Abstract
The overall understanding of the molecular etiologies of intellectual disability (ID) and developmental delay (DD) is increasing as next-generation sequencing technologies identify genetic variants in individuals with such disorders. However, detailed analyses conclusively confirming these variants, as well as the underlying molecular mechanisms explaining the diseases, are often lacking. Here, we report on an ID syndrome caused by de novo heterozygous loss-of-function (LoF) mutations in SON. The syndrome is characterized by ID and/or DD, malformations of the cerebral cortex, epilepsy, vision problems, musculoskeletal abnormalities, and congenital malformations. Knockdown of son in zebrafish resulted in severe malformation of the spine, brain, and eyes. Importantly, analyses of RNA from affected individuals revealed that genes critical for neuronal migration and cortex organization (TUBG1, FLNA, PNKP, WDR62, PSMD3, and HDAC6) and metabolism (PCK2, PFKL, IDH2, ACY1, and ADA) are significantly downregulated because of the accumulation of mis-spliced transcripts resulting from erroneous SON-mediated RNA splicing. Our data highlight SON as a master regulator governing neurodevelopment and demonstrate the importance of SON-mediated RNA splicing in human development.
Main Text
Recent advances in whole-exome and whole-genome sequencing have accelerated the identification of the genetic etiologies of intellectual disability (ID) and developmental delay (DD), facilitating appropriate care and therapy for affected individuals and their families. So far, mutations in more than 1,500 genes have been implicated in ID and DD disorders,1, 2, 3, 4, 5, 6, 7, 8, 9 and de novo single-nucleotide variants and copy-number variations (CNVs) have been identified as a major cause of severe ID and/or DD.5, 7 Recently, two independent studies reported on a single individual with ID and/or DD and a de novo mutation in SON (SON DNA binding protein [MIM: 182465]), which encodes a protein required for proper RNA splicing. However, the level of evidence required for securely implicating mutations in this gene as disease causing was lacking.5, 10, 11 Including these two individuals, we recruited a total of 20 unrelated individuals with mild to severe ID and/or DD (Figure 1A and Table S1) and report on the delineation of an ID syndrome caused by de novo LoF mutations in SON. This study was approved by the local institutes under the realm of diagnostic testing.
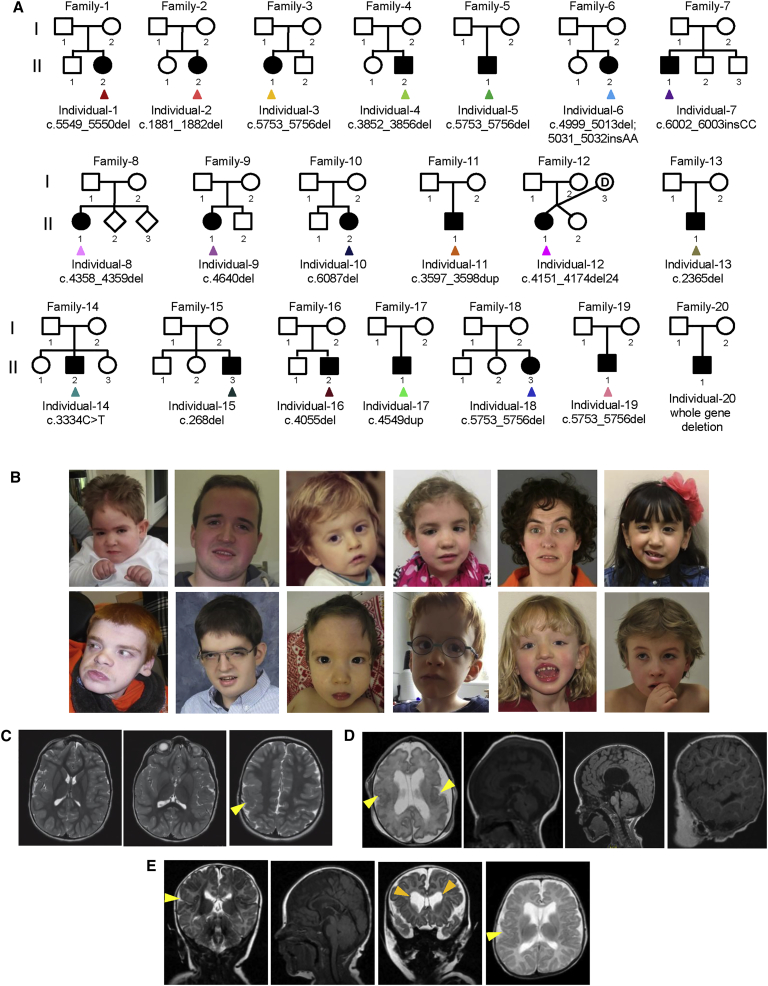
Figure 1.
