Abstract
Background
Many studies have examined the multiple correlates of non-adherence in Blacks. However, they are largely cross-sectional thus; these studies are unable to examine their predictive value on long-term medication adherence.
Purpose
To examine the predictive role of key psychosocial and interpersonal factors on changes in medication adherence over a one-year period.
Methods
Data were collected from 815 Black patients with hypertension followed in community health centers. Hypothesized predictor variables included self-efficacy, depressive symptoms, social support, and patient-provider communication measured at baseline, 6- and 12-months. The dependent variable, medication adherence was assessed at baseline, 6- and 12-months. Latent Growth Modeling was used to evaluate the pathways between the latent predictor variables and medication adherence.
Results
Participants were mostly female, low-income, with high school education or less, and mean age of 57 years. At baseline, high self-efficacy was associated with low depressive symptoms (β=−.22, p=0.05), collaborative patient-provider communication (β=.17, p=0.006), and better medication adherence (β=1.04, p<0.001). More social support and collaborative patient-provider communication were associated with low depressive symptoms (β=−.08, p=0.02; β=−.18, p=0.01). More social support was positively associated with collaborative patient-provider communication (β=.32, p<0.001). In the longitudinal model, increasing self-efficacy over time predicted improvements in medication adherence one year later (β=1.76, p<0.001; CFI=0.95; RMSEA=0.04; SRMR=0.04; Chi-Squared Index of Model Fit=1128.54).
Conclusions
Self-efficacy is a key predictor of medication adherence over time in Black patients with hypertension. Initial levels of self-efficacy are influenced by the presence of depressive symptoms as well as the perceived quality of patient-provider communication.
Keywords: Medication Adherence, Latent Growth Modeling, Self-Efficacy, Hypertension, African American
Background
The disproportionately higher rate of hypertension (HTN) and its related cardiovascular mortality between Black and White patients is well-documented.(1) Poor adherence to prescribed antihypertensive medications has been indicated as a major contributor to poor blood pressure (BP) control in Blacks.(2) As compared to their White counterparts, Blacks with HTN have been shown to be 1.81 to 4.30 times less likely to adhere to their antihypertensive medications, depending on the measure of adherence.(2-4) Given that sufficiently high levels of adherence is key for achieving adequate BP control, it follows that successful approaches to reducing the racial gap in HTN-related mortality that exists between Blacks and Whites must take into consideration the factors driving poor medication adherence in Blacks.
Previous studies have examined multiple correlates of poor medication adherence in Black patients with HTN including psychosocial and demographic characteristics of the patient, clinical characteristics of the medical regimen, and factors related to the healthcare environment.(5, 6) Although these studies provide some insight into the reasons for the higher rates of poor adherence in this patient population, the findings are largely limited to cross-sectional designs and thus, are unable to determine the predictive values of these factors on long-term medication adherence. Specifically, examining how these factors relate to adherence at one time point creates the assumption of constant adherence over time, which often does not hold. Rather, adherence behaviors are multi-dimensional thus; there is a need to examine the complex interactions between these various factors and their subsequent impact on changes in medication adherence over time.
The aim of the present study was to confirm and extend previous research by assessing the predictive role of key psychosocial (self-efficacy, depressive symptoms) and interpersonal factors (quality of patient-provider communication, social support) on changes in medication adherence among a sample of 815 hypertensive Black patients followed in 30 community health centers over a 12-month period. The direct and indirect effects of these factors on medication adherence were assessed using latent growth modeling.
Hypothesized Model of Medication Adherence
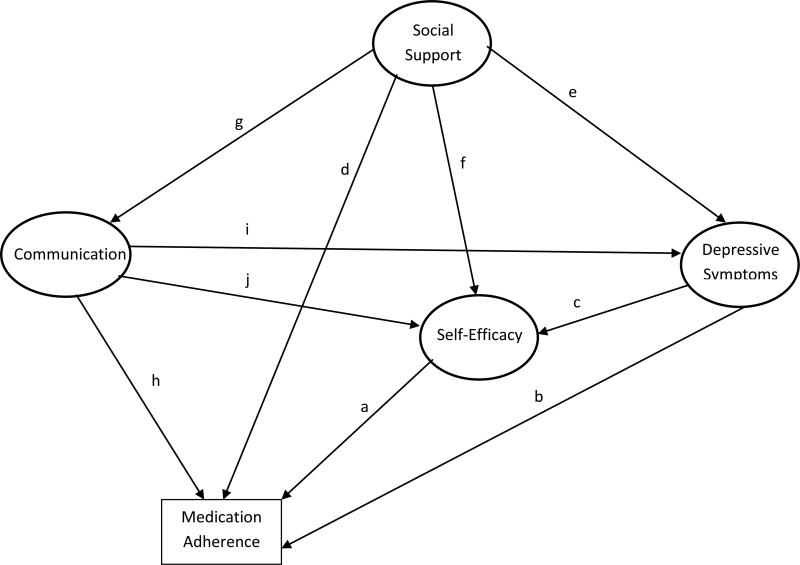
Salient findings from previous research guided the development of the hypothesized model of medication adherence tested in this study (Figure 1). Self-efficacy has consistently been associated with the adoption of, and adherence to, a variety of health-related behaviors including adherence to medications in patients with chronic diseases. (7) Individuals who have high levels of self-efficacy tend to be confident in their abilities to carry out a task (e.g., taking medications as prescribed), exert more effort when doing so, approach more challenging tasks, and persist longer in the face of obstacles and barriers. (7) As such, path a depicts the direct positive relationship between self-efficacy and medication adherence.
Figure 1.
Hypothesized (Measurement) Model of Medication Adherence
Depressive symptoms are another key psychosocial factor consistently found to be associated with medication adherence.(8) In patients with HTN, the presence of depressive symptoms is associated with significantly higher rates of poor medication adherence, after adjusting for the confounding effects of demographic, clinical, and other psychosocial variables.(9) In our previous work, we have also shown that Black hypertensive patients with depressive symptoms were less likely to adhere to their medications because of low self-efficacy.(10) Accordingly, path b depicts the direct negative effect of depressive symptoms on medication adherence, whereas path c represents the indirect impact of depressive symptoms on medication adherence through its negative relationship with self-efficacy.
