Abstract
Objectives
The majority of childhood cancer patients now achieve long-term survival, but the treatments that cured their malignancy often put them at risk of adverse health outcomes years later. New cancers are among the most serious of these late effects. The aims of this review are to compare and contrast radiation dose-response relationships for new solid cancers in a large cohort of childhood cancer survivors and to discuss interactions among treatment and host factors.
Methods
This review is based on previously published site-specific analyses for subsequent primary cancers of the brain, breast, thyroid gland, bone and soft tissue, salivary glands and skin among 12,268 five-year childhood cancer survivors in the Childhood Cancer Survivor Study. Analyses included tumor site-specific, individual radiation dose reconstruction based on radiotherapy records. Radiation-related second cancer risks were estimated using conditional logistic or Poisson regression models for excess relative risk (ERR).
Results
Linear dose-response relationships over a wide range of radiation dose (0–50 Gy) were seen for all cancer sites except the thyroid gland. The steepest slopes occurred for sarcoma, meningioma and non-melanoma skin cancer (ERR/Gy > 1.00), with glioma and cancers of the breast and salivary glands forming a second group (ERR/Gy=0.27–0.36). The relative risk for thyroid cancer increased up to 15–20 Gy and then decreased with increasing dose. Risk of thyroid cancer also was positively associated with chemotherapy, but the chemotherapy effect was not seen among those who also received very high doses of radiation to the thyroid. Excess risk of radiation-related breast cancer was sharply reduced among women who received 5 Gy or more to the ovaries.
Conclusions
Results suggest that the effect of high-dose irradiation is consistent with a linear dose-response for most organs but also reveal important organ- and host-specific differences in susceptibility and interactions between different aspects of treatment.
Introduction
More than 80% of childhood cancer patients now achieve long-term survival, but the curative treatments for their primary cancers often put them at risk of adverse health outcomes years later (1–4). New cancers are among the most life-threatening and psychologically traumatic of late effects and, even when not fatal, can cause serious lifelong morbidity. Radiation treatment is the most important therapy-related contributor to excess risk of new solid cancers among childhood cancer survivors. A challenge to understanding radiation-related risks is that radiation-induced solid cancers typically do not appear until a minimum of 5–10 years after treatment, meaning that contemporary studies are documenting effects of historical treatments (5). Analyses of risks of radiation-related second cancers in terms of a standard metric – radiation absorbed dose – can provide potentially generalizable understanding and a basis for predicting risks of new or hypothetical treatments without waiting for a minimum induction period to elapse.
Quantitative evaluation of the risks of subsequent cancers is complicated by the difficulty of assembling sufficiently large series of long-term survivors with adequate treatment information to allow for comprehensive, rigorous study. The Childhood Cancer Survivor Study (CCSS) meets these requirements. The original CCSS cohort includes 14,357 five year survivors of childhood cancer who were diagnosed between 1970 and 1986 with detailed information about treatment for the childhood cancer (6–8). Survivors remain under regular follow-up by questionnaires sent every 2–3 years to ascertain subsequent health outcomes, including new primary cancers, and sufficient time has elapsed for new, treatment-related solid cancers to develop (9). The combination of large study size, long follow-up, and detailed information about treatment creates unique opportunities for the study of treatment-related cancers and of host factors that might modify these risks.
Since 2005, the CCSS has published a series of analyses of treatment-related new primary cancers of the central nervous system (CNS) (10), female breast (11), thyroid gland (12–15), bone and soft tissue (16), salivary glands (17) and basal cell carcinoma (BCC) of the skin (18). These studies included detailed individual radiation dose reconstruction from radiotherapy records (19). Conducting site-specific second cancer studies within the same cohort and using consistent methods for radiation dosimetry enhances comparability of results across solid cancer types. Here, we compare and contrast results from these studies concerning radiation dose-response relationships and highlight selected results concerning interactions among treatment and host factors to illuminate factors associated with radiation carcinogenesis.
Methods
Study population
Persons eligible for the study included those who were originally diagnosed with leukemia, CNS cancer, Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), Wilms tumor, neuroblastoma, or soft tissue or bone sarcoma before age 21 at one of 26 hospitals in the US or Canada between 1970 and 1986 and who had survived at least five years (5, 6). Human subjects review boards at each participating center approved the study. A total of 20,276 eligible survivors were identified, of whom 14,357 were successfully located and agreed to participate. Initial contact and enrollment began in 1992 as a pilot with full implementation in 1994. Dose-response analyses described here are based on persons with radiation dose estimates (N=12,268). The usual reason for missing treatment information was lack of consent to review medical records. Informed consent was obtained from the survivor or parent for those who had died or were less than 18 years at the time of contact.
Treatment information
Medical records at participating hospitals were reviewed to obtain treatment information for the initial cancer, including radiotherapy. Records from radiation oncology departments were photocopied and forwarded to a collaborating radiation dosimetry group. Abstracted chemotherapy data included start and stop dates of all administered agents. Information about dose (mg/m2) and route of administration was obtained for 22 agents. An alkylator score for alkylating agents was calculated according to the method described by Tucker et al. (20).
