Abstract
The neuromuscular junction (NMJ) is one of the best-studied cholinergic synapses. Inherited defects of peripheral neurotransmission result in congenital myasthenic syndromes (CMSs), a clinically and genetically heterogeneous group of rare diseases with fluctuating fatigable muscle weakness as the clinical hallmark. Whole-exome sequencing and Sanger sequencing in six unrelated families identified compound heterozygous and homozygous mutations in SLC5A7 encoding the presynaptic sodium-dependent high-affinity choline transporter 1 (CHT), which is known to be mutated in one dominant form of distal motor neuronopathy (DHMN7A). We identified 11 recessive mutations in SLC5A7 that were associated with a spectrum of severe muscle weakness ranging from a lethal antenatal form of arthrogryposis and severe hypotonia to a neonatal form of CMS with episodic apnea and a favorable prognosis when well managed at the clinical level. As expected given the critical role of CHT for multisystemic cholinergic neurotransmission, autonomic dysfunctions were reported in the antenatal form and cognitive impairment was noticed in half of the persons with the neonatal form. The missense mutations induced a near complete loss of function of CHT activity in cell models. At the human NMJ, a delay in synaptic maturation and an altered maintenance were observed in the antenatal and neonatal forms, respectively. Increased synaptic expression of butyrylcholinesterase was also observed, exposing the dysfunction of cholinergic metabolism when CHT is deficient in vivo. This work broadens the clinical spectrum of human diseases resulting from reduced CHT activity and highlights the complexity of cholinergic metabolism at the synapse.
Main Text
The synapse is a highly specialized structure that is fundamental for the function of the neuron by mediating efficient chemical transmission to its postsynaptic cell. One of the best-studied synapses used as a model is the cholinergic neuromuscular junction (NMJ). Cholinergic transmission is unique among the neurotransmitter systems in that it is rapidly stopped not only by clearance but also by enzymatic cleavage of the neurotransmitter in the synaptic space by cholinesterases to ensure quick successive postsynaptic responses.1 Toward this aim, the nerve terminal takes up choline from the synaptic space through the presynaptic sodium-dependent high-affinity choline transporter 1 (CHT) to resynthesize ACh by the action of choline acetyltransferase (ChAT). The high-affinity choline uptake from the synaptic space by this hemicholinium-3 (HC-3)-sensitive transporter CHT represents the limiting step for sustained ACh synthesis and is tightly regulated with ChAT activity.2
The NMJ achieves action potential transmission from the motoneuron nerve terminal to the skeletal muscle fiber to control muscle contraction. Defective neurotransmission at the NMJ results in myasthenia, i.e., fluctuating fatigable muscle weakness.3 The inherited types of myasthenia form the group of congenital myasthenic syndromes (CMSs) characterized by an early age of onset, disease progression, no ACh receptor (AChR) antibodies, and responsiveness to treatments, especially acetylcholinesterase (AChE) inhibitors. Mutations in 23 genes are known to cause CMS. Among them is found CHAT (MIM: 118490) that is mutated in a recessive form of CMS with sudden episodes of apnea (CMS-EA [MIM: 254210]).4, 5 On the other hand, one dominant-negative truncating mutation in SLC5A7 (MIM: 608761) encoding CHT causes a distal hereditary motor neuronopathy, type VIIa (DHMN7A [MIM: 158580]) with progressive distal muscle wasting and vocal cord paresis, questioning the relationship of CHT with CMS.6 Worldwide, there are still many individuals with a clinical diagnosis of CMS who remain genetically undiagnosed. In this paper, we report loss-of-function mutations of SLC5A7 in six unrelated families as the underlying cause of a recessive form of CMS that clinically ranges from muscle hypotonia with early neonatal lethality to a neonatal form sharing striking similarities to CMS-EA resulting from CHAT mutations.