Cross-sectional studies also suggest that social support is an important interpersonal factor associated with better medication adherence.(11, 12) Evidence from a recent meta-analysis of social support indicates that functional support (e.g., help with medication reminders, picking up medications), as opposed to structural support (e.g., marital status, living arrangements) may be the most important aspect of support for adherence to antihypertensive medications.(11) Path d denotes the direct positive association between social support and medication adherence. While the moderating mechanisms between social support and adherence are not clearly defined in hypertensive patients, studies in other chronic illnesses indicate there are many possible explanations. Social support has been shown to increase patient satisfaction with medical care(13) as well as self-efficacy.(14) Social support is also believed to affect adherence through its relationship with depressive symptoms.(15-17) In line with these findings, we hypothesize that social support also operates indirectly on adherence through its relationships with depressive symptoms and self-efficacy as depicted in paths e and f, respectively.(18, 19) Path g denotes the bidirectional relationship between social support and patient-provider communication.
Finally, increasing evidence suggests that collaborative patient-provider relationships are associated with better medication adherence among patients with chronic diseases (20) and Black patients in particular.(20, 21) Thus, the role of patient's perception of his/her provider's communication on medication adherence was also assessed (path h). Although the potential mechanisms underlying this relationship are not clear, cross-sectional studies suggest psychosocial factors may play a role.(22, 23) For example, medication adherence self-efficacy served as a mediator between physician support and medication non-adherence in a sample of chronically ill patients, such that supportive physicians increased patient's self-efficacy, which decreased the chance of non-adherence.(24) Recent evidence also suggests that patients with depressive symptoms are less likely to report providers gave adequate explanations, were responsive to their preferences, and incorporated patient-centered communication into the visit than non-depressed patients.(25, 26) As such, patients with depressive symptoms may be less likely to adhere to their medications because they negatively perceive and evaluate the effectiveness of their provider's communication skills in facilitating medication adherence. Accordingly, it was hypothesized that collaborative patient-provider communication predicts lesser depressive symptoms (path i) and higher self-efficacy (path j), which in turn predicts better medication adherence.
Methods
Participants
This study was conducted as part of a cluster randomized clinical trial, Counseling African Americans to Control Hypertension (CAATCH), conducted in 30 community health centers in the New York metropolitan area from 2004 to 2009. (27) The purpose of CAATCH was to evaluate the effectiveness of a multi-level intervention, targeted at patients and physicians, in improving BP control among Blacks with uncontrolled HTN. Patient eligibility for CAATCH included: a) self-identification as Black/African American; b) having a diagnosis of HTN (defined as an average BP≥140/90 mmHg on at least two previous visits in the prior year); c) uncontrolled BP at the eligibility visit (BP ≥140/90 mmHg or ≥130/80 mmHg for those with kidney disease or diabetes) based on the mean of the final two of three BP measurements taken using a valid automated BP monitor (BPTru); d) taking at least 1 antihypertensive medication; e) age 18 years or older; and f) fluency in English language.
The patient intervention included: (1) interactive patient education on hypertension; (2) home BP monitoring; and (3) monthly lifestyle counseling sessions (e.g., dietary intake, physical activity, adherence to treatment recommendations). The physician intervention included: (1) monthly continuing medical education on HTN management and (2) provision of feedback on patients’ home BP readings and chart audits. Patients and physicians at the enhanced usual care sites received printed patient education material and HTN treatment guidelines, respectively. The results of the trial have been published elsewhere (28). Briefly, the CAATCH trial found no intervention effect on BP control assessed by an automated monitor at 12-months. However, the intervention was associated with greater BP control for patients without diabetes and small-sized community health centers in pre-specified subgroup analyses as well as mean 12-month office systolic BP. In the CAATCH trial, medication adherence, assessed at baseline did not moderate the intervention effects.
A total of 1,058 patients were enrolled in the CAATCH trial, of which 243 (23%) patients were excluded from this ancillary study due to incomplete data on one of the primary study measures. The baseline analyses were conducted using an analytical sample of 815 patients. When we began to model the longitudinal relations among the variables, additional subjects had missing values at 6 and 12 months. Initially, we attempted to fit the latent growth models to all subjects with any available data. However, we were not able to obtain model convergence and appropriate parameter estimates using this strategy. Thus we elected to use only subjects with complete data for longitudinal models (N=463). All patients provided written informed consent approved by the Institutional Review Boards of Columbia University and New York University Langone Medical Centers.
Predictor Variables
Self-efficacy was assessed at baseline, 6-, and 12-months with a 26-item behavior specific scale targeted at adherence to antihypertensive medications.(29) Patients were asked to rate their confidence in taking their BP medications under a variety of situations that may pose difficulties to them (e.g., when experiencing side effects, traveling, or the cost increase), using a 4-point Likert scale (range: not at all sure to extremely sure). Higher scores reflect higher self-efficacy. The scale has good reliability and test-retest properties in a similar group of black patients with HTN (29, 30; α=0.92). In our sample, the Cronbach's alpha was 0.96. For this analysis, latent constructs were represented by five indicators, based on means of four randomly created parcels of five items and one randomly created parcel of 6 items taken from the total 26-item pool.
Depressive Symptoms were assessed at baseline, 6- and 12-months, using the well-validated Patient Health Questionnaire (PHQ)-9, which is based directly on the diagnostic criteria for major depressive disorder in the Diagnostic and Statistical Manual Fourth Edition.(30) Higher scores are indicative of greater depressive symptomatology (α=0.87). The latent construct of depressive symptoms was represented by three randomly created parcels of three items each.
Social Support was assessed at baseline with the well-validated 19-item Medical Outcomes Study (MOS) Social Support Survey,(31) which assesses four dimensions of social support: emotional/informational, tangible, affectionate, and positive social interaction (αs >0.91). Respondents rate how often they receive support for a particular dimension using a 5-point Likert scale (range: none of the time to all of the time). Higher scores for an individual dimension indicate more support. The latent construct of social support was represented by the four observed social support subscales listed above.
Patient-provider communication was assessed at baseline with a self-report scale that assesses patients’ perception of the quality of their providers’ communication and the extent to which the provider encourages patient participation in the treatment process (α=0.94 for this study sample).(32) Responses were based on a Likert-type scale ranging from 1 = not at all to 4 = very much. Higher scores indicate more collaborative communication (33; range: 1- 4). The latent construct of patient-provider communication was represented by three randomly created item parcels. Of these three item parcels two contained four items and the third contained five items.
