Abstract
Inflammation, part of the body’s innate immune response, can lead to “sickness behaviors,” as well as alterations in social and affective experiences. Elevated levels of pro-inflammatory cytokines have been associated with increased neural sensitivity to social rejection and social threat, but also decreased neural sensitivity to rewards. However, recent evidence suggests that inflammation may actually enhance sensitivity to certain social rewards, such as those that signal support and care. Despite a growing interest in how inflammation influences neural reactivity to positive and negative social experiences, no known studies have investigated these processes in the same participants, using a similar task. To examine this issue, 107 participants were randomly assigned to receive either placebo or low-dose endotoxin, which safely triggers an inflammatory response. When levels of pro-inflammatory cytokines were at their peak, participants were scanned using fMRI while they received positive, negative, and neutral feedback from an “evaluator” (actually a confederate) about how they came across in an audio-recorded interview. In response to negative feedback (vs. neutral), participants in the endotoxin condition showed heightened neural activity in a number of threat-related neural regions (i.e., bilateral amygdala, dorsal anterior cingulate cortex) and a key mentalizing-related region (i.e., dorsomedial PFC), compared to placebo participants. Interestingly, when receiving positive feedback (vs. neutral), endotoxin led to greater neural activity in the ventral striatum and ventromedial PFC, regions often implicated in processing reward, compared to placebo. Together, these results reveal that individuals exposed to an inflammatory challenge are more “neurally sensitive” to both negative and positive social feedback, suggesting that inflammation may lead to a greater vigilance for both social threats and social rewards.
Keywords: inflammation, endotoxin, cytokines, social feedback, fMRI, threat, reward
Introduction
As part of the innate immune system, the inflammatory response, our “first line of defense” against foreign agents, is critical not only for protecting the body against injuries and infections, but also for altering behavior during times of illness. Specifically, pro-inflammatory cytokines can act on the brain to induce “sickness behavior,” a constellation of symptoms including loss of appetite, fatigue, achiness, and fever, which are thought to promote recovery and recuperation during illness and infection (Dantzer & Kelley, 2007). Indeed, a peripheral increase in levels of cytokines in the body can induce central cytokines in the brain, which can then influence neural reactivity and sickness behaviors (Dantzer et al., 2008). In the past decade, it has been demonstrated that inflammation can alter social and affective experiences as well, presumably also an adaptive response to maximize recovery from illness (Dantzer et al., 2008; Raison, Capuron, & Miller et al., 2006). In other words, in addition to the physical symptoms we typically think of as accompanying sickness, the inflammatory response also leads to a cascade of psychological changes that are just beginning to be fully explored.
Prior research suggests that increases in pro-inflammatory cytokines are associated with greater depressed mood (Eisenberger et al., 2010; Harrison et al., 2009; Reichenberg et al., 2001), heightened feelings of social disconnection (Eisenberger et al., 2010; Moieni et al., 2015), greater neural sensitivity to social rejection (Eisenberger et al., 2009) as well as more general social threat (Inagaki et al., 2012). These results make sense from an evolutionary perspective: When in a vulnerable state due to infection or injury, the ability to identify threatening individuals in the environment that may pose additional harm would be adaptive, and thus the brain may be “primed” to activate the “neural alarm system” (Eisenberger & Lieberman, 2004) in response to cues of potentially threatening conspecifics.
Less is known about the effects of an inflammatory response on neural responses to positive experiences. Two studies suggests that increases in levels of pro-inflammatory cytokines are associated with decreased neural sensitivity in reward-related neural regions to monetary rewards (Capuron et al., 2012; Eisenberger et al., 2010). These findings are consistent with animal research suggesting that inflammation leads to decreased consumption of palatable foods and lower likelihood of engaging in sexual behavior (de la Garza, 2005), as well as lower levels of activity in reward-related neural regions (Stone et al., 2006). This cytokine-induced “anhedonia” may also play an adaptive role during times of sickness, leading the organism to rest and recuperate rather than seeking out pleasurable stimuli that may serve to further weaken an already compromised physical condition.
However, recent findings have also shown that inflammation can sometimes lead to increases in reward processing, such as when viewing images of support givers (Inagaki et al., 2015) or when given the opportunity to affiliate with a familiar cage-mate (Yee & Prendergast, 2010). Greater sensitivity to “social rewards” during times of sickness may also serve an adaptive function, perhaps because individuals who mean to help or provide support are especially useful when one is in a vulnerable state (Cole, 2006; Hennessy et al., 2014). Yet, it is unclear if greater neural sensitivity to social rewards during sickness is specific to close-others, or if it is a more generalized response to other sources of positive social information. Hence, the present study aimed to examine how an inflammatory challenge alters neural sensitivity to negative and positive social feedback, in the same group of participants, and using similar tasks.
To accomplish this goal, we conducted a double-blind, placebo-controlled trial comparing neural responses to social feedback among individuals exposed to endotoxin (which safely triggers an increase in pro-inflammatory cytokines) versus those in a typical inflammatory state (i.e., placebo). Consistent with prior work, we hypothesized that endotoxin (vs. placebo) would lead to greater neural activity in brain regions that respond to threat and pain (i.e., dACC, amygdala, anterior insula) during negative social feedback (relative to neutral). We also examined how endotoxin (vs. placebo) affected activity in reward-related neural structures (i.e., ventral striatum, ventromedial prefrontal cortex [VMPFC]) during positive social feedback (compared to neutral). In addition, because the dorsomedial prefrontal cortex (DMPFC) is important for understanding the mental states of others (which is critical for a task in which one is being evaluated by others), and because there is increasing interest in evaluating the social cognitive impacts of the inflammatory response (Kullmann et al., 2014; Moieni et al., 2015), we also examined how endotoxin altered DMPFC activity in response to receiving both positive and negative feedback. Finally, we explored if, in addition to affecting neural responses, exposure to endotoxin was associated with self-reported affective responses to the social feedback.