Pedigree Structures, Photos, and Brain MRI of Individuals with SON Mutations
(A) Family pedigrees of individuals carrying mutations in SON.
(B) Top row from left to right: photos of individuals 2 (at age 5 years), 4 (age 19 years), 5 (age 2 years), 6 (age 6 years), 8 (age 34 years), and 10 (age 6 years). Bottom row from left to right: photos of individuals 11 (age 21 years), 13 (age 14 years), 15 (age 15 months), 16 (age 5 years), 18 (age 6 years), and 19 (age 10 years). Shared facial dysmorphisms include facial asymmetry, midface retraction, low-set ears, downslanting palpebral fissures, deep-set eyes, horizontal eyebrows, a broad and/or depressed nasal bridge, and a short philtrum.
(C) Axial T2-weighted fast spin-echo MRI of the brain of individual 1 at age 3 years. Three panels show ascending images (left to right) revealing that the individual’s insular cortex on the right is thickened and featureless. Less impressive areas of similar change were noted in the posterior aspect of the left insular cortex, which revealed bilateral perisylvian and parietal polymicrogyria (yellow arrowhead).
(D) Sagittal T1-weighted and axial T2-weighted MRI of the brain of individual 2. The two images on the left (age 1 day; gestational age 34 + 6 weeks) reveal enlarged lateral ventricles, cavum septum pellucidum, a hypoplastic cerebellar hemisphere, a broad cistern magna, a small fourth ventricle, and a thin corpus callosum. The cortex shows a simplified gyration pattern, and the perisylvian and frontotemporal areas are suspect for polymicrogyria (yellow arrowheads). The two panels on the right (age 2 years) show the fissure Sylvie with an abnormal cortical border, an Arnold Chiari malformation, and hydrocephalus.
(E) Frontal T2-weighted, sagittal T1-weighted, axial T2-weighted, and sagittal T1-weighted MRI of the brain of individual 7 (II-1 in family 7) at the age of 2 months. The cortex shows deep sulci and perisylvian areas suspect for polymicrogyria (yellow arrowheads), as well as discrete heterotopic nodules (orange arrowheads). A thin corpus callosum, a small fourth ventricle, enlarged frontal horns of the lateral ventricles, and cavum septum pellucidum are present.
We compared in detail the phenotypic characteristics of all 20 individuals with SON LoF mutations. Clinical examination showed that all affected individuals had mild to moderate facial dysmorphisms, including facial asymmetry, midface retraction, low-set ears, downslanting palpebral fissures, deep-set eyes, horizontal eyebrows, a broad and/or depressed nasal bridge, and a short philtrum (Figures 1B and Figure S1). Interestingly, brain MRI, available for 19 affected individuals, revealed that 17 of them had significant abnormalities, including abnormal gyration patterns (polymicrogyria, simplified gyria, and periventricular nodular heterotopia), ventriculomegaly, Arnold-Chiari malformations, arachnoid cysts, hypoplasia of the corpus callosum, hypoplasia of the cerebellar hemispheres, and loss of periventricular white matter (Figures 1C–1E). 11 of 20 individuals developed seizures and/or epilepsy with an age of onset ranging from 1 to 6 years. 17 of 20 individuals presented with musculoskeletal abnormalities, comprising hemivertebrae, scoliosis or kyphosis, contractures, hypotonia, and hypermobility of the joints. Vision problems, including cerebral visual impairment, hypermetropia, optic atrophy, and strabismus, were present in 15 of 20 individuals. In addition, the vast majority of individuals showed congenital malformations consisting of urogenital malformations (6/20), heart defects (5/20), gut malformations (3/20), and a high and/or cleft palate (2/20). Short stature was present in ten individuals, and craniosynostosis involving both the metopic (n = 1) and the sagittal sutures (n = 2) was noted in 3 of 20 individuals. Metabolic screening was performed in 9 of 20 individuals, confirming mitochondrial dysfunction in individuals 2 and 11 and an O-glycosylation defect in individual 20 (a clinical summary is provided in Table 1, and details are listed in Table S2). Apart from individuals 13 (II-1 in family 13; Figure 1A), 15 (II-3 in family 15), and 20 (II-1 in family 20), none of the individuals had additional coding-sequence mutations that explained (part of) the phenotype (Table S2). Individual 13 was clinically diagnosed with dyskeratosis congenita, for which a maternally inherited pathogenic TERT (MIM: 187270) mutation was identified (Table S2). Individual 13 was, however, more severely affected than could be explained by a TERT mutation alone. Similarly, none of the other coding variants identified in individual 15 or the additional genes deleted by the 384 kb deletion CNV in individual 20 were likely to explain the phenotype of these individuals (Table S2).