The six families were part of two distinct European CMS cohorts. Participants gave informed consent through a prospective donor scheme approved by national ethic committees (DC-2012-1535 and AC-2012-1536), and genomic DNA was isolated from blood samples. Medical histories were taken from all participants by neurologists. All parents displayed no peculiar medical history and were healthy at clinical examination. Electroneuromyography (ENMG) and repetitive nerve stimulation (RNS) were carried out under standardized protocols.7 A detailed clinical description of the two unrelated individuals (1 and 2) who underwent whole-exome sequencing is found in the Supplemental Note, and Table 1 summarizes the clinical features of the five isolated individuals (1, 4–7) with a neonatal form close to CMS-EA.5 To summarize, brief and recurrent EA occurred neonatally as the inaugural symptom in all subjects with the neonatal form. Other symptoms of CMS were present such as hypotonia, weakness and fatigability, hypomimia, and oculo-bulbar symptoms. A significant decrement in the EMG with RNS was observed in four individuals (1, 4, 5, 7). AChE inhibitors were highly efficient for three subjects (1, 4, and 7) with cessation of the EA and improvement of all myasthenic symptoms. Although individual 6 had no decrement in EMG with RNS, he had mild ptosis worsening at the end of the day, ophtalmoparesis (impaired abduction of left eye and impaired adduction of right eye) with variable strabismus, and striking limb fatigability, all features consistent with a diagnosis of CMS. The two sibs of family 2 displayed a more severe antenatal phenotype with hydramnios and arthrogryposis of fingers with knees and malformative features in individual 2 (see Supplemental Note). Death occurred at the age of 10 (individual 3) and 15 (individual 2) days.
Table 1.
Clinical Features of the Subjects with a Neonatal Form of CMS-EA due to SLC5A7 Mutations
| Individual 1 (France) | Individual 4 (Algeria) | Individual 5 (Italy) | Individual 6 (Italy) | Individual 7 (Romania) | |
|---|---|---|---|---|---|
| Sex; age at onset | male; birth | male; 2 months | female; birth | male; birth | male; birth |
| Mutations | c.194G>A (p.Gly65Glu), c.313C>T (p.Pro105Ser) | c.143A>G (p.Asp48Gly), c.143A>G (p.Asp48Gly) | c.524A>G (p.Tyr175Cys), c.1030G>C (p.Val344Leu) | c.331T>C (p.Tyr111His), c.1252T>G (p.Phe418Val) | c.872T>C (p.Ile291Thr), c.1336A>G (p.Arg446Gly) |
| Pregnancy | uneventful | uneventful | uneventful | uneventful | uneventful |
| First symptoms (age) | sudden apneas (b), hypotonia (b) | long meals (2 months), brief bradypneas (5 months) | respiratory distress (b), sudden apneas (b) | sudden apneas (b), weak cry (b) | sudden apneas (b), hypotonia, cyanosis (b), sucking difficulties (b) |
| Neonatal period | sudden apneas misdiagnosed as seizures; ventilation till 6 weeks; sucking, swallowing, and chewing difficulties; axial hypotonia | slow feeding; few minutes bradypneas; stridor; swallowing difficulties | frequent apneas requiring ventilation support | frequent apneas; dysphagia; acute ptosis | sudden apneas with cyanosis when crying misdiagnosed as breath holding |
| Motor delay | yes (walk 22 months) | yes (sit 9 months, walk 20 months) | yes (walking never gained) | yes (walk 17 months) | yes (sit 7 months, walk 18 months) |
| Symptoms during Course | |||||
| FUD | 16 years | 4 years | 5 years | 11 years | 3 years and 9 months |
| EA | yes, until starting AChEI at 18 months | 2 episodes | yes, till now | no | yes, until starting AChEI at 4 months |
| CH | yes | no | yes | no | ND |
| Ventilation; tracheostomy | yes (5 months); yes (from 6 months to 5 years) | no; no | yes; no | no; no | no; no |
| Bulbar weakness; facial weakness | sucking, swallowing, chewing till 10 months; facial weakness from 18 months till now (mild chewing, articulation) | intermittent stridor, no dysphagia; hypomimia | yes (tube feeding due to swallowing and chewing difficulties); constant facial weakness | yes (nasal voice, occasional dysphagia); no | dysphonia and dysphagia until 2 years; ND |
| Ptosis; OPH | yes; yes | yes (from 4 months); yes | yes; yes | yes; yes | yes; yes |
| Limb fatigability; weakness | yes; yes (Gowers till 28 months, run at 6 years), waddling gait | yes; no | yes; severe proximal | yes; yes (lower limb girdle) | yes; fatigability |