Cancer ascertainment and medical history
Information about new primary cancers, other aspects of medical history, and cancer risk factors was obtained from a baseline self-administered questionnaire in 1996 and periodic (~every 3 years) follow-up surveys thereafter. Each questionnaire asked about the occurrence of new primary cancers. Reports of new cancers were reviewed by a pediatric oncologist and pathologist. Pathology reports were requested in instances where a new primary cancer was deemed likely or possible. A small percentage of new cancers was ascertained through linkages with the National Death Index.
Radiation dosimetry
For studies of specific types of second (or subsequent) cancer, medical physicists estimated radiation doses to the organ or location of interest within an organ. For matched casecontrol studies of cancers of the brain (10), breast (11), skin (18), and bone or soft tissue (16), doses were estimated to the tumor site for the case and corresponding location in controls. For cohort analyses involving the thyroid gland (14, 15) and salivary glands (17), an average organ dose was estimated for each individual in the cohort. Doses were reconstructed from radiotherapy records concerning the initial cancer and information regarding location of the subsequent cancer, using methods described previously (19).
Statistical analysis
Conditional logistic regression (case-control analyses) or Poisson regression (cohort analyses) models were used to evaluate treatment effects and effect modification. Conditional regression analyses were conditioned on matching factors (age at diagnosis of the first cancer, survival and gender). Analyses were conducted for cancers of interest as second cancers only and as second or subsequent cancers. It made little difference in dose-response relationships, and the latter are reported here. The software packages EPICURE (Hirosoft International, Seattle, WA), STATA (College Station, TX) and SAS (Cary, NC) were used for these analyses.
For radiation effects, the excess relative risk (ERR) = RR-1 was modeled. Radiation dose-response models that were considered were simplifications of the general model:
in which D=dose, and β1–β4 are regression coefficients. The model ERR = β1D, corresponds to a straight-line dose-response relation, and β1 equals the slope [ERR per gray (Gy) of radiation]. Possible effect modifiers, such as age at exposure, time since exposure and gender, were evaluated by including them in the exponential term. Analyses concerning effects of radiation were adjusted for main effects of chemotherapy. Likelihood ratio tests (2-sided) were based on nested models, and profile likelihood-based 95% confidence intervals (CI) were calculated.
Results
Cohort characteristics
Baseline descriptive characteristics of the cohort are summarized in Table 1. More than 85% of the cohort was still alive as of the last follow-up. The median interval from diagnosis of the first cancer to end of follow-up for those still alive at the end of follow-up was 21 years. The median interval from first cancer to subsequent cancer ranged from 9 years for glioma to 19 years for breast cancer.
Table 1.
Demographic and clinical characteristics of 12,268 five-year survivors of childhood cancer diagnosed between 1970 and 1986 with estimated radiation doses [modified after Leisenring (6)] RT=radiotherapy; CT=chemotherapy.
| Characteristic | Number of Patients |
% |
|---|---|---|
| All patients | 12,268 | 100 |
| Gender | ||
| Male | 6,471 | 52.7 |
| Female | 5,797 | 47.3 |
| Race/ethnicity | ||
| White, not Hispanic | 11,055 | 90.1 |
