Abstract
Via whole-exome sequencing, we identified rare autosomal-recessive variants in UBA5 in five children from four unrelated families affected with a similar pattern of severe intellectual deficiency, microcephaly, movement disorders, and/or early-onset intractable epilepsy. UBA5 encodes the E1-activating enzyme of ubiquitin-fold modifier 1 (UFM1), a recently identified ubiquitin-like protein. Biochemical studies of mutant UBA5 proteins and studies in fibroblasts from affected individuals revealed that UBA5 mutations impair the process of ufmylation, resulting in an abnormal endoplasmic reticulum structure. In Caenorhabditis elegans, knockout of uba-5 and of human orthologous genes in the UFM1 cascade alter cholinergic, but not glutamatergic, neurotransmission. In addition, uba5 silencing in zebrafish decreased motility while inducing abnormal movements suggestive of seizures. These clinical, biochemical, and experimental findings support our finding of UBA5 mutations as a pathophysiological cause for early-onset encephalopathies due to abnormal protein ufmylation.
Main Text
Post-translational modifications (PTM) of proteins by ubiquitin and ubiquitin-like peptides increase the functional diversity of the proteome and are critical regulatory processes involved in many cellular functions including the control of cell cycle, stress response, signaling transduction, and immune response.1
The covalent attachment of the ubiquitin-fold modifier 1 (UFM1) to a target protein, also named ufmylation, is a recently identified ubiquitin-like PTM.2 Similarly to ubiquitination, ufmylation requires a series of enzymes referred to as E1 activating enzyme (UBA5), E2 conjugating enzyme (UFC1), and E3 ligase (UFL1) to transfer UFM1 to its targets.3 Most members of the UFM1 cascade and target proteins are localized in a large protein complex at the luminal site of the endoplasmic reticulum (ER) and are involved in the regulation of the unfolded protein response (UPR) and ER-stress-mediated apoptosis.4, 5 The UFM1 cascade has also been involved in the development of various cancers6, 7 and other diseases.5, 8, 9 However, the specific biological function of ufmylation and the clinical implications of its dysfunction remain largely uncharacterized, even though Duan et al.10 reported recently the putative involvement of UBA5 in a single family affected with recessive cerebellar ataxia. Here, we report the involvement of the ufmylation cascade in a severe autosomal-recessive early-onset neurological disorder through the identification of biallelic mutations in UBA5 (MIM: 610552) in four unrelated families.
This study was approved by the Angers University Hospital Ethics Committee (N° 2016-40). Participants or their parents provided informed, written consent for genetic studies. Using whole-exome sequencing (WES) as a clinical diagnostic tool, we identified rare variants in UBA5 (GenBank: NM_024818) in two children from a French family (family A, Figure 1). These children developed an early-onset severe neurological disorder consisting of infantile spasms followed by the development of intractable epilepsy, movement disorders, severe intellectual disability, acquired microcephaly, and failure to thrive (Table 1 and Supplemental Note). We excluded a dominant mutation with a germline mosaicism in one of the parents, and X-linked mutations. Then, in the absence of obvious consanguinity and blocks of homozygosity on the SNP array (data not shown), we prioritized WES data filtering for compound-heterozygous damaging variants and found that both children harbored rare biallelic UBA5 variants (Table 1).
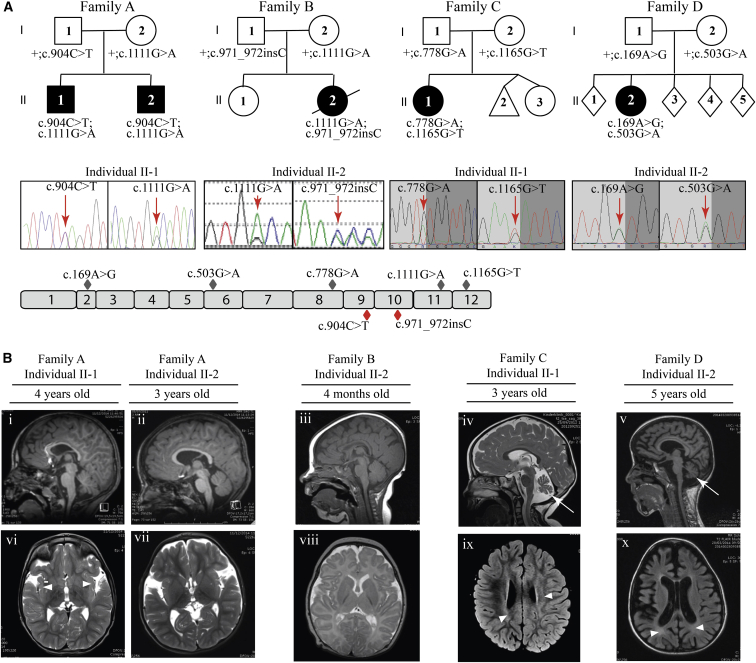
Figure 1.
Identification and Segregation of UBA5 Mutations and Brain MRI of the Five Affected Individuals
(A) Families with mutations in UBA5. Filled black symbols represent the affected individuals. Allelic status is given below each tested individual. Representative chromatograms show the compound heterozygous mutations for each family (family A: c.904C>T, c.1111G>A; family B: c.1111G>A, c.971_972insC; family C: c.778G>A, c.1165G>T; family D: c.169A>G, c.503G>A). Red arrows indicate the position of the nucleotide change. Schematic overview of the 12 exons (gray boxes) of human UBA5, with missense (gray diamonds) and nonsense (red diamonds) mutations.