Dependent Variable
Medication adherence was assessed as an observed variable at baseline, 6, and 12 months with the validated self-report scale developed by Morisky et al.,(34) which asks patients to respond “yes” or “no” to the following questions: a) Have you ever forgotten to take your medicine? b) Are you sometimes careless in regards to your medicine? c) Do you skip your medicine when you are feeling well? and d) When you feel badly due to the medicine, do you skip it? The Morisky scale has been used in other studies of black patients with HTN.(35-37) In our sample, the Cronbach's alpha was 0.67, which is consistent with the estimate of Morisky et al.(34)
Demographic data collected at the baseline visit included patient age, gender, marital status, employment status, educational and income level, and insurance status. Data on the number of prescribed antihypertensive medications was abstracted from patient's medical charts. Patient's systolic and diastolic BP was measured by trained research assistants using validated automated monitors and following standard American Heart Association guidelines.(38)
Analysis
Descriptive statistics and frequency distributions were used to describe all variables. Latent growth modeling was used to evaluate the pathways between the predictor variables and medication adherence depicted in the hypothesized model (Figure 1) with MPlus v.6.(39) Item parcels were created from the predictor scales and used as the observed indicants of the latent predictor variables. We chose to use parcels rather than individual items as indicators in the latent growth model because doing so help to reduce random error in indicators, leads to more reliable solutions, and maximizes model fit. (40) The use of parcels also helps to minimize the number of indicators required to define a latent variable in latent growth analyses. This is especially useful for measures such as the 26-item self-efficacy scale, which contains too many indicators to construct our latent self-efficacy terms.
With the exception of the social support measure, which had established subscales, the assignment of individual items to parcels was conducted using an online research randomizer. The dependent variable (medication adherence) was treated as an observed variable based on the sum of the four Morisky adherence items. The Morisky scale was treated as an observed variable because it has only four items and so cannot be parceled (at least three parcels are needed to provide an acceptable measurement model). Furthermore, the four items are dichotomous thus, if used individually in the analysis they must be treated as categorical items, which compromises the model fit. Using the hypothesized model delineated in Figure 1, the measurement model was evaluated based on the confirmatory factor analysis of the parcels with correlated factors. To test model fit, we constrained the residual variances of the indicators to the average of the three observed residual variances of medication adherence. The overall fit of the growth model for medication adherence was good (CFI=0.978, RMSEA=0.077, SRMR=0.070 and Chi-Square Index of Model Fit=11.246, p<.01) with all item parcels showing strong relationships to their designated latent constructs, indicating that the model is appropriate for the estimation in the latent growth model.
Latent growth modeling was then used to evaluate how changes in the predictor variables related to changes in adherence over the 12-month study period. The analytic process was as follows: First, baseline, 6-, and 12-month measures of self-efficacy, depressive symptoms and medication adherence were used as indicators for the latent slopes and intercepts of these variables in the model. In this analysis, the latent slope represents the change in medication adherence, depressive symptoms and self-efficacy as measured by the baseline, 6 month and 12 month values of these constructs. The latent intercept represents the baseline value of each construct based on the model of change of the construct. However, the actual baseline value of a construct (as represented in the cross-sectional model) and the initial level of a construct modeled by the intercept created from linear growth model are not necessarily equivalent. The baseline values in the cross sectional model are based on observed measures only. The intercept for the growth model is the predicted baseline value based on information from all three time points (baseline, 6 month, and 12 month). Since the intercept corresponds with the linear growth line fit during modeling, the intercept does not always exactly correspond to the observed baseline values of the construct.
Next, the latent slope and intercept of self-efficacy and depressive symptoms were regressed onto the slope and intercept of continuously measured medication adherence. Finally, baseline social support and patient-provider communication were entered as additional predictors of change in self-efficacy, depressive symptoms, and medication adherence. Patient age, gender, income level, and number of antihypertensive medications were also entered as covariates in the model. The Comparative Fit Index (CFI), Root Mean Square Error of Approximation (RMSEA), and Chi-Squared Index of Model Fit are reported as indices of model fit.
Since the current study was a sub-analysis of a multi-level intervention targeting therapeutic lifestyle changes for HTN control (e.g., dietary changes, physical activity, adherence to treatment recommendations), we also tested whether treatment group (intervention vs. control) affected the results. To examine whether the intervention had any effect on the psychosocial variables in this study we created a latent interaction variable between treatment group and the psychosocial constructs. This involved multiplying each item parcel by the dichotomous treatment variable and using the interaction terms as indicators of the latent interaction. Results of this analysis showed that self-efficacy, depressive symptoms, social support, and patient-provider communication did not consistently moderate the relation between intervention group and adherence at any of the time points (baseline, 6-months, and 12 months; ps>.05). The effect of treatment on medication adherence was also non-significant.
Complete data was available for 815 patients at baseline and 520 patients (64%) at 6-months and 593 patients (73%) at 12-months. Since complete data is required at all time-points to adequately measure latent growth, we calculated the Cohen's d to estimate the effect size difference due to missing data for each of the key predictor and socio-demographic variables (values ≤.20 are considered small). Patients with missing data were more likely to be younger (mean age: 56 vs. 28 years, p=.004, Cohen's d=.018), report having lower self-efficacy (mean: 2.37 vs. 2.24, p=.006, Cohen's d=.21) and being non-adherent to their medications at 12 months (Mean score: 4.05 vs. 3.7, p<.001, Cohen's d=.25).
Results
Patient demographics are shown in Table 1. Majority of the patients were female, with a mean age of 58 (SD±12.1) years. Approximately half had Medicaid, one-third had less than a high school education, two-thirds were unemployed, and most reported a household annual income < $20,000. The mean baseline BP was 151 (SD±20.9)/91 mmHg (SD±13.5) and mean number of prescribed antihypertensive medications was 2.19 (SD±1.04).
Table 1.
Characteristics of 815 Black Patients with Hypertensiona
| Characteristic | |
|---|---|
| Age, mean(SD) | 56.99 (12.18) |
| Gender, n (%) | 242 (29.1%) |
| Male | 589 (70.9%) |
| Female | |
| Race/Ethnicity, n (%) | |
| African American | 641 (76.9%) |
| West Indian/Caribbean | 143 (17.1%) |
| Multi-ethnic | 10 (1.2%) |
| Other | 19 (2.3%) |
| Marital Status, n (%) | |
| Never Married | 241 (28.9%) |
| Married | 208 (24.9%) |
| Divorced/Separated | 248 (29.8%) |
| Widowed | 128 (15.3%) |
| Education, n (%) | |
| Less than HS | 289 (34.7%) |
| High School/Technical School | 295 (35.4%) |
| Some College | 146 (17.5%) |
| College and above | 93(10.93%) |
| Employed, n (%) | |
| No | 550 (65.9%) |
| Yes | 259 (31.1%) |
| Baseline systolic blood pressure, mean (SD) | 149.45 (20.93) |
| Baseline diastolic blood pressure, mean (SD) | 89.78 (13.49) |
| Number of antihypertensive medications, mean (SD) | 2.19 (1.04) |
| Medication Adherence, mean (SD) | 1.07 (1.19) |
| Depressive Symptoms, mean (SD) | 4.74 (4.55) |
| Self-Efficacy, mean (SD) | 2.22 (0.59) |
| Social Support, mean (SD) | 68.21 (23.72) |
| Patient-provider Communication, mean (SD) | 2.20 (0.74) |
Race/ethnicity, marital status, education and employment do not add to 100% due to missing data at baseline
The mean medication adherence score at baseline was 1.07, with approximately half of the patients categorized as non-adherent, i.e. responded “yes” to at least one item (Table 1). At baseline, the mean PHQ-9 score was low (4.71, SD±4.55). The mean score on the medication adherence self-efficacy scale was 2.22 (range: 1 – 4); while the mean total score on the social support survey was 72.1 (SD±23.7, range: 0 – 100) and the mean patient-provider communication score was 2.16 (SD±0.89, range: 1 – 4).