Table 1.
Clinical Features of Individuals with SON Haploinsufficiency
| Percentage | Number of Affected Individuals | |
|---|---|---|
| Intellectual disability | 100% | 20/20 |
| Brain malformation | 89% | 17/19 |
| Ventricular enlargement | 74% | 14/19 |
| Corpus callosum abnormality | 53% | 10/19 |
| Cortex malformation | 37% | 7/19 |
| White-matter abnormalities | 21% | 4/19 |
| Cerebellar abnormalities | 21% | 4/19 |
| Other | 11% | 2/19 |
| Neurological features | 85% | 17/20 |
| Seizures | 55% | 11/20 |
| Hypotonia | 75% | 15/20 |
| Musculoskeletal abnormalities | 85% | 17/20 |
| Hypermobility | 40% | 8/20 |
| Scoliosis or kyphosis | 20% | 4/20 |
| Hemivertebrae | 10% | 2/20 |
| Contractures | 10% | 2/20 |
| Other | 85% | 17/20 |
| Eye and/or vision abnormality | 75% | 15/20 |
| Strabismus | 55% | 11/20 |
| Suspicion of CVI | 20% | 4/20 |
| Hypermetropia | 30% | 6/20 |
| Heart defect | 25% | 5/20 |
| Gastrointestinal malformation | 15% | 3/20 |
| Urogenital malformation | 30% | 6/20 |
| Horseshoe kidney | 10% | 2/20 |
| Other | 20% | 4/20 |
| Facial dysmorphism | 100% | 20/20 |
| Short stature | 50% | 10/20 |
| Craniosynostosis | 15% | 3/20 |
The following abbreviation is used: CVI, cortical visual impairment.
SON (GenBank: NM_138927.2) is composed of 12 exons (Figure 2A) and encodes a protein (GenBank: NP_620305.2) containing an arginine/serine (RS)-rich domain and two RNA-binding motifs (a G-patch and a double-stranded RNA binding motif) (Figure 2A).12, 13, 14 17 of 20 mutations are frameshift mutations, including a recurrent 4-bp deletion (c.5753_5756del) in four independent individuals (Table S1 and Figures 2A and 2B). The remaining mutations include a nonsense mutation, an in-frame deletion of eight amino acids, and a complete gene deletion (Table S1). Importantly, parental DNA was available for testing in 19 of 20 individuals and indicated that all mutations had occurred de novo (Figure S2 and Table S1). Interestingly, de novo truncating mutations in SON have not been observed in over 2,000 control individuals,4, 15, 16, 17, 18 and SON, with a Residual Variation Intolerance Score of −1.88, belongs to the 2% most intolerant human protein-coding genes.19 Furthermore, interrogation of large databases (such as the Exome Aggregation Consortium [ExAC] Browser) has shown that, after sequence context and mutability are considered, SON is significantly depleted of LoF variants according to multiple LoF metrics (pLI = 1.00, and the false-discovery rate of the LoF depletion score is p = 1.68 × 10−6).20, 21 Although these population genetic signatures of intolerance cannot be considered sufficient evidence of causality on their own, they support the hypothesis that SON LoF mutations are under strong purifying selection in the human population and that their occurrence most likely contributes to severe clinical phenotypes.
Figure 2.
SON Mutations and Their Functional Effect at the RNA and Protein Levels
(A and B) Schematic representation of SON (A) and SON (B) shows the position of the mutations identified in the 20 affected individuals with color-coded arrowheads. The locations of the PCR primer sets are indicated by black arrows.
(C) Real-time qPCR with three different primer pairs showed that SON mRNA from the affected individuals was overall downregulated in comparison to mRNA from the parents and unrelated normal individual. Error bars represent mean ± SD. ∗p < 0.001.
