Abstract
Adeno-associated viruses (AAVs) are promising viral vectors for therapeutic gene delivery, and the approval of an AAV1 vector for the treatment of lipoprotein lipase deficiency has heralded a new and exciting era for this system. However, preclinical and clinical studies show that neutralization from pre-existing antibodies is detrimental for medical application and this hurdle must be overcome before full clinical realization can be achieved. Thus the binding sites for capsid antibodies must be identified and eliminated through capsid engineering. Towards this goal and to recapitulate patient polyclonal responses, a panel of eight new mouse monoclonal antibodies (MAbs) has been generated against AAV8 and AAV9 capsids, two vectors in development for therapeutic application. Native (capsid) dot blot assays confirmed the specificity of these antibodies for their parental serotypes, with the exception of one MAb, HL2372, selected to cross-react against both capsids. Furthermore, in vitro assays showed that these MAbs are capable of neutralizing virus infection. These MAbs will be utilized for structural mapping of antigenic footprints on their respective capsids to inform development of the next generation of rAAV vectors capable of evading antibody neutralization while retaining parental tropism.
1. Introduction
Major advances have occurred in the development of Adeno-associated viruses (AAVs) as gene delivery vectors over the last two decades, including improvements in large scale vector production to support clinical trials (Chahal et al. 2014; Martin et al. 2013; Mietzsch et al. 2014). Significantly, recent successes in clinical trials worldwide have resulted in the approval of the use of the first AAV gene therapy product in Europe for the treatment of lipoprotein lipase deficiency (Pollack 2012), and numerous clinical trials are in progress for many other disease targets (Bainbridge et al. 2008; Brantly et al. 2009; Daniel Gaudet 2012; Ginn et al. 2013; Maguire et al. 2009; Maguire et al. 2008; Mendell et al. 2009; Smith et al. 2013; Wierzbicki et al. 2013). However, AAV elicits both a cellular and humoral immune response which must be overcome for improved vector efficacy. In the general population, ~40–70% of individuals have been exposed to AAVs (Blacklow et al. 1968; Boutin et al. 2010; Calcedo et al. 2011; Calcedo et al. 2009; Liu et al. 2013), and a significant number of potential patients already harbor pre-existing antibodies to AAVs (Ferreira et al. 2014; Halbert et al. 2006; Li et al. 2012; van der Marel et al. 2011). These pre-existing antibodies have been shown, even at low levels, to prevent successful gene delivery (Hurlbut et al. 2010; Manno et al. 2006; Scallan et al. 2006; Wang et al. 2011). In addition, the antibody response is likely to interfere with any re-administration of an AAV vector in the event that therapeutic levels are not maintained for the lifetime of the patient.
To understand AAV-antibody interactions and identify potential epitopes, the first step is to produce and have at hand a panel of anti-AAV antibodies. Here, we generated a panel of anti-AAV8 and anti-AAV9 mouse monoclonal antibodies (MAbs) using the hybridoma method to aid characterization of their capsid-antibody interactions. AAV8 is known for its enhanced hepatic cell transduction (Sands 2011) and has been used in numerous preclinical and clinical trials to target the liver (Bell et al. 2011; Nathwani et al. 2007; Nathwani et al. 2006; Nathwani et al. 2011). AAV9 has been reported to cross the blood-brain barrier (Bevan et al. 2011; Federici et al. 2012; Gray et al. 2011; Schuster et al. 2014; Zhang et al. 2011), and has become the vector of choice for treating genetic disease involving the central nervous system (CNS) (Cearley et al. 2007; Fu et al. 2011; Spampanato et al. 2011; Xue et al. 2010). However, despite the progress in AAV vector development, detailed antigenic footprint information is lacking for both AAV8 and AAV9. Until now, there has only been one MAb developed against AAV8 and AAV9, namely ADK8 and ADK9, respectively, and one cross-reactive MAb, ADK8/9 (Sonntag et al. 2011). The binding site of ADK8 on the AAV8 capsid surface has been identified, through cryo-electron microscopy (cryo-EM) reconstruction methods and confirmed by mutagenesis, to be located on the top of the protrusions surrounding the icosahedral 3-fold axes of the capsid (Gurda et al. 2012). However, the epitope for ADK9 on the AAV9 capsid surface remains unknown. The only available AAV9 antigenic information based on an in vivo library screening showed that residues 453–457, which are also located in the region of 3-fold protrusion, are important for antigenicity (Adachi et al. 2014). Since the antibody response in humans is polyclonal, the antigenic information from one monoclonal anti-AAV antibody is not sufficient to mimic patient responses. Therefore, in an effort to better understand the region(s) of the AAV8 and AAV9 capsids that are immunogenic/immunodominant, we have generated a panel of new antibodies to AAV8 and AAV9 capsids in mice using the hybridoma method. These antibodies will facilitate further studies, through molecular and structural biology, that will provide a better understanding of the antigenic regions of their respective capsids. This information can then be utilized to develop AAV8 and AAV9 variants, through rational sitedirected mutagenesis or structure guided directed evolution, with the ability to evade antibody neutralization while retaining parental tropism.
