Abstract
Type I interferon (IFNα/β)-driven immune responses to acute viral infection are critical to counter replication and prevent dissemination. However, the mechanisms underlying host resistance to herpes simplex virus type 1 (HSV-1) are incompletely understood. Here we show that mice with deficiencies in IFNα/β signaling or STING exhibit exacerbated neurovirulence and atypical lymphotropic dissemination of HSV-1 following ocular infection. Synergy between IFNα/β signaling and efficacy of early adaptive immune responses to HSV-1 were dissected using bone marrow chimeras and adoptive cell transfer approaches to profile clonal expansion, effector function, and recruitment of HSV-specific CD8+ T cells. Lymphotropic viral dissemination was commensurate with abrogated CD8+ T cell responses and pathological alterations of fibroblastic reticular cell (FRC) networks in the draining lymph nodes. Our results show that resistance to HSV-1 in the TG during acute infection is conferred in part by STING and IFNα/β-signaling in both bone marrow-derived and resident cells, which coalesce to support a robust HSV-1-specific CD8+ T cell response.
INTRODUCTION
Type I interferons (IFNα/β) are pleiotropic cytokines with diverse functional roles ranging from innate host defense to immunoregulation (1, 2). Acute viral infections stimulate rapid expression of IFNα/β through various pathogen recognition receptor pathways to induce an antiviral state and prime adaptive immune responses (3). Herpes simplex virus type 1 (HSV-1) is a prototypical, neurotropic member of the herpesvirus family, which includes eight human pathogens (e.g. HSV-2, varicella-zoster virus, Epstein-Barr virus, cytomegalovirus, etc.) that establish chronic infections with varying tissue tropisms and clinical consequences. Clinical manifestations of HSV-1 typically result from viral recrudescence in orofacial mucosal sites innervated by infected neurons within the trigeminal ganglia—the reservoir for HSV-1 latency.
Ocular morbidities arising from herpesvirus infections represent a particularly significant clinical concern, as diagnosis can be challenging (4–6). Herpesviruses are ubiquitous in the human population and often a danger for immunocompromised patients (7), thus identifying the molecular and cellular determinants of host resistance during acute infection could aid in the development of targeted therapies or vaccines.
The cytosolic DNA sensor signaling adaptor protein STING (stimulator of IFN genes) is paramount for host resistance to HSV-1 infection (8–12). However, the function of STING beyond immune surveillance and induction of IFNα/β during HSV-1 infection is incompletely understood. In the current investigation, we sought to identify the contributions of STING and IFNα/β signaling relative to innate and early adaptive immune responses to HSV-1 in the trigeminal ganglia (TG) to further define the host-pathogen interactions that arbitrate the severity of viral pathogenesis.
Animal models of ocular HSV-1 infection have long been utilized to investigate immunity and pathogenesis. We have recently identified the antiviral effector tetherin to be of particular importance in controlling HSV-1 neuroinvasion from the mucosal epithelium to the cornea-innervating TG in a STING-dependent manner (12). Along these lines, we hypothesized that STING counters HSV-1 replication within the TG by upregulating IFNα/β-dependent innate responses. Collectively, our results show that STING is dispensable for IFNα/β-dependent immunosurveillance in the peripheral nervous system, but that IFNα/β-induced innate responses are insufficient to control HSV-1 replication within the TG in the absence of STING. Furthermore, our data corroborate the longstanding principle that CD8+ T cell recruitment is essential for immunologic control of HSV-1 in the peripheral nervous system (13–16). This investigation also dissects the requirements of IFNα/β as a link between innate and adaptive immune responses to HSV-1 due to the established role of IFNα/β in driving optimal CD8+ T cell activation and clonal expansion in other models (2, 17–19). Finally, our investigation explores deficiencies in adaptive immunity resulting from atypical lymphotrophic dissemination of HSV-1 in mice deficient in STING or the IFNα/β receptor α chain (IFNAR1/CD118). Dissemination of virus to secondary lymphoid organs impairs HSV-specific CD8+ T cell responses by driving pathological alterations to the fibroblastic reticular cell (FRC) conduit system (20)—resulting in fewer HSV-specific CD8+ T cells in circulation.
MATERIALS AND METHODS
Mice and virus
C57BL/6 (WT), and STING−/− mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Colonies of CXCL10−/−, CXCR3−/−, CXCL10−/−CXCR3−/− DKO, STING−/−, CD118−/−, and gBT-I.1 mice were maintained in specific pathogen-free vivariums at the Dean McGee Eye Institute (DMEI) or the Rodent Barrier Facility (RBF) on the University of Oklahoma Health Sciences Center (OUHSC) campus (Oklahoma City, OK). CD118−/− and gBT-I.1 mice were backcrossed for four generations to generate gBT-I.1xCD118−/− mice. Progeny genotypes were verified by PCR. Mice were anesthetized via intraperitoneal (IP) injection of ketamine (100 mg/kg) and xylazine (6.6 mg/kg) for all procedures and exsanguinated via cardiac perfusion with 10–15 ml 1xPBS for tissue collection. Experimental procedures were conducted in accordance with protocols approved by Institutional Animal Care and Use Committees at DMEI and OUHSC. A neurovirulent HSV-1 (strain McKrae) was utilized for all experiments; the stock was propagated and maintained as previously described (21).
Infection and plaque assays
Six to twelve week old male and female mice were anesthetized for partial debridement of corneal epithelium with a 25-gauge needle prior to application of virus. Eyes were then blotted to remove the tear film and HSV-1 McKrae topically applied at an inoculum of 1000 plaque forming units (PFU) in 3 μl of saline. All infections were bilateral. Standard plaque assays on confluent CCL-81 Vero cell (ATCC, Manassas, VA) monolayers were utilized to quantify HSV-1 titers in homogenized tissue supernatants as described previously (21).
PCR
RNA was isolated from TG and converted into cDNA as described (9). Relative gene expression was calculated by the standard 2−ΔΔCt method, standardized reference genes, and normalized to WT UI controls following semi-quantitative real time PCR using a CFX Connect thermocycler (Bio-Rad, Hercules, CA). Viral transcripts were amplified using iTaq supermix (Biorad) with real-time primers from Integrated DNA Technologies (Coralville, IA). Primer sequences are listed in Supplemental Table I. PrimePCR technology (Bio-Rad) was utilized for ISG transcript expression studies according to the manufacturer’s instructions. Profiles of transcript expression represented by the cluster image map (or heat map) data supplement were generated using the National Cancer Institute’s CIMminer tool freely accessible online.
Immunoassays
For evaluation of cytokines and growth factors, tissue was harvested at the indicated times pi and homogenized using a Tissue-Tearor homogenizer (Biospec Products, Bartlesville, OK) in 500 μL 1× PBS containing 1× Calbiochem protease inhibitor set 1 (EMD Millipore, Billerica, MA), and supernatants clarified by centrifugation as described (21). ELISAs for IFNα and VEGF were obtained from eBioscience (San Diego, CA) and R&D Systems (Minneapolis, MN), respectively. All other analytes were assayed using Luminex-based multiplex platforms from BioRad (Bio-Plex) or EMD Millipore (Milliplex MAP). Data was transformed to reflect total analyte per mg tissue. For quantification of intracellular signaling proteins, tissues were mechanically dissociated in a Bullet Blender homogenizer (NextAdvance, Averill Park, NY) using 1.7 mL bead lysis tubes containing radioimmunoprecipitation assay lysis buffer supplied with protease inhibitor (Santa Cruz Biotechnology, Dallas, TX) and supplemented with PhosSTOP phosphatase inhibitor cocktail tablets (Roche, Indianapolis, IN) at 2× the suggested concentration. Lysates were incubated in a water-bath sonicator for 10 minutes to dissociate aggregates, supernatants collected following centrifugation at 15,000 × g for 10 minutes, and protein concentrations determined using a Pierce bicinchoninic acid (BCA) assay kit (ThermoFisher Scientific, Pittsburgh, PA). Total and phosphorylated proteins were quantified using Luminex-based Bio-Plex Pro magnetic cell signaling assays (BioRad); data reflect measured fluorescence obtained from 15 μg of sample protein input. All immunoassays were performed according to the manufacturers’ specifications.
Bone marrow chimeras
Chimeric mice were produced as previously described (22). Briefly CD45 congenic WT and CD118−/− mice subjected twice to 600 Gy γ-irradiation at a 4-hour interval. Irradiated mice were subsequently treated with 3×106 CD45 congenic bone marrow cells (BMC) intravenously to reconstitute the hematopoietic compartment. Ten weeks later, BMC grafts were verified by analysis of leukocytes in the blood, which showed a greater than 90% donor BMC composition relative to the CD45 congenic recipient allele. See Fig. 3 A for a schematic.
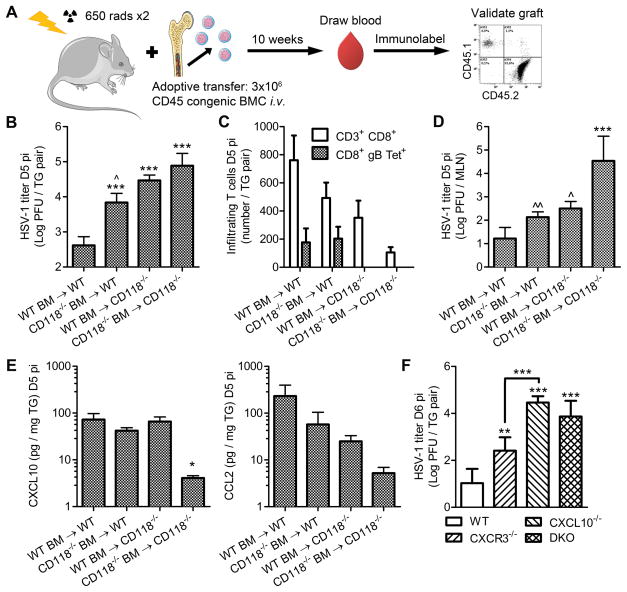
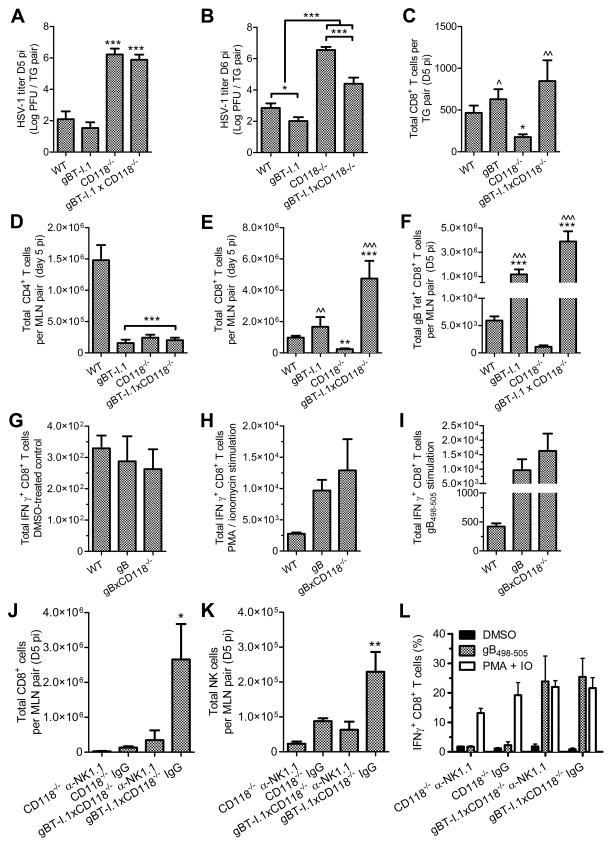
Figure 3. Resident and bone marrow-derived contributions of IFNα/β signaling.