(B) Brain MRI of the five affected individuals. Sagittal T1 (i, ii, iii, and v) and sagittal T2 (iv) images are shown. A thin corpus callosum in individuals II-1 from family A, II-2 from family B, and II-2 from family D (i, iii, v), cortical atrophy in individual II-2 from family D (v), and cerebellar atrophy (arrows) without brainstem anomalies in individuals II-1 from family C and II-2 from family D (iv, v) are observed. Axial T2 images (vi, vii, and viii) and T2 Flair (ix, x) are shown. White matter hyperintensities (arrowheads) in insula subcortical white matter (individual II-1 in family A [vi]), periventricular region (individuals II-1 in family C and II-2 in family D [ix, x]), and delayed myelination (individual II-2 in family B) are observed (viii). Widening of sylvian fissures (individuals II-1 and II-2 in family A [vi, vii]) and global cortical atrophy with ventricular dilation (individual II-2 in family D [x]) are observed.
Table 1.
Clinical Features of Individuals with UBA5 Mutations
| Individual | Age (Years) | Sex | Nucleotide and Amino Acid Changes | Hypotonia | Spasticity | Movement Disorder | Epilepsy | ID | Vision Defect | Microcephaly | Brain MRI | EEG Abnormalities | Failure to Thrive |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Family A: II-1 | 5 | M | c.1111G>A (p.Ala371Thr); c.904C>T (p.Gln302∗) | + | + | + | + | + | + | + | thin corpus callosum, hyperintensities subcortical white matter | + | + |
| Family A: II-2 | 4 | M | c.1111G>A (p.Ala371Thr); c.904C>T (p.Gln302∗) | + | + | − | + | + | + | + | widening of sylvian fissures | + | + |
| Family B: II-2 | 2.5 | F | c.1111G>A (p.Ala371Thr); c.971_972insC (p.Lys324Asnfs∗14) | + | − | − | + | + | + | + | delayed myelination, thin corpus callosum | + | + |
| Family C: II-1 | 6 | F | c.778G>A (p.Val260Met); c.1165G>T (p.Asp389Tyr) | + | − | + | − | + | + | + | mild cerebellar hypoplasia, white matter hyperintensities | − | + |
| Family D: II-2 | 6 | F | c.169A>G (p.Met57Val); c.503G>A (p.Gly168Glu) | + | + | + | − | + | + | + | severe cortical atrophy, thin corpus callosum, cerebellar atrophy | − | + |
Abbreviations are as follows: M, male; F, female; ID, intellectual deficiency; EEG, electroencephalogram.
We then evaluated UBA5 using Sanger sequencing in a cohort of 51 children affected with early-onset epileptic encephalopathy of unknown etiology, and we failed to find any additional case subjects. Next, we contacted several European genetic centers performing WES for genetic determination of unknown disorders and identified two additional unrelated families (C and D, Figure 1A) with rare biallelic variants of UBA5 (Table 1). In each of these families, the child had severe intellectual disability and movement disorder, but no epilepsy (Table 1 and Supplemental Note).
An additional child (family B) was identified through the family’s blog that mentioned the identification through WES of UBA5 variants. We contacted this family and the geneticists treating the child and obtained detailed clinical and molecular information (Table 1 and Supplemental Note).
WES was performed on affected children and on their both parents in families A and B and was performed only on affected children in families C and D. WES was performed at Integragen SA (family A), GeneDX (family B), and the Institute of Human Genetics, Helmholtz Center (families C and D). Whole-exome capture was performed using Agilent SureSelect Human All-Exon kit (v.2 for families A, B, and C and v.4 for family D). Sequencing was performed with Illumina Hiseq 2000 or 2500 generating paired-end reads. Average depth of coverage for families A, B, C, and D was 35×, 43×, 134×, and 78×, respectively. More than 91% of targeted regions were covered at least 10 times. DNA sequences were mapped to the reference human genome sequence (UCSC hg19) with Eland V2 for individuals from family A and BWA for individuals from families B, C, and D. CASAVA v.1.8 was used to perform variant calling and annotation for individuals from family A. SAMtools v.0.1.7 was used to detect single-nucleotide variants and small insertions and deletions for individuals from families B, C, and D.
Variants predicted to result in nonsynonymous or frameshift changes were further filtered to prioritize both rare variants with a minor allele frequency (MAF) of less than 1% and novel variants. None of the affected children had rare variant with a MAF of less than 0.1% in any known genes associated with early-onset encephalopathies.
All UBA5 variants were verified by Sanger sequencing. In all families, the segregation was consistent with a recessive mode of inheritance. We identified a total of seven rare UBA5 variants including five missense variants, one nonsense variant, and one 1-bp insertion resulting in a frameshift followed by a premature stop codon (Table 1). Notably, all missense variants affected highly conserved amino acid residues (Figure S1). All these variants but one are predicted to be damaging by SIFT, MutationTaster, PolyPhen-2 HVAR, and PROVEAN prediction algorithms (Table S1). The p.Val260Met change is predicted to be neutral and benign by PROVEAN and PolyPhen-2 HVAR but damaging and disease-causing by SIFT and MutationTaster, respectively. Two of the missense variants are recorded in dbSNP 141 and Exome Aggregation Consortium (ExAC) databases: c.1111G>A (p.Ala371Thr) and c.169A>G (p.Met57Val) (Table S1).