(D and E) Western blotting demonstrated reduced expression of SON. SON-N antibody (1:1,000) was generated against amino acids 74–88 of the human SON (amino acid sequence DTELRYKPDLKEGSR). The cocktail of WU SON antibodies was a mixture of three different SON antibodies (WU09 [1:100], WU14 [1:2,000], and WU21 [1:200]). The epitopes of WU SON antibodies were as follows: MDSQMLATSS for WU09, CEESESKTKSH for WU14, and SMMSAYERS for WU21. SON-N antibody (D) and the cocktail of SON WU antibodies (E) showed similar results. The bands indicated by the black arrow represent full-length SON. Other bands, which could represent potential isoforms, were also detected. Besides the bands present in samples from both normal and affected individuals, no other specific bands were detected in the affected individuals.
Transcripts bearing a premature stop codon are likely to be degraded by nonsense-mediated mRNA decay. To confirm that LoF mutations result in reduced dosage of SON, we used three different PCR primer sets (Table S3) to perform qRT-PCR to determine the amounts of the SON transcript in peripheral-blood mononuclear cells (PBMCs) isolated from trio 1 (I-1, I-2, and II-2 in family 1), trio 3 (I-1, I-2, and II-1 in family 3), individual 5 (II-1 in family 5; Figure 1A), and an unrelated healthy donor (Figure 2C). All three primer sets showed that compared to mRNA from the parental samples and the unrelated healthy donor, SON mRNA in the affected individuals was significantly downregulated (Figure 2C). Subsequent western blotting using PBMC lysates from trio 1 and two different SON antibodies consistently showed the reduction of SON in individual 1 (Figures 2D and 2E), indicating that de novo SON LoF mutations result in SON haploinsufficiency.
To examine the effect of SON haploinsufficiency on embryonic development, we utilized Danio rerio (zebrafish), which has a well-conserved homolog of human SON (NCBI Gene: LOC565999; Figures S3 and S4). We assessed the developmental effects of SON haploinsufficiency in vivo with morpholino (MO)-mediated knockdown of son in zebrafish embryos. Interestingly, embryos injected with a son MO had a host of developmental defects that ranged from bent tails (63.6%) to eye malformations and microcephaly (17.1%) and shortened or gnarled tails, deformed body axes, and massive body curvatures (2.1%) 24 hr post-injection (hpi) (Figure 3A and Figure S5). Embryos that survived 72 hpi progressed to more severe phenotypes including extreme spinal malformations (22.2%), head and eye malformations with brain edema (37.2%), and profound developmental abnormalities (10.1%; Figure 3B), mimicking features observed in the affected individuals.
Figure 3.
Targeted son Knockdown in Developing Zebrafish Causes Impaired Head Development and Spinal Malformations
(A) Zebrafish injected with a splice-blocking son morpholino (MO; 5′-TGGTCCTGGATATAACAGACAGATT-3′, 6.25 ng) that targeted the junction between intron 9 and exon 10, a control MO (5′-CCTCTTACCTCAGTTACAATTTATA-3′, 6.25 ng), or no MO showed a normal phenotype, a bent spine or tail, a head or eye defect, or a severe phenotype at 24 and 72 hpi. The percentages of embryos with each phenotype are shown in the bar graphs, and the number of embryos examined is listed next to each bar.
(B) Representative images of the phenotype observed 72 hr after MO injection (red arrow, bent spine or tail; white arrow, eye defects; and yellow arrow, brain edema).
SON is a nuclear speckle protein able to bind to both DNA and RNA, and its cellular functions include regulation of RNA splicing and gene transcription, as well as proper cell-cycle and embryonic stem cell maintenance.13, 22, 23, 24, 25 To identify molecular mechanisms underlying the clinical features of individuals with SON haploinsufficiency, we examined global expression patterns upon SON knockdown in cellular systems. Hereto, we re-analyzed microarray-based RNA-expression profiling and RNA-sequencing datasets generated upon SON knockdown in HeLa cells13, 22 and human embryonic stem cells.25 Surprisingly, from these previous datasets, we noticed that a group of genes playing pivotal roles in neuronal cell migration, embryonic survival, metabolism, and mitochondrial function, including TUBG1 (MIM: 191135), FLNA (MIM: 300017), PNKP (MIM: 605610), WDR62 (MIM: 613583), PSMD3, HDAC6 (MIM: 300272), PCK2 (MIM: 614095), PFKL (MIM: 171860), IDH2 (MIM: 147650), ACY1 (MIM: 104620), and ADA (MIM: 608958), showed significantly decreased expression upon SON knockdown (Tables S4 and S5).13, 22, 25 To investigate whether genes involved in regulating brain development and in metabolism are also downregulated in individuals with SON LoF mutations, we measured the levels of RNA expression of these genes in PBMCs from trio 1, trio 3, and individual 5, as well as from an unrelated healthy donor (primers are listed in Table S3). Using qPCR analysis, we confirmed that all 11 genes were indeed significantly downregulated in individuals with SON haploinsufficiency (Figures 4A and 4B).