| Axial weakness | yes (neck and spine), improved by AChEI | yes (neck intermittently) | yes | yes (neck, flexion and extension) | yes (normal walk but climbs stairs with one foot at time) |
| Scoliosis; cont. | mild; no | no; no | no; no | kyphosis; no | no; no |
| Amyotrophy | no | no | yes | no | no |
| DMN signs | no | no | no | pes planus | no |
| CD; behavior | no; normal | no; normal | yes; abnormal | yes; normal | yes; ND |
| Course Characteristics | |||||
| Evolution | marked improvement since AChEI administration at 18 months | improvement since AChEI administration at 18 months | stable with episodic crises, muscle weakness without progression | spontaneous recovery of respiratory crises | improved after increasing AChEI |
| Fluctuationsa | yes (few days) | yes (one day) | no | yes (diurnal) | yes (respiratory intercurrence) |
| Exacerb.; MC | no; no | no; no | no; no | yes; no | yes; ND |
| Last Examination | |||||
| Age | 16 years | 4 years | 5 years | 11 years | 3 years and 9 months |
| PV/NIV; T | no; no | no; no | PV; yes | no; no | no; no |
| Bulbar; facial | no; yes (minimal) | yes (mild stridor); yes (intermittently) | yes; yes | yes (nasal voice); yes | yes (nasal voice); yes |
| Ptosis; OPH | yes; yes (fluctuant in severity) | yes; yes (persistent) | yes; yes (persistent) | yes; yes (permanent) | yes; yes (permanent) |
| Limb weakness; fatigability | no; yes (moderate, lower limbs) | no; yes | generalized and constant severe weakness; no | yes (distal upper and proximal lower); no | yes; yes |
| Axial weakness | no | yes (neck, intermittent) | yes | yes (neck) | no |
| EMG (age) | 18 months | 16 months | 14 months | 3 years | 4 months |
| Decrement 3 Hz | 66% | no, but yes after a pulse of 10 s at 20 Hz | 70% | no (no challenging conditions done) | 10%–20% |
| Therapy and effect | AChEI, +++ | AChEI, +++ | AChEI, + | AChEI,−; salbutamol, −b | AChEI, ++ |
Abbreviations are as follows: b, birth; FUD, follow-up duration; EA, episodic apnea; AChEI, acetylcholinesterase inhibitors; OPH, ophthalmoparesis; CH, chronic hypoventilation; ND, not determined; cont., contractures; DMN, distal motor neuropathy; CD, cognitive deficit; MC, myasthenic crisis; Exacerb., exacerbation; PV, permanent ventilation; NIV, nasal intermittent ventilation; T, tracheostomy.
Except sudden apneas.
Adequate dose and duration of treatment were not optimal.
After exclusion of CHAT mutations by Sanger sequencing, whole-exome sequencing was performed in family 1 on blood genomic DNA though enrichment capture using the SureSelect Human All Exon v5 kit (Agilent Technologies) and paired-end massive parallel sequencing in a HiSeq 2000 machine (Illumina) with an average coverage of 60×. Whole-exome sequencing and variant analyses were performed for individual 2 (family 2) as previously described.8 Two heteroallelic candidate variants in SLC5A7 were found to be linked with the disease when searching for rare variations (less than 1% of frequency in control databases) with a recessive model of inheritance in the two families (Figure S1). In family 1, two missense variations (c.194G>A [p.Gly65Glu] and c.313C>T [p.Pro105Ser]; GenBank: NM_021815) segregated with the disease. In family 2, the two deceased infants were compound heterozygous for one missense (c.1082G>A [p.Arg361Gln]) and one nonsense (c.123_126del [p.Ile42∗]) variation inherited from their parents. No other candidate gene was retained in the two families when recessive inheritance and single-nucleotide variations not reported in polymorphic database were used as filtering parameters.
We then searched for SCL5A7 mutations by Sanger sequencing of its 10 exons in 95 unrecognized individuals with CMS-EA who did not have mutations in CHAT. Four isolated subjects were found to harbor two distinct SLC5A7 compound heterozygous candidate mutations with a recessive inheritance pattern (individuals 4–7, Table 1). All mutations were inherited except c.331T>C encoding the p.Tyr111His substitution (individual 6), with a maternal mutant allele arising de novo. None of the candidate mutations were reported in control databases (in-house, dbSNP, 1000 Genomes, ExAC), except c.1082G>A (p.Arg361Gln, family 2) and c.872T>C (p.Ile291Thr, individual 7) that were reported in 2 and 7 of 121,350 alleles in the heterozygous state (ExAC), respectively. All missense substitutions were reported as pathogenic by SIFT, PolyPhen-2, and MutationTaster.