2. Materials and Method
2.1. Expression and purification of AAV8 and AAV9 capsids
Recombinant AAV8 and AAV9 virus-like particles (VLPs) were expressed using the Bac-to- Bac baculovirus-Sf9 insect cell expression system (Gibco/ Invitrogen,Carlsbad, CA) and purified using a 20% sucrose cushion followed by sucrose gradient (5 to 40% [wt/ vol]) as previously reported (Lane et al. 2005; Mitchell et al. 2009). Purified AAV8 and AAV9 VLPs were concentrated to 1–3 mg/ml and buffer exchanged into 1X PBS, pH 7.4. The concentration of the samples was estimated by optical density measurements (using OD280 and E = 1.7 for calculation in mg/ml), as well as SDS-PAGE gel electrophoresis with BSA concentration standards. Prior to use, the purity and integrity of the VLPs were also monitored by SDS-PAGE and negative stain EM, respectively (data not shown).
2.2. Generation of AAV capsid specific monoclonal antibodies
The anti-AAV8 and anti-AAV9 hybridoma clones were generated in collaboration with the Interdisciplinary Center for Biotechnology Research (ICBR) Hybridoma Core Lab, University of Florida. Six-week-old female BALB/CByj mice were immunized three times with subcutaneous injections of 5, 10, 25, 50 or 75 μg of AAV capsids at 21-day intervals and one intraperitoneal injection on day 120 as the last boost. The first three subcutaneous injections were accompanied by a Sigma Adjuvant System (Sigma-Aldrich, St. Louis, MO), which contain 0.5 mg monophosphoryl lipid A, 0.5 mg synthetic trehalose dicorynomycolate in 44 μl squalene oil, 0.2% TWEEN 80 and water. Test bleeds from immunized animals were obtained 10–14 days after every booster injection, following animal care protocols. The collected sera were tested for high specific antibody response using ELISA and Dot Blot (against intact capsid) assays as described below. Four days after the final boost injection, the splenocytes of immunized mice were fused with mouse myeloma Sp2/0 cells using 50% PEG 1500 (polyethylene glycol) as the fusing agent. The fused hybrids were cultured in HAT (hypoxanthine-aminopterin-thymidine) (Sigma-Aldrich, St. Louis, MO) supplemented Dulbeccos Modified Eagles Medium (DMEM) to eradicate the unfused myeloma cells. To obtain the positive hybridoma clones, with highest specific anti-AAV capsid antibody response, the supernatants from the resulting hybridoma cells were collected and screened by total of 5 rounds of ELISA assays, as described below. Use of Animals in the UF Hybridoma Core Lab at University of Florida is under the guidelines of the Institutional Animal Care and Use Committee.
2.3. Screening of mice serum or hybridoma supernatants using VLP ELISA
The supernatants of hybridomas were screened in the Hybridoma Core Lab, ICBR, University of Florida, using AAV8 and AAV9 virus-like particles (VLPs) ELISA assays. Briefly, Nunc Maxisorp 96 well plates (Thermo Scientific, Rochester, NY) were coated with AAV VLPs at 4°C O/N prior to each ELISA assay. The plates were then blocked with 1% BSA in PBS at RT for 1 h, and then washed with washing buffer (1×PBS with 0.5% Tween 20). The immunized mouse serum or the hybridoma supernatants were applied to the plate and incubated at RT for 1 h. After washes, the secondary antibody, a rabbit anti-mouse IgG whole molecule AP (alkaline phosphatase), goat anti-mouse IgG gamma chain specific AP, or goat anti-mouse IgM mu chain specific AP (Sigma-Aldrich, St. Louis, MO) were added at 1:1000, 1:4000, and 1:4000 dilution in PBS with 1% BSA, respectively, for 1 h at RT. Finally after several washes, the substrates, p- Nitrophenyl Phosphate Disodium (Sigma), was applied to the plate and incubated for 1 h, then optical density readings were taken at 405 nm using a Molecular Devices SpectraMax 384 Plus (Sunnyvale, CA).