Figure 4.
Individuals Carrying Heterozygous SON LoF Mutations Have Defective RNA Splicing of Genes Associated with the Pathophysiology of ID and/or DD and Metabolic Disorders, Resulting in Their Reduced Expression
(A and B) Multiple genes associated with the pathophysiology of ID and/or DD (A) and metabolic disorders (B) in the affected individuals were downregulated in comparison to genes from parents and unrelated healthy individuals. TUBA1A mRNA served as a negative control (unaffected transcript). Error bars represent mean ± SD. ∗p < 0.001.
(C and D) Intron retention and exon skipping of genes involved in ID and/or DD when mutated (C) and genes involved in metabolic disorders when mutated (D) in the individuals with SON mutations. The locations of the primers used for PCR are marked by gray arrows above the exons. Analysis of TUBA1A pre-mRNA, which served as a negative control, demonstrated that splicing of this transcript is not impaired in the affected individuals. ∗, intron-retained products; #, exon-skipped products.
SON functions as a splicing co-factor that promotes correct and efficient RNA splicing of weak splice sites and alternative splice sites by facilitating spliceosome recruitment to the elongating RNA polymerase II complex.13 Prominent features observed upon SON knockdown in HeLa cells and human embryonic stem cells have included intron retention and exon skipping, which have been shown at the gene level for TUBG1, HDAC6, and ADA.13, 22, 25 We next sought to determine whether RNA splicing of these 11 genes is also impaired in our individuals with SON haploinsufficiency. To this end, we analyzed the pre-mRNA sequences of the remaining eight genes to predict weak splice sites that could be potential targets of SON-mediated RNA splicing (Table S6). We performed RT-PCR by using DNase-treated RNA samples isolated from trio 1, trio 3, individual 5, and an unrelated healthy donor and using primers designed to detect intron retention and exon skipping (Tables S6 and S7). We not only confirmed that these sites were indeed mis-spliced in HeLa cells upon SON knockdown (Figure S6) but also found that all three affected individuals showed significant intron retention (TUBG1, FLNA, PNKP, WDR62, PSMD3, PCK2, PFKL, IDH2, and ACY1) and exon skipping (HDAC6 and ADA) at the predicted sites of the target pre-mRNAs and that this resulted in the accumulation of mis-spliced products (Figures 4C and 4D). In contrast, mis-spliced RNA products were absent in the parents and unrelated donor (Figures 4C and 4D). Together, these results indicate that SON-mediated RNA splicing is severely compromised in individuals with SON haploinsufficiency.
Our data have revealed that the complex neurodevelopmental disorder observed in these affected individuals is due to compromised SON function, which causes insufficient production of downstream targets as a result of erroneous SON-mediated RNA splicing. Moreover, the roles of several downregulated genes are well-known causes of ID and/or DD in humans (Tables S4 and S5).4, 6, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35 For instance, FLNA haploinsufficiency is the most common cause of periventricular nodular heterotopia (MIM: 300049),35 a rare brain malformation that we also found among our cohort with SON LoF mutations. Similarly, de novo LoF mutations in TUBG1 are known to result in cortical malformations (MIM: 615412),32 also frequently observed in our cohort of affected individuals. Because we have shown that a substantial number of essential developmental genes are significantly downregulated upon SON haploinsufficiency, SON thus represents a master regulator of genes essential for human neurodevelopmental processes.
In summary, we have identified de novo LoF mutations in SON as a cause of a complex neurodevelopmental disorder associated with ID and/or DD and severe brain malformations. In addition, we have revealed the underlying molecular mechanism by showing that SON haploinsufficiency leads to defective RNA splicing of multiple genes critical for brain development, neuronal migration, and metabolism. Our findings thus greatly contribute to our understanding of how defective RNA splicing leads to human neurodevelopmental disorders.