(A) Schematic outlining generation of bone marrow (BM) chimeras using WT and CD118−/− mice as reciprocal or common donors and recipients. Figure was prepared using Servier Medical Art freely accessible on the public domain (www.servier.com) under a Creative Commons Attribution 3.0 Unported License. (B) Viral titer in the TG (n = 8–14/group; 3 independent experiments). (C) TG-infiltrating CD3+ T cells measured by flow cytometry depicting the total CD8+ population and a virus specific subset determined by MHC class I tetramer labeling for the immunodominant epitope of glycoprotein B (gB498-505; n = 4–12/group; 2–3 independent experiments). (D) Viral titer in the MLN (n = 5–12/group; 2–3 independent experiments). (E) Concentrations of CXCL10 and CCL2 in the TG (n = 6–12/group; 3 independent experiments). (F) Viral titer in the TG of WT, CXCL10−/−, CXCR3−/−, and CXCL10−/−CXCR3−/− double knock out (DKO) mice at day 6 pi (n = 3–11/group; 2–3 independent experiments). Samples were analyzed on a Beckman Coulter Epics XL flow cytometer; see (16) for gating strategies. Stastical differences were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests; significance thresholds are as follows: p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***; * and ^ reflect differences from WT→WT and CD118−/−→CD118−/−, respectively unless indicated otherwise. Bars represent mean ± SEM.
Cell isolation and adoptive transfer
For adoptive transfer experiments, CD8+ T cells were obtained from single cell suspensions of secondary lymphoid organs of naive or infected T cell receptor (TCR)-transgenic gBT-I.1 mice by MACS immunomagnetic isolation (Miltenyi Biotech, Auburn, CA). Isolated cells were incubated in 1 μM CFSE (eBioscience), washed, and 3×106 cells injected intravenously into recipients retro-orbitally without irradiation. Purities of immunomagnetically-enriched cells were evaluated by flow cytometry and found to be greater than 80% CD3+ CD8+ double positive cells relative to the total CD45+ population.
Flow cytometry
All tissues were dissociated in RPMI 1640 media supplemented with 10% heat-inactivated FBS, 10% heat-inactivated FBS, 1× antibiotic/antimycotic, and 10 μg/mL gentamicin (Gibco), i.e. ‘complete media.’ Lymph nodes were macerated into single cell suspensions over 40 μm mesh. TG specimens were mechanically dissociated using a Dounce homogenizer (ThermoFisher Scientific) and filtered through 40 μm mesh. For analysis of circulating leukocytes, 100 μL of blood was collected from the superficial temporal facial vein, mixed with 5 μL 0.5 M EDTA to prevent coagulation, and treated twice for two minutes with 1.0 mL lysing buffer (150 mM ammonium chloride, 10 mM postassium bicarbonate, 0.1 mM EDTA), and resuspended in complete media. Antibodies for flow cytometry were purchased from eBioscience, BD Biosciences (San Jose, CA), and Tonbo Biosciences (San Diego, CA). Mouse MHC Class I Tetramer [K(b)-SSIEFARL, HSV-1 gB498-505] was provided by the NIH Tetramer Core Facility (Atlanta, GA) and incubated with samples prior to the addition of antibody. Cells were incubated with anti-CD16/32 Fc block for 10 minutes prior to the addition of other antibodies. For surface antigen labeling and wash steps, cells were incubated with antibody in ‘wash buffer’ (1% bovine serum albumin in 1× PBS) for 30 minutes, washed twice, fixed in phosphate-buffered 1% paraformaldehyde, and resuspended in wash buffer for analysis. Intracellular immunolabeling was conducted using Cytofix/Cytoperm fixation permeabeabilization solution and Perm/Wash buffer (BD Biosciences) following surface labeling for functional assays. Lymphocyte viability was evaluated using the Miltenyi Biotec Annexin V-FITC kit according to the manufacturer’s directions. Samples were analyzed on a MACSQuant-10 flow cytometer with MacsQuantify software (Miltenyi Biotec) unless indicated otherwise. Flow plots substantiating gating strategies are shown in Supplemental Fig. 1.
T cell functional assays
Spleens were teased and filtered into single cell suspensions, subjected to 0.84% ammonium chloride for 2 minutes twice to lyse red cells, washed, and resuspended in complete media. For functional assays, 1×106 splenocytes were cultured in 1.0 mL complete media for 3 hours at 37°C, 5.0% CO2 in the presence of DMSO as a vehicle control, 2.0 μg gB498-505 peptide, or 50.0 ng PMA and 800 ng ionomycin as previously shown (21). After 1 hour in culture, 0.67 uL GolgiStop protein transport inhibitor (BD Biosciences) was added to facilitate intracellular IFNγ or IL-17 immunolabeling. Cells were fixed and labeled after a total of 4 hours in culture.
Lymphocyte depletion
Cell-specific or isotype control antibodies (Bio X Cell, West Lebanon, NH) were injected intraperitoneally for systemic delivery. For NK cell depletion, 300 μg of anti-NK.1.1 or IgG control antibody was injected one day before and after HSV-1 infection as reported by (23). For CD8+ T cell depletion, 200 μg of anti-CD8a or IgG control antibody was injected on days 3 and 5 pi. Depletion efficiency was confirmed by flow cytometry in tissues of interest.
Histology and microscopy
Lymph nodes harvested from HSV-1 infected mice and uninfected controls were fixed in Lymph-ID (BioSafe Supplies, LLC, Orlando, FL) prior to paraffin embedding and sectioning. Five-micron MLN sections were cut and mounted on glass slides. Hematoxylin and eosin (H&E) staining and a Modified Gomori’s Reticulum stain (StatLab, Baltimore, MD) were performed on sequential serial sections. Prepared slides were imaged on a Nikon E400 microscope outfitted with a 40× objective lens using MetaVue imaging software (Nikon, Melville, NY).
Statistical analysis
Normal distribution was assumed a priori for all data sets. Graphpad Prism 5 was used for statistical analysis. All data reflect mean ± SEM. One-way ANOVAs with Student-Newman-Keuls multiple comparisons tests or two-way ANOVAs with Bonferroni posttests were employed to assess data with one or two independent variables, respectively. Significance thresholds for each comparison are denoted as follows: p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***.
RESULTS
Loss of STING enhances HSV-1 neuropathogenesis independent of canonical IFNα/β responses
The neurovirulence of HSV-1 is determined in part by the efficiency of neuroinvasion and subsequent replicative proficiency within neuronal ganglia. Therefore, to investigate our hypothesis that STING is essential for host resistance to HSV-1 in the TG, we assessed viral titers and lytic gene expression following ocular HSV-1 infection of wild type (WT), STING deficient (STING−/−), and type I IFN receptor α chain deficient (CD118−/−) mice. By day 5 post-infection (pi), titers of infectious virus in the TG of STING−/− and CD118−/− mice were substantially higher than their WT counterparts (Fig. 1 A). We have previously shown that STING−/− and CD118−/− mice harbor more virus in the TG at day 3 pi than WT (12). Real-time PCR was performed on viral transcripts representing all three temporally regulated HSV-1 lytic gene classes to distinguish whether the increased viral burden was merely due to enhanced neuroinvasion from the infected cornea or compounded by efficient replication within the TG. Excised ganglia from each experimental group were analyzed for expression of infected cell protein 0 (ICP0), thymidine kinase (TK), and glycoprotein B (gB) as representatives of immediate-early (IE), early (E), and late (L) viral gene products, respectively (Supplemental Table I). Viral gene expression was more than 10-times greater in TG from STING−/− and CD118−/− mice relative to WT (Fig. 1 B). Thus, the elevated viral burden observed in STING−/− mice resulted not only from enhanced neuroinvasion (11, 12) but was augmented by efficient viral replication within the TG.
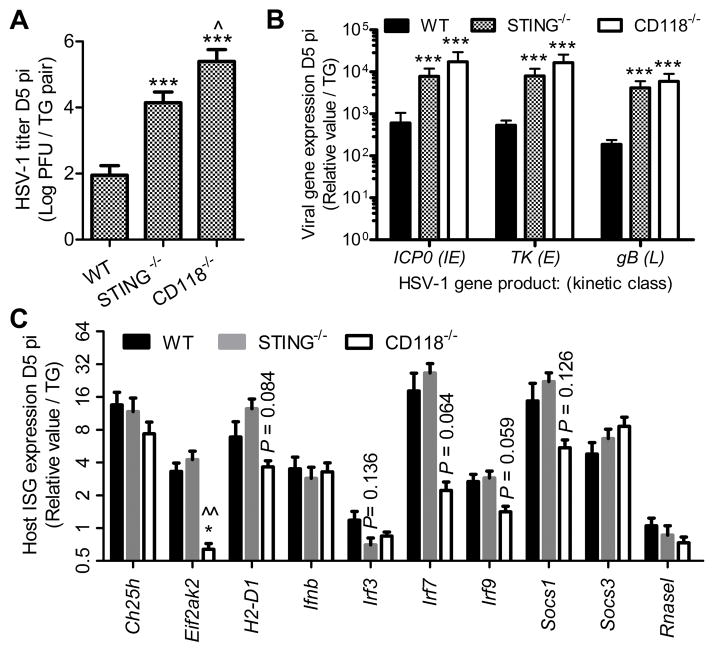
Figure 1. Roles of STING and IFNα/β in controlling HSV-1 in the TG.
(A) Viral titer at day 5 pi (n = 7–9 samples/group; 3 independent experiments). (B) Relative expression of viral genes including infected cell protein 0 (ICP0), thymidine kinase (TK), and glycoprotein B (gB) as representatives of immediate early (IE), early (E), and late (L) viral genes which mediate lytic commitment, DNA replication, and nascent virion assembly, respectively. Values are relative to phosphoglycerate kinase 1 (PGK1) expression and standardized to UI controls (n = 7–11 samples/group; 3 independent experiments). (C) Relative expression of interferon stimulated genes (ISG) in the TG including cholesterol 25-hydroxylase (Ch25h), eukaryotic translation initiation factor 2-alpha kinase 2 (Eif2ak2), histocompatibility 2, D region locus 1/MHC class I (H2-D1), IFN beta (Ifnb), IFN regulatory factor 3/7/9 (IRF3/7/9), and suppressor of cytokine signaling 1/3 (Socs1/3). Values are relative to the geometric mean of Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and TATA box binding protein (Tbp) expression and normalized to UI controls (n = 8–12 samples/group; 3 independent experiments). Stastical differences were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests; significance thresholds are as follows: p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***; * and ^ reflect differences from WT and STING−/−, respectively. Bars represent mean ± SEM. A heat map of gene expression data from Fig. 1B & C is shown in Supplemental Fig. 2.
Host resistance to acute HSV-1 infection is predominantly thought to be dependent upon induction of IFNα/β followed by transcriptional activation of interferon-stimulated genes (ISG), and generation of cell-mediated immunity. A PCR-based ISG array including antiviral effectors, positive regulators, and feedback inhibitors was utilized to substantiate whether enhanced viral replication in TG from STING−/− mice corresponded to diminished ISG responses. TG specimens from STING−/− mice exhibited an ISG profile remarkably similar to WT (Fig. 1C, Supplemental Fig. 2) despite the amplified HSV-1 neurovirulence. Furthermore, STING-dependent inflections in suppressor of cytokine signaling-1 (SOCS-1) and SOCS-3 expression previously reported in bone marrow-derived macrophages (24) were not observed in the TG during HSV-1 infection (Fig. 1 C). Collectively, our data show that STING-independent innate sensing pathways compensate for IFNα/β responses in the TG during HSV-1 infection, but that ISG induction alone is insufficient to suppress viral replication.
