimal” cbr1 promoter, because the gene reporter activities of both DNA fragments (i.e. “short” and “long”) were similar (Fig. 1). The chromatin immunoprecipitation assay provided further evidence in support of an interaction between Sp1 and the minimal canine cbr1 promoter (Fig. 2A).
Sp1 activates the transcription of promoters with several adjacent Sp1 motifs (Li et al., 2004). This capability appears to be exclusive of Sp1 and it has not been observed for other members of the Sp family (Yu et al., 2003). Transfection experiments with mutated canine cbr1 promoter constructs suggest that the consensus Sp1 sites A and G contribute to promoter activity. Simultaneous mutagenesis of both consensus sites does not completely suppress promoter activity suggesting that the non-consensus sites B to F may also contribute to regulate cbr1 expression (Fig. 2). Mutation of the non-consensus Sp1 sites B, C, D, E, and F modified cbr1 promoter activity. For example, the “hot spot” variant D is present in DNA samples from dogs from various breeds (approximate allele frequency, p ~ 0.22), including Poodles (p ~ 0.50) and Labrador Retrievers (p ~0.65). Mutagenesis of the Sp1 binding core in variant D led to a decrease in cbr1 promoter activity (Fig. 2A). This result lend support to the notion that multiple non-consensus Sp1 variants in the canine cbr1 promoter may contribute to differences in gene expression between individual dogs from various breeds (Cheng et al., 2012). We have also showed that Sp1-DNA binding blockade with mithramycin A and Sp1 overexpression impact canine cbr1 mRNA expression in MDCK cells (Fig. 3). In line, mithramycin A decreased cbr enzyme activity for the substrates daunorubicin and menadione, whereas the overexpression of Sp1 increased daunorubicin and menadione reductase activity (Fig. 4). Thus, these findings suggest that Sp1 regulates the transcription of canine cbr1 trough a tandem of Sp1 motifs located in the proximal promoter region. Studies are warranted to examine whether inter-breed variability in the number of Sp1 motifs in concert with polymorphic cbr1 variants that influence enzymatic activity (i.e. the functional cbr1 D218V non-synonymous polymorphism) impact the pharmacodynamics of anthracyclines in canine patients (Ferguson et al., 2015). During the mapping of the canine cbr1 locus, we identified a conserved xenobiotic response element (XRE, 5′-CACGCCA-3′) in the vicinity of the Sp1 cluster (~ 306 bp upstream of the translation initiation codon, A+1TG) (Cheng et al., 2012). Putative XREs (5′-CACGCNA/C-3′) overlapping some of the non-consensus Sp1 sites (e.g., site D) are also present in the proximal canine cbr1 promoter region. Although our current evidence suggests a role for the Sp1 sites during the regulation of cbr1, it is possible that the overlapping XRE sites would also be functional in specific contexts (e.g., in the presence of ligands of the aryl hydrocarbon receptor).
Supplementary Material
Acknowledgements
Research in this report was supported by awards from the National Institutes of Health National Institute of General Medical Sciences [R01GM073646], the Mae Stone Goode Trust, and the AKC Canine Health Foundation [Grant 1972]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the founding sources.
Abbreviations
- 5′-UTR
5′ untranslated region
- ACTB
beta-actin
- AKRs
aldo-keto reductases
- ALOX-5
Arachidonate 5-lipoxygenase
- CBR
carbonyl reductase
- CHF
Congestive heart failure
- ChIP
Chromatin Immunoprecipitation
- DMEM
Dulbecco's Modified Eagle Medium
- DMSO
Dimethyl sulfoxide
- MDCK
Madin-Darby canine kidney
- MTT
3-(4,5-dimethythiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- ROS
reactive oxygen species
- Sp1
specificity protein 1
Footnotes
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- Astra LI, Hammond R, Tarakji K, Stephenson LW. Doxorubicin-induced canine CHF: advantages and disadvantages. J Card Surg. 2003;18(4):301–306. doi: 10.1046/j.1540-8191.2003.02032.x. [DOI] [PubMed] [Google Scholar]
- Blume SW, Snyder RC, Ray R, Thomas S, Koller CA, Miller DM. Mithramycin inhibits SP1 binding and selectively inhibits transcriptional activity of the dihydrofolate reductase gene in vitro and in vivo. J Clin Invest. 1991;88(5):1613–1621. doi: 10.1172/JCI115474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broos S, Soete A, Hooghe B, Moran R, van Roy F, De Bleser P. PhysBinder: Improving the prediction of transcription factor binding sites by flexible inclusion of biophysical properties. Nucleic acids research. 2013;41(Web Server issue):W531–534. doi: 10.1093/nar/gkt288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Sanborn C, Ferguson D, Blanco JG. DNA sequence variants in the carbonyl reductase 1 (cbr1) gene in seven breeds of Canis lupus familiaris. Genet Mol Res. 2012;11(2):1109–1116. doi: 10.4238/2012.April.27.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic acids research. 1988;16(22):10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drazen JM, Yandava CN, Dube L, Szczerback N, Hippensteel R, Pillari A, Israel E, Schork N, Silverman ES, Katz DA, Drajesk J. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nature Genetics. 1999;22(2):168–170. doi: 10.1038/9680. [DOI] [PubMed] [Google Scholar]