Method
Participants
One hundred fifteen healthy participants (69 females; M age = 24.17, SD = 6.61) completed the study, as previously described (Inagaki et al., 2015; Moieni et al., 2015). Sixty-one participants were randomly assigned to receive low-dose endotoxin (0.8 ng/kg of body weight), while 54 were randomly assigned to receive placebo (0.9% saline), both administered by a nurse through intravenous bolus. All participants met common inclusion criteria for fMRI studies (i.e., no claustrophobia, current pregnancy, or metal implants), and were also confirmed to be free of Axis-I psychiatric conditions (via the SCID) as well as current physical health issues (i.e., allergies, autoimmune disease, BMI greater than 30, current prescription or recreational drug use). Of the 115 participants who completed overall study procedures, five did not complete fMRI scanning (two due to extreme sickness responses to the endotoxin, one due to claustrophobia, one due to a previously-unreported metallic implant, and one due to scanner technical issues), two had incomplete or unusable fMRI data (due to technical issues with stimulus presentation), one participant (from the endotoxin condition) was excluded from all fMRI analyses for being an outlier (more than 3 SD below the mean) on activity in multiple ROIs for contrasts of both negative and positive feedback, one participant (from the endotoxin condition) was excluded from correlation analyses involving IL-6 for being an outlier (more than 3 SD below the mean), and three participants (from the endotoxin condition) were excluded from correlation analyses involving TNF-α for being outliers (more than 3 SD below the mean). Thus, we had a final sample of 107 participants with usable fMRI data (endotoxin: n=56; placebo: n=51), 106 participants with both usable fMRI and plasma IL-6 data (endotoxin: n=55), and 104 participants with both usable fMRI and plasma TNF-α data (endotoxin: n=53). All participants provided written informed consent, and the UCLA IRB approved all study procedures. The study was registered as a Clinical Trial (#NCT01671150).
Procedures
Complete details of the overall study procedures are described elsewhere (Moieni et al., 2015) but are summarized here. Participants completed both telephone and in-person screening sessions to confirm they met the eligibility criteria described above. On the day of the experimental session, participants arrived at the UCLA Clinical and Translational Research Center (CTRC), where a nurse inserted a catheter into the dominant forearm for hourly blood draws and a catheter into the non-dominant forearm for continuous saline flush and drug administration. During a 90-minute acclimation period, participants completed an audio-recorded interview in which they were asked questions about themselves for approximately 10 minutes (e.g., “What is your best quality?,” and “What are you most afraid of?”). Participants were told that, during the MRI scan, trained evaluators would listen to and evaluate their interview. After the 90 minute acclimation period, participants received either the endotoxin or placebo injection, and approximately 2 hours later (when the inflammatory response begins to peak for those in the endotoxin condition; Eisenberger et al., 2009, 2010; Moieni et al., 2015), participants underwent an fMRI scan during which they received feedback on their interview from an evaluator (see “Social Feedback fMRI Task” section below for more details), among other neuroimaging tasks not reported here (see Inagaki et al., 2015). Hourly blood draws were taken throughout the session to assess levels of pro-inflammatory cytokines (at baseline prior to endotoxin/placebo administration and then approximately every hour after drug/placebo administration for a total of six hours post administration). Cytokine analyses for the current study focus on the baseline time point and the pre-scan time point (approximately 2 hours post-injection) because this second time point was closest to when the fMRI task was collected and because our prior work has shown sustained increases in cytokines (relative to baseline) at this time (Eisenberger et al., 2009, 2010).
Measures
Social Feedback fMRI Task
The social feedback fMRI task was adapted from tasks used previously to study neural responses to social evaluation (Eisenberger et al., 2011; Muscatell et al., 2015). Upon arrival at the MRI scanning center, participants met two other individuals (actually confederates; one male, one female) who they believed they would be interacting with during the MRI tasks. Specifically for the present task, participants were told that while they were in the MRI scanner, one of the evaluators would be seated in the scanner control room and would listen to the participant’s interview and provide feedback about how the participant came across in the interview. Meanwhile, the participant would be able to see the evaluator’s feedback through the MRI goggles that were connected to a computer in the control room. In actuality, during the scan participants viewed a pre-recorded video that showed a mouse cursor moving around a grid containing 24 “adjective buttons” and clicking on a new adjective every 10 sec. During the scan, participants received 15 instances each of positive (e.g., “interesting”, “kind”), neutral (e.g., “sensible”, “reserved”), and negative feedback (e.g., “boring”, “annoying”) from the evaluator. All feedback was presented in a pseudorandom order with the constraint that no more than two adjectives of the same valence could be presented consecutively.
fMRI Data Acquisitio
Imaging data were acquired on a Siemens 3 Tesla “Tim Trio” MRI scanner housed at UCLA’s Staglin IMHRO Center for Cognitive Neuroscience. Foam padding was placed around the participants’ heads for comfort and to constrain head movement. A high-resolution T1-weighted echo-planar imaging volume (spin-echo, TR=5000ms; TE=33ms; matrix size 128×128; 36 axial slices; FOV=20cm; 3-mm thick, skip 1-mm) and T2-weighted, matched-bandwidth anatomical scan (slice thickness=3mm, gap=1mm, 36 slices, TR=5000ms, TE=34ms, flip angle=90°, matrix size 128×128, FOV=20 cm) were acquired for each participant, followed by a functional scan lasting 8 min, 38 sec (echo planar T2-weighted gradient-echo, TR=2000ms, TE=25ms, flip angle=90°, matrix size 64×64, 36 axial slices, FOV=20 cm; 3-mm thick, skip 1-mm).
Self-Reported Affect
Participants were asked to indicate how they felt (1 = really bad, 4 = really good) each time an adjective was selected during the feedback task by pressing a button on a scanner-compatible button box held in the dominant hand, which formed a measure of trial-by-trial affect. Participants were also asked to indicate how they felt at the very beginning and very end of the task (i.e., immediately before and immediately after the evaluation, also measured on a 4-point scale). These pre/post ratings formed a measure of overall affective responses to the task. Trial-by-trial affect ratings were missing for two participants due to computer problems, leaving a total sample of 106 participants with these ratings. Overall affect measures were missing from seven participants (leaving a total of 101), due either to computer problems, or the participant not making a response to either the pre- or post-task affect measure.