Conflicts of Interest
D.N.S., S.T., and D.E. are employees of Ambry Genetics, Inc. M.T.C., K.M., G.D., B.B., A.B., and T.F. are employees of GeneDx, Inc.
Acknowledgments
We are deeply grateful to all individuals and their families for participating in this study. We thank Dr. Michael Markey (Wright State University) for transcript splicing analysis, Dr. David Goldstein, Jennifer Sullivan, and Nicole Walley (Duke University) for their help in collecting clinical information, Petra de Vries (Radboudumc) and Hermann-Josef Lüdecke (University Duisburg-Essen) for technical support, and Dr. Christian Gilissen (Radboudumc) for bioinformatic support. We thank the University of Washington Center for Mendelian Genomics (funded by the National Human Genome Research Institute and National Heart, Lung, and Blood Institute grant 1U54HG006493 to Drs. Debbie Nickerson, Jay Shendure, and Michael Bamshad) and the Radboudumc Genomics Technology Center for family-based sequencing. This work was supported by NIH grants (CA190688 and CA185818 to E.-Y.E.A. and GM084407 to P.A.B.), a start-up fund from the University of South Alabama Mitchell Cancer Institute (to E.-Y.E.A.), the Ohio Board of Regents (to P.A.B.), a California State University Chico Internal Research Grant (to D.L.S.), grants from Stichting ODAS and Vereniging Bartiméus-Sonneheerdt (5781251 to B.B.A.d.V. and D.G.M.B.), and a grant from the Dutch Organization for Health Research and Development (912-12-109 to B.B.A.d.V. and J.A.V.). This study used data generated by the DECIPHER Consortium. A full list of centers who contributed to the generation of the data is available at http://decipher.sanger.ac.uk and via email at decipher@sanger.ac.uk. Funding for the DECIPHER project was provided by the Wellcome Trust.
Published: August 18, 2016
Footnotes
Supplemental Data include six figures and seven tables and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.06.029.
Contributor Information
Lisenka E.L.M. Vissers, Email: lisenka.vissers@radboudumc.nl.
Eun-Young Erin Ahn, Email: eahn@health.southalabama.edu.
Web Resources
Clinical Genome Database, http://research.nhgri.nih.gov/CGD/
DECIPHER, https://decipher.sanger.ac.uk/
ESEfinder, http://rulai.cshl.edu/tools/ESE/
ExAC Browser, http://exac.broadinstitute.org/
Genic Intolerance, http://genic-intolerance.org/
NCBI Gene, http://www.ncbi.nlm.nih.gov/gene
OMIM, http://www.omim.org/
Supplemental Data
References
- 1.Ropers H.H. Genetics of early onset cognitive impairment. Annu. Rev. Genomics Hum. Genet. 2010;11:161–187. doi: 10.1146/annurev-genom-082509-141640. [DOI] [PubMed] [Google Scholar]
- 2.Mefford H.C., Batshaw M.L., Hoffman E.P. Genomics, intellectual disability, and autism. N. Engl. J. Med. 2012;366:733–743. doi: 10.1056/NEJMra1114194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vissers L.E., de Ligt J., Gilissen C., Janssen I., Steehouwer M., de Vries P., van Lier B., Arts P., Wieskamp N., del Rosario M. A de novo paradigm for mental retardation. Nat. Genet. 2010;42:1109–1112. doi: 10.1038/ng.712. [DOI] [PubMed] [Google Scholar]
- 4.Rauch A., Wieczorek D., Graf E., Wieland T., Endele S., Schwarzmayr T., Albrecht B., Bartholdi D., Beygo J., Di Donato N. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 5.Gilissen C., Hehir-Kwa J.Y., Thung D.T., van de Vorst M., van Bon B.W., Willemsen M.H., Kwint M., Janssen I.M., Hoischen A., Schenck A. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. doi: 10.1038/nature13394. [DOI] [PubMed] [Google Scholar]
- 6.Deciphering Developmental Disorders Study Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519:223–228. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ligt J., Willemsen M.H., van Bon B.W., Kleefstra T., Yntema H.G., Kroes T., Vulto-van Silfhout A.T., Koolen D.A., de Vries P., Gilissen C. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 2012;367:1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 8.Vissers L.E., Gilissen C., Veltman J.A. Genetic studies in intellectual disability and related disorders. Nat. Rev. Genet. 2016;17:9–18. doi: 10.1038/nrg3999. [DOI] [PubMed] [Google Scholar]