Baseline Model
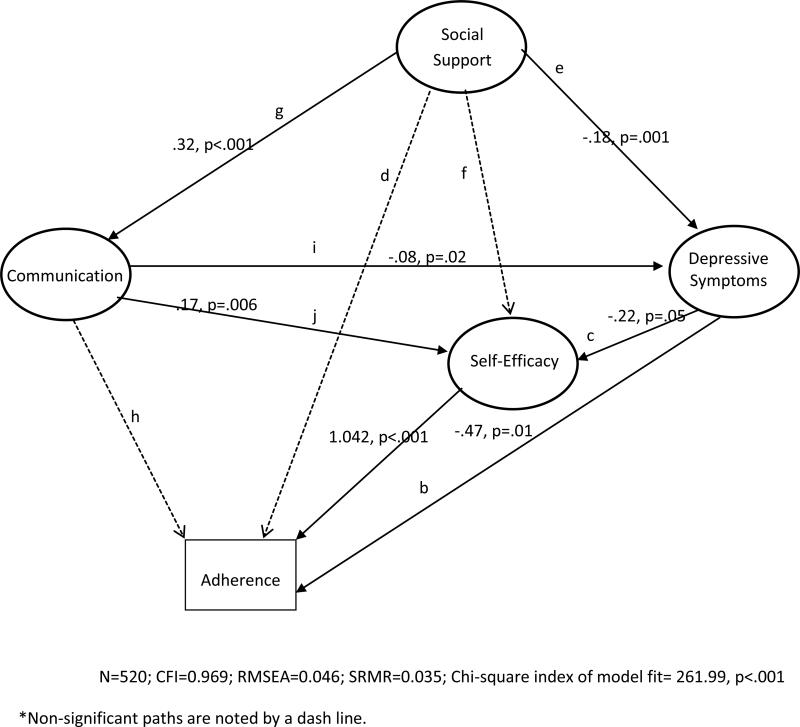
As shown in Figure 2, the baseline model yielded a well-fitting model (n=815, CFI=0.969, RMSEA=0.046, SRMR=0.035, Chi-square index of model fit=261.99, p<.001). As hypothesized, high self-efficacy and low depressive symptoms were associated with better medication adherence (path a; β=1.04, p<.001 and path b; β= −0.47, p=0.01, respectively). Depressive symptoms also exerted an indirect effect on medication adherence through its influence on self-efficacy; patients with low depressive symptoms at baseline also reported high self-efficacy (path c; β=−.22; p=.05). Social support and patient-provider communication were not directly associated with medication adherence at baseline (path d; β= −0.20, p=0.22 and path h; β=.06, p=0.57, respectively), as hypothesized. Examining the individual subscales of the social support measure did not change this finding (data not shown). Collaborative patient-provider communication was associated with high self-efficacy (path j; β=.17, p=0.006), low depressive symptoms (path i; β= −0.08, p=0.02), and more social support (path g; β=0.32, p<0.001). Social support was negatively associated with depressive symptoms (path e; β= −0.18, p=.001); there was no association with self-efficacy (path f; β=−0.11; p=.26).
Figure 2.
Baseline Model of Medication Adherence
Latent Growth Model
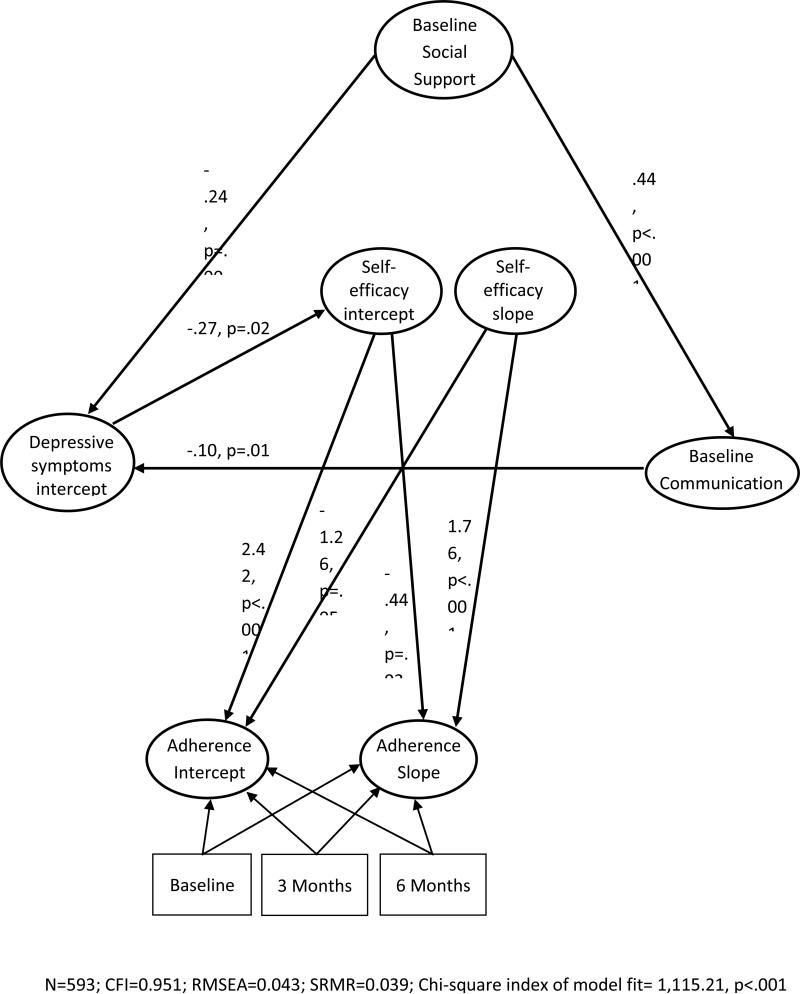
Latent growth models were conducted using the 6-month and 12-month data in addition to the baseline data (Figure 3). Results of this model had good indices of fit (n=463; CFI=0.951; RMSEA=0.043; SRMR=0.039; Chi-Squared Index of Model Fit=1115.21, p<0.001). Examination of the model paths showed that both the initial level (latent intercept) of self-efficacy as well as changes in self-efficacy (slope) over time predicted changes in medication adherence over the 12-month study period (intercept: β=−0.44, p=.03 and slope: β=1.76, p<.001). The positive regression path between the slopes of self-efficacy slope and medication adherence indicates that as self-efficacy increases over time, medication adherence also increases. The negative regression path between the intercept of self-efficacy and medication adherence slope indicates that individuals who start with higher levels of self-efficacy at baseline are less likely to show improvements in medication adherence over time.