Inflammatory Measures
Whole blood samples were collected in pre-chilled EDTA tubes. After collection, the samples were centrifuged at 4 °C, plasma was harvested into multiple aliquots, and then stored in a -70 °C freezer until the completion of the study. Using a Bio-Plex 200 (Luminex) Instrument, Bio-Plex software v4.1, and a 5-parameter logistic curve fit, plasma levels of IL-6 and TNF-α were quantified by means of high sensitivity bead-based multiplex immunoassays (Performance High Sensitivity Human Cytokine, R&D Systems, Minneapolis, MN). This R&D Systems multiplex assay has been shown to have excellent intra- and inter-assay reproducibility for these two analytes in a recent temporal stability study of circulating cytokine levels (Epstein et al., 2013), and very strong correlations (r>.94) across a wide range of concentrations with high sensitivity ELISA kits from the same manufacturer (Breen et al., 2014). All multiplex assays were performed on plasma samples diluted 2-fold according to the manufacturer’s protocol, and all calculated concentrations generated by the BioPlex Manager software were included in data analyses. Paired samples from each subject (baseline and the pre-scan time point) were assayed on the same 96-well plate; multiplex assays were chosen for the analyses because of the large dynamic range necessary to evaluate both low physiologic (baseline) and very high post-endotoxin (pre-scan) cytokine concentrations in the same assay. The ranges of detection for IL-6 and TNF-α were 0.2–3800 pg/mL and 0.8–3100 pg/mL, respectively, and no samples exceeded the upper limit of detection for either analyte. Two participants were below the lower limit of detection on baseline IL-6 levels and are thus not included in IL-6 analyses. The mean intra-assay CV% of the standards was <8% for IL-6 and TNF-α; the inter-assay CV% of an internal laboratory quality control sample was <13% for both analytes.
Data Analysis
Inflammatory data
Full details of the overall effects of endotoxin administration on levels of pro-inflammatory cytokines are presented elsewhere (see Moieni et al., 2015). For between-group and correlational analyses in the present study, we focus on changes in IL-6 and TNF-α from the baseline timepoint (prior to injection) to the timepoint immediately preceding the MRI scan (approximately 2 hours after injection), given that levels of pro-inflammatory cytokines at this timepoint are likely contributing to differences in neural responses between the conditions. All cytokine data were natural log transformed prior to analyzing; however, for ease of interpretation, raw values are used for means and standard deviations reported in text and tables.
fMRI data
The preprocessing stream followed the DARTEL (Diffeomorphic Anatomical Registration Through Exponential Lie Algebra) procedure in SPM8 (Wellcome Department of Imaging Neuroscience, London) and involved realignment to correct for head motion, normalization of the T2-weighted matched bandwidth to warp the images into Montreal Neurologic Institute (MNI) space (resampled at 3x3x3mm) and spatial smoothing using an 8 mm Gaussian kernel, full width at half maximum, to increase signal-to-noise ratio. First-level effects were estimated using the general linear model to investigate neural activity to each type of feedback (positive, neutral, negative). Each block lasted approximately 10 seconds, starting from when the adjective button was selected by the confederate and lasting until the next button was selected. Random effects analyses of the group were then computed using the first-level contrast images for each participant.
Given our a-priori hypotheses regarding the role of threat- and pain-related “neural alarm system” regions in responding to negative social feedback (compared to neutral), we conducted region-of-interest (ROI) analyses examining neural activity in neural regions previously associated with social threat and rejection: dorsal anterior cingulate cortex (dACC), bilateral anterior insula, and bilateral amygdala. Analyses examining neural responses to positive social feedback (compared to neutral) focused on regions previously implicated in reward processing: the bilateral ventral striatum, and the ventromedial prefrontal cortex (VMPFC). In addition, because the dorsomedial prefrontal cortex (DMPFC) is important for understanding the mental states of others (which is critical for a task in which one is being evaluated), we also examined how exposure to endotoxin altered DMPFC activity in response to receiving both positive and negative feedback (vs. neutral).
The dACC, anterior insula, amygdala, and ventral striatum ROIs were defined structurally based on the Automated Anatomical Labeling Atlas (Tzourio-Mazoyer et al., 2002) of the Wakeforest University Pickatlas (Maldjian et al., 2003). The boundaries for the dACC ROI used a rostral boundary of y=+32 on the basis of criteria established by Vogt and colleageus (Vogt et al., 2003) and a caudal boundary of y=0 (Way et al., 2009). For the anterior insula ROI, the caudal boundary was y=+8 to correspond with agranular insula (Ongur et al., 2003). The boundaries for the amygdala were: -32<x<-12, -12<y<4, -24<z<-8 for the left, and 12<x<32, -21<y<4, -24<z<-8 for the right. The boundaries for the ventral striatum ROI were defined by combing the caudate and putamen and then constraining the regions to -10<x<10, 4<y<18, -12<z<0 (Inagaki et al., 2015). VMPFC and DMPFC ROIs were constructed manually on a voxel-by-voxel basis in FSLview using AAL for reference, guided primarily by divisions outlined by Amodio & Frith (2006) of functional boundaries within PFC. The boundaries for the VMPFC were: -18<x<18, 36<y<68, -34<z<-12, and for the DMPFC were: -18<x<18, 30<y<60, 24<z<58.
Because we did not have hypotheses regarding laterality effects in the limbic regions (anterior insula, amygdala, ventral striatum), we averaged activity in the left and right hemispheres for these regions. To assess any potential sex differences in the current results, preliminary analyses included sex as an independent variable. Sex did not moderate any of the observed associations between feedback type and condition for any of the ROIs, inflammatory responses, or affective responses (all ps> .15), and as such, analyses reported here examine both males and females together.