- 9.Lelieveld S.H., Reijnders M.R.F., Pfundt R., Yntema H.G., Kamsteeg E.-J., de Vries P., de Vries B.B.A., Willemsen M.H., Kleefstra T., Löhner K. Meta-analysis of 2,104 trios provides support for 10 new genes for intellectual disability. Nat. Neurosci. 2016 doi: 10.1038/nn.4352. Published online August 1, 2016. [DOI] [PubMed] [Google Scholar]
- 10.Zhu X., Petrovski S., Xie P., Ruzzo E.K., Lu Y.F., McSweeney K.M., Ben-Zeev B., Nissenkorn A., Anikster Y., Oz-Levi D. Whole-exome sequencing in undiagnosed genetic diseases: interpreting 119 trios. Genet. Med. 2015;17:774–781. doi: 10.1038/gim.2014.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacArthur D.G., Manolio T.A., Dimmock D.P., Rehm H.L., Shendure J., Abecasis G.R., Adams D.R., Altman R.B., Antonarakis S.E., Ashley E.A. Guidelines for investigating causality of sequence variants in human disease. Nature. 2014;508:469–476. doi: 10.1038/nature13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma A., Takata H., Shibahara K., Bubulya A., Bubulya P.A. Son is essential for nuclear speckle organization and cell cycle progression. Mol. Biol. Cell. 2010;21:650–663. doi: 10.1091/mbc.E09-02-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn E.Y., DeKelver R.C., Lo M.C., Nguyen T.A., Matsuura S., Boyapati A., Pandit S., Fu X.D., Zhang D.E. SON controls cell-cycle progression by coordinated regulation of RNA splicing. Mol. Cell. 2011;42:185–198. doi: 10.1016/j.molcel.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickey C.J., Kim J.H., Ahn E.Y. New discoveries of old SON: a link between RNA splicing and cancer. J. Cell. Biochem. 2014;115:224–231. doi: 10.1002/jcb.24672. [DOI] [PubMed] [Google Scholar]
- 15.Iossifov I., O’Roak B.J., Sanders S.J., Ronemus M., Krumm N., Levy D., Stessman H.A., Witherspoon K.T., Vives L., Patterson K.E. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genome of the Netherlands Consortium Whole-genome sequence variation, population structure and demographic history of the Dutch population. Nat. Genet. 2014;46:818–825. doi: 10.1038/ng.3021. [DOI] [PubMed] [Google Scholar]
- 17.Gulsuner S., Walsh T., Watts A.C., Lee M.K., Thornton A.M., Casadei S., Rippey C., Shahin H., Nimgaonkar V.L., Go R.C., Consortium on the Genetics of Schizophrenia (COGS) PAARTNERS Study Group Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell. 2013;154:518–529. doi: 10.1016/j.cell.2013.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu B., Ionita-Laza I., Roos J.L., Boone B., Woodrick S., Sun Y., Levy S., Gogos J.A., Karayiorgou M. De novo gene mutations highlight patterns of genetic and neural complexity in schizophrenia. Nat. Genet. 2012;44:1365–1369. doi: 10.1038/ng.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petrovski S., Wang Q., Heinzen E.L., Allen A.S., Goldstein D.B. Genic intolerance to functional variation and the interpretation of personal genomes. PLoS Genet. 2013;9:e1003709. doi: 10.1371/journal.pgen.1003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrovski S., Gussow A.B., Wang Q., Halvorsen M., Han Y., Weir W.H., Allen A.S., Goldstein D.B. The Intolerance of Regulatory Sequence to Genetic Variation Predicts Gene Dosage Sensitivity. PLoS Genet. 2015;11:e1005492. doi: 10.1371/journal.pgen.1005492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Exome Aggregation Consorsium Analysis of protein-coding genetic variation in 60,706 humans. bioRxiv. 