Figure 3.
Latent Growth Model of 12 Month Medication Adherence
Contrary to the cross-sectional results, depressive symptoms were not directly associated with medication adherence in the longitudinal model. Rather, depressive symptoms continued to exert an indirect effect through its influence on patients’ initial level of self-efficacy (β = −0.27, p=0.02), which in turn predicted medication adherence. Finally, the associations between baseline patient-provider communication, social support, and depressive symptoms remained significant in the longitudinal model (communication and support: β=0.44, p<.001; communication and depressive symptoms: β=−.10, p=.01; support and depressive symptoms: β=−0.24, p=.003). Specifically, patients who rated communication with their provider as collaborative and had more social support also reported lower levels of depressive symptoms. Patient-provider communication was no longer associated with initial levels of self-efficacy (β=0.08, p=.16) or with changes in self-efficacy over time (β=−0.22, p=.54).
To determine the amount of change in the self-efficacy scale needed to exert a positive effect on changes in medication adherence, we ran secondary analysis that isolated these variables in the latent growth model. Results of this analyses showed that a one standard deviation increase on the self-efficacy scale over the 12-month period equates to a proportional 0.89 of a standard deviation increase in adherence on the Morisky scale. For reference, the mean standard deviation for medication adherence across all three time points was 1.13 units in the current analysis. This indicates that for every standard deviation increase in latent self-efficacy, adherence on the Morisky scale will improve by approximately one unit. Our findings suggest that patients who reported low self-efficacy and poor medication adherence at baseline experienced the greatest improvements in both variables over the 12-month period. Alternatively, patients who reported high self-efficacy and better medication adherence at baseline showed minimal increases in their scores over time (as indicated by the negative relationship between self-efficacy intercept and medication adherence slope). Finally, the average slope for medication adherence was 0.24 indicating that patient's self-reported adherence improved, on average, one-quarter of a point every three months. The average slope for the self-efficacy scale was 0.036, indicating that improvements in this scale over time were not as substantial.
To evaluate the causal direction of this pathway, we conducted a sensitivity analysis reversing the role of medication adherence and self-efficacy to test the alternative hypothesis that medication adherence behavior drives self-efficacy beliefs for adherence. To do so, medication adherence was entered as a predictor variable whereas the latent construct of self-efficacy at 12-months was entered as the primary outcome variable in the latent growth model. This reverse causation model resulted in non-convergence. To obtain convergence we had to eliminate covariates, but even then this led to over-estimation of the slope/intercept of self-efficacy (predicting more than 100% of the variance) and estimates that were not statistically possible (Heywood cases), which is usually caused by having too many factors in the model or a misspecification. We suspect this was due to the slope and intercept of medication adherence being derived from three observed variables whereas the slope and intercept of self-efficacy was derived from three latent variables (created with multiple parcels). In sum, this analysis indicates that the model is most reasonably estimated when changes in self-efficacy predict changes in medication adherence and not the reverse.
Discussion
This study assessed the predictive role of self-efficacy, depressive symptoms, social support and quality of patient-provider communication on medication adherence among a sample of 815 Black patients with uncontrolled HTN followed in community health centers during a 12-month period. By using longitudinal analyses, this study provides some clarification into the role of key psychosocial and interpersonal factors on medication adherence in hypertensive Blacks. Results of our study showed that among patients with initially low levels of self-efficacy, increases in self-efficacy over time predicted improvements in medication adherence one year later. Patients with high levels of initial self-efficacy also reported low levels of depressive symptoms and collaborative patient-provider communication. Depressive symptoms at baseline were also associated with poor medication adherence. However, in contrast to our hypothesis, depressive symptoms did not exert a direct effect on medication adherence at 12 months, when accounting for the other predictor variables in the model.(41,42) Depressive symptoms played an indirect role through its effect on patients’ initial level of self-efficacy (as modeled by the latent intercept of self-efficacy), which in turn was associated with changes in medication adherence over time. Finally, more social support was associated with patient-provider communication rated as collaborative; both of these interpersonal factors were also associated with low depressive symptoms at baseline as well as in the latent growth model.
Self-efficacy is a well-documented factor associated with an individual's ability to enact and maintain self-management behaviors such as, adhering to antihypertensive medications.(38) For example, a recent systematic review that combined 20 years of research on medication adherence in patients with chronic disease (12% of studies in patients with HTN) identified self-efficacy as the most prominent predictor of adherence across all of the included studies.(43) Among hypertensive Black patients, higher levels of self-efficacy has also been shown to have a direct association with better medication adherence in cross-sectional analyses.(5) We extend these findings by also demonstrating that increasing levels of self-efficacy over time predict improvements in medication adherence over a one-year period. More importantly, the greatest improvements were seen among patients who initially reported low self-efficacy and poor medication adherence at baseline. Conversely, patients who initially reported high levels of self-efficacy as well as better medication adherence showed smaller improvements in adherence over time. In addition, our findings show that patients’ initial level of self-efficacy is affected by the presence of depressive symptoms. In combination, these variables determined patients’ baseline adherence behaviors and how they changed over time.
The inability to find a direct relationship between depressive symptoms and medication adherence in the longitudinal model may seem surprising when compared to previous research in hypertensive patients however; there are several differences in study designs that may explain these findings.(8) First, a majority of previous studies were conducted in predominately white populations. For example, the Cohort Study of Medication Adherence among Older Adults (CoSMO) study, one of the few longitudinal studies to also assess the predictive value of psychosocial factors such as depressive symptoms on adherence, included predominately white, older adults (mean age: 75 years; 30% Black).(41,42) Second, previous studies, including the CoSMO study,(41,42) have primarily used multivariable regression models to examine associations with adherence. The latent growth modeling approach of the current study allowed for the examination of complex relationships between changing levels of the key predictor variables (i.e., depressive symptoms and self-efficacy) on changes in medication adherence while adjusting for baseline levels of the constructs. Third, differences in measurement of depressive symptoms and medication adherence across studies may explain divergent results. This is exemplified by a recent meta-analysis that showed the association between depressive symptoms and adherence was dependent on the type of adherence assessment used.(8) Finally, the mean score on the PHQ-9 in this study was low (4.71) thus, we may have failed to find an association due to few patients meeting the criteria for depression.