Results
Inflammatory Responses
As reported previously (Moieni et al., 2015), there was a significant time (baseline vs. ~2 hours post injection) by condition (endotoxin vs. placebo) interaction for levels of IL-6 and TNF-α (for IL-6: F(1,104)=294.96, p<.001; for TNF-α: F(1,106)=587.70, p<.001). Specifically, participants in the endotoxin condition showed a greater increase from baseline to approximately 2 hours post-injection in both IL-6 (M diff=141.85, SD=164.56) and TNF-α (M diff=146.03, SD=105.11), compared to those in the placebo condition (M diff IL-6=0.58, SD=1.58, t(104)=17.17, p<.001; M diff TNF-α =-.19, SD=3.78; t(106)=-24.24, p<.001).
Self-Reported Affective Responses
Next, we examined if the different types of feedback provided during the social feedback task were associated with trial-by-trial changes in self-reported affect. A mixed ANOVA with feedback type (positive, negative, neutral) as a within-subjects factor and group (endotoxin vs. placebo) as a between-subjects factor revealed a significant main effect of feedback type (F(2,208)=662.74, p<.001), but no main effect of condition (F(1,104)=.012, p=.91) or feedback by condition interaction (F(2,208)=.87, p=.42). To ensure that the feedback was eliciting the intended affective responses, we conducted follow-up tests comparing responses to the negative vs. neutral feedback, and the positive vs. neutral feedback. Participants reported feeling more negative in response to the negative feedback compared to the neutral feedback (t(105)=-24.16, p<.001) and more negative in response to the neutral feedback compared to the positive feedback (t(105)=13.04, p<.001). Thus, participants in both the endotoxin and placebo group reported feeling worse in response to the negative feedback (compared to neutral), and better in response to the positive feedback (compared to neutral; see Table 1 for all self-report comparisons).
Table 1.
Effects of an inflammatory challenge (endotoxin vs. placebo) on levels of pro-inflammatory cytokines, as well as affective responses to the social feedback task.
| Measure | Endotoxin (Mean, SD) | Placebo (Mean, SD) |
|---|---|---|
| Baseline (pre-injection) IL-6 | 2.68 (3.80) | 2.14 (1.64) |
| Pre-Scan (post-injection) IL-6 | 129.73 (124.07) | 2.72 (1.64) |
| Baseline (pre-injection) TNF-α | 9.30 (13.91) | 7.54 (4.83) |
| Pre-Scan (post-injection) TNF-α | 156.36 (105.05) | 7.35 (2.64) |
| Pre-Social Feedback Task Affect | 2.98 (.86) | 3.29 (.54) |
| Post-Social Feedback Task Affect | 2.65 (.87) | 2.82 (.73) |
| Affective Response to Negative Feedback Trials | 1.47 (.46) | 1.56 (.60) |
| Affective Response to Positive Feedback Trials | 3.66 (.34) | 3.61 (.36) |
| Affective Response to Neutral Feedback Trials | 3.14 (.47) | 3.09 (.45) |
Note. IL-6= interleukin-6; TNF-α= tumor necrosis factor alpha. All cytokine values are in pg/mL. Affective responses were assessed via responses to the question “How do you feel right now?” rated on a 1-4 scale (1=really bad, 4=really good).
Turning to the overall affective response to the task (i.e., pre/post scan ratings), we conducted mixed ANOVAs with time as a within-subjects factor (pre/post task) and condition (endotoxin vs. placebo) as the between-subjects factor. Results revealed a significant main effect of time, F(1, 99)=34.23, p<.001, reflecting the fact that negative affect increased from pre- to post-task. There was also no main effect of condition (F(1,99)=3.24, p=.065), and no interaction between time and condition (F(1,99)=.65, p=.42), indicating that participants in both conditions showed a similar increase in negative affect in response to the feedback.
Neural Responses to Negative Social Feedback
Next, we examined if exposure to an inflammatory challenge (endotoxin) led to differences in neural activity to negative feedback vs. neutral feedback. To do this, we conducted a mixed ANOVA with feedback type (negative, neutral) as a within-subject factor and group (endotoxin vs. placebo) as the between-subjects factor, for neural activity in “neural alarm system” regions (i.e., dACC, bilateral anterior insula, bilateral amygdala; Eisenberger & Lieberman, 2004) and the DMPFC, a region critical for thinking about the thoughts and feelings of others. We hypothesized that participants in the endotoxin condition would show greater activity in these regions during negative feedback (vs. neutral) compared to those in the placebo condition. Because we were particularly interested in the feedback by group interactions (to examine if the inflammatory challenge was affecting neural responses to the different types of feedback), we list and decompose (with paired-samples t-tests within each group) any significant interactions first, followed by listing any significant main effects.
dACC Responses
For the dACC, we observed a significant feedback by condition interaction (F(1,105)=4.40, p=.019). Follow-up t-tests within each condition revealed that participants in the endotoxin condition showed significantly greater dACC activity to negative vs. neutral feedback (t(55)=1.95, p=.028), whereas participants in the placebo condition showed no difference in dACC activity to negative vs. neutral feedback (t(50)=-1.58, p=.11). There was no significant main effect of feedback type on dACC activity (F(1,105)=.005, p=.95), and there was no significant main effect of condition (F(1,105)=1.95, p=.083). (See Figure 1 and Table 2a for all the ROI analyses for negative vs. neutral feedback listed below).
Figure 1.