2.4. Anti-AAV VLP dot blot analysis
AAV VLPs were allowed to adsorb onto supported nitrocellulose membranes (Bio-Rad, Hercules, CA) in the dot blot manifold (Schleicher and Schuell, Dassel, Germany). Excess fluid was drawn through the membrane by vacuum filtration. The membrane was removed from the manifold and blocked with 10% milk in PBS, pH 7.4 for 1 h. Primary antibody in the form of anti-AAV mouse serum, hybridoma supernatant, or the purified MAbs, in different dilutions depending on the sample being tested, was applied to the membrane in PBS with 5% milk and incubated for 1 h. Following this, the membrane was washed with PBS and horse radish peroxidase (HRP)-linked secondary antibody was applied at a dilution of 1:5000 in PBS and incubated for 1 h. The membrane was washed with PBS and then Super Signal West Pico Chemiluminescent Substrate (ThermoFisher, Waltham, WA) was applied to the membrane and the signal detected on X-ray film. The B1 antibody, which binds to the C terminus of the viral capsid proteins in all the AAV serotypes except for AAV4 (Wistuba et al. 1995), was used as a control to confirm the presence of AAV capsid proteins using denatured capsids (boiled and blotted). ADK8 and ADK9 (Sonntag et al. 2011) were used as positive controls for AAV8 and AAV9, respectively, to detect in intact (non-boiled) capsids.
2.5. Determination of the isotypes for the anti-AAV MAbs
The isotypes of the newly generated anti-AAV antibodies were determined in the ICBR Hybridoma Core Lab, University of Florida, using the IsoStrip Mouse Monoclonal Antibody Isotyping Kit (Satnta Cruz Biotechnology, Santa Cruz, CA). The supernatant of the hybridoma cell cultures were diluted 1:10 to 1:100 in PBS depending on the concentration of antibodies in the supernatant. The diluted samples were loaded onto the development tube provided in the kit and incubated for 30 s. One isotyping strip was placed in each development tube and incubated for 5–10 min until appearance of the blue bands for which their positions indicate the isotype (IgG1, IgG2a, IgG2b, IgG3, IgM or IgA) and the light chain type (kappa or lambda) of the MAb.
2.6. Neutralization assay
The neutralization abilities of the newly generated anti-AAV8 and anti-AAV9 antibodies were assayed in HeLa cells (Veron et al. 2012). Briefly, luciferase gene packaged recombinant rAAV8-Luc or rAAV9-Luc vectors were mixed with different hybridoma supernatants in a volume ratio 1:1, and then used to infect HeLa cells at a 10,000 MOI (multiplicity of infection). After 24 hours, the cells were lysed and the expressed luciferase activity was assayed for each complex using a luciferase assay (Promega, Madison, WI) as described in the manufacturer's protocol. In this assay, ADK4, a MAb that specifically recognizes AAV4 (Kuck et al. 2007) was used as a negative control while ADK8 and ADK9, known to neutralize infection by AAV8 (Gurda et al. 2012) and AAV9 (Sonntag et al. 2011), respectively, were used as positive control.
3.0 Results
3.1. New panel of anti-AAV8 and anti-AAV9 antibodies
Two anti-AAV8 (designated HL2381 and HL2383; HL stands for Hybridoma Lab) and four anti-AAV9 (HL2368, HL2370, HL2372, and HL2374) IgG antibodies were generated in mice by 3 subcutaneous and 1 intraperitoneal immunization of AAV8 or AAV9 capsids, respectively, in 6 week old BLAB/CByj mice (Table 1). The clones were selected from fused hybridoma hybrids after capsid ELISA and dot blot screenings. All the 6 antibodies generated only bind to intact capsids (Figure 1) not the linear viral proteins when tested in Western blot assays (data not shown). The isotypes of these new anti-AAV antibodies were identified: the 2 anti-AAV8 antibodies, HL2381 and HL2383, are both IgG3; the 4 anti-AAV9 antibodies, HL2368, HL2370, HL2372, and HL2374, are IgG3, IgG2a, IgG2a, and IgG3, respectively (Table 1).
Table 1.