| Black, not Hispanic | 469 | 3.8 |
| Hispanic | 322 | 2.6 |
| Other or unknown | 422 | 3.4 |
| Age at diagnosis of the first cancer (years) | ||
| ≤ 4 | 4,959 | 40.4 |
| 5–9 | 2,697 | 22.0 |
| 10–14 | 2,458 | 20.0 |
| 15–20 | 2,154 | 17.6 |
| First cancer diagnosis | ||
| Acute lymphoblastic leukemia | 3,561 | 29.0 |
| Other leukemia | 590 | 4.8 |
| Hodgkin lymphoma | 1,638 | 13.4 |
| Non-Hodgkin lymphoma | 911 | 7.4 |
| Astrocytoma | 1,003 | 8.2 |
| Other CNS cancer | 586 | 4.8 |
| Kidney cancer (Wilms tumor) | 1,068 | 8.7 |
| Soft tissue cancer | 1,061 | 8.6 |
| Bone cancer | 1,028 | 8.4 |
| Neuroblastoma | 822 | 6.7 |
| Treatment modality (CT and RT) | ||
| No RT, No CT | 922 | 7.5 |
| CT, No RT | 3,090 | 25.2 |
| RT, No CT | 1,423 | 11.6 |
| Both RT and CT | 6,667 | 54.3 |
| RT, unknown CT | 166 | 1.4 |
Radiation-dose-response
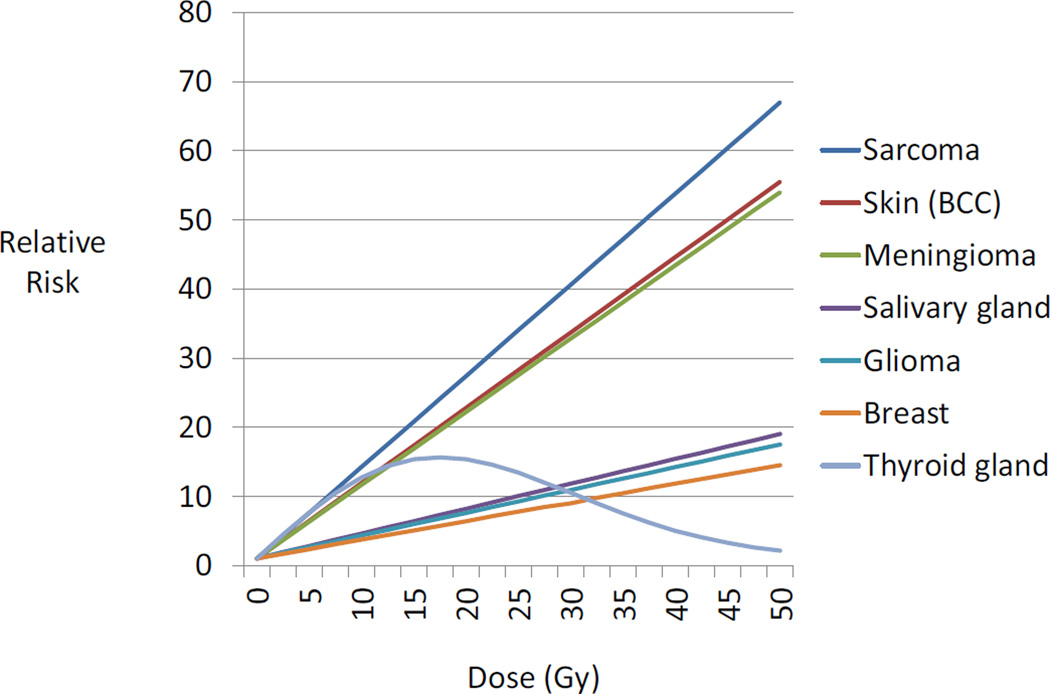
Results of dose-response analyses from previous CCSS studies of radiation-related subsequent cancer risks are summarized in Table 2 and Figure 1. Glioma, meningioma and cancers of the breast, thyroid gland, bone and soft tissue, skin (BCC) and salivary glands all showed significant positive linear terms for the radiation dose-response. Only for thyroid cancer was there statistically significant evidence of a departure from a straight line relationship, with a downturn at high doses beginning between 15 and 20 Gy. This relationship was best described by a linear-exponential-quadratic dose-response [(β1D)exp(β4D2)]. For the tumor types not showing departure from linearity, the lines clustered in two groups with differing slopes, with sarcoma, BCC and meningioma having a steeper slope (ERR/Gy > 1.00) and salivary gland cancer, glioma and breast cancer having flatter slopes (ERR/Gy=0.27–0.36). Dose-response relationships for these six types of neoplasm were consistent with linearity for doses up to 50 Gy. The slope of the ascending arm of the dose-response for thyroid cancer resembled slopes for sarcomas, BCC and meningioma for doses less than 10 Gy.
Table 2.
Radiation-related risks of second (or subsequent) primary cancers among childhood cancer survivors, by type of subsequent cancer.
| Site (Type) of Subsequent Cancer |
Number of.Cases (with dose) |
ERR/Gy (95% CI) | P-value* | Statistical Evidence of Curvilinearity of Dose- Response? |
Usual Types of First Cancer Among Cases |
Reference Number |
|---|---|---|---|---|---|---|
| Brain/CNS (glioma) |
35 | 0.33¥ (0.07–1.71) | < .001 | No | Leukemia, CNS | 10 |
| Brain/CNS (meningioma) |
58 | 1.06¥ (0.21–8.15) | < .001 | No | Leukemia, CNS | 10 |
| Breast | 107 | 0.27¥¥ (0.10–0.67) | < .0001 | No | HL, bone | 11 |
| Thyroid gland | 115 | 1.38# | < .001* | Yes (downward at very high doses) |
HL, leukemia, CNS, bone |
12–15 |
| Bone/soft tissue |
84 | 1.32¶ (0.44–4.22) | <.001 | No | HL, STS, bone | 16 |
| Skin (BCC) | 179 | 1.09** (0.49–2.64) | < .001 | No | HL, leukemia | 18 |
| Salivary glands |
20 | 0.36‖ (0.06–2.50) | .005 | No | Leukemia, HL, NHL |
17 |
Abbreviations: BCC=basal cell carcinoma, CI=confidence interval, Gy=gray, CNS=central nervous system, ERR=excess relative risk; HL=Hodgkin lymphoma, NHL=non-Hodgkin lymphoma;STS=soft tissue sarcoma
P-value is for contrast of linear dose-response model with null model, except for thyroid cancer for which the P-value is for a contrast of a linear-exponential-quadratic model with a model that includes only the linear term.
Adjusted for age at first cancer diagnosis, time since first cancer diagnosis, and gender. ERR/Gy is averaged over all ages at exposure.