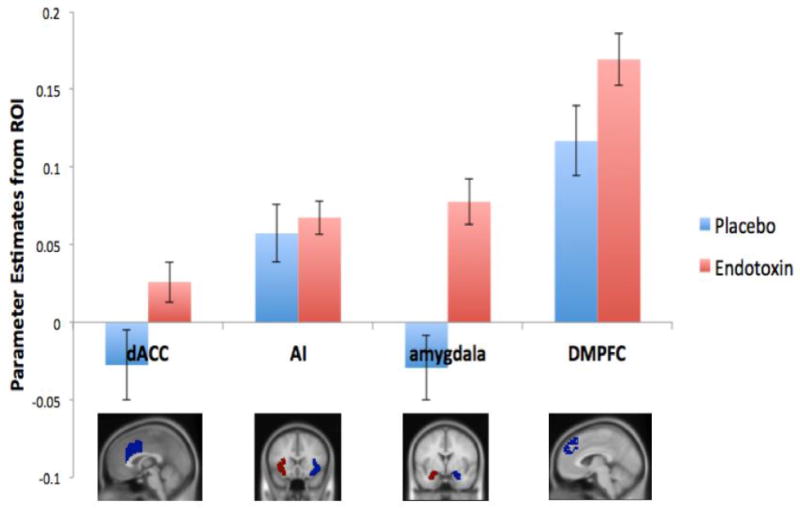
Neural responses in the dACC, anterior insula (AI), amygdala, and DMPFC to negative social feedback (vs. neutral) as a function of condition (endotoxin vs. placebo).
Table 2.
Neural responses to: A) negative vs. neutral feedback in threat/pain-related (dACC, anterior insula, amygdala) and mentalizing-related neural regions (DMPFC) and B) positive vs. neutral feedback in reward-related (ventral striatum, VMPFC) and mentalizing-related neural regions (DMPFC).
| A) Neural Region-of-Interest | Negative Feedback (Mean, SD) | Neutral Feedback (Mean, SD) |
|---|---|---|
| Endotoxin | ||
| dACC | .08 (.55) | .05 (.54) |
| Anterior Insula | .14 (.45) | .07 (.44) |
| Amygdala | .13 (.69) | .05 (.70) |
| DMPFC | .18 (.63) | .01 (.62) |
| Placebo | ||
| dACC | .20 (.59) | .23 (.57) |
| Anterior Insula | .29 (.48) | .23 (.51) |
| Amygdala | .11 (.65) | .14 (.66) |
| DMPFC | .26 (.67) | .14 (.69) |
| B) Neural Region-of-Interest | Positive Feedback (Mean, SD) | Neutral Feedback (Mean, SD) |
| Endotoxin | ||
| Ventral Striatum | -.12 (.47) | -.16 (.49) |
| VMPFC | -.07 (.77) | -.17 (.78) |
| DMPFC | .04 (.61) | .01 (.62) |
| Placebo | ||
| Ventral Striatum | -.10 (.62) | -.09 (.66) |
| VMPFC | -.17 (.73) | -.17 (.77) |
| DMPFC | .07 (.65) | .14 (.69) |
Anterior Insula Responses
For the anterior insula, there was no significant feedback by condition interaction (F(1,105)=.24, p=.31), indicating that participants in the endotoxin and placebo conditions showed similar patterns of anterior insula activity to the negative and neutral feedback. There was a significant main effect of feedback type (F(1,105)=35.50, p<.001), with greater neural activity to negative compared to neutral feedback, as well as a significant main effect of condition (F(1,105)=2.93, p=.045), with placebo participants again showing overall greater activity in the anterior insula (regardless of feedback type) compared to endotoxin participants.
Amygdala Responses
For the amygdala, we observed a significant feedback by condition interaction (F(1,105)=18.08, p<.001), which was driven by participants in the endotoxin condition showing significantly greater amygdala activity to the negative vs. neutral feedback (t(55)=5.24, p<.001), whereas participants in the placebo condition showed no difference in amygdala activity to the neutral vs. negative feedback (t(50)=1.41, p=.082). There was also a significant main effect of feedback type (F(1,105)=3.70, p=.029), with greater amygdala activity to the negative compared to neutral feedback, and no main effect of condition (F(1,105)=.081, p=.39).
DMPFC Responses
Finally, there was also a significant feedback by condition interaction for activity in the DMPFC (F(1,105)=3.58, p=.031). Follow-up repeated-measures t-tests revealed that endotoxin participants showed a larger increase in DMPFC activity to negative vs. neutral feedback (t(55)=10.05, p<.001) compared to placebo participants (t(50)=5.19, p<.001). In terms of main effects, there was significant main effect of feedback (F(1,105)=105.95, p<.001), with greater DMPFC activity to negative compared to neutral feedback, but no main effect of condition (F(1,105)=.79, p=.19).
Neural Responses to Positive Social Feedback
We also examined if inflammation led to differences in neural activity to positive feedback vs. neutral feedback. Thus, we conducted a mixed ANOVA with feedback type (positive, neutral) as a within-subject factor and group (endotoxin vs. placebo) as the between-subjects factor, for neural activity in reward-related structures (i.e., bilateral ventral striatum, VMPFC), and the DMPFC, a key mentalizing-related neural region.
Ventral Striatum Responses
For the ventral striatum, we observed a significant feedback by condition interaction (F(1,105)=4.24, p=.021), which was driven by the fact that participants in the endotoxin condition showed significantly greater ventral striatum activity to the positive vs. neutral feedback (t(55)=2.89, p<.001), while participants in the placebo condition showed no difference in ventral striatum activity to positive vs. neutral feedback (t(50)=.41, p=.34). There was no main effect of feedback type (F(1,105)=.94, p=.083), and no main effect of condition (F(1,105)=.19, p=.33). (See Figure 2 and Table 2b for all the ROI analyses for positive vs. neutral feedback listed below).
Figure 2.