- Ferguson DC, Cheng Q, Blanco JG. Characterization of the Canine Anthracycline-Metabolizing Enzyme Carbonyl Reductase 1 (cbr1) and the Functional Isoform cbr1 V218. Drug metabolism and disposition: the biological fate of chemicals. 2015;43(7):922–927. doi: 10.1124/dmd.115.064295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzPatrick WM, Dervisis NG, Kitchell BE. Safety of concurrent administration of dexrazoxane and doxorubicin in the canine cancer patient. Vet Comp Oncol. 2010;8(4):273–282. doi: 10.1111/j.1476-5829.2010.00225.x. [DOI] [PubMed] [Google Scholar]
- Gillings S, Johnson J, Fulmer A, Hauck M. Effect of a 1-hour IV infusion of doxorubicin on the development of cardiotoxicity in dogs as evaluated by electrocardiography and echocardiography. Vet Ther. 2009;10(1-2):46–58. [PubMed] [Google Scholar]
- Gonzalez-Covarrubias V, Ghosh D, Lakhman SS, Pendyala L, Blanco JG. A functional genetic polymorphism on human carbonyl reductase 1 (CBR1 V88I) impacts on catalytic activity and NADPH binding affinity. Drug metabolism and disposition: the biological fate of chemicals. 2007;35(6):973–980. doi: 10.1124/dmd.107.014779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Bae JS, Suh CH, Nahm DH, Holloway JW, Park HS. Polymorphism of tandem repeat in promoter of 5-lipoxygenase in ASA-intolerant asthma: a positive association with airway hyperresponsiveness. Allergy. 2005;60(6):760–765. doi: 10.1111/j.1398-9995.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- Latchman DS. Eukaryotic Transcription Factors. Biochemical Journal. 1990;270(2):281–289. doi: 10.1042/bj2700281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, He S, Sun JM, Davie JR. Gene regulation by Sp1 and Sp3. Biochem Cell Biol. 2004;82(4):460–471. doi: 10.1139/o04-045. [DOI] [PubMed] [Google Scholar]
- Mordente A, Meucci E, Martorana GE, Giardina B, Minotti G. Human heart cytosolic reductases and anthracycline cardiotoxicity. IUBMB Life. 2001;52(1-2):83–88. doi: 10.1080/15216540252774829. [DOI] [PubMed] [Google Scholar]
- Mordente A, Minotti G, Martorana GE, Silvestrini A, Giardina B, Meucci E. Anthracycline secondary alcohol metabolite formation in human or rabbit heart: biochemical aspects and pharmacologic implications. Biochemical pharmacology. 2003;66(6):989–998. doi: 10.1016/s0006-2952(03)00442-8. [DOI] [PubMed] [Google Scholar]
- Olson RD, Mushlin PS, Brenner DE, Fleischer S, Cusack BJ, Chang BK, Boucek RJ., Jr. Doxorubicin cardiotoxicity may be caused by its metabolite, doxorubicinol. Proc Natl Acad Sci U S A. 1988;85(10):3585–3589. doi: 10.1073/pnas.85.10.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones-Lombrana A, Ferguson D, Hageman Blair R, Kalabus JL, Redzematovic A, Blanco JG. Interindividual variability in the cardiac expression of anthracycline reductases in donors with and without Down syndrome. Pharm Res. 2014;31(7):1644–1655. doi: 10.1007/s11095-013-1267-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiber EA, Kranaster R, Lam E, Nikan M, Balasubramanian S. A non-canonical DNA structure is a binding motif for the transcription factor SP1 in vitro. Nucleic acids research. 2012;40(4):1499–1508. doi: 10.1093/nar/gkr882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratterree W, Gieger T, Pariaut R, Saelinger C, Strickland K. Value of Echocardiography and Electrocardiography as Screening Tools Prior to Doxorubicin Administration. J Am Anim Hosp Assoc. 2012;48(2):89–96. doi: 10.5326/JAAHA-MS-5680. [DOI] [PubMed] [Google Scholar]
- Schaupp CM, White CC, Merrill GF, Kavanagh TJ. Metabolism of doxorubicin to the cardiotoxic metabolite doxorubicinol is increased in a mouse model of chronic glutathione deficiency: A potential role for carbonyl reductase 3. Chem-Biol Interact. 2015;234:154–161. doi: 10.1016/j.cbi.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Smale ST. Transcription initiation from TATA-less promoters within eukaryotic protein-coding genes. Bba-Gene Struct Expr. 1997;1351(1-2):73–88. doi: 10.1016/s0167-4781(96)00206-0. [DOI] [PubMed] [Google Scholar]
- Tamaki T, Ohnishi K, Hartl C, LeRoy EC, Trojanowska M. Characterization of a GC-rich region containing Sp1 binding site(s) as a constitutive responsive element of the alpha 2(I) collagen gene in human fibroblasts. The Journal of biological chemistry. 1995;270(9):4299–4304. doi: 10.1074/jbc.270.9.4299. [DOI] [PubMed] [Google Scholar]
- Yu B, Datta PK, Bagchi S. Stability of the Sp3-DNA complex is promoter-specific: Sp3 efficiently competes with Sp1 for binding to promoters containing multiple Sp-sites. Nucleic acids research. 2003;31(18):5368–5376. doi: 10.1093/nar/gkg706. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.