The anti-AAV8 and anti-AAV9 HL series of antibodies
| Antibody ID | Against serotype | Isotype |
|---|---|---|
| HL2368 | AAV9 | IgG3 |
| HL2370 | AAV9 | IgG2a |
| HL2372 | AAV9 | IgG2a |
| HL2374 | AAV9 | IgG3 |
| HL2381 | AAV8 | IgG3 |
| HL2383 | AAV8 | IgG3 |
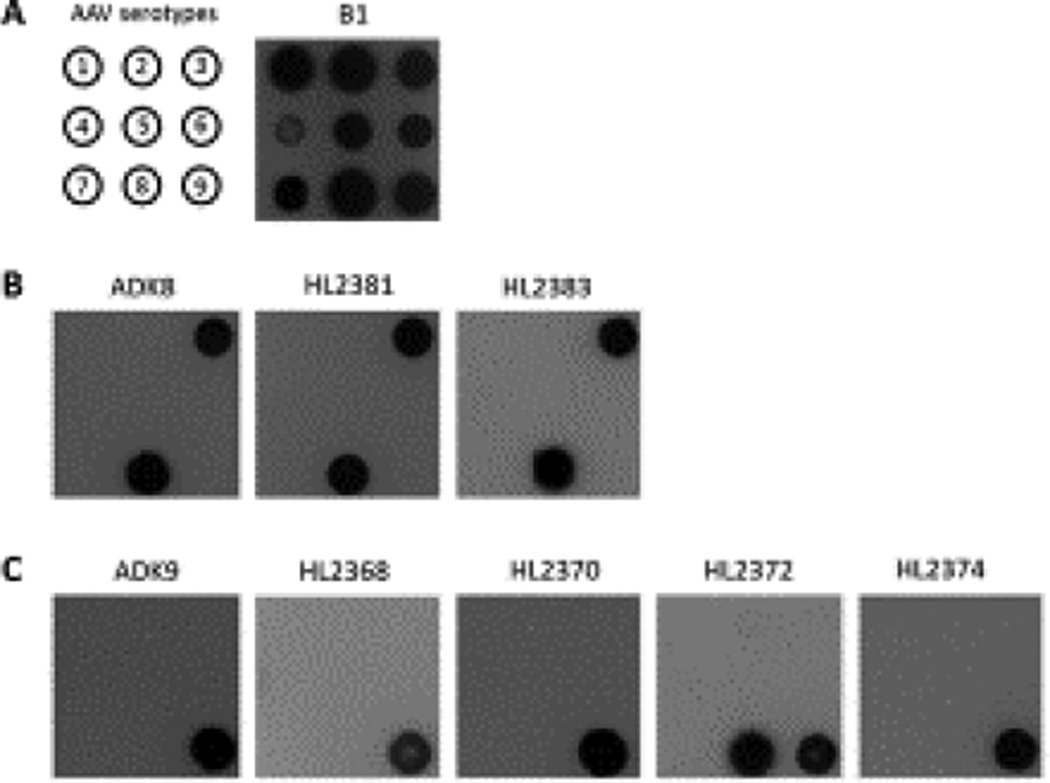
Figure 1.
Cross reactivity of HL antibodies. (A) Position map of AAV serotypes and B1 antibody (Wobus et al. 2000) reactivity of AAV1-AAV9, the representative members of the AAV antigenic clades and clonal isolates (Gao et al. 2004). (B) The cross-reactivity the anti-AAV8 antibodies against different AAV serotypes: ADK8 (Sonntag et al. 2011) as a positive control, and the new anti-AAV8 HL antibodies, HL2381 and HL2383. (C) The cross-reactivity of the anti-AAV9 antibodies against different AAV serotypes: ADK9 (Sonntag et al. 2011) as a positive control, and the anti-AAV9 HL antibodies, HL2368, HL2370, HL2372, and HL2374.
3.2. The HL MAbs are serotype specific, with the exception of HL2372 that cross-reacts with AAV8 and AAV9
To check the cross-reactivity of the MAbs against different AAV serotypes, the anti-AAV8 and anti-AAV9 antibodies were used as primary antibodies to probe the intact capsids of AAV1- AAV9 in a native dot blot assay (Fig. 1). The B1 antibody confirmed the presence of AAV capsid proteins, with the exception of AAV4 (Fig.1 A), while ADK8 and ADK9 confirmed the presence of AAV8 and AAV9 capsids (Fig.1 B and C). Two anti-AAV8 antibodies, HL2381 and HL2383, were able to recognize AAV8 as predicted, but also cross reacted with AAV3B (Fig.1 B), which has also been observed for the ADK8 antibody (Sonntag et al. 2011). During the clonal selection, one anti-AAV9 clone, HL2372, exhibited binding to both AAV8 and AAV9. This cross-reactivity was confirmed by dot blot assay (Fig. 1 C). All other anti-AAV9 antibodies, HL2368, HL2370, and HL2374, bound to AAV9 only (Fig. 1 C).