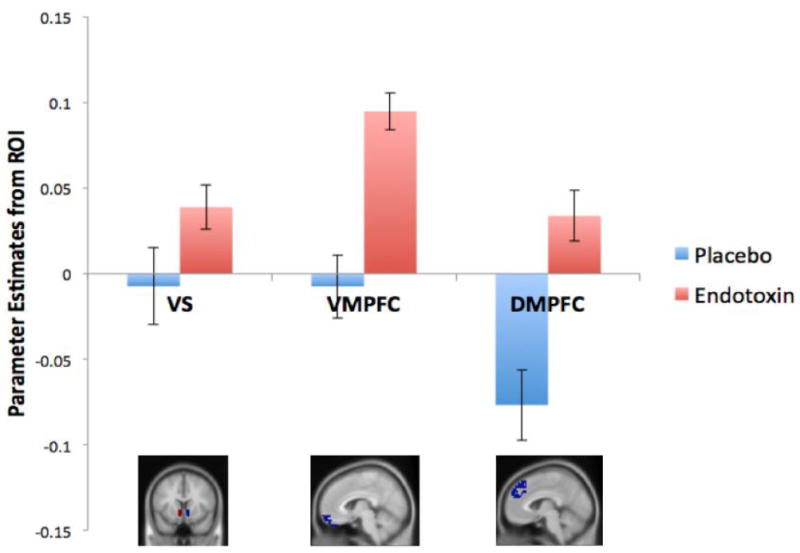
Neural responses in the ventral striatum (VS), VMPFC, and DMPFC to positive social feedback (vs. neutral) as a function of condition (endotoxin vs. placebo)
VMPFC Responses
Turning to the VMPFC, there was again a significant feedback by condition interaction (F(1,105)=9.39, p<.001). Similar to the VS findings above, the interaction was driven by participants in the endotoxin condition showing significantly greater VMPFC activity to the positive vs. neutral feedback (t(55)=4.09, p<.001), while participants in the placebo condition showed no difference in VMPFC activity to positive vs. neutral feedback (t(50)=.31, p=.38). In addition, there was also a significant main effect of feedback type (F(1,105)=6.82, p=.005), with greater VMPFC activity to the positive feedback compared to the neutral feedback, and no main effect of condition (F(1,105)=.13, p=.36).
DMPFC Response
Finally, for the DMPFC, there was also a significant feedback by condition interaction (F(1,105)=15.43, p<.001). Follow-up t-tests within each condition revealed that participants in the endotoxin condition showed significantly greater DMPFC activity to the positive vs. neutral feedback (t(55)=1.90, p=.032), whereas participants in the placebo condition showed significantly greater DMPFC activity to the neutral feedback (compared to positive; t(50)=-3.48, p<.001). There was no main effect of feedback type (F(1,105)=2.33, p=.065), and no main effect of condition (F(1,105)=.45, p=.25).
Correlations Between Neural and Inflammatory Responses
To examine the relationship between inflammatory reactivity to endotoxin and neural responses to negative and positive social feedback, we conducted correlational analyses for participants in the endotoxin condition. Specifically, we examined correlations between changes in levels of pro-inflammatory cytokines (IL-6, TNF-α) in response to endotoxin (pre-scan – baseline), and neural activity in each of the ROIs. In response to negative (vs. neutral) feedback, we observed significant correlations between levels of IL-6 and neural activity in the anterior insula (r(53)=.25, p=.03), the amygdala (r(53)=.30, p=.01), and the DMPFC (r(53)=.27, p=.02; see Figure 3). There was no significant relationship between levels of IL-6 and neural activity in the dACC in response to negative feedback (r(53)=.13, p=.17). There were also no relationships between levels of TNF-α and activity in any of the ROIs in response to negative feedback (all r <.12, p>.19). With regard to positive feedback, there were no significant relationships between levels of IL-6 or TNF-α with activity in the ventral striatum, VMPFC, or DMPFC (all r<.11, p>.21).
Figure 3.
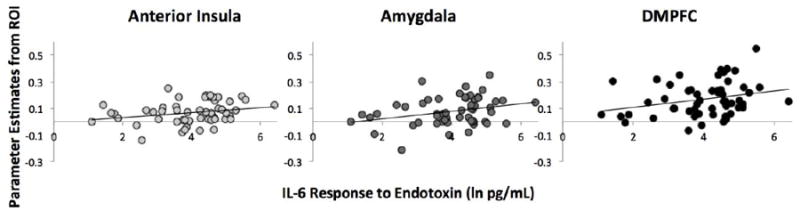
Scatter plot showing the significant associations between levels of IL-6 and activity in the anterior insula, amygdala, and DMPFC in response to negative social feedback (vs. neutral) for participants in the endotoxin condition.
Discussion
Results from the present study suggest that individuals exposed to an inflammatory challenge show greater neural sensitivity to both positive and negative social feedback. Specifically, individuals exposed to endotoxin exhibited greater neural activity in the dACC and amygdala, key pain- and threat-related neural regions involved in a “neural alarm system,” (Eisenberger & Lieberman, 2004) in response to receiving negative (vs. neutral) feedback. Interestingly, endotoxin also led to greater neural activity in reward-related neural regions (i.e., ventral striatum, VMPFC) in response to receiving positive (vs. neutral) social feedback. Finally, exposure to the inflammatory challenge was also associated with heightened DMPFC reactivity to both negative and positive social feedback (compared to neutral), highlighting the possible role of inflammation in modulating activity of this key mentalizing-related neural region in response to valenced social feedback. Taken together, these results add to a growing literature suggesting that sickness affects the way the brain responds to social and affective information, which has important implications for our understanding of the consequences of inflammatory-induced illness.
With regard to negative social feedback, we found that while placebo participants showed no significant difference in activity in the dACC or amygdala in response to negative (vs. neutral) feedback, individuals in the endotoxin condition showed significantly greater activity in these regions in response to the negative (vs. neutral) feedback. These results are consistent with prior work that has also shown that endotoxin leads to greater amygdala reactivity to socially threatening images (Inagaki et al., 2012), and that greater inflammatory responses to endotoxin are associated with greater dACC activity to social exclusion (Eisenberger et al., 2010). Thus, it appears that an inflammatory challenge increases neural reactivity to negative social experiences and information. This enhanced neural sensitivity to social threat may be adaptive, given that threatening conspecifics may signal the potential for further injury or infection for an already sick individual, and thus the brain may be especially “primed” during sickness to detect negative, threatening social information.