Multiple studies have confirmed the importance of canonical IFN signaling through STAT1 with respect to ISG-mediated resistance to HSV-1 in the TG (25–27). Ancillary IFN signaling also propagates physiological changes through STAT1-independent mediators such as the mitogen-activated protein kinase (MAPK), extracellular signal-regulated kinase (ERK), and protein kinase B (PKB/AKT) pathways to elicit antiviral defenses (28–31). Though these signaling pathways are also active in healthy nerve ganglia, modulation of such responses is likely important for control of HSV-1 in the peripheral nervous system. For example, IFNα/β/γ-dependent STAT1 phosphorylation is reportedly subdued in neurons relative to mitotically-active cells as a measure of protection against cytotoxicity (31, 32).
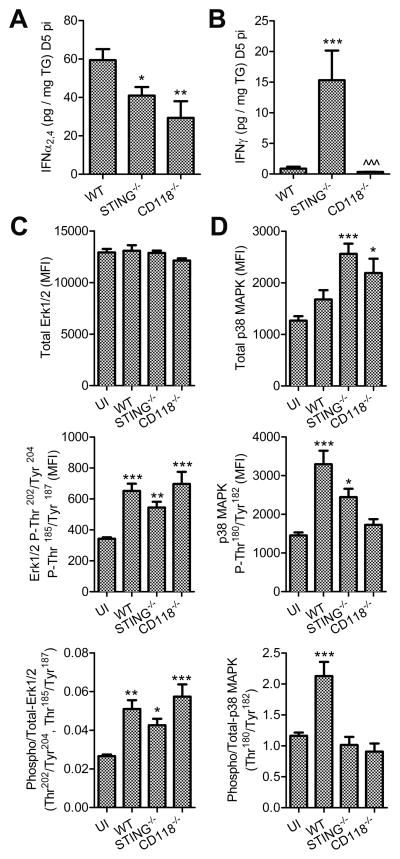
To further analyze IFN-related signal transduction in TG from STING−/− mice during HSV-1 infection, concentrations of IFNα and IFNγ were evaluated along with phosphorylation of ancillary downstream signaling mediators at day 5 pi in TG homogenates (Fig. 2). Briefly, IFNα protein levels were higher in WT than STING−/− samples, while the concentration of IFNγ was elevated in STING−/− mice relative to WT (Fig. 2, A and B). Though IFNγ-induced neuroprotective signaling through Erk1/2 has been described (31), Erk1/2 phosphorylation was increased in TG from all experimental groups pursuant to HSV-1 infection irrespective of measured IFN ligand availability (Fig. 2 C). No changes in AKT phosphorylation were detected in parallel (data not shown). However, efficient phosphorylation of p38 MAPK was only observed in WT samples in terms of phosphorylated-to-total protein ratios (Fig. 2 D). Multiple studies have reported protective mechanisms for IFN- and stress-associated p38 MAPK signal transduction including regulation of the balance between apoptosis and autophagy (33–35). Autophagy was recently inferred to be an important STING-dependent protective countermeasure against HSV-1 in the nervous system (11). Here, susceptibility to HSV-1 neuropathogenesis in the TG of STING−/− mice was essentially independent of local ISG induction through canonical IFN-signaling pathways. Taken together, ancillary STAT1-independent IFN-signal transduction involving p38 MAPK may confer protection against HSV-1 in the TG through an unknown mechanism possibly involving autophagy—as reflected in other models (11, 34, 36). Furthermore, the early contributions of CD8+ T cells with respect to control of HSV-1 remained to be explored in STING−/− mice.
Figure 2. IFN signal transduction.
Protein concentrations of IFNα2,4 (A) and IFNγ (B) in TG at day 5 pi (n = 6–20/group; 2–5 independent experiments). Total and phosphorylated Erk1/2 (C), and p38 MAPK (D) protein in TG measured by suspension array on tissue homogenates (n = 8–11/group; 3 independent experiments). Values reflect median fluorescence intensity (MFI). The phosphorylated to total ratios are also shown. Stastical differences were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests (A, B) or with Dunnetts multiple comparisons tests compring experimental groups to UI controls (C–E); significance thresholds are as follows: p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***; For A & B, * and ^ reflect differences from WT and STING−/−, respectively; * indicates differences from UI for C–E. Bars represent mean ± SEM.
IFNα/β-signaling in resident & bone marrow-derived cells contributes to resistance against HSV-1 neuropathogenesis via CD8+ T cell recruitment
Bone marrow (BM) chimeras were generated (Fig. 3 A) in an effort to dissect the contributions of IFNα/β-signalling in resident and BM-derived cells with respect to host resistance to HSV-1 at day 5 pi. Intact IFNα/β-signaling was required in both resident and BM-derived cells for optimal resistance to HSV-1 in terms of viral burden in the TG (Fig. 3 B). However, loss of IFNα/β signaling in the resident cell population (i.e. CD118−/− recipients) compromised recruitment of gB498-505-specific CD8+ T cells to the TG (Supplemental Figure 1, Fig. 3 C), reflecting the immunodominant CD8+ T cell epitope of HSV-1 in C57BL/6 mice based on MHC class I tetramer labeling (37, 38). Neurotropism is not the exclusive route of HSV-1 dissemination, as infectious virus is also detectable in the draining mandibular lymph nodes (MLN; Fig. 3 D) through a mechanism involving hematogenous and/or lymphatic spread where it can disrupt lymph node integrity and generation of effective T cell responses (22, 39). Consistent with this notion, total CD8+ T cell infiltration into the TG of BM-chimeric mice was inversely proportional to the amount of virus recovered from the MLN (Fig. 3, C and D). Upwards of 80% of the CD8+ T cell infiltrate in the TG during the early stages of acute HSV-1 infection responds to known viral epitopes, and 50% are specific for the gB498-505 epitope (38). However, 10–20% of the CD8+ T cells reflect bystander-activated cells that do not target HSV-1 based on analysis of TCR specificity for known epitopes and observations of OVA-specific transgenic T cells within HSV-1 infected TG in co-transfer experiments (38, 40). The phenomenon of cytokine-driven bystander CD8+ T cell activation is well documented in other contexts (41–43).
Concentrations of IFN-driven chemokines associated with recruitment of effector CD8+ T cells (16, 44–46), specifically C-X-C motif chemokine ligand 10 (CXCL10) and C-C motif chemokine ligand 2 (CCL2), were produced in the TG by resident cells and/or BM-derived cells (Fig. 3 E) in a compensatory manner largely dependent upon IFNα/β signaling in one component or the other. However, no apparent differences in CXCL1 or CCL5 concentrations were observed among experimental groups (data not shown). To further substantiate the importance of CXCL10, viral titers in the TG were compared in mice deficient in CXCL10, its receptor CXCR3, or both. Global loss of CXCR3 had a modest effect on viral burden in the TG; however, CXCL10−/− mice exhibited a magnified susceptibility to HSV-1 in the TG that was paralleled in double-knockout (DKO) mice (Fig. 3 F). Taken together, these data support a paradigm in which IFNα/β signaling in tissue-resident and BM-derived cell populations is important for countering viral dissemination, preserving early adaptive CD8+ T cell responses, and facilitating lymphocyte recruitment to infected tissues.
IFNα/β-signaling is dispensable in CD8+ T cells without a concomitant loss in effector function against HSV-1
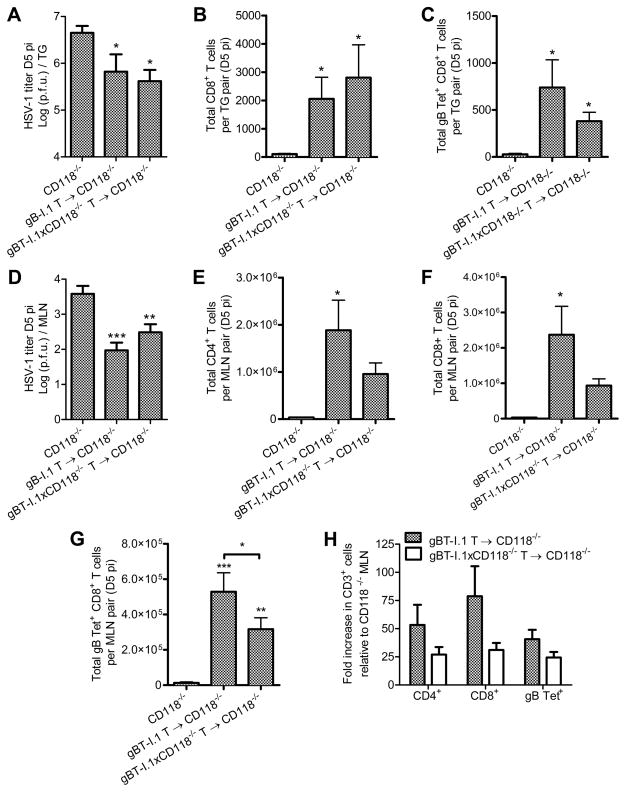
T cell receptor (TCR) transgenic mice (gBT-I.1) in which nearly all CD8+ T cells recognize the gB498-505 epitope of HSV-1 (47) were back-crossed with CD118−/− mice (gBT-I.1xCD118−/−) to further identify the impact of IFNα/β signaling in CD8+ T cells relative to early immunologic control of HSV-1 in the TG. Adoptive transfer of naive HSV-1 specific CD8+ T cells isolated from gBT-I.1 or gBT-I.1xCD118−/− mice into virally infected CD118−/− mice corroborated the importance of CD8+ T cell responses with respect to IFNα/β-independent mechanisms of protection against acute HSV-1 infection (Fig. 4). Specifically, addition of HSV-specific CD8+ T cells into highly immunocompromised CD118−/− mice lowered virus titers in the TG approximately 10-fold (Fig. 4 A) correlative with a restoration of T cell infiltration into the TG (Fig. 4, B and C). This treatment also lowered viral titers in the MLN (Fig. 4 D) and restored T cell populations therein (Fig. 4, E–H) independent of IFNα/β-signaling in the exogenous CD8+ T cells. Furthermore, recovery of T cell responses in recipient mice (Fig. 4 H) was principally attributed to restoration of the endogenous host immune response, as the majority of T cells contained in the MLN or infiltrating into the TG of recipient mice were not exogenous CFSE-labeled TCR-transgenic cells which co-label with the gB498-505 tetramer (Fig. 4, B–C and F–G; quantification of CFSE+ cells not shown).
Figure 4. Adoptive transfer of gBT-I.1 CD8+ T cells into CD118−/− mice.
(A) Viral titer in the TG of CD118−/− mice or CD118−/− mice receiving 3×106 CD8+ T cells intravenously (IV) from gBT-I.1 or gBT-I.1xCD118−/− mice 24 hours pi; titers reflect day 5 pi (n = 9–14/group; 3–4 independent experiments). (B) TG-infiltrating CD8+ T cells and (C) HSV-1 specific CD8+ T cells by tetramer labeling (n = 5–6/group; 2 independent experiments). (D) Viral titer in the MLN (n = 17–19/group; 4 independent experiments). (E) Total CD4+ T cells, (F) CD8+ T cells, and (G) HSV-1 specific CD8+ T cells by tetramer labeling in MLN pairs (n = 5–8/group; 3 independent experiments). (H) Fold change in T cell numbers in the MLN relative to CD118−/− mice not receiving CD8+ T cells from gBT-I.1 or gBT-I.1xCD118−/− mice. See Supplmental Fig. 1 for flow cytometry gating strategies. Stastical differences were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests; significance thresholds are as follows: p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***; * reflects differences from CD118−/− controls unless indicated otherwise. Bars represent mean ± SEM.