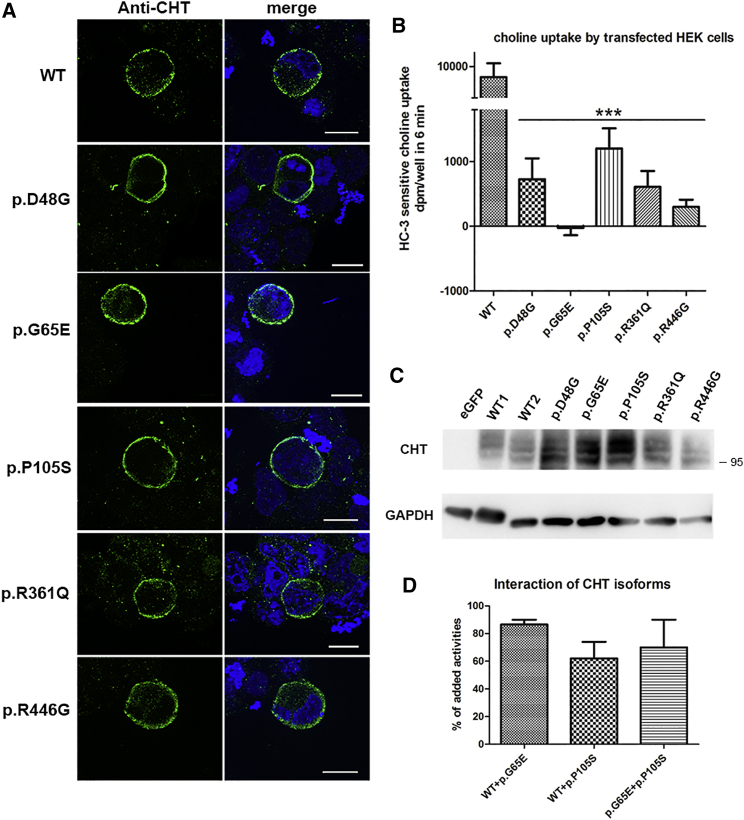
The 10 missense mutations were located all along the 580 amino acid residues composing CHT that consists of 13 transmembrane segments (Figure 1A).9 All substituted for an amino acid residue that is highly conserved during evolution (Figure 1B). Four variations affected amino acids that have been demonstrated to be critical for choline uptake activity (Asp48, Tyr111, Arg361, and Arg446).2, 10 We investigated the pathogenicity of five of these ten missense substitutions that were distributed all along the transporter (c.143A>G [p.Asp48Gly], c.194G>A [p.Gly65Glu], c.313C>T [p.Pro105Ser], c.1082G>A [p.Arg361Gln], and c.1336A>G [p.Arg446Gly]) on CHT activity in vitro to determine their functional effect. We introduced the single-nucleotide changes into the human cDNA encoding CHT (OriGene Technologies) by site-directed mutagenesis (GenScript). The WT and mutant cDNA constructs were transiently transfected into HEK293T cells using Fugene6 reagent (Promega), and membrane expression as well as choline uptake were evaluated. Figure 2A shows typical immunostaining patterns (ApoTome 2 acquisition, Carl Zeiss) obtained on permeabilized cells stained with a monoclonal antibody directed against human CHT (clone 62-2E8, Sigma-Aldrich). We observed CHT membrane immunolabeling for the five investigated missense mutations, suggesting conserved cell trafficking of the mutated CHT compared to control.
Figure 1.
Schematic Representation of CHT and Position of the Variants Linked to the Antenatal Form with Arthrogryposis and to the Neonatal Form of CMS
(A) The substitutions affecting an amino acid residue already described as critical for CHT activity are in red. All amino acid changes result from mutations that were in the heterozygous state except c.143A>G (p.Asp48Gly), which was homozygous (circle). The variants studied at the functional level are underlined. The changes resulting in DHMN7A (p.Lys499Asnfs∗13) and linked to ADHD (p.Ile89Val) are also indicated.
(B) The alignment of human CHT with the sequences of five model species (rat, mouse, zebrafish, Torpedo mamorata, and Caenorhabditis elegans with the percent of amino acid identities in brackets) demonstrates the high conservation of the substituted residues.
Figure 2.
Heterologous Expression and Transporter Activity of CHT in Transfected HEK293T Cells
(A) Immunostaining of heterologous CHT using anti-SLC5A7 antibodies showed a membranous staining of the wild-type (WT) and the five variant CHT investigated in HEK293T cells transiently transfected with hSLC5A7 cDNA constructs (c.143A>G [p.Asp48Gly], c.194G>A [p.Gly65Glu], c.313C>T [p.Pro105Ser], c.1082G>A [p.Arg361Gln], and c.1336A>G [p.Arg446Gly]). Nuclei are stained with DAPI (blue). Scale bars represent 10 μm.