Although inflammation has well-known anhedonic effects such that increased inflammation is associated with reduced reward-related responding (Capuron et al., 2012; Eisenberger et al., 2010), the current results suggest that the role of inflammation in reward processing may be more nuanced. Indeed, we have previously shown that experimentally induced inflammation (vs. placebo) leads to greater, rather than less, ventral striatum activity in response to images of close, supportive others (Inagaki et al., 2015) and in the present study we found that endotoxin (vs. placebo) was associated with greater ventral striatum activity to positive, rewarding feedback from a stranger. Why might an inflammatory challenge lead to seemingly opposite effects on reward-related responding? Neural responding may be more sensitive to the context under which stimuli are being processed. That is, exposure to endotoxin in the current paradigm leaves one sick and potentially vulnerable to threats in the environment. Thus, this state may lead to heightened rather than reduced sensitivity to cues of potential care, closeness, and safety (such as positive feedback from another person), while simultaneously leading to reduced sensitivity to other rewarding outcomes (i.e., winning money) that are not immediately relevant to recovery. However, future work is needed to assess the behavioral implications of heightened reward-related responding to socially relevant rewards, to know whether inflammation also leads to altered behavior in the face of positive social feedback.
Interestingly, results from the present study also add to a growing literature suggesting that inflammation also influences neural activity in regions involved in mentalizing, or attending to the thoughts and feelings of others. Specifically, we found that individuals in the endotoxin condition showed greater activity in the DMPFC, a core node of the mentalizing network, in response to both negative and positive social feedback (vs. neutral), compared to those in the placebo condition. These results are consistent with recent work by Kullmann and colleagues (Kullman et al., 2015), which also found that individuals exposed to an inflammatory challenge showed greater activity in neural regions implicated in mentalizing (i.e., MPFC, superior temporal sulcus, temporal parietal junction) during a social cognition task. However, another recent study suggests that inflammation is related to poorer behavioral performance on a task involving identifying the emotional states of others (Moieni et al., 2015), suggesting a possible divergence between neural and behavioral findings. It may be the case that inflammation leads to greater engagement of neural regions involved in mentalizing, but that this activation does not necessarily lead to more accurate social cognition. Much more work is needed to fully specify how inflammation affects social cognition and activation in neural systems supporting such processes, but data from the present study and some past research suggest that exposure to an inflammatory challenge may lead to greater activation in brain regions involved in attending to the thoughts and feelings of others.
In addition to exploring group differences in neural responses to social feedback as a function of endotoxin exposure compared to placebo, we also examined if, within individuals exposed to endotoxin, there was a relationship between circulating levels of pro-inflammatory cytokines and neural reactivity. We found that individuals who showed a larger increase in levels of IL-6, a key inflammatory mediator, also showed greater neural responses in the anterior insula, the amygdala, and the DMPFC in response to the negative (vs. neutral) social feedback. We did not find a relationship between TNF-α levels and neural activity, nor did we find an association between levels of pro-inflammatory cytokines (IL-6 or TNF-α) and neural responses to the positive (vs. neutral) social feedback. One possible reason for a lack of relationship between TNF-α responses to endotoxin and neural reactivity is related to variability; there was much more variability in participants’ IL-6 responses (range=.05-934.11 pg/mL) compared to their TNF-α responses (range=17.79-619.11 pg/mL) to endotoxin, thus leaving a somewhat more restricted range within which to examine relationships between TNF-α and neural activity. It is also interesting that we did not see a relationship between plasma levels of cytokines and neural reactivity to the positive social feedback, despite seeing an overall difference in ventral striatum, VMPFC, and DMPFC activation between those in the endotoxin group compared to placebo. It is possible that endotoxin-induced changes in other markers of immune activation not measured in the current study (i.e., anti-inflammatory cytokines, chemokines) or in other physiological parameters (e.g., metabolic changes, hormonal changes) may be more tightly linked with neural responses to positive feedback, though future research is needed to address this possibility.
While the present study presents some intriguing associations between exposure to an inflammatory challenge and alterations in neural responses to social feedback, it remains unclear, what, exactly, is being altered in the body and the brain to bring about these changes in neural reactivity. One possibility that has received some attention in the animal literature suggests that exposure to endotoxin activates indoleamine 2,3-dioxygenase (IDO), an enzyme that is induced by pro-inflammatory cytokines and is important for the degradation of tryptophan, the primary amino-acid precursor of serotonin (O’Connor et al., 2009; Raison et al., 2010). It is also possible that individual-differences in cholinergic signaling may modulate the association between peripheral levels of pro-inflammatory cytokines and neural reactivity, as vagus nerve stimulation and corresponding increases in acetylcholine have been shown to have anti-inflammatory effects (Pavlov & Tracey, 2005). While we did not measure levels of tryptophan, vagal tone, or acetylcholine in the present study, it will be interesting for future work to explore these additional physiologic pathways as mechanisms and modulators of immune-to-brain signaling in humans, and their potential role in influencing neural responses to social information during times of sickness.
In sum, results from this study suggest that an acute inflammatory challenge may sensitize individuals to evaluative feedback communicated by social agents, and thus lead to greater activation in both threat/pain and reward-related neural regions, in addition to mentalizing regions, in response to negative and positive social feedback. This heightened sensitivity to social information makes sense from an adaptive perspective, as when in a vulnerable state, individuals should be especially attuned to cues of possible additional threats (i.e., negative social feedback), but also to signals of possible help or support (i.e., positive social feedback).