Direct infection of TCR-transgenic mice reflected a delayed yet important contribution of CD8+ T cells with respect to HSV-1 titers in the TG among CD118-sufficient and -deficient mice (Fig. 5, A–C). Virus was not detected in the MLN of WT or gBT-I.1 mice at day 5 pi. However, HSV-1 disseminated to the MLN in gBT-I.1xCD118−/− and CD118−/− mice by day 5 pi with 10- to 100- fold higher titers in the CD118−/− mice (Fig. 4 D, data not shown). As previously noted (47), TCR transgenic mice exhibit deficiencies in the total numbers of CD4+ T cells (Fig. 5 D). Consistent with the observed reduction in viral burden, CD8+ T cell responses were restored in the MLN of gBT-I.1xCD118−/− mice compared to CD118−/− mice (Fig. 5, E and F). Analysis of IFNγ production in TCR-transgenic CD8+ T cells following in vitro stimulation with PMA and ionomycin or SSIEFARL peptide corresponding to the gB498-505 epitope indicated no functional abnormalities (Fig. 5, G–I).
Figure 5. Direct infection of gBT-I.1 and gBT-I.1xCD118−/− mice.
(A) Viral titer in the TG at day 5 pi (n = 6–7 mice/group; 3 independent experiments). (B) Viral titer in the TG at day 6 pi (n = 9–11 mice/group; 3–4 independent experiments). (C) Numbers of CD8+ T cells infiltrating into the TG at day 5 pi (n = 7–17 mice/group; 3–5 independent experiments). Total numbers of CD4+ (D), CD8+ (E), and HSV-1 specific CD8+ T cells by tetramer labeling for gB498-505 (F) in the MLN at day 5 pi (n = 6–28 mice/group; 2–6 independent experiments). T cell responses in splenocyte cultures at day 5 pi showing basal IFNγ production following exposure to the DMSO vehicle control (G), IFNγ production following PMA and ionomycin stimulation (H), or IFNγ production following exposure to SSIEFARL peptide (I) corresponding to the gB498-505 epitope of HSV-1 (n = 5–6 mice per group; 2 independent experiments). (J) Numbers of NK cells in the MLN following injection of NK1.1-specific depleting antibody or IgG isotype control antibody. (K) Total CD8+ T cells in the MLN at day 5 pi following NK depletion. (L) Functional responses of CD8+ T cells in splenocyte cultures as described above. For J–L, n = 4 mice/group; 2 independent experiments). See Supplmental Fig. 1 for flow cytometry gating strategies. Stastical differences were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests; significance thresholds are as follows: p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***; For A–F, * and ^ reflect differences from WT and CD118−/−, respectively, while * indicates differences from all other groups for J–L. Bars represent mean ± SEM.
Elevated NK cell counts were observed in the MLN of the gBT-I.1xCD118−/− animals relative to their CD118−/− counterparts (data not shown). NK cell-mediated fratricide of CD8+ T cells in secondary lymphoid organs is observed in models of LCMV infection in the absence of IFNα/β signaling, such that NK cell depletion enhances the number of LCMV-specific CD8+ T cells (23, 48). We sought to identify the impact of NK cells on CD8+ T cell fratricide in CD118−/− and gBxCD118−/− mice following HSV-1 dissemination to the MLN. In contrast to LCMV models, NK cell depletion did not enhance the total number or affect the functional capacity of CD8+ T cells in CD118−/− or gBTxCD118−/− mice infected with HSV-1 (Fig. 5, J–L). Rather, depletion of NK cells reduced the number of HSV-specific CD8+ T cells. This data is consistent with established findings showing that NK cells facilitate HSV-1 containment to reduce viral dissemination (49, 50). These results also support another report suggesting that NK cell depletion does not enhance CD8+ T cell responses to adenovirus in the absence of IFNα/β signaling (51).
CD8+ T cell responses are distorted in HSV-1 infected STING−/− mice
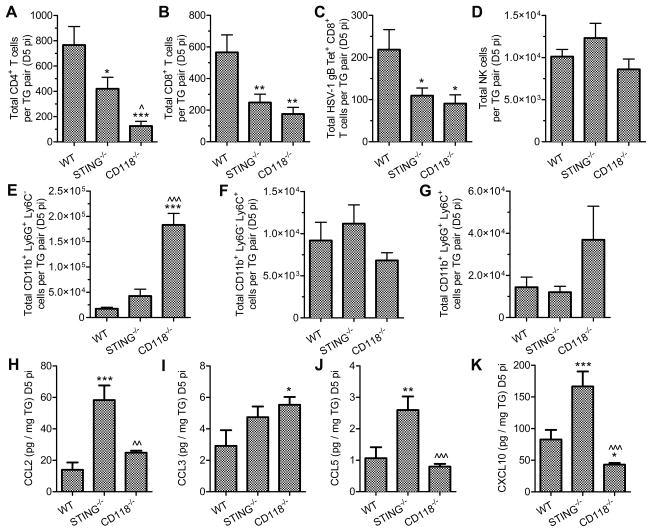
Since host resistance in STING−/− mice was lost despite preservation of IFNα/β signaling within the TG, we investigated early adaptive and cellular innate immune responses in these animals. Infiltration of CD4+, CD8+, and gB498-505-tetramer+ CD8+ T cells into the TG was reduced in STING−/− mice relative to WT such that the numbers of TG-infiltrating cells in STING−/− mice were indistinguishable from that observed in the highly immunocompromised CD118−/− mice (Fig. 6, A–C). However, NK cell infiltration was not impacted by deficiencies in STING or CD118 mice (Fig. 6 D). Patterns in TG-infiltrating CD11b+ myeloid cells produced several noteworthy observations but were not associated with the susceptibility of STING−/− mice. Consistent with the reported repressive role of IFNα/β signaling (52), neutrophil infiltration was limited in TG from STING−/− mice (Fig. 6 E) despite a high viral burden (Fig. 1 A). Additionally, the phenotypes of TG-infiltrating monocytes diverged from what is seen in mucosal tissues in CD118−/− mice. Specifically, Ly6G− Ly6C+ inflammatory monocytes are virtually absent from the corneas and lungs of HSV-1 and influenza infected CD118−/− mice, respectively (12, 53, 54). In contrast, inflammatory monocytes were abundant in the TG of CD118−/− mice following HSV-1 infection (Fig. 6 F). However, there were no significant differences in total numbers of infiltrating monocyte populations among TG specimens from WT, STING−/−, or CD118−/− mice (Fig. 6, F and G). Distinct anatomical host niches differ in profiles of neutrophil recruitment and monocyte differentiation during inflammation and infection, as indicated above comparing cellular innate responses in nervous and mucosal tissue and validated by others (52, 55, 56).
Figure 6. Leukocyte infiltration in TG from STING−/− mice.
Lymphoid infiltrate in the TG at day 5 pi including (A) CD4+ T cells, (B) CD8+ T cells, (C) gB498-505 tetramer+ CD8+ T cells, and (D) NK cells (n = 8–15 mice per group; 3–5 independent experiments). Myeloid cell infiltrate in the TG at day 5 pi including (E) neutrophils, (F) Ly6C+ Ly6G− monocytes, and (G) Ly6C+ Ly6G+ monocytes (n = 7–12 mice per group; 3–4 independent experiments). T cell chemoattractant concentrations in the TG at day 5 pi including (H) CCL2, (I) CCL3, (J) CCL5, and (K) CXCL10 (n = 8–20 TG per group; 3 independent experiments). See Supplmental Fig. 1 for flow cytometry gating strategies. Stastical differences were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests; significance thresholds are as follows: p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***; * and ^ reflect differences from WT and STING−/−, respectively. Bars represent mean ± SEM.
Because differences in viral burden in the TG of STING−/− mice could not be readily interpreted as a consequence of overt deficits in IFNα/β signaling or cellular innate responses, the observed defect in CD8+ T cell infiltration into the TG of STING−/− mice was explored further. Chemokines associated with CD8+ T cell recruitment were analyzed in the TG at day 5 pi (Fig. 6, H–K). Concentrations of CCL2, CCL5, and CXCL10 were significantly elevated in TG from STING−/− mice relative to WT (Fig. 6, H,J,K); thus, the availability of chemotactic factors in the TG microenvironment did not substantiate the dearth of CD8+ T cell infiltration in these animals.
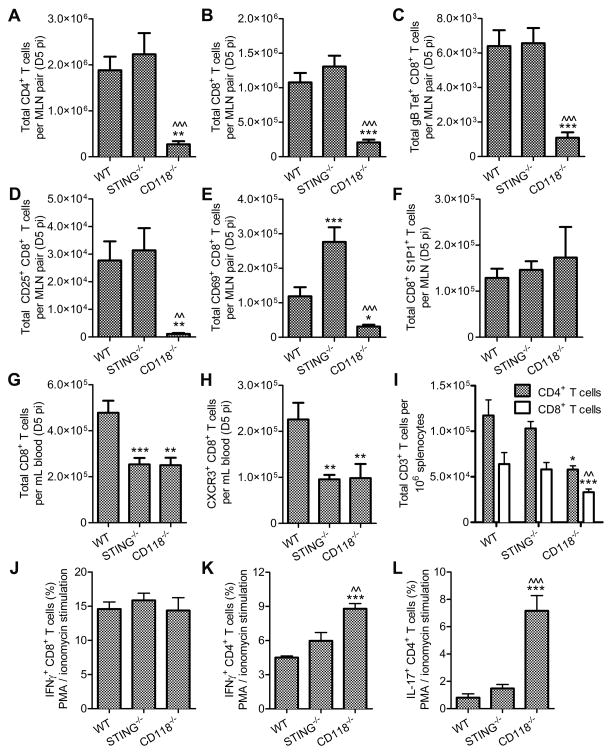
T cell responses to HSV-1 infection have not been described in STING−/− mice despite their elevated susceptibility to multiple routes of infection (8, 11). Immune responses were consequently evaluated to compare profiles of T cell generation, circulation, and function in WT, STING−/−, and CD118−/− mice. Flow cytometry-based analysis of T cell populations in the MLN revealed that CD4+, CD8+, and gB498-505-tetramer+ CD8+ T cells in STING−/− mice expand identically to WT following infection (Fig. 7, A–C). In contrast, T cells precipitously decline in the MLN of HSV-1-infected CD118−/− mice concomitant with viral dissemination to the MLN (39) as was observed in the present investigation. Further phenotypic characterization of STING−/− CD8+ T cells in the MLN revealed proficient activation as determined by up-regulation of the IL-2 receptor CD25 (Fig. 7 D), yet a two-fold increase in proliferating CD69+ CD8+ T cells relative to WT (Fig. 7 E). No differences were noted in sphingosine-1-phosphate receptor 1 (S1P1)+ CD8+ T cells as a transient marker of lymph node egress (Fig. 7 F). In addition, a 2- to 3- fold increase in CD44high CD62Llow effector memory CD8+ T cells was observed in MLN of STING−/− relative to WT with no observable differences in the CD44high CD62Lhigh central memory population (data not shown). However, the number of circulating CD8+ T cells in the blood were substantially lower in STING−/− and CD118−/− mice compared to WT (Fig. 7 G), with a similar trend in activated CXCR3+ CD8+ T cells (Fig. 7 H). Approximately 50% of the circulating CXCR3+ CD8+ T cells co-expressed high levels of CD44 in WT and STING−/− mice, though only 30% of the CXCR3+ CD8+ T cells in CD118−/− exhibited the same phenotype (data not shown).
Figure 7. T cell generation, mobilization, and function.