In ExAC, the allele frequency of the c.169A>G variant is 8.3 × 10−5, which is compatible with the expected very low prevalence of the disease. However, the allele frequency of c.1111G>A is 0.0046 in the Finnish population, 0.0028 in the remaining European population, and 0.00297 in the French Exome (FREX) Project database. The calculated prevalence of individuals who are homozygous for this variant would therefore vary from 1/113.370 and 1/128.460 in the French and European populations, respectively, to 1/47,260 in the Finnish population. However, c.1111G>A may act as a hypomorphic allele, as one individual who is homozygous for this allele was identified among 10,490 persons enrolled in the Finnish Sequencing Initiative Suomi (SISu) database. Importantly, this individual, who is in his fifties, is free from any neurological disorder (A.-E. Lehesjoki, personal communication). Nevertheless, in the three individuals with the most severe phenotypes of this study (families A and B), the c.1111G>A allele is associated with a loss-of-function (LOF) allele. Five LOF variants are recorded for UBA5 in the ExAC database (Table S2) and none in the FREX database. Given the allele frequency of these variants in the European population, the maximum probability that compound heterozygosity of c.1111G>A and a LOF allele would occur by chance is 1/3.06 × 106 (Table S2), whereas that of c.1111G>A and c.169A>G would be 1/2.62 × 106. These associations are absent in the ExAC and FREX databases.
Similarly, homozygosity or compound heterozygosyty of LOF alleles would have a maximum frequency of 1/73 × 106 in the European population. However, the combination of two LOF alleles is likely to be embryonically lethal, as reported for Uba5 KO mouse11 and Drosophila10 models. Finally, any other association of pathogenic missense variants cannot be calculated, as they are not recorded in any database.
To analyze the consequences of the seven UBA5 mutations identified in this study on UFM1 activation, a time-dependent in vitro thioester formation assay was performed as previously described2 (Figures 2A and S2). We used recombinant UBA5 isoforms reproducing the seven mutations and UFM1 (MIM: 610553), synthesized in fusion with Glutathion-S-Transferase (GST). Reaction mixtures containing one of each UBA5 proteins, UFM1, and ATP were incubated during 0, 2, and 20 min before analyses. Conjugates of UFM1 to UBA5 variants were observed for all UBA5 proteins, except for the p.Gln302∗ and p.Gly168Glu mutants. Nevertheless, we observed a significantly delayed activity for the p.Met57Val and p.Val260Met proteins and a slightly reduced activity for the p.Ala371Thr and p.Asp389Tyr proteins. Surprisingly, we also found a residual activity for the p.Lys324Asnfs∗14 truncated protein.
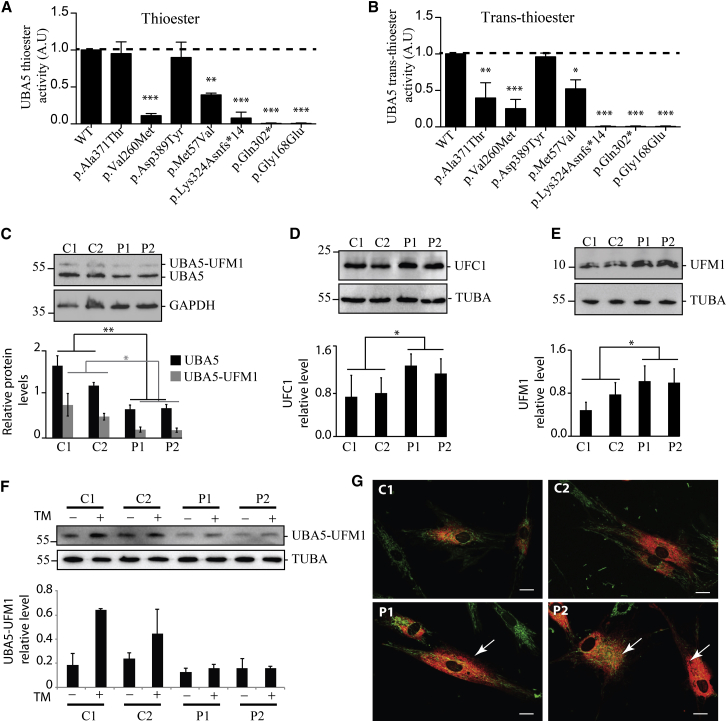
Figure 2.
Biochemical and Cellular Characterization of the UBA5 Disease-Causing Variants
(A and B) UBA5, UFM1, and UFC1 recombinant proteins were produced in E. coli, purified, and used for in vitro UFM1 thioester activation and trans-thioester conjugation reactions, according to published protocols.2
(A) In vitro assessment of UFM1 thioester activation by the UBA5-Glutathion-S-Transferase (GST) fusion proteins, each carrying one of the mutations of the affected families. The thioester formations were monitored after 2 min of incubation.
(B) Assessment of UFM1 trans-thioester conjugation to UFC1 by the UBA5-GST fusion proteins carrying each one of the mutations of the affected families. The trans-thioester formations were monitored after 3 min of incubation.
Three independent experiments were used for quantification and statistical analyses by one-way ANOVA, Bonferroni’s multiple comparison test, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
(C–G) Skin fibroblasts from the two affected individuals from family A (P1 equate to II-1 and P2 equate to II-2) and from two control subjects were grown in DMEM-F12 for 24 hr and used for assessing the UFM1 cascade.
(C) Representative western blots (top) of free (lower bands) and ufmylated (upper bands) UBA5 (Proteintech cat# 12093-1-AP, RRID: AB_2211743) and GAPDH (GeneTex cat# GTX100118, RRID: AB_1080976) proteins in control (C1, C2) and UBA5 mutant (P1, P2) fibroblast protein extracts, and quantification (bottom) of the relative levels of UBA5/GAPDH and UBA5-UFM1/GAPDH.
(D) Representative western blots (top) of UFC1 (Abcam cat# ab189251) and quantification (bottom) of UFC1 expression level related to TUBA (Sigma-Aldrich cat# T9026, RRID: AB_477593).