(B) Evaluation of the choline transporter activity sensitive to HC-3, corresponding to the heterologous expression of CHT activity in transiently transfected HEK293T cells. The y axis bar is interrupted for a better visualization of the residual transporter activity of the variant proteins.
(C) Western blot analysis of heterologous CHT in total cell extracts of HEK293 cells transiently transfected with the wild-type or mutant hSLC5A7 constructs performed in denaturating conditions. Three main bands (95 kDa) were observed using a polyclonal antibody directed against human CHT. Anti-GAPDH antibody was used as loading control. No band specific to CHT was observed in extracts from cells transfected with eGFP alone (eGFP), confirming the specificity of the antibody. The size and amount of the bands specific to heterologous CHT were similar between the wild-type (lines WT1 and 2) and the five variants.
(D) Evaluation of HC-3-sensitive choline transport when coexpressing the wild-type and the c.313C>T (p.Pro105Ser) and c.194G>A (p.Gly65Glu) mutant constructs in transiently transfected HEK293T cells. The activities recorded in cells coexpressing the indicated combinations of cDNA are compared to the summation of the individual activities obtained when the cells were transfected with the wild-type or the mutant constructs.
The results are expressed as mean ± SEM. Statistical significance was calculated by Student’s t test with a level of statistical significance set at p < 0.05 (∗∗∗p < 0.001).
We then determined whether the choline uptake by CHT was altered by the amino acid substitutions. HC-3-sensitive choline transport by transfected cells was evaluated on a period of 6 min using 1 μM 14C-choline (PerkinElmer) subtracting the uptake observed with 1 μM HC-3 (Sigma-Aldrich), since choline uptake by mock-transfected HEK293 cells was not sensitive to this HC-3 concentration.10 Whole-cell lysates were assayed for accumulated radioactivity by liquid scintillation spectrometry (Packard Tri-carb Liquid Scintillation Counter). Specific CHT-mediated choline uptake was severely impaired for all mutants tested, with residual activity varying between 0% and 10% of control levels (Figure 2B). Although total choline uptake by mock-transfected cells was not saturated in the low micromolar range of choline concentrations, the wild-type CHT-mediated component of choline transport was characterized by a Km of 2.5 μM and a Vmax of 75 pmol/well (0.5 nmol/mg protein) over 6 min. When choline uptake by the p.Asp48Gly and p.Pro105Ser isoforms of CHT was analyzed at this range of concentrations, the low level of uptake did not allow convergence (p.Asp48Gly) or gave a much lower estimate of Vmax at 5 pmol/well, with a Km of 0.8 μM (p.Pro105Ser, data not shown). The other variants were not tested because their residual activity was even less than that of p.Asp48Gly. Western blot analyses of total cell extracts using an affinity purified polyclonal anti-CHT antibody (Abcam) confirmed that the amino acid substitutions did not affect the size of CHT and that the drastic reduction of choline transport activity of mutated CHT was not due to reduced protein level compared to the wild-type CHT (Figure 2C). The size and pattern of the detected bands closely resembled those observed previously for homo-oligomers.10 CHT functions as a homo-oligomer, and two amino acid substitutions (c.265A>G [p.Ile89Val] and c.1497delG [p.Lys499Asnfs∗13]) observed in humans have been reported to exert a dominant-negative effect on the wild-type CHT.6, 11 We therefore coexpressed the cDNAs containing the c.194G>A (p.Gly65Glu) and the c.313C>T (p.Pro105Ser) mutations—the two compound heterozygous mutations identified in individual 1—with the wild-type cDNA or with each other. The CHT activity of co-transfected cells was less than that calculated from the addition of CHT activities obtained when the cDNA were transfected individually (Figure 2D). However, the impact of the variants on the WT transporter activity was low, concordant with the recessive nature of the tested mutations.