Lymphocyte profile in the MLN including (A) CD4+ T cells, (B) CD8+ T cells, and (C) gB498-505 tetramer+ CD8+ T cells at day 5 pi (n = 12–22 mice per group; 3–6 independent experiments). Profile of CD8+ T cells in the MLN expressing (D) CD25, (E) CD69, and (F) sphingosine-1-phosphate receptor 1 (S1P1) to evaluate activation, proliferation, and egress, respectively at day 5 pi (n = 6–16 mice per group; 3–4 independent experiments). (G) Total number of CD8+ T cells and (H) activated CXCR3+ CD8+ T cells in the peripheral circulation at day 5 pi (n = 9–16 mice per group; 4–5 independent experiments). Functional analysis of T cells in mixed splenocyte cultures at day 5 pi showing (I) T cell ratios, IFNγ production in (J) CD8+ and (K) CD4+ T cells over background in PMA and ionomycin-stimulated cultures, (L) IL-17 production in stimulated CD4+ T cells over background (n = 6–11 mice per group; 3–4 independent experiments). See Supplmental Fig. 1 for flow cytometry gating strategies. Stastical differences were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests; significance thresholds are as follows: p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***; * and ^ reflect differences from WT and STING−/−, respectively. Bars represent mean ± SEM.
In contrast to T cell responses observed in DNA plasmid-vaccinated and tumor-bearing STING−/− mice (8, 57), in vitro stimulation revealed no intrinsic defect in the ability of CD4+ or CD8+ T cells recovered from HSV-1 infected STING−/− mice to produce IFNγ in mixed splenocyte cultures containing WT-equivalent T cell frequencies (Fig. 7 I) following PMA and ionomycin stimulation (Fig. 7, J and K). However, it was noted that CD4+ T cells obtained from HSV-1 infected CD118−/− mice were hyper-responsive with respect to IFNγ and IL-17 production (Fig. 7, K and L). Furthermore, TCR-dependent IFNγ production by CD8+ T cells did not differ between WT and STING−/− splenocyte cultures pulsed with SSIEFARL peptide corresponding to the gB498-505 epitope of HSV-1 (data not shown).
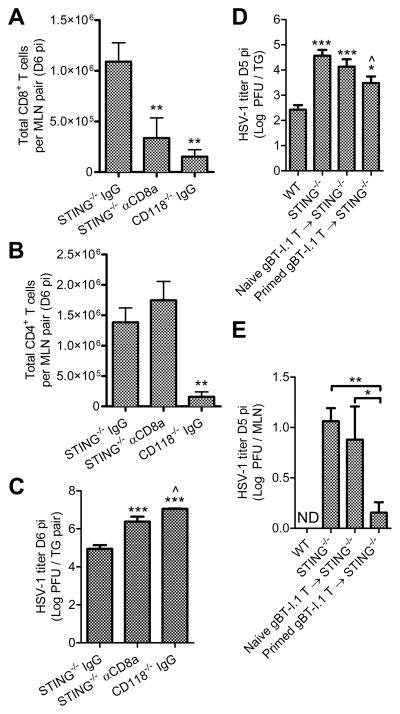
Because CD8+ T cells were functional in STING−/− mice, systemic depletion of CD8+ T cells was pursued to determine whether the reduced number of responding CD8+ T cells in the TG impacted HSV-1 titers. Antibody-mediated CD8+ T cell depletion was effective based on cell counts in the MLN reaching levels comparable to the CD118−/− mice, yet the CD4+ T cell population was unaffected (Fig. 8A, B). Relative to IgG isotype control-treated STING−/− mice, CD8+ T cell-depleted STING−/− mice displayed an increase in viral titer in the TG comparable to CD118−/− mice (Fig. 8 C).
Figure 8. Augmentation of CD8+ T cells in STING−/− mice.
Antibody-mediated depletion of CD8+ T cells by administration of 200 μg anti-CD8a or IgG isotype control antibody at days 3 and 5 pi showing (A) CD8+ and (B) CD4+ T cells in the MLN at day 6 pi, (C) HSV-1 titers in the TG following CD8+ T cell depletion (n = 5–7 mice/group; 3 independent experiments). Adoptive transfer of 3×106 naive CD8+ T cells isolated from uninfected gBT-I.1 mice or primed CD8+ T cells isolated from HSV-1 infected gBT-I.1 mice and injected IV into STING−/− mice 24 hours post infection showing impact on viral titers in the (D) TG and (E) MLN at day 5 pi relative to WT controls (n = 6–12 mice per group; 3–5 independent experiments; ND, not detected). See Supplmental Fig. 1 for flow cytometry gating strategies. Stastical differences were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests; significance thresholds are as follows: p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***; for A–C * and ^ reflect differences from STING−/− IgG and STING−/− αCD8a, respectively. In panels D–E, * and ^ reflect differences from WT and STING−/− controls, respectively, unless indicated otherwise. Bars represent mean ± SEM.
T cell complementation studies were subsequently explored in STING−/− mice. Adoptive transfer of CD8+ T cells from naive gBT-I.1 mice into STING−/− mice had no effect on HSV-1 titers in the TG by day 5 pi (Fig. 8 D) despite seeding of exogenous T cells in the MLN (data not shown). However, adoptive transfer of in vivo-activated (i.e. ‘primed’) CD8+ T cells from HSV-1 infected gBT-I.1 mice into STING−/− mice led to a 10-fold reduction of HSV-1 titers in the TG relative to STING−/− controls (Fig. 8 D). Consistent with this result, adoptive transfer of primed, but not naive, gBT-I.1 CD8+ T cells also reduced viral titers in the MLN (Fig. 8 E). Collectively, our results substantiate the importance of early responding CD8+ T cells in the peripheral nervous system to control primary acute HSV-1 infection and suggest that the MLN microenvironment is affected by viral dissemination such that mobilization of recently activated CD8+ T cells is compromised.
HSV-1 affects T cell responses in the MLN by modulating the supportive network of fibroblastic reticular cells
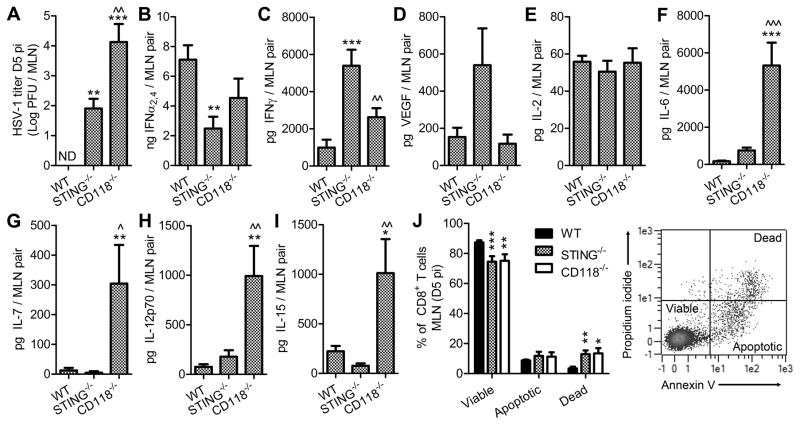
MLN were surveyed for HSV-1 titers and cytokines or growth factors associated with T cell survival to identify whether the discrepancy between the numbers of CD8+ T cells in the MLN and in circulation in STING−/− mice were elicited by pathological alterations within the MLN following viral dissemination, as has been reported in CD118−/− mice (22, 39). Specifically, we hypothesized that fibroblastic reticular cell (FRC) networks are lost in HSV-1 infected lymph nodes, leading to compromised leukocyte trafficking and survival. Viral dissemination to the MLN was not observed in WT mice by day 5 pi, but HSV-1 was readily detectable in MLN from STING−/− mice albeit at lower titers than was observed in CD118−/− mice (Fig. 9 A). Variances in IFNα2,4 and IFNγ were noted comparing STING−/− and WT MLN (Fig. 9, B and C) in addition to a trend in increased levels of VEGF in STING−/− MLN (Fig. 9 D). The total quantity of IL-2 was consistent in the MLN of all groups (Fig. 9 E). However, a conspicuous elevation of IL-6, IL-7, IL-12p70, and IL-15 was noted in CD118−/− mice despite the synchronous loss of T cells in these animals (Fig. 9, F–I). Moreover, the abundance of these cytokines did not differ between STING−/− and WT mice (Fig. 9, C–F). Propidium iodide and annexin V dyes were utilized to assess survival of CD8+ T cells in the MLN. Though STING−/− mice had a total CD8+ T cell count in the MLN similar to WT (Fig. 7 B), the number of dead CD8+ T cells in STING−/− mice was disproportionately high with a parallel reduction in the viable cell population (Fig. 9 J). Thus CD8+ T cell viability in STING−/− mice is compromised following viral dissemination to the MLN, as was previously reported for CD118−/− mice (39) and corroborated herein.
Figure 9. Survival of CD8+ T cells following viral dissemination to the MLN.
(A) HSV-1 titer in the MLN at day 5 pi (n = 7–8 mice per group; 3 independent experiments; ND, not detected). Total amounts of cytokines associated with antiviral defense including IFNα2,4 (B) and IFNγ (C); the immunomodulatory growth factor VEGF A (D); T cell differentiation or survival including IL-2 (E), IL-6 (F), IL-7 (G), IL-12p70 (H), and IL-15 (I) were evaluated at day 5 pi (n = 9 mice per group; 3 independent experiments). Stastical differences for panels A – I were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests; * and ^ reflect differences from WT and STING−/−, respectively. (J) Viability of CD8+ T cells recovered from MLN on day 5 pi assessed by propidium iodide and Annexin V staining (n = 5–9 mice per group; 2–3 independent experiments); parameters for determination of cell viability are shown in inset dot plot; differences were determined by two- way ANOVA with Bonferroni post tests; significance thresholds are as follows: p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***. Bars represent mean ± SEM.
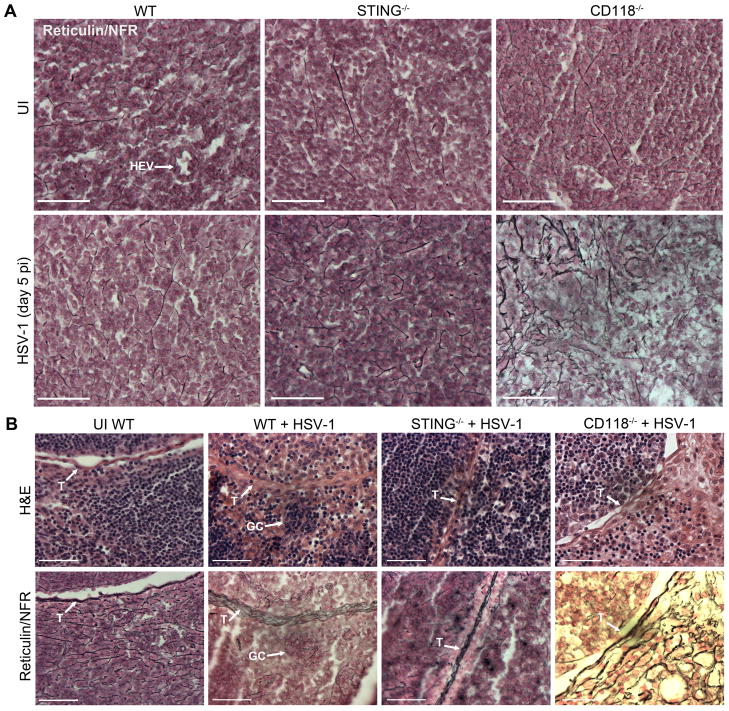
Gross visual examination revealed that MLN excised from STING−/− and CD118−/− mice were morphologically hypertrophic compared to WT specimens (data not shown). Pathologic edema is prevalent in MLN of CD118−/− mice following HSV-1 dissemination to the MLN, as previously shown by T-2 weighted magnetic resonance imaging highlighting gross lymphadenopathy (22). Diffuse hypocellularity and edema were substantiated histologically in MLN sections from CD118−/− mice within the central paracortical-medullary zone (Fig. 10 A) and peripheral subcapsular-perifollicular zone (Fig. 10 B) following HSV-1 dissemination. In contrast, MLN from STING−/− mice exhibited WT-equivalent densities of mononuclear cells without extensive edema at day 5 pi (Fig. 10, A and B). No hypoplasia or other aberrant pathology were observed in UI MLN from any experimental group (Fig. 10 A). While edema in the corneas and lymph nodes of HSV-1 infected CD118−/− mice is associated with the loss of lymphatic vessels (22), we have recently shown that lymphatic vessels are preserved in the mucosae of HSV-1 infected STING−/− mice (12).