(E) Representative western blots (top) in non-reducing conditions (using Optiblot LDS Sample buffer, Abcam) of free UFM1 (Abcam cat# ab109305, RRID: AB_10864) and quantification (bottom) of UFM1 expression level related to TUBA.
(F) Representative western blots (top) of UBA5 ufmylation with (+) and without (−) 10 μg/mL of tunicamycin (TM, BML-CC104, Enzo Life Sciences) treatment for 24 hr and quantification (bottom) related to TUBA. Three independent experiments were used for quantification and statistical analyses using Student’s t test, ∗p < 0.05; ∗∗p < 0.01.
(G) Confocal live images of WT (C1, C2) and UBA5 mutant (P1, P2) fibroblasts transduced by an RFP-tagged ER red probe (C10591, CellLight ER-RFP, BacMam 2.0, Thermo Fischer Scientific), and stained with MitoTracker-green (M7514, Thermofisher) to reveal the ER and mitochondria. Arrows highlight expanded ER network in UBA5 mutant fibroblasts. Scale bar represents 10 μm.
We also investigated whether UBA5 mutations affect UFM1 conjugation to UFC1 using a trans-thioester assay.2 We incubated UBA5 isoforms with UFM1, UFC1, and ATP for 0, 3, and 30 min (Figures 2B and S3). UBA5-p.Asp389Tyr conjugation activity was similar to WT UBA5, whereas p.Met57Val, p.Val260Met, and p.Ala371Thr proteins had significantly delayed trans-thiol activities and p.Lys324Asnfs∗14, p.Gln302∗, and p.Gly168Glu proteins had no detectable activity (Figure 2B).
Thus, in vitro UBA5 activation and conjugation assays show that p.Gln302∗ and p.Gly168Glu proteins are catalytically inactive, whereas p.Met57Val, p.Val260Met, and p.Lys324Asnfs∗14 proteins have drastic reductions in catalytic activities. In contrast, the p.Ala371Thr and p.Asp389Tyr protein activities are barely reduced which is consistent with the fact that both amino acid changes are located out of the catalytic domain (aa. 57–329) and which gives an additional argument in favor of hypomorphic alleles.
Notably, the clinical severity of the disorder appears to correlate with residual UBA5 activity. Infantile spasms and pharmacoresistant epilepsy were observed in the three children with a null allele (families A and B) associated with drastically decreased UBA5 activity, whereas children with two missense variants (families C and D) associated with milder impairment of UBA5 activity had developmental delays and movement disorder but no epilepsy.
We also analyzed skin fibroblasts from the two affected children in family A. In a western blot assay, fibroblasts from these individuals, compared to WT fibroblast, showed markedly decreased expression of UBA5 (50% decrease) and ufmylated UBA5 (60% decrease) (Figure 2C). Expression of UFC1, encoding the E2 enzyme of the ufmylation cascade, and UFM1 were increased in the individual’s fibroblasts compared to WT fibroblasts (Figure 2D and 2E). UBA5 ufmylation after a 24 hr-long tunicamycin (TM) treatment was increased in WT cells, while mutant cells were unable to increase UBA5 ufmylation (Figure 2F). In addition, mutant cells were resistant to TM long exposure and showed similar pro-apoptotic staurosporine sensitivity as WT cells (Figure S4). Confocal live cell imaging revealed an expanded ER network in UBA5 mutant compared to WT fibroblasts (Figure 2G). Taken together, these results indicate that the ufmylation pathway is defective in UBA5 mutant fibroblasts, with decreased content of UBA5 and increased content of UFC1 and UFM1.
At a cellular level in mutant fibroblasts, the ER volume is increased and response to TM treatment is deficient. Both of these features are typically observed in cells suffering from ER stress.
The Ufm1 cascade in C. elegans is evolutionarily conserved, with similar targets as in humans.12 Thus, we analyzed the neurotransmission capacity of worm carrying deletions in the genes encoding for the E1 enzyme Uba5, the protease Ufsp2, and for the ufmylation targets Ufbp1 or Cdkr3, using aldicarb, an Ach esterase inhibitor, and pentylenetetrazole (PTZ), a GABA receptor antagonist.13
Wild-type (N2 Bristol)14 and Ufm1 cascade mutant worms (Table S3) did not show a seizure phenotype in the presence of PTZ, whereas PTZ-sensitive worms (unc-43)15 showed severe seizure phenotypes (data not shown). However, compared to WT animals, all Ufm1 cascade mutants displayed a significantly higher rate of pharynx grinder paralysis in the presence of aldicarb (Figure 3A), pointing to a pathophysiological mechanism involving an increased amount of ACh in the neuromuscular junctions.13 Our data are consistent with those obtained in a Drosophila model in which UBA5 knockdown induced locomotive defects, a shortened lifespan, and aberrant neuromuscular junctions (NMJs).10 Treatment with levamisole, an ACh receptor agonist known to paralyze worms,16 did not induce differences in motility between