Data from the present study should be interpreted in light of some important limitations. First, we did not have comparable non-social threat or reward tasks, so the extent to which the present effects are specific to positive and negative social feedback remains unknown. It will be important for future research to directly compare neural reactivity to social vs. non-social threat and reward, to examine the specificity of these effects to social stimuli. Second, we did not find any interactions between condition and feedback on self-reported affective responses, suggesting that while there was greater neural activity in threat and reward-related regions to the valenced social feedback among individuals exposed to endotoxin, this did not translate into differences in self-reported feelings. It is possible our measure of self-reported affect was not sensitive enough to capture the effects of inflammation on affective experience, that participants in the endotoxin condition were experiencing ceiling effects on negative affect, or perhaps the effects of inflammation operate at a more automatic level (reflected in differential patterns of neural activity) but not at the level of awareness. Despite these limitations, the present results add to a growing literature suggesting that endotoxin influences the way the brain responds to social information, increasing activity in regions involved in threat and pain during negative social feedback, while simultaneously increasing activity in regions involved in reward during positive social feedback. These data converge on the intriguing possibility that inflammation may increase neural sensitivity to social information, which may be adaptive for promoting recuperation and recovery following injury or infection.
Acknowledgments
This research was funded by an R01 from NIMH to NIE (5R01MH091352). The authors also acknowledge the additional support provided by R01AG034588; R01AG026364; R01CA160245-01; R01CA119159; R01HL095799; R01DA032922-01; P30AG028748 to M.R.I., as well as the National Center for Advancing Translational Sciences UCLA CTSI Grant UL1TR000124, and the Cousins Center for Psychoneuroimmunology. We would like to thank the staff and support of the UCLA Clinical and Translational Research Center, the UCLA Staglin IMHRO Center for Cognitive Neuroscience, and Anthony Suffredini, M.D. at the National Institutes of Health, Warren Grant Magnuson Clinical Center, for providing the standard reference endotoxin. We also acknowledge the contributions of Kira Adsit, Kyla Agbayani, Matthew Dutcher, Elizabeth Glanzer, Ross Perry and Lauren Yang, who served as research assistants on the project.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Breen EC, Perez C, Olmstead R, Eisenberger NI, Irwin MR. Comparison of multiplex immunoassays and ELISAs for the determination of circulating levels of inflammatory cytokines. Brain, Behavior, and Immunity. 2014;40:e39. [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Miller AH, et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Archives of General Psychiatry. 2012;69:1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. The complexity of dynamic host networks. In: Deisboeck TS, Kresh JY, editors. Complex Systems Science in BioMedicine. Kluwer Academic; New York: 2006. pp. 605–629. [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior, and Immunity. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: When the immune system subjugates the brain. Nature Reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Garza R. Endotoxin-or pro-inflammatory cytokine-induced sickness behavior as an animal model of depression: focus on anhedonia. Neuroscience & Biobehavioral Reviews. 2005;29:761–770. doi: 10.1016/j.neubiorev.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: Endotoxin reduces ventral striatum responses to reward. Biological Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Mashal NM, Irwin MR. Inflammation and social experience: An inflammatory challenge induces feelings of social disconnection. Brain, Behavior, and Immunity. 2010;24:558–563. doi: 10.1016/j.bbi.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. An fMRI study of cytokine-induced depressed mood and social pain: The role of sex differences. NeuroImage. 2009;47:881–890. doi: 10.1016/j.neuroimage.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: A common neural alarm system for physical and social pain. Trends in Cognitive Sciences. 2004;8:264–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Epstein MM, Breen EC, Magpantay L, Detels R, Lepone L, Penugonda S, Birmann BM. Temporal stability of serum concentrations of cytokines and soluble receptors measured across to years in low-risk HIV-seronegative men. Cancer Epidemiology, Biomarkers, and Prevention. 2013;22:2009–2015. doi: 10.1158/1055-9965.EPI-13-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Cercignani M, Voon V, Critchley HD. Effects of inflammation on hippocampus and substantia nigra responses to novelty in healthy human participants. Neuropsychopharmacology. 2015;40:831–838. doi: 10.1038/npp.2014.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Cole SW, Eisenberger NI. Inflammation selectively enhances amygdala activity to socially threatening images. NeuroImage. 2012;59:3222–3226. doi: 10.1016/j.neuroimage.2011.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki TK, Muscatell KA, Irwin MR, Moieni M, Dutcher JM, Jevtic I, Breen EC, Eisenberger NI. The role of the ventral striatum in inflammatory-induced approach toward support figures. Brain, Behavior, and Immunity. 2015;44:247–252. doi: 10.1016/j.bbi.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann JS, Grigoleit JS, Wolf OT, Engler H, Oberbeck R, Elsenbruch S, et al. Experimental human endotoxemia enhances brain activity during social cognition. Social Cognitive and Affective Neuroscience. 2014;9:786–793. doi: 10.1093/scan/nst049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JB, Kraft RA. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Breen EC, Eisenberger NI. Inflammation impairs social cognitive processing: A randomized controlled trial of endotoxin. Brain, Behavior, and Immunity. 2015;48:132–138. doi: 10.1016/j.bbi.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: Implications for sex differences in depression. Neuropsychopharmacology. 2015;40:1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. Journal of Comparative Neurology. 2003;460:425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-diosygenase activation in mice. Molecular Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain, Behavior, and Immunity. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Nature Reviews Immunology. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G, Spivey JR, Saito K, Miller AH. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-α: Relationship to CNS immune responses and depression. Molecular Psychiatry. 2010;15:393–403. doi: 10.1038/mp.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, et al. Cytokine-associated emotional and cognitive disturbances in humans. Archives of General Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Stone EA, Lehmann ML, Lin Y, Quartermain D. Depressive behavior in mice due to immune stimulation is accompanied by reduced neural activity in brain regions involved in positively motivated behavior. Biological Psychiatry. 2006;60:803–811. doi: 10.1016/j.biopsych.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. European Journal of Neuroscience. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Taylor SE, Eisenberger NI. Variation in the mu-opioid receptor gene (OPRM1) is associated with dispositional and neural sensitivity to social rejection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15079–15084. doi: 10.1073/pnas.0812612106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee JR, Prendergast BJ. Sex-specific regulation of inflammatory responses and sickness behaviors. Brain, Behavior, and Immunity. 2010;24:942–951. doi: 10.1016/j.bbi.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