Figure 10. Histological evaluation of reticular fiber network remodeling in the MLN following HSV-1 infection.
(A) Color micrographs of MLN paracortical-medulary T cell zones in fixed 5 μm sections from UI (top) WT, STING−/−, and CD118−/− mice and infected (bottom) animals at day 5 pi showing changes in the organization and density of reticular fiber conduits (silver stain) and mononulcear cells (nuclear fast red; NFR); the high endothelial venule in the WT UI image is labeled HEV. (B) Serial sections of H&E (top) and reticulin/NFR (bottom) stained MLN subcapsular-perifollicular zones showing changes in the reticular fiber meshwork; notations for the following strutures are included: trabeculae, ‘T’ and germinal center, ‘GC.’ Images reflect representative images from 5 mice per group; 2 independent experiments. All images were captured at 40× magnification; scale bar = 50 μm.
Lymph node architecture and adaptive immune responses are also modulated by changes within the FRC network during infection (20, 58). Histologic examination of FRC networks within MLN sections from WT, STING−/−, and CD118−/− mice was achieved using a classical silver stain technique to identify reticulin fibers (Fig. 10, A and B). While no differences were observed in the FRC network microarchitecture in UI MLN samples (Fig. 10 A), distinct alterations following infection were observed in STING−/− and CD118−/− mice (Fig. 10, A and B). Extended tracks of fine branching fibers were observed in WT MLN before and after infection (Fig. 10, A and B) with clear compartmentalization of germinal centers (Fig. 10 B). In contrast, the FRC network within the MLN of STING−/− mice was predominately composed of discontinuous bone spicule-like formations indicative of FRC damage at day 5 pi (Fig. 10 A). Contrary to our hypothesis, the FRC network in CD118−/− MLN exhibited an exceptionally dense, continuous labyrinthine phenotype following infection consistent with FRC proliferation and fibrosis (Fig. 10, A and B). However, changes in FRC networks were not observed along MLN trabeculae (Fig. 10 B).
Collectively, these observations support disparate mechanisms of FRC-associated T cell dysfunction in STING−/− and CD118−/− mice resulting in impaired immunity to HSV-1. High concentrations of cytokines associated with lymphocyte survival (Fig. 9, F–I) despite concurrent loss of both CD4+ and CD8+ T cells (Fig. 7, A–C) in the MLN of CD118−/− mice is consistent with reticulin fibrosis (Fig. 10) and loss of lymphatic vessels within the MLN (22). Soluble factors in fibrotic tissues are prone to entrapment in the extracellular matrix, and T cells require efferent lymphatic vessels to enter the bloodstream from lymph nodes. FRCs respond to IFNγ and IFNβ by increasing MHC class I expression, are able to activate naive CD8+ T cells, but restrict effector memory differentiation via up-regulation of the co-inhibitory molecule programmed death-ligand 1 (PD-L1) (59). Based on the high concentration of IFNγ (Fig. 9 C) and observations of FRC damage (Fig. 10 A) in the MLN of STING−/− mice, we attribute the lack of effector CD8+ T cell mobilization (Fig. 7 H) to discontinuity of the FRC network within the MLN. Another possible mechanism may involve FRC-mediated inhibition of proliferating or recently activated effector CD8+ T cells responding to antigen within the lymph node, as substantiated by the high proportion of dying/dead CD8+ T cells therein (Fig. 9J) in STING−/− mice relative to WT. Consistent with this, adoptive transfer of primed but not naive HSV-1 specific CD8+ T cells were able to reduce HSV-1 titers in STING−/− mice (Fig. 8, D and E). Thus, virus-associated pathological alteration of the FRC network is a likely (but not necessary a causal) mechanism responsible for the T cell immunodeficiency. However, it is conceivable that T cell priming is also impaired in STING−/− mice as deficiencies in innate immunity involving antigen presenting cells have been observed (8, 12, 57, 60).
DISCUSSION
The current investigation provides insight into host countermeasures that facilitate immunologic resistance to acute HSV-1 infection in divergent susceptible cell populations and anatomic compartments. Notably, our data suggest that IFNα/β-mediated resistance to HSV-1 replication in the peripheral nervous system involves STING and non-canonical IFNα/β signaling that we speculate includes the p38 MAPK pathway. Modulation of MAPK/ERK and AKT signaling pathways by HSV has been reported with varied results primarily in mitotically active cells, yet the contributions of IFN to these pathways has been neglected (61–65). Downstream signaling pathways mediated by IFNα/β signaling and STING are highly regulated (66). Accumulating evidence suggests that neurons exhibit a predilection for alternative IFN signaling cascades relative to mitotically active cells—a strategy of protection against cytotoxicity (31, 32); this differs from the orthodox supposition that STAT1-dependent ISG responses arbitrate antiviral defenses ubiquitously.
The protective effects of IFNα/β signaling and CD8+ T cell responses during the acute phase of HSV-1 infection have been well documented individually; however, the mechanistic link between these countermeasures is incompletely understood. Here, we show that a deficiency in immunosurveillance against HSV-1 via loss of STING or CD118 leads to atypical viral dissemination, lymphadenitis, and defective adaptive immune responses. Our experiments detail the impact of IFNα/β and STING with respect to activation, function, mobilization, and recruitment of effector CD8+ T cells during acute infection. We and others have demonstrated previously that CD118−/− and STING−/− mice succumb to encephalitis by day 5–7 pi when ocularly infected with neurovirulent HSV-1 strains such as McKrae or strain 17 (11, 67). Thus, the experiments conducted here reflect early stages of the adaptive CD8+ T cell responses to infection rather than the peak of T cell infiltration into the TG, which occurs around days 12–14 pi in WT mice (14, 15). Our data show that early T cell responses help control viral replication in the TG; however, CD8+ T cell responses do not restrict viral dissemination to the central nervous system or prevent lethality due to encephalitis in CD118−/− or STING−/− mice (data not shown).
Naive CD8+ T cells require antigen presentation, co-stimulation, and a cytokine-dependent signal for activation and optimal effector function. Moreover, IFNα/β serves as one cytokine that supports CD8+ T cell activation (17). Our data contribute two seminal observations that challenge current understandings of IFNα/β signaling on T cell responses to viral infection (23, 48). First, our data show that IFNα/β-signaling enhances but is not requisite for CD8+ T cell effector function during HSV-1 infection. Secondly, we show that immunodeficiency can manifest as a secondary outcome of pathological alterations to FRC networks pursuant to HSV-1 dissemination into secondary lymphoid organs. This is consistent with other models showing abrogated T cell responses resulting from virus-associated damage to FRCs (59, 68–70). One caveat to our studies is that adoptive transfer of HSV-specific TCR-transgenic CD8+ T cells creates a high precursor frequency that may circumvent physiological T cell priming.
Lymph nodes enlarge during immune responses to provide for optimal engagement between foreign antigens and lymphocytes. This process involves the coordinated activities of many constituents ranging from hematopoietic cells including macrophages, dendritic cells, mast cells, and lymphocytes, to non-hematopoietic structures including lymphatic vessels and FRC conduits for antigen transport and leukocyte migration (71–74). Within the lymph node microenvironment, FRCs provide structural support (75), are a prominent source of the T cell survival factor IL-7 (76), promote interactions between dendritic cells and T cells (77), and are critical for antiviral immunity (78). As noted, FRCs are targets of multiple viral infections and can adversely affect T cell responses in the virally infected lymph node (20). Mechanisms of FRC-mediated T cell attenuation involve PD-L1, inducible nitric oxide synthase (iNOS), and arguably indoleamine 2,3-dioxygenase (IDO) (59, 68, 79–82). Notably, experimental neutralization of PD-L1 ameliorates the neurovirulence of HSV-1 and abrogates HSV-1-specific CD8+ T cell responses (83). Consistent with this, anti-PD-L1 antibody treatment contributes to immunopathology and disruption of FRC networks in secondary lymphoid organs during LCMV infection (68).
Here, we show that adoptive transfer of HSV-1 specific CD8+ T cells into CD118−/− mice can reduce viral burden and partially restore endogenous T cell responses (Fig. 4). Though the mechanism driving this phenomenon remains to be elucidated, we speculate that reducing the number of infected FRCs and/or limiting virus-associated disruption of the lymph node microarchitecture is responsible for restoration and mobilization of the endogenous T cell responses. Titrations of different numbers of adoptively transferred TCR transgenic T cells are known to modulate the gB-precursor frequency during acute HSV-1 infection (84); however, regulatory mechanisms are thought to control the size of the CD8+ T cell effector population based on antigen availability (85). One caveat to our adoptive transfer studies using 3×106 gB-specific CD8+ T cells is that it reportedly leads to a three-fold higher effector population by day 5 pi relative to the physiological response observed in immunologically naive WT mice (84). While the increase in effector cell frequency is much greater than 3-fold in CD118−/− mice following adoptive transfer (Fig. 4H), it is important to note that this is in comparison to a substantial pathological loss of endogenous lymphocytes due to virus-associated disruption of the lymph node integrity in CD118−/− mice as characterized previously (39). However, IFNα/β-signaling enhances but is not requisite for CD8+ T cell effector responses and viral clearance as demonstrated by reductions in HSV-1 titers in the MLN and TG of CD118−/− mice receiving gBT-I.1 or gBT-I.1xCD118−/− T cells.
While the contributions of STING or IFNα/β to FRC physiology have not been explored in depth, the effects of STING and CD118−/− on T cell responses are of considerable interest. Rational vaccine design for HSV-1/2 has primarily yet unsuccessfully sought the development of a strong T cell response to confer protection (6, 86). Furthermore, cyclic dinucleotide STING agonists and STING ligand-inducing adjuvants have been utilized to improve the efficacy of vaccine-elicited TH1 responses to viral, bacterial, and tumor antigens (87–90). Furthermore, various models have produced mixed evidence for STING and IFNα/β-dependent T cell responses. CD8+ T cell responses are reportedly muted in STING−/− mice following DNA plasmid vaccination (8) and in response to solid tumor challenge (57) or baculovirus infection (60). Consistent with these reports, STING−/− macrophages fail to upregulate MHC class II following stimulation (8, 12). In contrast, cell-mediated immunity induced upon Listeria monocytogenes infection is enhanced in STING−/− mice (91). Moreover, CD8+ T cell responses are largely unaffected in CD118−/− and STING−/− mice inoculated with recombinant attenuated adenoviral vectors (51). Here, we show efficient activation and proliferation of HSV-specific CD8+ T cells in the MLN of STING−/− mice with defective mobilization during HSV-1 infection. This data correlates with pathologic alteration of the MLN FRC network. Moreover, adoptive transfer of primed but not naive HSV-1 specific CD8+ T cells into STING−/− mice leads to a reduction in viral burden (Fig. 8 D, E), suggesting a defect in appropriate T cell priming in this model. As such, we suggest the lymph node serves as a trap for which T cells exposed to antigen are unable to egress due to disorganization of the FRC network. Collectively, our data definitively highlight the importance of IFNα/β and STING-mediated immune responses relative to control and susceptibility to acute HSV-1 infection. While not necessarily unexpected, we show that early responding CD8+ T cells are necessary to repress viral replication within neuronal ganglia. Finally, we posit that future HSV vaccine development should entertain specific targeting of the STING-IFNα/β pathway to elicit protective immunity, as this signaling pathway is essential for the generation of protective countermeasures against HSV-1 in the naive host.