Cholinergic metabolism is critical for NMJ formation, and knock-out Slc5a7 mice lacking CHT display defective innervation patterns at the NMJ.12 To determine whether the NMJ structure was impacted when CHT activity is deficient in human, we investigated the NMJs in muscle biopsy samples available for the 16-year-old individual 1 (deltoid muscle) and for the deceased newborn 2 (autopsy material) via standard protocols.13 CHT was immunostained at the NMJ in the muscle biopsies of the two individuals at a level similar to the control muscle biopsy using a polyclonal antibody (Abcam) as expected from the in vitro analyses of CHT protein levels (Figure 3A). Denervation-reinnervation processes at the NMJs were evident in the muscle sample of individual 1 (Figure 3B and Table S1). Electron microscopy further confirmed the occurrence of a partial denervation-reinnervation process with small nerve terminals and empty synaptic gutters (n = 5; Figure S2A). When present, nerve terminals contained synaptic vesicles that appeared normal in quantity (Figure S2B). Well-defined secondary synaptic folds were observed, suggesting normal formation of the post-synaptic element. Cytoplasmic immunostaining of terminal Schwann cells using S100 antibodies did not detect major abnormalities except for a less well-defined staining pattern compared to control (Figure S3A), which was confirmed at the ultrastructural level (Figure S2A). Autopsy material of newborn 2 showed immature NMJs with thin and unbranched terminal axons contacting undefined subneural folds that sometimes appeared to be polyneuronally innervated (Figure 3C). This is consistent with delayed NMJ development in this newborn, since elimination of polyneuronal innervation should be completed during the 25th week in utero.14
Figure 3.
Immunostaining Analyses of NMJs in Muscle Samples from Individuals 1 and 2
(A) CHT immunostaining (green) and post-synaptic nAChR fluorescent staining (red in b, d, f) on transversal muscle sections showed the presence of CHT at NMJs in individuals 1 (c, d) and 2 (e, f) with a staining intensity similar to the adult control (a, b).
(B and C) Representative pictures of muscle biopsies stained for the motor axons with an anti-neurofilament (in green) and for post-synaptic nAChR with α-bungarotoxin (in red).
(B) The staining pattern of the two synaptic elements in the young adult individual 1 led us to classify the NMJs in three categories depending upon the innervation status of each NMJ: denervated (b), remodeled (c), or neoformed (d, d′, d′′; d is the merged representation of d′ and d′′). One NMJ from the adult control (a) with well-defined synaptic gutters responsible for the well-circumscribed post-synaptic nAChR fluorescent staining is shown for comparison.
(C) Three representative images of immature NMJs observed in the autopsy material of the deceased newborn (individual 2) with no well-differentiated pattern for the postsynaptic apparatus (a, b, c), evidence for accumulation of neurofilament staining in nerve terminal (arrow in b and c), and polyinnervation (arrowhead in c).
Scale bars represent 10 μm.
To determine whether critical synaptic actors of cholinergic metabolism were impacted by defective CHT activity, we investigated their localization by immunostaining. ChAT (polyclonal antibody, Abcam) and the vesicular acetylcholine transporter VAChT (monoclonal antibody, clone S6-38, Abcam)—which allows the storage of ACh into synaptic vesicles1—were present in levels that look similar to controls (Figure S3B). Enzymatic (Koelle-Friedenwald) and fluorescent staining of AChE using fasciculin-2 also appeared similar between case and control subjects (Figure S4A and data not shown). By contrast, the immunostaining of butyrylcholinesterase (BChE, 11D8 clone)15—the cholinesterase bound to perisynaptic Schwann cells in mature NMJs16—was abnormally strong in the NMJs of both subjects compared to control samples (Figure S4B). These data indicate synaptic remodeling when CHT activity is impaired at the human peripheral cholinergic synapse.
Our data bring the total number of presynaptic forms of CMSs to five and demonstrate that SLC5A7 is the second most frequent mutated gene linked to a presynaptic form after CHAT. The phenotype of the five children presenting with the neonatal form who are reported in this paper is fully reminiscent of recessive CMS-EA due to ChAT deficiency.5, 17 CHAT mutations lead to impaired synthesis of ACh by reduced ChAT protein level or kinetic defects depending upon the mutation.18 The similarity of CMS-EA resulting from SLC5A7 or CHAT loss-of-function mutations is to be expected because both diminish the synthesis of ACh in the nerve terminal, thereby limiting the ability of the cholinergic synapse to continue functioning when the demand of ACh release is high.2 The association of a lethal antenatal form could be expected because CHT is detected at high levels in E14 rodent spinal cord when NMJ begins to form.19
One interesting observation was the strong immunostaining of synaptic BChE observed in the two analyzed muscle biopsy samples. BChE has not been well investigated as an actor in ACh metabolism at the NMJ. This cholinesterase is bound to perisynaptic Schwann cells, and its absence does not cause disease in mice or humans.16, 20, 21, 22 The upregulation of BChE does not seem to be a secondary consequence of NMJ remodeling with denervation-reinnervation events because these are frequently observed when analyzing NMJs of individuals with CMS. Conceivably, BChE finely tunes neuromuscular transmission, probably by regulating the binding of ACh to α7 nAChR located on the terminal Schwann cells.16
Interestingly, the sole inherited human disease resulting from one nucleotide deletion (c.1497delG [p.Lys499Asnfs∗13]) in SLC5A7 already reported in the literature is DHMN7A with no signs of myasthenia.6, 23, 24 The individuals reported here did not present any features of neuropathy at last examination, but we cannot exclude the development of neuropathic signs with age. The residual CHT activity in DHMN7A was estimated to be 25% in vitro, which is higher than that observed for the CMS-EA-associated mutations.6 This may point to a relationship between the residual CHT activity and the associated phenotype that deserves further investigations.