3.3. The HL series of anti-AAV8 and anti-AAV9 MAbs are neutralizing
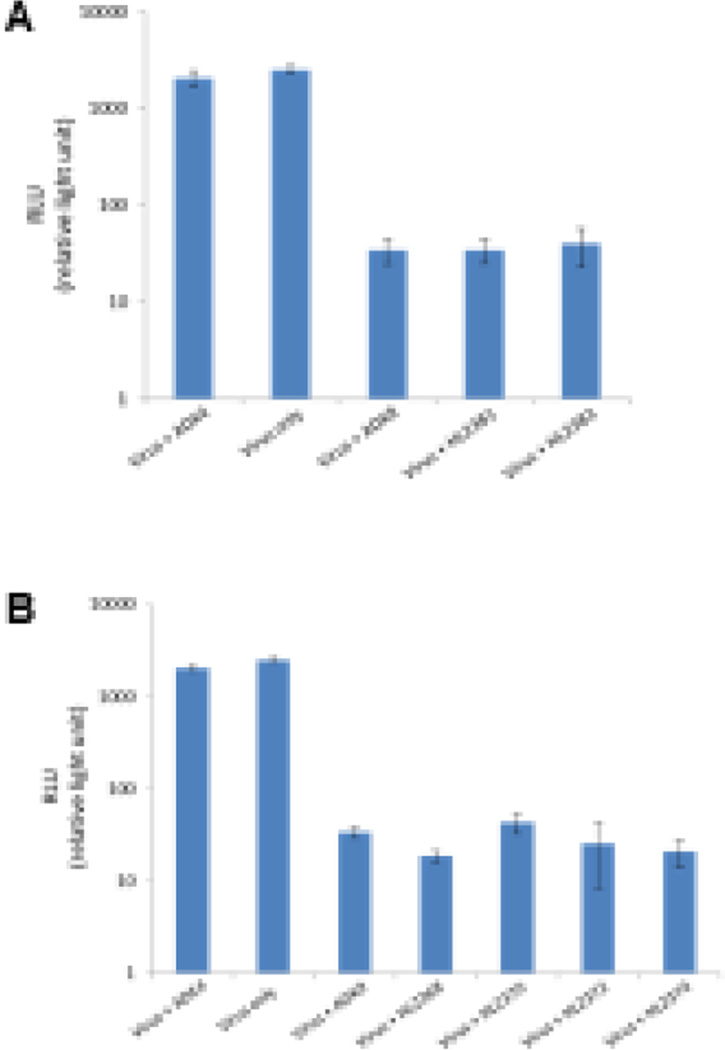
The in vitro neutralization abilities of the anti-AAV8 and anti-AAV9 antibodies were assayed in HeLa cells (Fig. 2). The ADK8 and ADK9 MAbs (Sonntag et al. 2011) used as positive controls were observed to neutralize infection. However, ADK4, a MAb that specifically recognizes AAV4 (Kuck et al. 2007), was used as a negative control and was not able to neutralize the infection of rAAV8 (Fig. 2 A) or rAAV9 (Fig. 2 B), with the resulting luciferase activities being similar in the virus+ADK4 and virus-only groups (Fig. 2). In the virus+ADK8 and virus+ADK9 groups, the luciferase activities were decreased ~20 fold in relative light units (RLUs) compared to the virus-only groups (Fig. 2 A and B, respectively). Similar results were observed with virus mixed with HL2368, HL2370, HL2372, HL2374, HL2381, and HL2383, suggesting that all the antibodies can neutralize infection in cells, as observed with ADK8 (Fig. 2 A) and ADK9 (Fig. 2 B).
Figure 2.
In vitro neutralization by HL antibodies. (A and B) The transduction efficiency of rAAV8 and rAAV9, respectively, as measured by luciferase activity, in the absence/presence of the HL antibodies. The ADK4 antibody was used as a negative control, while ADK8 and ADK9 were used as positive controls, respectively.
4. Discussion
With recent advances in the development of AAVs as gene therapy vectors or for use as active (protein expression) and passive (antibody expression) delivery systems for vaccination, there is a need for a better understanding of their antigenic reactivity and anti-body binding site structures. In addition, there is an ever expanding pool of AAV variants being developed for specific tissue targeting and improved efficacy. Advancing these studies requires the availability of more anti-AAV capsid antibodies as detecting tools as well as for elucidating antigenic footprints for engineering host immune recognition escape. The developed HL panel of anti-AAV8 and anti-AAV9 hybridoma clones described expands the pool of known anti-AAV8 and anti-AAV9 antibodies. These new antibodies showed in vitro neutralizing ability at the same level as ADK8 and ADK9, indicative of binding sites that enable functions required for infection (Fig. 2). Structural characterization of the binding site(s) for these MAbs will aid the design of neutralization escaping vectors to evade the polyclonal antibody response that is pre-existing in the human population.