Adjusted for age at first cancer diagnosis and time since first cancer diagnosis. ERR/Gy is averaged over all ages at exposure.
Adjusted for attained age, gender and type of first cancer. ERR/Gy is for the linear term in a linear-exponential-quadratic model [(β1D) exp(β4D2)] (Bhatti et al. 2010); P-value is for a contrast of a linear-exponential-quadratic model with a model that includes only the linear term. ERR/Gy is averaged over all ages at exposure.
Adjusted for age at first cancer diagnosis, time since first cancer diagnosis, sex, any anthracyclines, any chemotherapy and type of first cancer.
Adjusted for age at first cancer diagnosis, time since first cancer diagnosis, gender, race, HL as first cancer diagnosis, and calendar year of first cancer diagnosis
Adjusted for attained age, gender, race and attained calendar period.
Figure 1.
Fitted radiation dose-response by type of second cancer, based on results from published studies described in Table 2. The order of second cancers from top to bottom in the graph is the same as in the key to the right of the panel. BCC=basal cell carcinoma.
Effect modification
The examples described below are intended as being illustrative of types of effect modification observed in CCSS second cancer studies rather than as a comprehensive review of the topic. They exemplify the potential and sometimes striking effects of host factors, combined effects of radiotherapy and chemotherapy, and indirect effects of irradiation of sites other than the second cancer site of interest.
Radiation and host factors
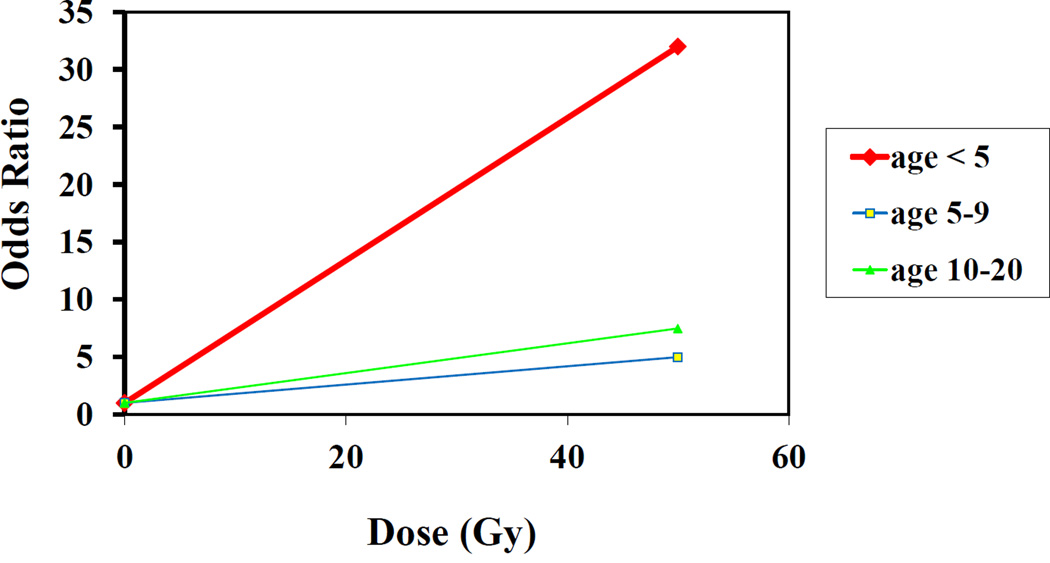
In general, the linear component of the radiation dose-response did not vary significantly by age at exposure or gender, with the exception of thyroid cancer and glioma. Thyroid cancer showed the expected inverse association with age at exposure for the linear term of the dose-response (P<0.001) (14). For glioma, the overall test for trend for an effect of age at exposure was not significant (P=0.15); however, radiation-related risk was inversely associated with age at exposure, and the dose-response was significant only among persons irradiated prior to age 5; fitted slopes were nearly flat for those irradiated between the ages of 5–9 or 10–20 (Figure 2) (10). Meningioma showed little relationship with age at irradiation (10). For breast cancer, there was insufficient variation in age at diagnosis of first cancer among cases to permit evaluation by age at exposure, as there were strong correlations among type of first cancer, radiation dose to the breast and age at exposure (11). Approximately two-thirds of the breast cancers occurred among HL patients, who had the highest breast doses, but HL rarely occurs before age 10.
Figure 2.
Risk of glioma following radiotherapy for first cancer, by age at first cancer [data from Neglia et al. (10)]
Combined effects of radiotherapy and chemotherapy
Among non-irradiated patients, there was an indication of increased risk of thyroid cancer associated with prior treatment with alkylating agents (in particular, procarbazine); however, this association was dampened among persons who also received high-dose radiation to the thyroid gland and absent among those with thyroid doses of 20 Gy or more (Table 3) (15).
Table 3.