Supplementary Material
Acknowledgments
We acknowledge the NIH Tetramer Core Facility for provision of the MHC class I tetramer (National Institute of Allergy and Infectious Diseases, contract # HHSN272201300006C). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors would like to thank the DMEI Vivarium staff for their help maintaining animals; Meghan Carr, Jeremy Jinkins, and Min Zheng for technical assistance; and Linda Boone for her histological expertise and assistance. We also recognize the original providers of our transgenic and knockout mice: Helen Rosenberg, CD118−/−; Francis Carbone, gBT-I.1; Bao Lu, CXCR3−/−; and Andrew Luster, CXCL10−/−.
Footnotes
Grant support: This work was supported by National Institutes of Health (NIH) Grants R01 AI053108, P30 EY021725, and T32 EY023202. Additional support was provided by an unrestricted grant from Research to Prevent Blindness.
References
- 1.Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2013;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crouse J, Kalinke U, Oxenius A. Regulation of antiviral T cell responses by type I interferons. Nat Rev Immunol. 2015;15:231–242. doi: 10.1038/nri3806. [DOI] [PubMed] [Google Scholar]
- 3.McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma S. Diagnosis of infectious diseases of the eye. Eye. 2012;26:177–184. doi: 10.1038/eye.2011.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu L, Zhu H. Ocular herpes: the pathophysiology, management and treatment of herpetic eye diseases. Virol Sin. 2014;29:327–342. doi: 10.1007/s12250-014-3539-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Royer DJ, Cohen AW, Carr DJ. The current state of vaccine development for ocular HSV-1 infection. Expert Rev Ophthalmol. 2015;10:113–126. doi: 10.1586/17469899.2015.1004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Virgin HW, Wherry EJ, Ahmed R. Redefining Chronic Viral Infection. Cell. 2009;138:30–50. doi: 10.1016/j.cell.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conrady CD, Zheng M, Fitzgerald KA, Liu C, Carr DJJ. Resistance to HSV-1 infection in the epithelium resides with the novel innate sensor, IFI-16. Mucosal Immunol. 2012;5:173–183. doi: 10.1038/mi.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalamvoki M, Roizman B. HSV-1 degrades, stabilizes, requires, or is stung by STING depending on ICP0, the US3 protein kinase, and cell derivation. Proc Natl Acad Sci U S A. 2014;111:E611–617. doi: 10.1073/pnas.1323414111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker ZM, Murphy AA, Leib DA. Role of the DNA Sensor STING in Protection from Lethal Infection following Corneal and Intracerebral Challenge with Herpes Simplex Virus 1. J Virol. 2015;89:11080–11091. doi: 10.1128/JVI.00954-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Royer DJ, Carr DJJ. A STING-dependent innate-sensing pathway mediates resistance to corneal HSV-1 infection via upregulation of the antiviral effector tetherin. Mucosal Immunol. 2016;9:1065–1075. doi: 10.1038/mi.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simmons A, Tscharke DC. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wuest TR, Carr DJJ. Dysregulation of CXCR3 signaling due to CXCL10 deficiency impairs the antiviral response to herpes simplex virus 1 infection. J Immunol Baltim Md 1950. 2008;181:7985–7993. doi: 10.4049/jimmunol.181.11.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol Baltim Md 1950. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 18.Aichele P, Unsoeld H, Koschella M, Schweier O, Kalinke U, Vucikuja S. Cutting Edge: CD8 T Cells Specific for Lymphocytic Choriomeningitis Virus Require Type I IFN Receptor for Clonal Expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 19.Starbeck-Miller GR, Xue HH, Harty JT. IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. J Exp Med. 2014;211:105–120. doi: 10.1084/jem.20130901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fletcher AL, Acton SE, Knoblich K. Lymph node fibroblastic reticular cells in health and disease. Nat Rev Immunol. 2015;15:350–361. doi: 10.1038/nri3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Royer DJ, Zheng M, Conrady CD, Carr DJJ. Granulocytes in Ocular HSV-1 Infection: Opposing Roles of Mast Cells and Neutrophils. Invest Ophthalmol Vis Sci. 2015;56:3763–3775. doi: 10.1167/iovs.15-16900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryant-Hudson KM, Chucair-Elliott AJ, Conrady CD, Cohen A, Zheng M, Carr DJJ. HSV-1 targets lymphatic vessels in the eye and draining lymph node of mice leading to edema in the absence of a functional type I interferon response. Am J Pathol. 2013;183:1233–1242. doi: 10.1016/j.ajpath.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crouse J, Bedenikovic G, Wiesel M, Ibberson M, Xenarios I, Von Laer D, Kalinke U, Vivier E, Jonjic S, Oxenius A. Type I interferons protect T cells against NK cell attack mediated by the activating receptor NCR1. Immunity. 2014;40:961–973. doi: 10.1016/j.immuni.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Sharma S, Campbell AM, Chan J, Schattgen SA, Orlowski GM, Nayar R, Huyler AH, Nündel K, Mohan C, Berg LJ, Shlomchik MJ, Marshak-Rothstein A, Fitzgerald KA. Suppression of systemic autoimmunity by the innate immune adaptor STING. Proc Natl Acad Sci U S A. 2015;112:E710–717. doi: 10.1073/pnas.1420217112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin BA, James C, Silverman RH, Carr DJJ. Critical role for the oligoadenylate synthetase/RNase L pathway in response to IFN-beta during acute ocular herpes simplex virus type 1 infection. J Immunol Baltim Md 1950. 2005;175:1100–1106. doi: 10.4049/jimmunol.175.2.1100. [DOI] [PubMed] [Google Scholar]
- 26.Halford WP, Weisend C, Grace J, Soboleski M, Carr DJJ, Balliet JW, Imai Y, Margolis TP, Gebhardt BM. ICP0 antagonizes Stat 1-dependent repression of herpes simplex virus: implications for the regulation of viral latency. Virol J. 2006;3:44. doi: 10.1186/1743-422X-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosato PC, Leib DA. Neuronal Interferon Signaling Is Required for Protection against Herpes Simplex Virus Replication and Pathogenesis. PLOS Pathog. 2015;11:e1005028. doi: 10.1371/journal.ppat.1005028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gil MP, Bohn E, O’Guin AK, Ramana CV, Levine B, Stark GR, Virgin HW, Schreiber RD. Biologic consequences of Stat1-independent IFN signaling. Proc Natl Acad Sci U S A. 2001;98:6680–6685. doi: 10.1073/pnas.111163898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 30.Pasieka TJ, Collins L, O’Connor MA, Chen Y, Parker ZM, Berwin BL, Piwnica-Worms DR, Leib DA. Bioluminescent Imaging Reveals Divergent Viral Pathogenesis in Two Strains of Stat1-Deficient Mice, and in αβγ Interferon Receptor-Deficient Mice. PLoS ONE. 2011;6:e24018. doi: 10.1371/journal.pone.0024018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Donnell LA, Henkins KM, Kulkarni A, Matullo CM, Balachandran S, Pattisapu AK, Rall GF. Interferon gamma induces protective non-canonical signaling pathways in primary neurons. J Neurochem. 2015;135:309–322. doi: 10.1111/jnc.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yordy B, Iijima N, Huttner A, Leib D, Iwasaki A. A Neuron-Specific Role for Autophagy in Antiviral Defense against Herpes Simplex Virus. Cell Host Microbe. 2012;12:334–345. doi: 10.1016/j.chom.2012.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webber JL, Tooze SA. New insights into the function of Atg9. FEBS Lett. 2010;584:1319–1326. doi: 10.1016/j.febslet.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 34.Matsuzawa T, Kim BH, Shenoy AR, Kamitani S, Miyake M, MacMicking JD. IFN- Elicits Macrophage Autophagy via the p38 MAPK Signaling Pathway. J Immunol. 2012;189:813–818. doi: 10.4049/jimmunol.1102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sui X, Kong N, Ye L, Han W, Zhou J, Zhang Q, He C, Pan H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014;344:174–179. doi: 10.1016/j.canlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Sridharan S, Jain K, Basu A. Regulation of Autophagy by Kinases. Cancers. 2011;3:2630–2654. doi: 10.3390/cancers3022630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wallace ME, Keating R, Heath WR, Carbone FR. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J Virol. 1999;73:7619–7626. doi: 10.1128/jvi.73.9.7619-7626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.St Leger AJ, Peters B, Sidney J, Sette A, Hendricks RL. Defining the herpes simplex virus-specific CD8+ T cell repertoire in C57BL/6 mice. J Immunol Baltim Md 1950. 2011;186:3927–3933. doi: 10.4049/jimmunol.1003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conrady CD, Thapa M, Wuest T, Carr DJJ. Loss of Mandibular Lymph Node Integrity Is Associated with an Increase in Sensitivity to HSV-1 Infection in CD118-Deficient Mice. J Immunol. 2009;182:3678–3687. doi: 10.4049/jimmunol.0803878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheridan BS, Cherpes TL, Urban J, Kalinski P, Hendricks RL. Reevaluating the CD8 T-Cell Response to Herpes Simplex Virus Type 1: Involvement of CD8 T Cells Reactive to Subdominant Epitopes. J Virol. 2009;83:2237–2245. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tough DF, Borrow P, Sprent J. Induction of Bystander T Cell Proliferation by Viruses and Type I Interferon in Vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 42.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander Activation of CD8+ T Cells Contributes to the Rapid Production of IFN- in Response to Bacterial Pathogens. J Immunol. 2001;166:1097–1105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 43.Gilbertson B, Germano S, Steele P, Turner S, de St Groth BF, Cheers C. Bystander Activation of CD8+ T Lymphocytes during Experimental Mycobacterial Infection. Infect Immun. 2004;72:6884–6891. doi: 10.1128/IAI.72.12.6884-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carr MW, Roth SJ, Luther E, Rose SS, Springer TA. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol Baltim Md 1950. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 46.Wuest TR, Thapa M, Zheng M, Carr DJJ. CXCL10 expressing hematopoietic-derived cells are requisite in defense against HSV-1 infection in the nervous system of CXCL10 deficient mice. J Neuroimmunol. 2011;234:103–108. doi: 10.1016/j.jneuroim.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller SN, Heath W, McLain JD, Carbone FR, Jones CM. Characterization of two TCR transgenic mouse lines specific for herpes simplex virus. Immunol Cell Biol. 2002;80:156–163. doi: 10.1046/j.1440-1711.2002.01071.x. [DOI] [PubMed] [Google Scholar]