The six new HL MAbs are either IgG2a or IgG3. In a previous study to create anti-AAV2 MAbs, although both Balb/c and C57B1/6 mice were intramuscularly (i.m) immunized with capsids, high titers of IgG2a and IgG3 were induced in the Balb/c mice but only IgG2a isotypes were observed in the C57B1/6 mice (Chirmule et al. 2000). Other studies have shown that C57B1/6 mice tend to generate IgG2a and IgG2b antibodies when immunized with AAVs (Kuck et al. 2007; Sonntag et al. 2011; Zhang et al. 2005). In this study, IgG2a and IgG3 antibodies were also generated in Balb/c mice (Table 1), similar to the observation by Chirmule et al in Balb/c mice despite different immunization routes: subcutaneous (this study) versus i.m (Chirmule et al. 2000). This suggests that AAVs have an IgG isotype preference. However, in another study using subcutaneous immunization in Balb/c mice, a spectrum of MAb isotypes were selected, including IgG1, IgG2a, IgG2b, IgG3, and IgM (Harbison et al. 2012). The only significant difference between this work and that by Harbison et al was the administration route of the final boost. Rather than using intraperitoneal injections, Harbison et al used intravenous injections. Interestingly, structure characterization of the antibodies generated in their study showed the fragment antibody binding footprints to be mostly overlapping on the capsid surface and a large number of the MAbs were IgM suggesting that further maturation was required or perhaps further clonal selection would have narrowed the spectrum of isotypes observed. These observations indicate that the relationship between virus and infected host is complex with respect to MAb generation, maturation, and isotype switching and requires further investigation. It is likely that the genetic backgrounds of animal strains, antigen dose, and routes of antigen administrations may cause different IgG subclass profiles to be generated.
The six hybridoma clones reported in this study were generated from mice immunized with either AAV8 or AAV9. However, three of the antibodies generated bind to more than one AAV serotype: HL2381 and HL2383 bind to both AAV8 and AAV3B, and HL2372 generated against AAV9 also binds to AAV8 (Fig. 1). Other known anti-AAV monoclonal antibodies shown to be cross-reactive to AAV capsids that were not used for generation include ADK1a and ADK1b, isolated from spleen cells of mice immunized with AAV1 yet bind to AAV6 capsids (Kuck et al. 2007). This is likely due to the high sequence identity (~99%) between the AAV1 and AAV6 capsid viral proteins, where only six amino acids differ between them (Ng et al. 2010), and the fact that the footprint mapped for ADK1a and ADK1b do not contain these differing residues. Similarly AAV1/AAV6 cross reactivity has also been observed for the 4E4 and 5H7 anti-AAV1 antibodies generated against AAV1 (Harbison et al. 2012). Unlike AAV1 and AAV6, the sequence identity between AAV3B and AAV8 is ~83% and between AAV8 and AAV9 is ~82%, and the overall structural overlay identity is slightly higher at ~86% between AAV3 and AAV8, and ~88% between AAV8 and AAV9. However, AAV8 and AAV9 both also show ~88% structural identity to AAV1, AAV2, and AAV6, and these new antibodies did not exhibit cross reactivity to these three serotypes. These observations suggest common surface properties between the serotypes for which cross reactivity is observed. In addition, it is noteworthy that ADK8, generated from a different mice strain, with different immunization protocol (Sonntag et al. 2011), also binds to AAV3B (Fig. 1B). All three antibodies, HL2381, HL2383, and ADK8, only bind to intact AAV capsids, suggesting that there must be common antigenic structural characteristics shared between the AAV3B and AAV8 capsid surfaces. These observations generate a need to better understand the antigenic properties of these AAV serotypes to enable antibody escape vectors because of the polyclonal anti-AAV response observed in the human population.
In summary, we have expanded the number of anti-AAV8 and anti-AAV9 antibodies available to the AAV community which can be used as tools to study the antigenic properties of wild type AAV8 and AAV9. They will also serve as reagents for characterizing variants being developed for improved therapeutic efficacy.
Highlights.
A panel of mouse monoclonal antibodies were generated against AAV8 and AAV9
The new MAbs recognize conformational epitopes
In vitro virus neutralization by new antibodies suggests recognition of functional regions
The new MAbs provide tools for characterization of AAV8 and AAV9 variants being developed for improved therapeutic efficacy
Acknowledgments
We thank the UF Interdisciplinary Center for Biotechnology Research (ICBR) Hybridoma Core Lab for their collaboration to generate the AAV8 and AAV9 MAbs. We thank James Wilson (U. Pennsylvania) for providing the AAV8 and AAV9 DNA used for generating the VLPs used for creating the AAV8 and AAV9 MAbs. This project was funded in by NIH R01 grants GM082946 (MAM and RM) and HL089221 (MAM and AA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement of Conflict: M. Agbandje-McKenna (MAM) is an SAB member for Voyager Therapeutics, Inc., and has a sponsored research agreement with AGTC and Avalanche Biotechnologies. Both companies have interest in the development of AAV for gene delivery applications. MAM, Aravind Asokan (AA), and Barry Byrne (BB) are inventors of AAV patents licensed to various biopharmaceutical companies. MAM and AA are co-founders of Stride Therapeutics, LLC, and BB is a co-founder of AGTC. These are biopharmaceutical companies with interest in developing AAV vectors for gene delivery application.