Incidence rate ratio (RR) of thyroid cancer by level of exposure to alkylating agents (alkylating agent score) and dose of radiation to the thyroid gland. Modified after Veiga et al. (15)
| Radiation Dose (Gy) | ||||
|---|---|---|---|---|
| Exposure to Alkylating Agents (Score) |
≤20 | |||
| 0 RRa |
0–5 RRb |
0–20 RRb |
>20 RRb |
|
| Not exposed (0) | 1.0c | 1.0c | 1.0c | 1.0c |
| Low/medium(1–2) | 1.8 | 2.5 | 2.3 | 1.0 |
| Highly exposed (3) | 9.4 | 5.5 | 2.8 | 1.0 |
| P (trend) | 0.08 | 0.02 | 0.009 | 0.99 |
Adjusted for sex, natural log of attained age, type of first cancer (HL, leukemia, others)
Adjusted for sex, natural log of attained age, type of first cancer and radiation dose on continuous scale
Reference category
Indirect effects of radiation
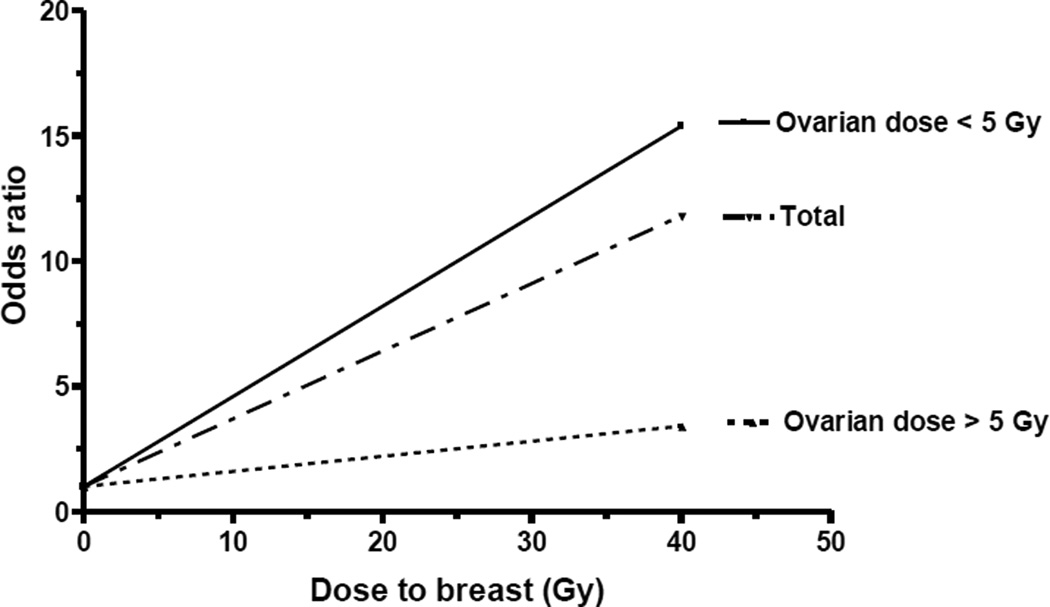
Because it is known that ablation of ovarian function by oophorectomy or radiation doses to the ovary > 5 Gy at an early age reduces the risk of breast cancer in women not receiving chest radiotherapy (21–24), we evaluated the influence of ovarian radiation on radiation-related breast cancer risk (11). The excess risk associated with breast irradiation was attenuated among women who also received ovarian radiation doses in excess of 5 Gy (P for interaction=0.002) (Figure 3).
Figure 3.
Breast cancer risk by radiation dose to the breast and ovary. Reproduced from Inskip et al. (11).
Effect of type of first cancer
Among specific types of first cancer, HL survivors generally showed the highest second cancer risks, but differences in risk by type of first cancer narrowed considerably once radiation treatment was taken into account. With adjustment for radiation dose, type of first cancer was not significantly associated with risk of subsequent glioma, meningioma, breast cancer or salivary gland cancer (10, 11, 17). There were exceptions to this general finding. For example, after adjustment for radiation dose and age at irradiation, risk of thyroid cancer remained elevated among persons with an initial HL or neuroblastoma, with the latter association based on small numbers (9 cases) (12, 14). A possible effect of heightened surveillance among HL survivors was suggested by the smaller average thyroid cancer size at diagnosis (12); however, this finding was not confirmed in a subsequent larger series (14). When survivors diagnosed with a first HL were excluded from the analysis, the thyroid cancer dose-response relationship was essentially unchanged (14). After adjustment for radiation dose and treatment with anthracyclines, risk of a second primary bone or soft tissue sarcoma was increased among survivors of an initial HL or sarcoma relative to those with initial diagnosis of leukemia (16).
Discussion
In a series of analyses of second primary cancers in the CCSS cohort, significant radiation dose-response relationships were apparent for glioma, meningioma, carcinomas of the breast, thyroid gland, salivary glands and skin, and sarcomas of bone and soft tissue, all of which are considered as radiosensitive tissues (25, 26). Only for thyroid cancer was there statistical evidence of departure from a linear dose-response, with downward curvature at high doses, likely due to radiation-induced cell-sterilization (14). Also of note is the apparent clustering of linear dose-response relationships into two groups, one consisting of sarcoma, meningioma and BCC, and the other of glioma, breast and salivary gland cancer. The former group includes two types of cancers of mesenchymal tissue and one of epithelial origin; the latter includes two cancers arising from epithelial tissue and one from neuroepithelial tissue.