control and mutant worms (Figure S5), suggesting that the alteration of Ufm1 cascade and of Ufbp1 and Cdkr3 induce increased ACh release in neuromuscular junctions. Although it is premature to directly extrapolate these results to brain dysfunction in humans, it is interesting to note the established associations between ACh neuron function and severe central neurological disorders. First, mutations in CHRNA4 (MIM: 118504), CHRNB2 (MIM: 118507), and CHRNA2 (MIM: 118502), encoding nicotinic acetylcholine receptor (nAChR) α and β subunits, cause autosomal-dominant nocturnal frontal lobe epilepsy (ADNFLE [MIM: 600513]), an epileptic disorder with clusters of motor seizures occurring predominantly during sleep.17, 18 In addition, neuronal nAChRs participating in synaptic plasticity are involved in learning, memory, and brain development.19 Second, disruptions or alterations of nicotinic cholinergic mechanisms are involved in neurological disorders, including epilepsy, schizophrenia, Parkinson disease, dementia with Lewy bodies, and Alzheimer disease.19 Finally, a selective reduction in acetylcholinergic neurons (AChNs) has been found in the pedunculopontine tegmental nucleus of people with refractory epilepsy including infantile spasms.20 Although these findings support a role for AChNs dysfunction in neurologic disorders (including some with epilepsy), it is fair to conclude that the specific chain of events from UBA5 dysfunction to the associated clinical condition reported here (with refractory epilepsy, movement disorders, and severe developmental delay) remains largely uncharacterized—an unsurprising situation given the complexity of the cholinergic regulation in both developing and mature brains.21
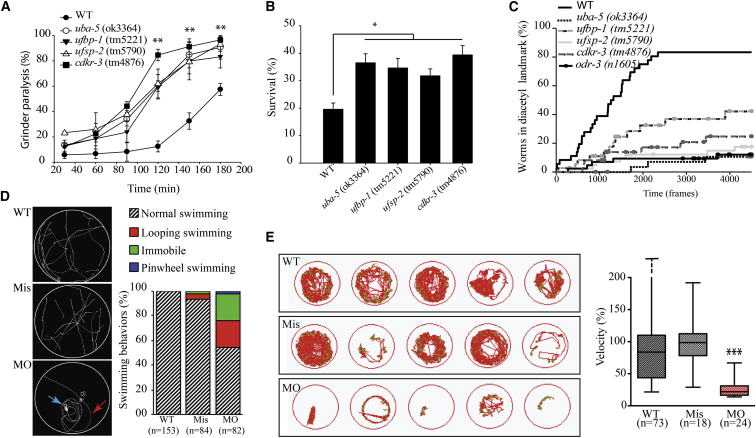
Figure 3.
Characterization of C. elegans Strains Deleted for Genes Involved in Ufmylation and Zebrafish Silenced for uba5
(A–C) C. elegans UFM-1 cascade deletion strains uba-5(ok3364), ufbp-1(tm5221), ufsp-2(tm5790), and cdkr-3(tm4876) were used for the following experiments and compared to the wild-type (WT) strain (N2 Bristol).
(A) Assessment of grinder paralysis of the UFM-1 cascade deletion strains treated with 0.5 mM aldicarb for 60 min reveal that the mutants display a highly significant increase in grinder paralysis, witnessing failure to negatively regulate acetylcholine release (ANOVA, ∗∗p < 0.01).
(B) Assessment of survival of the UFM-1 cascade deletion strains, after an 18 hr treatment with the ER-stressor dithiothreitol (DTT, 11 mM), show that mutants are resistant to ER stress, compared to WT animals (ANOVA, ∗p < 0.05).
(C) Assessment of chemotaxis toward the attractant diacetyl of the UFM-1 cascade deletion strains shows that the percentage of animals entering the landmark containing diacetyl (1/1,000) was significantly lower for the UFM-1 cascade deletion mutants compared to WT (Kaplan-Meier curve, p < 0.01). Negative control odr-3(n1605) worms were unable to sense diacetyl. Trajectories of the worms were recorded for 7.5 min with 10 frames per second via the FTIR-based Imaging Method (FIM).26
(D and E) Zebrafish from the AB genetic background were maintained at 28°C on a 14 hr light and 10 hr dark cycle, and fertilized eggs were injected with the antisense uba5 morpholino oligonucleotide (MO) and the mismatch oligonucleotide (Mis) at a concentration of 0.35 pM.
(D) Assessment of 72 hpf larvae motility, after a slight mechanical stimulation on the tail. The motion of individual larvae was examined (left) and scored (right) as normal swimming, looping swimming (red arrow), pinwheel swimming (blue arrow), or immobile and revealed a significant and specific alteration of swimming in uba5-depleted animals (MO), compared to larvae injected with the mismatch morpholino (Mis) and control larvae (WT). Quantification of the swimming behavior showed a normal motility for WT and Mis-injected larvae, whereas 45.1% of the MO showed impaired motility: 22% were immobile, 20.7% exhibited looping swimming, and 2.4% showed pinwheel swimming.
(E) Spontaneous motility of zebrafish monitored at 5 days post fertilization (dpf) using the Zebrabox recording system (Viewpoint). The cumulative movement of representative WT, Mis, and MO larvae is represented (left). The quantification of the velocity (right, mm/s), expressed as percentages of the WT values, revealed a significant decreased motility in uba5-depleted zebrafish (∗∗∗p < 0.001 between WT/MO and between Mis/MO but not significant between WT/Mis; statistical significance was inferred with the non-parametric Mann-Whitney test). Horizontal bars in the boxes represent the median values, horizontal bars outside the boxes represent the maximal and minimal individual values (in the WT, the maximal individual value was 676%).