Reference
- Adachi K, Enoki T, Kawano Y, et al. Drawing a high-resolution functional map of adeno-associated virus capsid by massively parallel sequencing. Nat Commun. 2014;5:3075. doi: 10.1038/ncomms4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Ali RR. Success in sight: The eyes have it! Ocular gene therapy trials for LCA look promising. Gene Ther. 2008;15:1191–1192. doi: 10.1038/gt.2008.117. [DOI] [PubMed] [Google Scholar]
- Bell P, Gao G, Haskins ME, et al. Evaluation of adeno-associated viral vectors for liver-directed gene transfer in dogs. Hum Gene Ther. 2011;22:985–997. doi: 10.1089/hum.2010.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan AK, Duque S, Foust KD, et al. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther. 2011;19:1971–1980. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacklow NR, Hoggan MD, Rowe WP. Serologic evidence for human infection with adenovirus-associated viruses. J Natl Cancer Inst. 1968;40:319–327. [PubMed] [Google Scholar]
- Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types, 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- Brantly ML, Chulay JD, Wang L, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A. 2009;106:16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R, Morizono H, Wang L, et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo R, Vandenberghe LH, Gao G, et al. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199:381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27:9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal PS, Schulze E, Tran R, et al. Production of adeno-associated virus (AAV) serotypes by transient transfection of HEK293 cell suspension cultures for gene delivery. J Virol Methods. 2014;196:163–173. doi: 10.1016/j.jviromet.2013.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirmule N, Xiao W, Truneh A, et al. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol. 2000;74:2420–2425. doi: 10.1128/jvi.74.5.2420-2425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel Gaudet JMT, Kastelein John. Gene therapy for lipoprotein lipase deficiency. Hyperlipidaemia and cardiovascular disease. 2012;23:310–320. doi: 10.1097/MOL.0b013e3283555a7e. [DOI] [PubMed] [Google Scholar]
- Federici T, Taub JS, Baum GR, et al. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2012;19:852–859. doi: 10.1038/gt.2011.130. [DOI] [PubMed] [Google Scholar]
- Ferreira V, Twisk J, Kwikkers K, et al. Immune responses to intramuscular administration of alipogene tiparvovec (AAV1-LPL(S447X)) in a phase II clinical trial of lipoprotein lipase deficiency gene therapy. Hum Gene Ther. 2014;25:180–188. doi: 10.1089/hum.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Dirosario J, Killedar S, et al. Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood-brain barrier gene delivery. Mol Ther. 2011;19:1025–1033. doi: 10.1038/mt.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH, Alvira MR, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginn SL, Alexander IE, Edelstein ML, et al. Gene therapy clinical trials worldwide to 2012 - an update. J Gene Med. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- Gray SJ, Matagne V, Bachaboina L, et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurda BL, Raupp C, Popa-Wagner R, et al. Mapping a neutralizing epitope onto the capsid of adeno-associated virus serotype 8. J Virol. 2012;86:7739–7751. doi: 10.1128/JVI.00218-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert CL, Miller AD, Mcnamara S, et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum Gene Ther. 2006;17:440–447. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison CE, Weichert WS, Gurda BL, et al. Examining the cross-reactivity and neutralization mechanisms of a panel of mAbs against adeno-associated virus serotypes 1 and 5. J Gen Virol. 2012;93:347–355. doi: 10.1099/vir.0.035113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbut GD, Ziegler RJ, Nietupski JB, et al. Preexisting immunity and low expression in primates highlight translational challenges for liver-directed AAV8-mediated gene therapy. Mol Ther. 2010;18:1983–1994. doi: 10.1038/mt.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuck D, Kern A, Kleinschmidt JA. Development of AAV serotype-specific ELISAs using novel monoclonal antibodies. J Virol Methods. 2007;140:17–24. doi: 10.1016/j.jviromet.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lane MD, Nam HJ, Padron E, et al. Production, purification, crystallization and preliminary X-ray analysis of adeno-associated virus serotype 8. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2005;61:558–561. doi: 10.1107/S1744309105014132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Narkbunnam N, Samulski RJ, et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 2012;19:288–294. doi: 10.1038/gt.2011.90. [DOI] [PubMed] [Google Scholar]