While depressive symptoms did not exert a direct effect on long-term medication adherence in this study, the importance of screening for depressive symptoms in Black patients with HTN in primary care should not be minimized. Specifically, depressive symptoms played an important role in this study through its negative impact on patients’ initial level of self-efficacy, which predicted worse medication adherence over time. Thus, identification and management of depressive symptoms is needed in order to bolster the positive effects of self-efficacy on long-term medication adherence.
Similar to the above findings, patient-provider communication indirectly affected adherence through its association with patients’ initial level of self-efficacy (in the baseline cross-sectional model) and initial level of depressive symptoms (latent growth model intercept as well as the baseline cross-sectional model). This finding replicates those of similar studies, which have shown that when patients and providers engage in collaborative discussions within the medical encounter, patients feel more self-efficacious,(44,45) and are more likely to adhere to treatment recommendations.(46) A growing body of evidence has also found a robust relationship between depressive symptoms and interpersonal difficulties in the patient-provider relationship.(25, 26, 47) For example, using data from the 2003 Health Information National Trends Survey, patients with depressive symptoms were significantly more likely than their non-depressed counterparts to rate communication with their provider as poor.(47) In a sample of 231 ethnically-diverse diabetes patients, those exhibiting severe depressive symptoms were significantly more likely to report suboptimal patient-provider communication, with the lowest ratings given to patient-centered and collaborative communication behaviors.(26) These findings not only reinforce the importance of facilitating collaborative patient-provider communication in the medical encounter but also help to identity the mechanisms through which this relationship operates to affect medication adherence.
Finally, as with patient-provider communication, social support did not directly affect medication adherence. Instead, social support acted as an external ‘protective factor’ that buffered the negative effects that an individual-level psychosocial factor, depressive symptoms, had on medication adherence. Taken together, these findings suggest that intervention efforts that seek to improve patient adherence behaviors must simultaneously support the patient within the context of their medical care and the community they reside. This is particularly important for disadvantaged populations who are least likely to discuss medication-taking behaviors with their providers (48) and lack access to resources that facilitate medication adherence due to social (e.g., lack of transportation) and financial barriers (e.g., high co-pays).(49)
Several limitations are worth noting. Medication adherence was assessed by self-report, which may have resulted in an overestimation of adherence levels. However, the prevalence of self-reported non-adherence in this study is similar to the estimated 50-70% range documented by the World Health Organization.(50) Future studies should utilize a more objective measure of adherence, such as electronic monitoring devices to confirm our findings. This study was a secondary analysis of a larger group randomized control trial thus; we were limited to the self-report measures collected within that trial and may not have captured all the factors predictive of medication adherence. Moreover, patient-provider communication and social support were only collected at baseline, which may provide a limited view of how these variables affect adherence over time. Future research should test additional patient (e.g., beliefs, health literacy), physician (e.g., prescribing behaviors, therapeutic inertia) health care system (e.g., medication costs), and disease-related (e.g., complexity of the medical regimen) factors in order to provide a more comprehensive understanding of the barriers to medication adherence in this patient population. Another limitation is that due to incomplete data, with a smaller analytic sample size available at 12 months. Patients with missing data at 12 months were generally similar to patients with complete data with the exception of small differences in age (mean age: 56 vs. 58 years, p=.004, Cohen's d=.018), self-efficacy (mean: 2.24 vs. 2.37, p=.006, Cohen's d=.21), and 12 month adherence to medications (Mean score: 3.7 vs. 4.05, p<.001, Cohen's d=.25). Although the differences are few and small, we nonetheless recognize that this is a limitation in the current research. We also acknowledge that patient attrition from the parent trial may have influenced our results. As we do not know the adherence behaviors of these individuals, this is another limitation in the current findings. Individuals that enroll in clinical trials may be different from the general patient population thus; future research should evaluate the replicability of these results. Finally, this study was conducted in a relatively homogenous sample of Black hypertensive patients and findings may not generalize to other racial/ethnic groups. While previous studies in hypertensive patients suggest that self-efficacy and depressive symptoms are potent predictors of adherence behaviors (8, 9, 43), future studies should replicate these findings in a sample of patients of diverse racial/ethnic backgrounds as well as in other disease states.
In conclusion, increasing self-efficacy over time predicted improvements in medication adherence one year later among a sample of 815 Black patients with uncontrolled HTN followed in community health centers. Individuals who initially displayed lower levels of self-efficacy were the most likely to benefit from improvements in self-efficacy and thereby report better medication adherence at 12-months. Depressive symptoms indirectly affected medication adherence over time through its association with self-efficacy, with individuals who reported lower initial depressive symptoms being more likely to report higher initial self-efficacy. Patients who reported more social support and collaborative patient-provider communication at baseline also reported lower depressive symptoms. Finally, more social support was associated with ratings of collaborative communication.
The excess burden of uncontrolled HTN in Blacks remains one of the most vexing public health problems in the US. The age-adjusted prevalence estimate of HTN in Blacks is 42.1%, compared to 28% for Whites, making Blacks the most highly afflicted group in the US. Moreover, among Blacks, HTN develops at an earlier age, is more aggressive, and less well managed than in Whites.(51) Thus, it is not surprising that the single most common explanation for cardiovascular mortality disparities between Blacks and Whites is HTN.(52) While poor adherence to prescribed antihypertensive medications is a major contributing factor to poorer HTN-related outcomes in Blacks (2) our current knowledge is limited to correlational models that cannot help elucidate the causal drivers of poor adherence. Moreover, few studies have exclusively focused on Blacks despite a disproportionately higher disease burden. Thus, a strength of this study was the use of latent growth modeling to delineate the process by which Blacks are less likely to take their medications over a one year period.