If our interpretation is correct, and the downturn in the radiation dose- response for thyroid cancer at high doses is attributable to cell-killing, then our observations suggest that high doses of radiation also “protect” against thyroid cancers initiated by other exposures, such as chemotherapy, and not simply by potentially oncogenic thyroid damage caused by radiation. This argues for a reformulation of the general relative risk model from:
to:
which allows for a cell-killing effect of high-dose irradiation on thyroid cells transformed from any cause.
It is not clear why this downturn in dose-response is not seen for other solid cancers; however, it should be noted that a linear dose-response relation does not necessarily imply absence of cell killing. Fractionation of dose over a period of weeks allows time for radiation-induced stem cell repopulation, which can offset radiation-induced cell killing (27). Replenishment of stem cells over time puts more cells at risk of transformation relative to numbers at risk in the case of acute radiation exposures and can yield linear dose-response relations extending to high doses, even when considerable high-dose cell killing is occurring (27).
A higher ERR/Gy for meningioma than glioma following therapeutic cranial irradiation during childhood is a consistent finding (5, 28, 29). An inverse association between age at radiation exposure and risk of glioma also has been seen for other irradiated populations, including exposures from radiotherapy for benign or malignant disease during childhood (28–30), as well as atomic bomb survivors (31). This observation could extend to other human neurocarcinogens, including those associated with gliomas in adults, making exposures during this early window of radio-sensitivity important in determining the level of risk in adults. This might be a reason why epidemiologic studies of gliomas in adults have been so unsuccessful in identifying causal environmental exposures (32). The experimental chemical neuro-oncology literature shows a strong effect of timing of exposure, beginning in utero and extending through the post-natal period, not only on CNS tumor yield but also on histologic type (33–36).
The observation of a protective effect of high-dose ovarian irradiation on the subsequent risk of breast cancer is not surprising and has been reported previously in the context of artificial menopause (21, 22), pelvic radiotherapy for malignant or benign disease (23, 24), and irradiation of young adults for HL (37, 38). The CCSS findings extend this observation to adolescence, a time when the ovaries are thought to be more radio-resistant as compared to adults (39–41). They also demonstrate the combined effects of high-dose breast and ovarian irradiation. Such patients still experience excess breast cancer risk, but the excess is less than it would have been absent high-dose ovarian irradiation. The relevant target cells in the ovary for radiologic menopause are likely those involved in estrogen production. High-dose ovarian radiation may suppress estrogen production, as well as that of cells producing androgens that may be converted peripherally to estrogens (24). In the absence of estrogenic stimulation, the tumorigenic potential of radiation-induced breast cellular damage might not be realized.
The generalizability of radiation risk estimates based on five-year childhood cancer survivors is unclear. Persons who develop a first childhood cancer may be at different, most likely increased, inherent risk of developing a new cancer in comparison with the general population due to a higher likelihood of genetic predisposition. Persons who survive five or more years after a first cancer are a selected sample of all childhood cancer patients. They may also be under increased surveillance, at least temporarily, relative to the general population. Survivors of a first cancer may alter their lifestyle in ways that affect, in either direction, their risks due to other, non-treatment exposures.
Strengths of the CCSS studies include individualized dose reconstruction based on radiotherapy records, consistency of dose reconstruction methods across studies, the availability of chemotherapy data, the relatively large size of the cohort, long-term follow-up and high participation proportions on follow-up surveys.
Nonetheless, these studies have limitations. We cannot exclude the possibility that successful tracing of eligible survivors or their decision to participation in the study was related to the occurrence of subsequent cancers (42, 43). Subsequent cancers in the CCSS cohort are largely self-reported. Study collaborators sought pathologic confirmation of self-reported cancers and, when doing so, searched broadly so as to include possible cancers. Nonetheless, there is a possibility of under-ascertainment. Results give an incomplete picture of risks of new primary cancers insofar as the cohort is limited to five-year survivors of the initial cancer. This is more of an issue for treatment-related leukemia, which commonly occurs before the five-year time point, than for solid cancers. Cancer treatment for recurrent disease or intervening new primary cancers was not effectively ascertained, particularly when it occurred at an institution other than the original treating hospital or more than five years following diagnosis of the first cancer.