- Liu Q, Huang W, Zhao C, et al. The prevalence of neutralizing antibodies against AAV serotype 1 in healthy subjects in China: implications for gene therapy and vaccines using AAV1 vector. J Med Virol. 2013;85:1550–1556. doi: 10.1002/jmv.23647. [DOI] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- Martin J, Frederick A, Luo Y, et al. Generation and characterization of adeno-associated virus producer cell lines for research and preclinical vector production. Hum Gene Ther Methods. 2013;24:253–269. doi: 10.1089/hgtb.2013.046. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009;66:290–297. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietzsch M, Grasse S, Zurawski C, et al. OneBac: platform for scalable and high-titer production of adeno-associated virus serotype 1–12 vectors for gene therapy. Hum Gene Ther. 2014;25:212–222. doi: 10.1089/hum.2013.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M, Nam HJ, Carter A, et al. Production, purification and preliminary X-ray crystallographic studies of adeno-associated virus serotype 9. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2009;65:715–718. doi: 10.1107/S1744309109021460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, Mcintosh J, et al. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood. 2007;109:1414–1421. doi: 10.1182/blood-2006-03-010181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, Ng CY, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107:2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia. BN Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng R, Govindasamy L, Gurda BL, et al. Structural characterization of the dual glycan binding adeno-associated virus serotype 6. J Virol. 2010;84:12945–12957. doi: 10.1128/JVI.01235-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack A. European Agency Backs Approval of a Gene Therapy. New York: New York Times; 2012. [Google Scholar]
- Sands MS. AAV-mediated liver-directed gene therapy. Methods Mol Biol. 2011;807:141–157. doi: 10.1007/978-1-61779-370-7_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan CD, Jiang H, Liu T, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- Schuster DJ, Dykstra JA, Riedl MS, et al. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat. 2014;8:42. doi: 10.3389/fnana.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BK, Collins SW, Conlon TJ, et al. Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum Gene Ther. 2013;24:630–640. doi: 10.1089/hum.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag F, Kother K, Schmidt K, et al. The assembly-activating protein promotes capsid assembly of different adeno-associated virus serotypes. J Virol. 2011;85:12686–12697. doi: 10.1128/JVI.05359-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampanato C, De Leonibus E, Dama P, et al. Efficacy of a combined intracerebral and systemic gene delivery approach for the treatment of a severe lysosomal storage disorder. Mol Ther. 2011;19:860–869. doi: 10.1038/mt.2010.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Marel S, Comijn EM, Verspaget HW, et al. Neutralizing antibodies against adeno-associated viruses in inflammatory bowel disease patients: implications for gene therapy. Inflamm Bowel Dis. 2011;17:2436–2442. doi: 10.1002/ibd.21673. [DOI] [PubMed] [Google Scholar]
- Veron P, Leborgne C, Monteilhet V, et al. Humoral and cellular capsid-specific immune responses to adeno-associated virus type 1 in randomized healthy donors. J Immunol. 2012;188:6418–6424. doi: 10.4049/jimmunol.1200620. [DOI] [PubMed] [Google Scholar]
- Wang L, Calcedo R, Bell P, et al. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther. 2011;22:1389–1401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki AS, Viljoen A. Alipogene tiparvovec: gene therapy for lipoprotein lipase deficiency. Expert Opin Biol Ther. 2013;13:7–10. doi: 10.1517/14712598.2013.738663. [DOI] [PubMed] [Google Scholar]
- Wistuba A, Weger S, Kern A, et al. Intermediates of adeno-associated virus type 2 assembly: identification of soluble complexes containing Rep and Cap proteins. J Virol. 1995;69:5311–5319. doi: 10.1128/jvi.69.9.5311-5319.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobus CE, Hugle-Dorr B, Girod A, et al. Monoclonal antibodies against the adeno-associated virus type 2 (AAV-2) capsid: epitope mapping and identification of capsid domains involved in AAV-2-cell interaction and neutralization of AAV-2 infection. J Virol. 2000;74:9281–9293. doi: 10.1128/jvi.74.19.9281-9293.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue YQ, Ma BF, Zhao LR, et al. AAV9-mediated erythropoietin gene delivery into the brain protects nigral dopaminergic neurons in a rat model of Parkinson's disease. Gene Ther. 2010;17:83–94. doi: 10.1038/gt.2009.113. [DOI] [PubMed] [Google Scholar]
- Zhang H, Yang B, Mu X, et al. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol Ther. 2011;19:1440–1448. doi: 10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HG, High KA, Wu Q, et al. Genetic analysis of the antibody response to AAV2 and factor IX. Mol Ther. 2005;11:866–874. doi: 10.1016/j.ymthe.2005.02.014. [DOI] [PubMed] [Google Scholar]