In addition, because C. elegans uba-5(ok3364) mutants are resistant to ER stress,12 we monitored the effect of dithiothreitol (DDT) treatment on all Ufm1 cascade mutants and found that, compared to WT, they were resistant to ER stress (Figure 3B). Therefore, both in mutated fibroblasts and in C. elegans, alteration of the Ufm1 cascade induces ER-stress resistance, although without triggering the unfolded protein response (UPR; data not shown). Together, these data suggest that a close connection exists between ufmylation and ER physiology, without pointing to the UPR as the culprit mechanism in this disease. Our results further suggest that the ufmylation targets UFBP1 and CDK5RAP3, which are involved in functions downstream of UFM1 cascade,5, 6, 22, 23, 24 might play a crucial role in the pathogenicity of the disease. Indeed, these proteins aggregate in a large complex with UFL1 and UFSP2 at the ER membrane,5, 6, 23, 25 and CDK5RAP3 decreases the kinase activity of CDK5, which mutations are responsible for a severe form of epileptic encephalopathy associated with lissencephaly.26
In a next step, using a computer-aided chemotaxis assay, we analyzed the effects of the Ufm1 cascade on C. elegans sensorial behavior by a chemotaxis assay with the attractant diacetyl.27 Results revealed a decreased ability in sensing diacetyl in all mutants compared to WT animals (Figure 3C), pointing to a failure in the C. elegans sensory system in the absence of the Ufm1 cascade and its targets.
Finally, we examined the phenotype of zebrafish (Danio rerio) silenced for uba5. We first identified the single uba5 ortholog gene in the zebrafish genome (GenBank: NP_001292546.1), which encodes a protein sharing 80% identity with human UBA5. The uba5 morpholinos (MOs) and mismatch MOs (Mis) (Gene Tools) were designed against the splice junction between intron 1 and exon 2 of the zebrafish uba5. Embryos injected with uba5-MO or control uba5-Mis did not reveal morphological alteration in comparison to non-injected WT embryos at 24, 48, and 72 hr post fertilization (hpf) (data not shown), indicating a normal development of uba5-depleted larvae. Nevertheless, the motility of the morphants was considerably altered, as suspected by their decreased movements and difficulty to exit the chorion (data not show). To quantify the motility defect, we examined the touch-escape response of larvae at 72 hpf. Although WT and uba5-Mis injected larvae exhibited linear movement upon stimulation, morphants were either unable to move or exhibited looping or pinwheel swimming (Figure 3D, showing representative traces of Movies S1, S2, and S3). The analysis of large populations revealed a pronounced impaired motility in 45.1% of the uba5-MO-injected larvae (Figure 3D, Movies S4, S5, and S6), with predominant looping (37% of the mobile morphants). To further characterize the motor activity of uba5-depeleted zebrafishes, we tracked the spontaneous motility of 5-day-old larvae, which revealed a severe and specific decrease in morphant motility (Figure 3E). Thus, silencing of uba5 in zebrafish resulted in seizure-like behavior28, 29 and decreased locomotor function, both reminiscent of clinical phenotypes in the most severely affected children in this study. The findings underscore the crucial importance of UBA5 activity and thereby protein ufmylation in central nervous system function.
Further suggestive evidence linking ufmylation to neurological disorders derives from the observation that UFC1 and UFM1 colocalize with NCAM on the cell surface of neurons.30 NCAM is a neural cell adhesion molecule implicated in axon growth, neuronal differentiation, and synaptic plasticity in brain. UFM1 overexpression also increases the endocytosis of NCAM, a process that has to be fine-tuned for proper nervous system development.31 Finally, interactors of the ufmylation cascade have been found using mass spectrometry,32 including USP9X, which is required for proper neural cell migration and axonal growth. Loss-of-function mutations of USP9X (MIM: 300072) cause an X-linked intellectual disability.33
In summary, we report clinical, molecular, and experimental evidence indicating that biallelic UBA5 mutations cause early-onset encephalopathy characterized by seizures, developmental delays, and movement disorders. We suggest that adding UBA5 to gene panels targeted to individuals with early-onset refractory seizures or movement disorders accompanied by severe intellectual deficiency could increase the diagnostic yield and help with clinical management and genetic counseling.
Consortia
The FREX Consortium’s principal investigators are Emmanuelle Génin, Dominique Campion, Jean-François Dartigues, Jean-François Deleuze, Jean-Charles Lambert, and Richard Redon. Collaborators are as follows: bioinformatics group (Thomas Ludwig, Benjamin Grenier-Boley, Sébastien Letort, Pierre Lindenbaum, Vincent Meyer, Olivier Quenez), statistical genetics group (Christian Dina, Céline Bellenguez, Camille Charbonnier-Le Clézio, Joanna Giemza), data collection (Stéphanie Chatel, Claude Férec, Hervé Le Marec, Luc Letenneur, Gaël Nicolas, Karen Rouault), and sequencing (Delphine Bacq, Anne Boland, Doris Lechner).
Acknowledgments
We thank all the families for their participation in this study. We thank INSERM and CNRS, the Region Pays de la Loire, Angers Loire-Métropole, University of Angers, and University Hospital of Angers for their financial support to the PREMMI project, the University of Montpellier for institutional support, the German Bundesministerium für Bildung und Forschung through the German Network for mitochondrial disorders (mitoNET, 01GM1113C to T.M. and H.P.), and the E-Rare project GENOMIT (01GM1207 for T.M. and H.P.). T.B.H. was supported by the BMBF through the Juniorverbund in der Systemmedizin “mitOmics” (FKZ 01ZX1405C). This work was also supported by France Génomique National infrastructure, funded as part of “Investissement d’avenir” program managed by Agence Nationale pour la Recherche. We thank the Caenorhabditis Genetics Center and the National Bioresource Project for the Experimental Animal Nematode C. elegans (NBRP) for providing us with knockout strains. We thank C. Klämbt, B. Risse, N. Otto, and D. Berh for the opportunity to use the FIMtrack system for chemotaxis assay on C. elegans and for their support in data analysis. We thank Mr. Cubedo and Dr. Rossel for hosting zebrafish (INSERM U710) and C. Conan for performing the touch-response test. A.S. is funded by the Région Languedoc-Roussillon and P.B. is supported by grants from the Avenir-Atip program from INSERM and the CNRS, the Région Languedoc-Roussillon, and the Association Française contre les Myopathies. We thank Delphine Heron for having tested UBA5 in children affected with early encephalopathy and Joanne Walker and Kanaya Malkani for critical reading of the manuscript.