There are uncertainties in dose reconstruction even when complete treatment records were available. Dosimetry was estimated based on pictures, diagrams and descriptions rather than film confirmation; if the second tumor was not located accurately within an organ, tumor site-specific dose estimates would carry imprecision. In addition, the radiotherapy often occurred at an age when organ size and position in the body were changing and can only be inferred. To adjust for this, tumor location in an adult needed to be extrapolated backwards in time, with estimates about where the progenitor cell might have been located at the time of irradiation. Even when doses can be well characterized, dose distributions across the study population were not ideal for evaluating the shape of the dose-response, organ-specific doses tended to be clustered at high- or low-dose extremes, with limited information for intermediate dose ranges. Furthermore, correlations among type of first cancer, organ-specific radiation dose and age at exposure makes it difficult to distinguish effects of each factor on second cancer risk. Aspects of radiation treatment other than total radiation dose could not be evaluated due to lack of variation; nearly all treatments involved high energy photons, and total dose and number of dose fractions tend to be highly correlated.
In summary, quantitative study of radiation-related second cancers, including both dose-response and factors that modify dose-response, provides some basis for generalization regarding new types of radiotherapy and for projecting risks associated with contemporary or hypothetical future treatment regimens. The fact that adjustment for radiation dose and chemotherapy greatly attenuates associations between subsequent cancer risk and type of first cancer suggests that treatment effects in most instances predominate over inherent susceptibility factors related to type of first cancer, and that meaningful inferences can be drawn about radiation effects from studies of new cancers among persons with different types of first cancer. Results of such studies also are relevant to radiation protection and to hypotheses about mechanisms of radiation carcinogenesis. Thus, inclusion of detailed radiation dosimetry in studies of second cancers not only provides information relevant to the clinical management and follow-up of cancer survivors but also allows for potentially generalizable insight into radiation epidemiology and radiobiology that may be applicable in different, and possibly novel, settings. With continued follow-up and study of the CCSS cohort as it reaches the ages at which the incidence rates of typical adult cancers increase sharply, a more comprehensive picture of radiation-related cancer risks among childhood cancer survivors will emerge.
Supplementary Material
The majority of childhood cancer patients now achieve long-term survival, but the treatments that cured their primary malignancy often put them at risk of adverse health outcomes years later. New primary cancers are among the most serious of these late effects. The aims of this review are to compare and contrast radiation dose-response relationships for new primary solid cancers in a large cohort of childhood cancer survivors and discuss interactions among treatment and host factors.
Acknowledgments
This work was supported by the National Cancer Institute (CA55727; G.T. Armstrong, Principal Investigator) and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics. Dr Ronckers is supported by the Dutch Cancer Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Notification: No actual or potential conflicts of interest exist.
References
- 1.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 2.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, Altekruse SF, Feuer EJ, Huang L, Mariotto A, Miller BA, Lewis DR, Eisner MP, Stinchcomb DG, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2006. Bethesda (MD): National Cancer Institute; 2009. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009. [Google Scholar]
- 5.Berrington de Gonzalez A, Gilbert E, Curtis R, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys. 2013;86:224–233. doi: 10.1016/j.ijrobp.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;10(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102:1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98:1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 11.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:3901–3907. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigurdson AJ, Ronckers CM, Mertens AC, et al. Second primary thyroid cancer after a first childhood malignancy: a report from the Childhood Cancer Survivor Study. Lancet. 2005;365:2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 13.Ronckers C, Sigurdson AJ, Stovall M, et al. Thyroid cancer in childhood cancer survivors: A detailed evaluation of radiation dose-response and its modifiers. Radiat Res. 2006;166:618–628. doi: 10.1667/RR3605.1. [DOI] [PubMed] [Google Scholar]