These findings provide key insights for the development of intervention approaches that can build and maintain a patient's level of self-efficacy, within a supportive environment, in order to facilitate future medication adherence in this high risk population. In the research setting, innovative study designs that leverage technology could be used to generate individualized psychosocial profiles to identify the specific, most important causes of poor adherence for patients. Tailored adherence-promoting strategies can then be used to address the barriers identified from the individualized profiles. In the clinical setting, healthcare practices would benefit from integrating evidence-based teaching models into the culture of the organization that offer providers multiple avenues to learn, as well as practice, tangible, and effective communication skills that encourage their patients to discuss both their physiological and psychosocial health as well as offer resources to support patients’ adherence behaviors across clinic and community-based settings. Integral members of the care team such as, Nurses and Medical Assistants could also be leveraged to provide brief adherence counseling to assist patients in developing problem solving skills to identify and overcome situations that pose difficulties to taking medications regularly.
Acknowledgements
This study was supported by K23 HL 098564-A1, K24 HL 111315, and R01HL078566 from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict of Interest Statement: The authors have no conflict of interest to disclose.
Compliance with Ethical Standards
The authors have no potential conflicts of interest to disclose. The content of the manuscript is original and has not been submitted in part or whole elsewhere. All content in the manuscript were created by the authors specifically for this manuscript and have not been published elsewhere. No data has been fabricated or manipulated to support our conclusions. The authors have full control of the data and it may be requested by the journal for review, if needed. No data, text, or theories by others are presented as if they were our own and proper acknowledgements to other works have been referenced throughout the manuscript. Each author listed on the manuscript has made significant contributions to the design, conceptualization or implementation of the study, and have seen and approved the final manuscript.
This manuscript reports on research involving human participants. All patients provided written informed consent approved by the Institutional Review Boards of Columbia University and New York University Langone Medical Centers.
References
- 1.Centers for Disease Control and Prevention Racial/Ethnic disparities in the awareness, treatment, and control of hypertension - United States, 2003-2010. MMWR. 2013;62:351–355. [PMC free article] [PubMed] [Google Scholar]
- 2.Bosworth HB, Dudley T, Olsen MK, et al. Racial differences in blood pressure control: potential explanatory factors. Am J of Med. 2006;119:70, e79–15. doi: 10.1016/j.amjmed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 3.Hyre AD, Krousel-Wood MA, Muntner P, Kawasaki L, DeSalvo KB. Prevalence and predictors of poor antihypertensive medication adherence in an urban health clinic setting. J Clin Hypertens. 2007;9:179–186. doi: 10.1111/j.1524-6175.2007.06372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charles H, Good CB, Hanusa BH, Chang CC, Whittle J. Racial differences in adherence to cardiac medications. J Nat Med Assoc. 2003;95:17–27. [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis LM. Factors associated with medication adherence in hypertensive blacks: a review of the literature. J Cardiov Nurs. 2012;27:208–219. doi: 10.1097/JCN.0b013e318215bb8f. [DOI] [PubMed] [Google Scholar]
- 6.Lewis LM, Ogedegbe C, Ogedegbe G. Enhancing adherence of antihypertensive regimens in hypertensive African-Americans: current and future prospects. Exp Rev Cardiov Ther. 2012;10:1375–1380. doi: 10.1586/erc.12.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandura . Self-efficacy and health behaviour. In: Baum A, Wienman J, West R, McManus C, editors. Cambridge Handbook of Psychology, Health and Medicine. Cambridge University Press; Cambridge: 1997. pp. 160–162. [Google Scholar]
- 8.Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26:1175–1182. doi: 10.1007/s11606-011-1704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang PS, Bohn RL, Knight E, Glynn RJ, Mogun H, Avorn J. Noncompliance with antihypertensive medications: the impact of depressive symptoms and psychosocial factors. J Gen Intern Med. 2002;17:504–511. doi: 10.1046/j.1525-1497.2002.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoenthaler A, Allegrante J, Ogedgegbe G. Self-efficacy mediates the relationship between depressive symptoms and medication adherence among hypertensive African Americans. Health Educ Behav. 2009;36:127–137. doi: 10.1177/1090198107309459. [DOI] [PubMed] [Google Scholar]
- 11.Magrin ME, D'Addario M, Greco A, et al. Social support and adherence to treatment in hypertensive patients: a meta-analysis. Ann Behav Med. 2015;49:307–318. doi: 10.1007/s12160-014-9663-2. [DOI] [PubMed] [Google Scholar]
- 12.DiMatteo MR. Social support and patient adherence to medical treatment: a meta-analysis. Health Psychol. 2004;23:207–218. doi: 10.1037/0278-6133.23.2.207. [DOI] [PubMed] [Google Scholar]
- 13.Da Costa D, Clarke AE, Dobkin PL, et al. The relationship between health status, social support and satisfaction with medical care among patients with systemic lupus erythematosus. Int J Qual Health Care. 1999;11:201–207. doi: 10.1093/intqhc/11.3.201. [DOI] [PubMed] [Google Scholar]
- 14.Taal E, Rasker JJ, Seydel ER, Wiegman O. Health status, adherence with health recommendations, self-efficacy and social support in patients with rheumatoid arthritis. Patient Educ Couns. 1993;20:63–76. doi: 10.1016/0738-3991(93)90122-d. [DOI] [PubMed] [Google Scholar]
- 15.Holahan CJ, Moos RH, Holahan CK, Brennan PL. Social support, coping, and depressive symptoms in a late-middle-aged sample of patients reporting cardiac illness. Health Psychol. 1995;14:152–163. doi: 10.1037//0278-6133.14.2.152. [DOI] [PubMed] [Google Scholar]
- 16.Connell CM, Davis WK, Gallant MP, Sharpe PA. Impact of social support, social cognitive variables, and perceived threat on depression among adults with diabetes. Health Psychol. 1994;13:263–273. doi: 10.1037//0278-6133.13.3.263. [DOI] [PubMed] [Google Scholar]
- 17.Penninx BW, van Tilburg T, Boeke AJ, Deeg DJ, Kriegsman DM, van Eijk JT. Effects of social support and personal coping resources on depressive symptoms: different for various chronic diseases? Health Psychol. 1998;17:551–558. doi: 10.1037//0278-6133.17.6.551. [DOI] [PubMed] [Google Scholar]
- 18.Simoni JM, Frick PA, Lockhart D, Liebovitz D. Mediators of social support and antiretroviral adherence among an indigent population in New York City. AIDS Patient Care STDS. 2002;16:431–439. doi: 10.1089/108729102760330272. [DOI] [PubMed] [Google Scholar]