Published: August 18, 2016
Footnotes
Supplemental Data include a clinical note, five figures, three tables, and six movies and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.06.030.
Contributor Information
Dominique Bonneau, Email: dobonneau@chu-angers.fr.
The FREX Consortium:
Emmanuelle Génin, Dominique Campion, Jean-François Dartigues, Jean-François Deleuze, Jean-Charles Lambert, Richard Redon, Thomas Ludwig, Benjamin Grenier-Boley, Sébastien Letort, Pierre Lindenbaum, Vincent Meyer, Olivier Quenez, Christian Dina, Céline Bellenguez, Camille Charbonnier -Le Clézio, Joanna Giemza, Stéphanie Chatel, Claude Férec, Hervé Le Marec, Luc Letenneur, Gaël Nicolas, Karen Rouault, Delphine Bacq, Anne Boland, and Doris Lechner
Accession Numbers
Whole-exome sequencing data for family A have been deposited in the European Nucleotide Archive (ENA) with accession PRJEB9854. The GenBank accession number for zebrafish mmp21 is KT207790.
Web Resources
Ensembl Genome Browser, http://www.ensembl.org/index.html
European Nucleotide Archive, http://www.ebi.ac.uk/ena
ExAC Browser, http://exac.broadinstitute.org/
France Génomique (FREX) Project, https://www.france-genomique.org/spip/
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
GeneCards, http://www.genecards.org
Morpholinos (Gene Tools), http://www.gene-tools.com/morpholino_antisense_oligos
MutationTaster, http://www.mutationtaster.org/
OMIM, http://www.omim.org/
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
Primer3, http://bioinfo.ut.ee/primer3
PROVEAN, http://provean.jcvi.org
SISu Project, http://www.sisuproject.fi/
The Human Protein Atlas, http://www.proteinatlas.org/
ZFIN: BLAST Query, https://zfin.org/action/blast/blast
Supplemental Data
References
- 1.van der Veen A.G., Ploegh H.L. Ubiquitin-like proteins. Annu. Rev. Biochem. 2012;81:323–357. doi: 10.1146/annurev-biochem-093010-153308. [DOI] [PubMed] [Google Scholar]
- 2.Komatsu M., Chiba T., Tatsumi K., Iemura S., Tanida I., Okazaki N., Ueno T., Kominami E., Natsume T., Tanaka K. A novel protein-conjugating system for Ufm1, a ubiquitin-fold modifier. EMBO J. 2004;23:1977–1986. doi: 10.1038/sj.emboj.7600205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniel J., Liebau E. The ufm1 cascade. Cells. 2014;3:627–638. doi: 10.3390/cells3020627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang M., Zhu X., Zhang Y., Cai Y., Chen J., Sivaprakasam S., Gurav A., Pi W., Makala L., Wu J. RCAD/Ufl1, a Ufm1 E3 ligase, is essential for hematopoietic stem cell function and murine hematopoiesis. Cell Death Differ. 2015;22:1922–1934. doi: 10.1038/cdd.2015.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemaire K., Moura R.F., Granvik M., Igoillo-Esteve M., Hohmeier H.E., Hendrickx N., Newgard C.B., Waelkens E., Cnop M., Schuit F. Ubiquitin fold modifier 1 (UFM1) and its target UFBP1 protect pancreatic beta cells from ER stress-induced apoptosis. PLoS ONE. 2011;6:e18517. doi: 10.1371/journal.pone.0018517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J., Lei G., Mei M., Tang Y., Li H. A novel C53/LZAP-interacting protein regulates stability of C53/LZAP and DDRGK domain-containing Protein 1 (DDRGK1) and modulates NF-kappaB signaling. J. Biol. Chem. 2010;285:15126–15136. doi: 10.1074/jbc.M110.110619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo H.M., Park J.H., Jeon Y.J., Chung C.H. Ubiquitin-fold modifier 1 acts as a positive regulator of breast cancer. Front. Endocrinol. (Lausanne) 2015;6:36. doi: 10.3389/fendo.2015.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H., Li J., Tillman B., French B.A., French S.W. Ufmylation and FATylation pathways are downregulated in human alcoholic and nonalcoholic steatohepatitis, and mice fed DDC, where Mallory-Denk bodies (MDBs) form. Exp. Mol. Pathol. 2014;97:81–88. doi: 10.1016/j.yexmp.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azfer A., Niu J., Rogers L.M., Adamski F.M., Kolattukudy P.E. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am. J. Physiol. Heart Circ. Physiol. 2006;291:H1411–H1420. doi: 10.1152/ajpheart.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duan R., Shi Y., Yu L., Zhang G., Li J., Lin Y., Guo J., Wang J., Shen L., Jiang H. UBA5 mutations cause a new form of autosomal recessive cerebellar ataxia. PLoS ONE. 2016;11:e0149039. doi: 10.1371/journal.pone.0149039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatsumi K., Yamamoto-Mukai H., Shimizu R., Waguri S., Sou Y.-S., Sakamoto A., Taya C., Shitara H., Hara T., Chung C.H. The Ufm1-activating enzyme Uba5 is indispensable for erythroid differentiation in mice. Nat. Commun. 2011;2:181. doi: 10.1038/ncomms1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hertel P., Daniel J., Stegehake D., Vaupel H., Kailayangiri S., Gruel C., Woltersdorf C., Liebau E. The ubiquitin-fold modifier 1 (Ufm1) cascade of Caenorhabditis elegans. J. Biol. Chem. 2013;288:10661–10671. doi: 10.1074/jbc.M113.458000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locke C., Berry K., Kautu B., Lee K., Caldwell K., Caldwell G. Paradigms for pharmacological characterization of C. elegans synaptic transmission mutants. J. Vis. Exp. 2008;18:e837. doi: 10.3791/837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vashlishan A.B., Madison J.M., Dybbs M., Bai J., Sieburth D., Ch’ng Q., Tavazoie M., Kaplan J.M. An RNAi screen identifies genes that regulate GABA synapses. Neuron. 