CMS-EA is one of the most lethal CMS types due to the sudden episodes of apnea in neonates. The most frequent cause of obstructive apneas in newborns is a passive pharyngeal collapse during inspiration due to low muscle tone, which is probably favored by the congenital muscle weakness. Sudden apneic episodes have been sometimes reported in fast-channel syndrome and forms of CMS resulting from mutations in the genes encoding the skeletal muscle sodium channel and rapsyn.25, 26, 27, 28 However, sudden EA as a constant feature is specific to forms of CMS resulting from ChAT and CHT deficiency, highlighting a relationship between congenital dysfunction of choline homeostasis and EA. ACh deficit also has probable dysautonomic effects in line with the established importance of cholinergic pathways in the autonomic nervous system. This is supported by the tachycardia and hypertension in mice heterozygous for a null allele of Slc5a7.29, 30 In vivo administration of HC-3—the specific inhibitor of CHT—leads to respiratory paralysis from central dysfunction, and cholinergic agonists as well as AChE inhibitors exert significant effects on respiratory rhythm.31, 32 The strong link observed between CHT deficiency and the occurrence of sudden EA therefore underlines the still not well-characterized role of central cholinergic transmission in the fine control of respiratory function.33
CHT is present in central cholinergic neurons and cholinergic neurotransmission is important for cognitive and behavioral functions.19 One hypomorphic polymorphism in SLC5A7 (rs1013940 corresponding to the c.265A>G [p.Ile89Val] variation) is associated with pediatric attention deficit-hyperactivity disorder (ADHD [MIM: 143465]).34 Among the individuals with CMS-EA due to recessive SLC5A7 mutations reported here, three (5, 6, and 7) present cognitive deficits. If the multifactorial etiology of cognition must be considered before evoking any strong risk of cognitive or behavioral deficits in subjects with impaired CHT, our observation leads to the recommendation of carefully following up the subjects to detect and adequately manage any central phenotype due to CHT deficiency.
To summarize, our present work demonstrates the existence of a clinical spectrum resulting from CHT dysfunction that highlights the complexity of synaptic cholinergic metabolism and its genetics in human diseases with possible multisystem involvement that deserves further investigation.
Acknowledgments
The authors are grateful to the families for their invaluable participation in this study. This work was supported by AFM-Téléthon (grant ID 20030), the programs “Investissements d’avenir” ANR-10-IAIHU-06 (IHU-A-ICM) and ANR-10-INBS-09 (France Génomique National infrastructure), and Fondation Maladies Rares within the Myocapture sequencing project. The study was also supported by the Medical Research Council UK (reference G1002274, grant ID 98482) and by the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement No. 305444 (RD-Connect) and 305121 (Neuromics) to H.L. We thank Dr. Eric Krejci for his generous gift of toxins and antibodies specific to cholinesterases and the Plateforme d’Imagerie Cellulaire Pitié Salpêtrière (PICPS) and the Service Commun de Microscopie Université PARIS DESCARTES for confocal microscopy. We thank the DNA and Cell Banks of Genethon and CRB-REFGENSEP, and the Celis, Histomics and Bioinformatics/Biostatistics (iCONICS) core facilities of the ICM for precious support. We wish to thank Drs. Rolf Stucka, Marina Dusl, Angela Abicht, and Jan Senderek, all at Ludwig-Maximilians-University Munich, Germany, for their contributions to exclude known genes linked to CMS in two families described in this study and for providing DNA samples, and Dr. Patricia Boffi, Turino, Italy, for the initial referral of one of the subjects.
Published: August 25, 2016
Footnotes
Supplemental Data include a supplemental note, four figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.ajhg.2016.06.033.