- 48.Xu HC, Grusdat M, Pandyra AA, Polz R, Huang J, Sharma P, Deenen R, Köhrer K, Rahbar R, Diefenbach A, Gibbert K, Löhning M, Höcker L, Waibler Z, Häussinger D, Mak TW, Ohashi PS, Lang KS, Lang PA. Type I interferon protects antiviral CD8+ T cells from NK cell cytotoxicity. Immunity. 2014;40:949–960. doi: 10.1016/j.immuni.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Frank GM, Buela KAG, Maker DM, Harvey SAK, Hendricks RL. Early Responding Dendritic Cells Direct the Local NK Response To Control Herpes Simplex Virus 1 Infection within the Cornea. J Immunol. 2012;188:1350–1359. doi: 10.4049/jimmunol.1101968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim M, Osborne NR, Zeng W, Donaghy H, McKinnon K, Jackson DC, Cunningham AL. Herpes simplex virus antigens directly activate NK cells via TLR2, thus facilitating their presentation to CD4 T lymphocytes. J Immunol Baltim Md 1950. 2012;188:4158–4170. doi: 10.4049/jimmunol.1103450. [DOI] [PubMed] [Google Scholar]
- 51.Quinn KM, Zak DE, Costa A, Yamamoto A, Kastenmuller K, Hill BJ, Lynn GM, Darrah PA, Lindsay RWB, Wang L, Cheng C, Nicosia A, Folgori A, Colloca S, Cortese R, Gostick E, Price DA, Gall JGD, Roederer M, Aderem A, Seder RA. Antigen expression determines adenoviral vaccine potency independent of IFN and STING signaling. J Clin Invest. 2015;125:1129–1146. doi: 10.1172/JCI78280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stock AT, Smith JM, Carbone FR. Type I IFN suppresses Cxcr2 driven neutrophil recruitment into the sensory ganglia during viral infection. J Exp Med. 2014;211:751–759. doi: 10.1084/jem.20132183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conrady CD, Zheng M, Mandal NA, van Rooijen N, Carr DJJ. IFN-α-driven CCL2 production recruits inflammatory monocytes to infection site in mice. Mucosal Immunol. 2013;6:45–55. doi: 10.1038/mi.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seo SU, Kwon HJ, Ko HJ, Byun YH, Seong BL, Uematsu S, Akira S, Kweon MN. Type I interferon signaling regulates Ly6C(hi) monocytes and neutrophils during acute viral pneumonia in mice. PLoS Pathog. 2011;7:e1001304. doi: 10.1371/journal.ppat.1001304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Powell DR, Huttenlocher A. Neutrophils in the Tumor Microenvironment. Trends Immunol. 2016;37:41–52. doi: 10.1016/j.it.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MYK, Duggan R, Wang Y, Barber GN, Fitzgerald KA, Alegre ML, Gajewski TF. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown FD, Turley SJ. Fibroblastic reticular cells: organization and regulation of the T lymphocyte life cycle. J Immunol Baltim Md 1950. 2015;194:1389–1394. doi: 10.4049/jimmunol.1402520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ng CT, Nayak BP, Schmedt C, Oldstone MBA. Immortalized clones of fibroblastic reticular cells activate virus-specific T cells during virus infection. Proc Natl Acad Sci. 2012;109:7823–7828. doi: 10.1073/pnas.1205850109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hervas-Stubbs S, Riezu-Boj JI, Mancheno U, Rueda P, Lopez L, Alignani D, Rodriguez-Garcia E, Thieblemont N, Leclerc C. Conventional but Not Plasmacytoid Dendritic Cells Foster the Systemic Virus-Induced Type I IFN Response Needed for Efficient CD8 T Cell Priming. J Immunol. 2014;193:1151–1161. doi: 10.4049/jimmunol.1301440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hargett D, McLean T, Bachenheimer SL. Herpes simplex virus ICP27 activation of stress kinases JNK and p38. J Virol. 2005;79:8348–8360. doi: 10.1128/JVI.79.13.8348-8360.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wagner MJ, Smiley JR. Herpes Simplex Virus Requires VP11/12 To Activate Src Family Kinase-Phosphoinositide 3-Kinase-Akt Signaling. J Virol. 2011;85:2803–2812. doi: 10.1128/JVI.01877-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qin D, Feng N, Fan W, Ma X, Yan Q, Lv Z, Zeng Y, Zhu J, Lu C. Activation of PI3K/AKT and ERK MAPK signal pathways is required for the induction of lytic cycle replication of Kaposi’s sarcoma-associated herpesvirus by herpes simplex virus type 1. BMC Microbiol. 2011;11:240. doi: 10.1186/1471-2180-11-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chuluunbaatar U, Roller R, Mohr I. Suppression of Extracellular Signal-Regulated Kinase Activity in Herpes Simplex Virus 1-Infected Cells by the Us3 Protein Kinase. J Virol. 2012;86:7771–7776. doi: 10.1128/JVI.00622-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheshenko N, Trepanier JB, Stefanidou M, Buckley N, Gonzalez P, Jacobs W, Herold BC. HSV activates Akt to trigger calcium release and promote viral entry: novel candidate target for treatment and suppression. FASEB J Off Publ Fed Am Soc Exp Biol. 2013;27:2584–2599. doi: 10.1096/fj.12-220285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ma F, Li B, Yu Y, Iyer SS, Sun M, Cheng G. Positive feedback regulation of type I interferon by the interferon-stimulated gene STING. EMBO Rep. 2015;16:202–212. doi: 10.15252/embr.201439366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conrady CD, Zheng M, van Rooijen N, Drevets DA, Royer D, Alleman A, Carr DJJ. Microglia and a functional type I IFN pathway are required to counter HSV-1-driven brain lateral ventricle enlargement and encephalitis. J Immunol Baltim Md 1950. 2013;190:2807–2817. doi: 10.4049/jimmunol.1203265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mueller SN, Matloubian M, Clemens DM, Sharpe AH, Freeman GJ, Gangappa S, Larsen CP, Ahmed R. Viral targeting of fibroblastic reticular cells contributes to immunosuppression and persistence during chronic infection. Proc Natl Acad Sci. 2007;104:15430–15435. doi: 10.1073/pnas.0702579104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G, Lifson JD, Carlis JV, Haase AT. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng M, Paiardini M, Engram JC, Beilman GJ, Chipman JG, Schacker TW, Silvestri G, Haase AT. Critical role of CD4 T cells in maintaining lymphoid tissue structure for immune cell homeostasis and reconstitution. Blood. 2012;120:1856–1867. doi: 10.1182/blood-2012-03-418624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Girard JP, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- 72.Germain RN, Robey EA, Cahalan MD. A Decade of Imaging Cellular Motility and Interaction Dynamics in the Immune System. Science. 2012;336:1676–1681. doi: 10.1126/science.1221063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell–derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–1205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 74.Rantakari P, Auvinen K, Jäppinen N, Kapraali M, Valtonen J, Karikoski M, Gerke H, Iftakhar-E-Khuda I, Keuschnigg J, Umemoto E, Tohya K, Miyasaka M, Elima K, Jalkanen S, Salmi M. The endothelial protein PLVAP in lymphatics controls the entry of lymphocytes and antigens into lymph nodes. Nat Immunol. 2015;16:386–396. doi: 10.1038/ni.3101. [DOI] [PubMed] [Google Scholar]
- 75.Astarita JL, Cremasco V, Fu J, Darnell MC, Peck JR, Nieves-Bonilla JM, Song K, Kondo Y, Woodruff MC, Gogineni A, Onder L, Ludewig B, Weimer RM, Carroll MC, Mooney DJ, Xia L, Turley SJ. The CLEC-2–podoplanin axis controls the contractility of fibroblastic reticular cells and lymph node microarchitecture. Nat Immunol. 2014;16:75–84. doi: 10.1038/ni.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Onder L, Narang P, Scandella E, Chai Q, Iolyeva M, Hoorweg K, Halin C, Richie E, Kaye P, Westermann J, Cupedo T, Coles M, Ludewig B. IL-7-producing stromal cells are critical for lymph node remodeling. Blood. 2012;120:4675–4683. doi: 10.1182/blood-2012-03-416859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bajénoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal Cell Networks Regulate Lymphocyte Entry, Migration, and Territoriality in Lymph Nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chai Q, Onder L, Scandella E, Gil-Cruz C, Perez-Shibayama C, Cupovic J, Danuser R, Sparwasser T, Luther SA, Thiel V, Rülicke T, Stein JV, Hehlgans T, Ludewig B. Maturation of Lymph Node Fibroblastic Reticular Cells from Myofibroblastic Precursors Is Critical for Antiviral Immunity. Immunity. 2013;38:1013–1024. doi: 10.1016/j.immuni.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, Collier A, Turley SJ. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 2011;12:1096–1104. doi: 10.1038/ni.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Siegert S, Huang HY, Yang CY, Scarpellino L, Carrie L, Essex S, Nelson PJ, Heikenwalder M, Acha-Orbea H, Buckley CD, Marsland BJ, Zehn D, Luther SA. Fibroblastic Reticular Cells From Lymph Nodes Attenuate T Cell Expansion by Producing Nitric Oxide. PLoS ONE. 2011;6:e27618. doi: 10.1371/journal.pone.0027618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dai X, Zhu BT. Indoleamine 2,3-Dioxygenase Tissue Distribution and Cellular Localization in Mice: Implications for Its Biological Functions. J Histochem Cytochem. 2010;58:17–28. doi: 10.1369/jhc.2009.953604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siegert S, Luther SA. Positive and negative regulation of T cell responses by fibroblastic reticular cells within paracortical regions of lymph nodes. Front Immunol. 2012:3. doi: 10.3389/fimmu.2012.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bryant-Hudson KM, Carr DJJ. PD-L1-Expressing Dendritic Cells Contribute to Viral Resistance during Acute HSV-1 Infection. Clin Dev Immunol. 2012;2012:1–9. doi: 10.1155/2012/924619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stock AT, Mueller SN, Kleinert LM, Heath WR, Carbone FR, Jones CM. Optimization of TCR transgenic T cells for in vivo tracking of immune responses. Immunol Cell Biol. 2007;85:394–396. doi: 10.1038/sj.icb.7100076. [DOI] [PubMed] [Google Scholar]
- 85.Kemp RA, Powell TJ, Dwyer DW, Dutton RW. Cutting Edge: Regulation of CD8+ T Cell Effector Population Size. J Immunol. 2004;173:2923–2927. doi: 10.4049/jimmunol.173.5.2923. [DOI] [PubMed] [Google Scholar]
- 86.Halford WP. Antigenic breadth: a missing ingredient in HSV-2 subunit vaccines? Expert Rev Vaccines. 2014;13:691–710. doi: 10.1586/14760584.2014.910121. [DOI] [PubMed] [Google Scholar]
- 87.Chandra D, Quispe-Tintaya W, Jahangir A, Asafu-Adjei D, Ramos I, Sintim HO, Zhou J, Hayakawa Y, Karaolis DKR, Gravekamp C. STING ligand c-di-GMP improves cancer vaccination against metastatic breast cancer. Cancer Immunol Res. 2014;2:901–910. doi: 10.1158/2326-6066.CIR-13-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, Mechette K, Leong JJ, Lauer P, Liu W, Sivick KE, Zeng Q, Soares KC, Zheng L, Portnoy DA, Woodward JJ, Pardoll DM, Dubensky TW, Kim Y. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7:283ra52. doi: 10.1126/scitranslmed.aaa4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J Clin Invest. 2015;125:2532–2546. doi: 10.1172/JCI79915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carroll EC, Jin L, Mori A, Muñoz-Wolf N, Oleszycka E, Moran HBT, Mansouri S, McEntee CP, Lambe E, Agger EM, Andersen P, Cunningham C, Hertzog P, Fitzgerald KA, Bowie AG, Lavelle EC. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity. 2016 doi: 10.1016/j.immuni.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Archer KA, Durack J, Portnoy DA. STING-Dependent Type I IFN Production Inhibits Cell-Mediated Immunity to Listeria monocytogenes. PLoS Pathog. 2014;10:e1003861. doi: 10.1371/journal.ppat.1003861. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.