2008;58:346–361. doi: 10.1016/j.neuron.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Martin R.J., Robertson A.P., Buxton S.K., Beech R.N., Charvet C.L., Neveu C. Levamisole receptors: a second awakening. Trends Parasitol. 2012;28:289–296. doi: 10.1016/j.pt.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinlein O.K., Mulley J.C., Propping P., Wallace R.H., Phillips H.A., Sutherland G.R., Scheffer I.E., Berkovic S.F. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat. Genet. 1995;11:201–203. doi: 10.1038/ng1095-201. [DOI] [PubMed] [Google Scholar]
- 18.Aridon P., Marini C., Di Resta C., Brilli E., De Fusco M., Politi F., Parrini E., Manfredi I., Pisano T., Pruna D. Increased sensitivity of the neuronal nicotinic receptor alpha 2 subunit causes familial epilepsy with nocturnal wandering and ictal fear. Am. J. Hum. Genet. 2006;79:342–350. doi: 10.1086/506459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dani J.A., Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi M., Nakajima K., Miyata R., Tanuma N., Kodama T. Lesions of acetylcholine neurons in refractory epilepsy. ISRN Neurol. 2012;2012:404263. doi: 10.5402/2012/404263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becchetti A., Aracri P., Meneghini S., Brusco S., Amadeo A. The role of nicotinic acetylcholine receptors in autosomal dominant nocturnal frontal lobe epilepsy. Front. Physiol. 2015;6:22. doi: 10.3389/fphys.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim C.H., Nam H.-S., Lee E.H., Han S.H., Cho H.J., Chung H.J., Lee N.S., Choi S.J., Kim H., Ryu J.S. Overexpression of a novel regulator of p120 catenin, NLBP, promotes lung adenocarcinoma proliferation. Cell Cycle. 2013;12:2443–2453. doi: 10.4161/cc.25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shiwaku H., Yoshimura N., Tamura T., Sone M., Ogishima S., Watase K., Tagawa K., Okazawa H. Suppression of the novel ER protein Maxer by mutant ataxin-1 in Bergman glia contributes to non-cell-autonomous toxicity. EMBO J. 2010;29:2446–2460. doi: 10.1038/emboj.2010.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai Y., Pi W., Sivaprakasam S., Zhu X., Zhang M., Chen J., Makala L., Lu C., Wu J., Teng Y. UFBP1, a key component of the Ufm1 conjugation system, is essential for ufmylation-mediated regulation of erythroid development. PLoS Genet. 2015;11:e1005643. doi: 10.1371/journal.pgen.1005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon J., Cho H.J., Han S.H., No J.G., Kwon J.Y., Kim H. A novel LZAP-binding protein, NLBP, inhibits cell invasion. J. Biol. Chem. 2010;285:12232–12240. doi: 10.1074/jbc.M109.065920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magen D., Ofir A., Berger L., Goldsher D., Eran A., Katib N., Nijem Y., Vlodavsky E., Tzur S., Behar D.M. Autosomal recessive lissencephaly with cerebellar hypoplasia is associated with a loss-of-function mutation in CDK5. Hum. Genet. 2015;134:305–314. doi: 10.1007/s00439-014-1522-5. [DOI] [PubMed] [Google Scholar]
- 27.Risse B., Thomas S., Otto N., Löpmeier T., Valkov D., Jiang X., Klämbt C. FIM, a novel FTIR-based imaging method for high throughput locomotion analysis. PLoS ONE. 2013;8:e53963. doi: 10.1371/journal.pone.0053963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baraban S.C., Taylor M.R., Castro P.A., Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience. 2005;131:759–768. doi: 10.1016/j.neuroscience.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 29.Stewart A.M., Desmond D., Kyzar E., Gaikwad S., Roth A., Riehl R., Collins C., Monnig L., Green J., Kalueff A.V. Perspectives of zebrafish models of epilepsy: what, how and where next? Brain Res. Bull. 2012;87:135–143. doi: 10.1016/j.brainresbull.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Homrich M., Wobst H., Laurini C., Sabrowski J., Schmitz B., Diestel S. Cytoplasmic domain of NCAM140 interacts with ubiquitin-fold modifier-conjugating enzyme-1 (Ufc1) Exp. Cell Res. 2014;324:192–199. doi: 10.1016/j.yexcr.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat. Rev. Neurosci. 2008;9:26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- 32.Havugimana P.C., Hart G.T., Nepusz T., Yang H., Turinsky A.L., Li Z., Wang P.I., Boutz D.R., Fong V., Phanse S. A census of human soluble protein complexes. Cell. 2012;150:1068–1081. doi: 10.1016/j.cell.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homan C.C., Kumar R., Nguyen L.S., Haan E., Raymond F.L., Abidi F., Raynaud M., Schwartz C.E., Wood S.A., Gecz J., Jolly L.A. Mutations in USP9X are associated with X-linked intellectual disability and disrupt neuronal cell migration and growth. Am. J. Hum. Genet. 2014;94:470–478. doi: 10.1016/j.ajhg.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.