- 19.Cha E, Erlen JA, Kim KH, Sereika SM, Caruthers D. Mediating roles of medication-taking self-efficacy and depressive symptoms on self-reported medication adherence in persons with HIV: a questionnaire survey. Int J Nurs Stud. 2008;45:1175–1184. doi: 10.1016/j.ijnurstu.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciechanowski PS, Katon WJ, Russo JE, Walker EA. The patient-provider relationship: attachment theory and adherence to treatment in diabetes. Am J Psychiatry. 2001;158:29–35. doi: 10.1176/appi.ajp.158.1.29. [DOI] [PubMed] [Google Scholar]
- 21.Schoenthaler A, Chaplin WF, Allegrante JP, et al. Provider communication effects medication adherence in hypertensive African Americans. Patient Educ Couns. 2009;75:185–191. doi: 10.1016/j.pec.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piette JD, Schillinger D, Potter MB, Heisler M. Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J Gen Intern Med. 2003;18(8):624–633. doi: 10.1046/j.1525-1497.2003.31968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider J, Kaplan SH, Greenfield S, Li W, Wilson IB. Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J Gen Intern Med. 2004;19:1096–1103. doi: 10.1111/j.1525-1497.2004.30418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carpenter DM, DeVellis RF, Fisher EB, DeVellis BM, Hogan SL, Jordan JM. The effect of conflicting medication information and physician support on medication adherence for chronically ill patients. Patient Educ Couns. 2010;81:169–176. doi: 10.1016/j.pec.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schenker Y, Stewart A, Na B, Whooley MA. Depressive symptoms and perceived doctor-patient communication in the heart and soul study. J Gen Intern Med. 2009;24:550–556. doi: 10.1007/s11606-009-0937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swenson SL, Rose M, Vittinghoff E, Stewart A, Schillinger D. The influence of depressive symptoms on clinician-patient communication among patients with type 2 diabetes. Med Care. 2008;46:257–265. doi: 10.1097/MLR.0b013e31816080e9. [DOI] [PubMed] [Google Scholar]
- 27.Ogedegbe G, Tobin JN, Fernandez S, et al. Counseling African Americans to Control Hypertension (CAATCH) trial: a multi-level intervention to improve blood pressure control in hypertensive blacks. Circ Cardiovasc Qual Outcomes. 2009;2:249–56. doi: 10.1161/CIRCOUTCOMES.109.849976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogedegbe G, Tobin JN, Fernandez S, et al. Counseling African Americans to Control Hypertension: cluster-randomized clinical trial main effects. Circulation. 2014;129:2044–51. doi: 10.1161/CIRCULATIONAHA.113.006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogedegbe G, Mancuso CA, Allegrante JP, Charlson ME. Development and evaluation of a medication adherence self-efficacy scale in hypertensive African-American patients. J Clin Epidemiol. 2003;56:520–529. doi: 10.1016/s0895-4356(03)00053-2. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez S, Chaplin W, Schoenthaler A, Ogedegbe G. Revision and validation of the medication adherence self-efficacy scale (MASES) in hypertensive African Americans. J Behav Med. 2008;31:453–462. doi: 10.1007/s10865-008-9170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 33.Bultman DC, Svarstad BL. Effects of physician communication style on client medication beliefs and adherence with antidepressant treatment. Patient Educ Couns. 2000;40:173–185. doi: 10.1016/s0738-3991(99)00083-x. [DOI] [PubMed] [Google Scholar]
- 34.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Marcum ZA, Zheng Y, Perera S, et al. Prevalence and correlates of self-reported medication non-adherence among older adults with coronary heart disease, diabetes mellitus, and/or hypertension. Res Social Adm Pharm. 2013;9:817–827. doi: 10.1016/j.sapharm.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson MP, Waring ME, Wang ML, et al. Weight-based discrimination and medication adherence among low-income African Americans with hypertension: how much of the association is mediated by self-efficacy? Ethn Dis. Spring. 2014;24:162–168. [PubMed] [Google Scholar]
- 37.Ogedegbe G, Schoenthaler A, Fernandez S. Appoint-keeping behavior is not related to medication adherence in hypertensive African Americans. J Gen Intern Med. 2007;22:1176–1179. doi: 10.1007/s11606-007-0244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 39.MPLUS (Version 6.11). [Computer Software] Muthén & Muthén; Los Angeles, CA: [Google Scholar]
- 40.Little TD, Cunningham WA, Shahar G. To Parcel or Not to Parcel: Exploring the Question, Weighting the Merits. Struct Equ Modeling. 2002;9:151–173. [Google Scholar]
- 41.Krousel-Wood M, Islam T, Muntner P, et al. Association of depression with antihypertensive medication adherence in older adults: cross-sectional and longitudinal findings from CoSMO. Ann Behav Med. 2010;40:248–257. doi: 10.1007/s12160-010-9217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krousel-Wood M, Joyce C, Holt E, et al. Predictors of decline in medication adherence: results from the cohort study of medication adherence among older adults. Hypertension. 2011;58:804–810. doi: 10.1161/HYPERTENSIONAHA.111.176859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holmes EA, Hughes DA, Morrison VL. Predicting adherence to medications using health psychology theories: a systematic review of 20 years of empirical research. Value Health. 2014;17:863–876. doi: 10.1016/j.jval.2014.08.2671. [DOI] [PubMed] [Google Scholar]
- 44.Zachariae R, Pedersen CG, Jensen AB, Ehrnrooth E, Rossen PB, von der Maase H. Association of perceived physician communication style with patient satisfaction, distress, cancer-related self-efficacy, and perceived control over the disease. Br J Cancer. 2003;88:658–665. doi: 10.1038/sj.bjc.6600798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002;17:243–252. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zolnierek KB, Dimatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826–834. doi: 10.1097/MLR.0b013e31819a5acc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rutten LJ, Augustson E, Wanke K. Factors associated with patients' perceptions of health care providers' communication behavior. J Health Commun. 2006;11(Suppl 1):135–146. doi: 10.1080/10810730600639596. [DOI] [PubMed] [Google Scholar]
- 48.Street RL, Jr., Gordon H, Haidet P. Physicians' communication and perceptions of patients: is it how they look, how they talk, or is it just the doctor? Soc Sci Med. 2007;65:586–598. doi: 10.1016/j.socscimed.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose LE, Kim MT, Dennison CR, Hill MN. The contexts of adherence for African Americans with high blood pressure. J Adv Nurs. 2000;32:587–594. doi: 10.1046/j.1365-2648.2000.01538.x. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization . Adherence to long-term therapies: Evidence for action. World Health Organization; Geneva: 2003. [Google Scholar]
- 51.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 52.Fiscella K, Holt K. Racial Disparity in Hypertension Control: Tallying the Death Toll. Ann Fam Med. 2008;6:497–502. doi: 10.1370/afm.873. [DOI] [PMC free article] [PubMed] [Google Scholar]