2016 doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma A., Markey M., Torres-Muñoz K., Varia S., Kadakia M., Bubulya A., Bubulya P.A. Son maintains accurate splicing for a subset of human pre-mRNAs. J. Cell Sci. 2011;124:4286–4298. doi: 10.1242/jcs.092239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J.H., Baddoo M.C., Park E.Y., Stone J.K., Park H., Butler T.W., Huang G., Yan X., Pauli-Behn F., Myers R.M. SON and Its Alternatively Spliced Isoforms Control MLL Complex-Mediated H3K4me3 and Transcription of Leukemia-Associated Genes. Mol. Cell. 2016;61:859–873. doi: 10.1016/j.molcel.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn E.E., Higashi T., Yan M., Matsuura S., Hickey C.J., Lo M.C., Shia W.J., DeKelver R.C., Zhang D.E. SON protein regulates GATA-2 through transcriptional control of the microRNA 23a∼27a∼24-2 cluster. J. Biol. Chem. 2013;288:5381–5388. doi: 10.1074/jbc.M112.447227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu X., Göke J., Sachs F., Jacques P.E., Liang H., Feng B., Bourque G., Bubulya P.A., Ng H.H. SON connects the splicing-regulatory network with pluripotency in human embryonic stem cells. Nat. Cell Biol. 2013;15:1141–1152. doi: 10.1038/ncb2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bilgüvar K., Oztürk A.K., Louvi A., Kwan K.Y., Choi M., Tatli B., Yalnizoğlu D., Tüysüz B., Cağlayan A.O., Gökben S. Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature. 2010;467:207–210. doi: 10.1038/nature09327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J.F., Zhang Y., Wilde J., Hansen K.C., Lai F., Niswander L. Microcephaly disease gene Wdr62 regulates mitotic progression of embryonic neural stem cells and brain size. Nat. Commun. 2014;5:3885. doi: 10.1038/ncomms4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jamuar S.S., Lam A.T., Kircher M., D’Gama A.M., Wang J., Barry B.J., Zhang X., Hill R.S., Partlow J.N., Rozzo A. Somatic mutations in cerebral cortical malformations. N. Engl. J. Med. 2014;371:733–743. doi: 10.1056/NEJMoa1314432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jensen L.R., Lenzner S., Moser B., Freude K., Tzschach A., Wei C., Fryns J.P., Chelly J., Turner G., Moraine C. X-linked mental retardation: a comprehensive molecular screen of 47 candidate genes from a 7.4 Mb interval in Xp11. Eur. J. Hum. Genet. 2007;15:68–75. doi: 10.1038/sj.ejhg.5201714. [DOI] [PubMed] [Google Scholar]
- 30.Nicholas A.K., Khurshid M., Désir J., Carvalho O.P., Cox J.J., Thornton G., Kausar R., Ansar M., Ahmad W., Verloes A. WDR62 is associated with the spindle pole and is mutated in human microcephaly. Nat. Genet. 2010;42:1010–1014. doi: 10.1038/ng.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nofech-Mozes Y., Blaser S.I., Kobayashi J., Grunebaum E., Roifman C.M. Neurologic abnormalities in patients with adenosine deaminase deficiency. Pediatr. Neurol. 2007;37:218–221. doi: 10.1016/j.pediatrneurol.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Poirier K., Lebrun N., Broix L., Tian G., Saillour Y., Boscheron C., Parrini E., Valence S., Pierre B.S., Oger M. Mutations in TUBG1, DYNC1H1, KIF5C and KIF2A cause malformations of cortical development and microcephaly. Nat. Genet. 2013;45:639–647. doi: 10.1038/ng.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen J., Gilmore E.C., Marshall C.A., Haddadin M., Reynolds J.J., Eyaid W., Bodell A., Barry B., Gleason D., Allen K. Mutations in PNKP cause microcephaly, seizures and defects in DNA repair. Nat. Genet. 2010;42:245–249. doi: 10.1038/ng.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu T.W., Mochida G.H., Tischfield D.J., Sgaier S.K., Flores-Sarnat L., Sergi C.M., Topçu M., McDonald M.T., Barry B.J., Felie J.M. Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat. Genet. 2010;42:1015–1020. doi: 10.1038/ng.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox J.W., Lamperti E.D., Ekşioğlu Y.Z., Hong S.E., Feng Y., Graham D.A., Scheffer I.E., Dobyns W.B., Hirsch B.A., Radtke R.A. Mutations in filamin 1 prevent migration of cerebral cortical neurons in human periventricular heterotopia. Neuron. 1998;21:1315–1325. doi: 10.1016/s0896-6273(00)80651-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.