- 14.Bhatti P, Veiga L, Ronckers C, et al. Radiotherapy for childhood cancer and risk of thyroid cancer in a large cohort study: an update from the Childhood Cancer Survivor Study. Radiat Res. 2010;174:741–752. doi: 10.1667/RR2240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veiga L, Bhatti P, Ronckers CM, et al. Chemotherapy and the risk of thyroid cancer after a childhood malignancy: an update from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2012;21:92–101. doi: 10.1158/1055-9965.EPI-11-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson T, Rajaraman P, Stovall M, et al. Risk factors associated with secondary sarcomas in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. Int J Rad Oncol Biol Phys. 2012;84:224–230. doi: 10.1016/j.ijrobp.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boukheris H, Stovall M, Gilbert ES, et al. Risk of salivary gland cancer following childhood cancer: a report from the Childhood Cancer Survivor Study. Int J Radiat Biol Oncol Phys. 2012 Jul 24; doi: 10.1016/j.ijrobp.2012.06.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watt TC, Inskip PD, Stratton K, et al. Radiation-related risk of basal cell carcinoma in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2012;104:1240–1250. doi: 10.1093/jnci/djs298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 20.Tucker MA, Meadows AT, Boice JD, Jr, et al. Leukemia after therapy with alkylating agents for childhood cancer. J Natl Cancer Inst. 1987;78:459–464. [PubMed] [Google Scholar]
- 21.Feinleib M. Breast cancer and artificial menopause: a cohort study. J Natl Cancer Inst. 1968;41:315–329. [PubMed] [Google Scholar]
- 22.Trichopoulos D, MacMahon B, Cole P. Menopause and breast cancer risk. J Natl Cancer Inst. 1972;48:605–613. [PubMed] [Google Scholar]
- 23.Boice JD, Jr, Blettner M, Kleinerman RA, et al. Radiation dose and breast cancer risk in patients treated for cancer of the cervix. Int J Cancer. 1989;44:7–16. doi: 10.1002/ijc.2910440103. [DOI] [PubMed] [Google Scholar]
- 24.Inskip PD. Pelvic radiotherapy, sex hormones, and breast cancer. Cancer Causes Control. 1994;5:471–478. doi: 10.1007/BF01694761. [DOI] [PubMed] [Google Scholar]
- 25.NRC (National Research Council) BEIR VII Phase 2. Washington, DC: National Academies Press; 2006. Health risks from exposure to low levels of ionizing radiation. [PubMed] [Google Scholar]
- 26.UNSCEAR (United Nations Scientific Committee on the Effects of Atomic Radiation) Sources, effects and risks of ionizing radiation. UNSCEAR 2013 Report Volume II Scientific Annex B: Effects of radiation exposure of children. 2013 http://www.unscear.org/docs/reports/2013/UNSCEAR_2013_Report_Annex_B_Children.pdf. [Google Scholar]
- 27.Sachs RK, Brenner DJ. Solid tumor risks after high doses of ionizing radiation. Proc Natl Acad Sci USA. 2005;102:13040–13045. doi: 10.1073/pnas.0506648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sadetzki S, Chetrit A, Freedman L, et al. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;63:424–432. doi: 10.1667/rr3329. [DOI] [PubMed] [Google Scholar]
- 29.Taylor AJ, Little MP, Winter DL, et al. Population-based risks of CNS tumors in survivors of childhood cancer: the British Childhood Cancer Survivor Study. J Clin Oncol. 2010;28:5287–5293. doi: 10.1200/JCO.2009.27.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlsson P, Holmberg E, Lundell M, et al. Intracranial tumors after exposure to ionizing radiation during infancy: a pooled analysis of two Swedish cohorts of 28,008 infants with skin hemangioma. Radiat Res. 1998;150:357–364. [PubMed] [Google Scholar]
- 31.Preston DL, Ron E, Yonehara S, et al. Tumors of the nervous system and pituitary gland associated with atomic bomb radiation exposure. J Natl Cancer Inst. 2002;94:1555–1563. doi: 10.1093/jnci/94.20.1555. [DOI] [PubMed] [Google Scholar]
- 32.Inskip PD, Linet MS, Heineman EF. Etiology of brain tumors in adults. Epidemiol Rev. 1995;17:382–414. doi: 10.1093/oxfordjournals.epirev.a036200. [DOI] [PubMed] [Google Scholar]
- 33.Druckrey H. Specific carcinogenic and teratogenic effects of 'indirect' alkylating methyl and ethyl compounds, and their dependency on stages of ontogenic developments. Xenobiotica. 1973;3:271–303. [Google Scholar]
- 34.Kleihues P, Lantos PL, Magee PN. Chemical carcinogenesis in the nervous system. Int Rev Exp Pathol. 1976;15:153–232. [PubMed] [Google Scholar]
- 35.Kleihues P, Aguzzi A, Wiestler OD. Cellular and molecular aspects of neurocarcinogenesis. Toxicol Pathol. 1990;18:193–203. doi: 10.1177/019262339001800125. [DOI] [PubMed] [Google Scholar]
- 36.Rice JM, Rehm S, Donovan PJ, et al. Comparative transplacental carcinogenesis by directly acting and metabolism-dependent alkylating agents in rodents and nonhuman primates. In: Napalkov NP, Rice JM, Tomatis L, Yamasaki H, editors. Perinatal and multigeneration carcinogenesis. Lyon: IARC; 1989. pp. 17–34. [PubMed] [Google Scholar]
- 37.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among women with Hodgkin’s disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 38.van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer following Hodgkin’s disease. J Natl Cancer Inst. 2003;95:971–980. doi: 10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- 39.Damewood MD, Grochow LB. Prospects for fertility after chemotherapy or radiation for neoplastic disease. Fertil Steril. 1986;45:443–459. doi: 10.1016/s0015-0282(16)49268-x. [DOI] [PubMed] [Google Scholar]
- 40.Chemaitilly W, Mertens AC, Mitby P, et al. Acute ovarian failure in the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2006;91:1723–1728. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- 41.Sklar C. Maintenance of ovarian function and risk of premature menopause related to cancer treatment. J Natl Cancer Inst Monogr. 2005;34:25–27. doi: 10.1093/jncimonographs/lgi018. [DOI] [PubMed] [Google Scholar]
- 42.Mertens AC, Walls RS, Taylor L, et al. Characteristics of childhood cancer survivors predictive of their successful tracing. J Clinical Epidemiol. 2004;57:933–944. doi: 10.1016/j.jclinepi.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Ness KK, Leisenring W, Goodman P, et al. Assessment of selection bias in clinic-based populations of childhood cancer survivors: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2009;52:379–386. doi: 10.1002/pbc.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.