Web Resources
1000 Genomes, http://www.1000genomes.org
ExAC Browser, http://exac.broadinstitute.org/
GenBank, http://www.ncbi.nlm.nih.gov/genbank/
Human Splicing Finder, http://www.umd.be/HSF3/HSF.html
MutationTaster, http://www.mutationtaster.org/
OMIM, http://www.omim.org/
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
Supplemental Data
References
- 1.Black S.A., Rylett R.J. Choline transporter CHT regulation and function in cholinergic neurons. Cent. Nerv. Syst. Agents Med. Chem. 2012;12:114–121. doi: 10.2174/187152412800792724. [DOI] [PubMed] [Google Scholar]
- 2.Haga T. Molecular properties of the high-affinity choline transporter CHT1. J. Biochem. 2014;156:181–194. doi: 10.1093/jb/mvu047. [DOI] [PubMed] [Google Scholar]
- 3.Engel A.G., Shen X.M., Selcen D., Sine S.M. Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol. 2015;14:420–434. doi: 10.1016/S1474-4422(14)70201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byring R.F., Pihko H., Tsujino A., Shen X.M., Gustafsson B., Hackman P., Ohno K., Engel A.G., Udd B. Congenital myasthenic syndrome associated with episodic apnea and sudden infant death. Neuromuscul. Disord. 2002;12:548–553. doi: 10.1016/s0960-8966(01)00336-4. [DOI] [PubMed] [Google Scholar]
- 5.Ohno K., Tsujino A., Brengman J.M., Harper C.M., Bajzer Z., Udd B., Beyring R., Robb S., Kirkham F.J., Engel A.G. Choline acetyltransferase mutations cause myasthenic syndrome associated with episodic apnea in humans. Proc. Natl. Acad. Sci. USA. 2001;98:2017–2022. doi: 10.1073/pnas.98.4.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barwick K.E., Wright J., Al-Turki S., McEntagart M.M., Nair A., Chioza B., Al-Memar A., Modarres H., Reilly M.M., Dick K.J. Defective presynaptic choline transport underlies hereditary motor neuropathy. Am. J. Hum. Genet. 2012;91:1103–1107. doi: 10.1016/j.ajhg.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicole S., Chaouch A., Torbergsen T., Bauché S., de Bruyckere E., Fontenille M.J., Horn M.A., van Ghelue M., Løseth S., Issop Y. Agrin mutations lead to a congenital myasthenic syndrome with distal muscle weakness and atrophy. Brain. 2014;137:2429–2443. doi: 10.1093/brain/awu160. [DOI] [PubMed] [Google Scholar]
- 8.Thevenon J., Duffourd Y., Masurel-Paulet A., Lefebvre M., Feillet F., El Chehadeh-Djebbar S., St-Onge J., Steinmetz A., Huet F., Chouchane M. Diagnostic odyssey in severe neurodevelopmental disorders: toward clinical whole-exome sequencing as a first-line diagnostic test. Clin. Genet. 2016;89:700–707. doi: 10.1111/cge.12732. [DOI] [PubMed] [Google Scholar]
- 9.Okuda T., Haga T., Kanai Y., Endou H., Ishihara T., Katsura I. Identification and characterization of the high-affinity choline transporter. Nat. Neurosci. 2000;3:120–125. doi: 10.1038/72059. [DOI] [PubMed] [Google Scholar]
- 10.Okuda T., Osawa C., Yamada H., Hayashi K., Nishikawa S., Ushio T., Kubo Y., Satou M., Ogawa H., Haga T. Transmembrane topology and oligomeric structure of the high-affinity choline transporter. J. Biol. Chem. 2012;287:42826–42834. doi: 10.1074/jbc.M112.405027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okuda T., Okamura M., Kaitsuka C., Haga T., Gurwitz D. Single nucleotide polymorphism of the human high affinity choline transporter alters transport rate. J. Biol. Chem. 2002;277:45315–45322. doi: 10.1074/jbc.M207742200. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson S.M., Bazalakova M., Savchenko V., Tapia J.C., Wright J., Blakely R.D. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc. Natl. Acad. Sci. USA. 2004;101:8762–8767. doi: 10.1073/pnas.0401667101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauché S., Boerio D., Davoine C.S., Bernard V., Stum M., Bureau C., Fardeau M., Romero N.B., Fontaine B., Koenig J. Peripheral nerve hyperexcitability with preterminal nerve and neuromuscular junction remodeling is a hallmark of Schwartz-Jampel syndrome. Neuromuscul. Disord. 2013;23:998–1009. doi: 10.1016/j.nmd.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Hesselmans L.F., Jennekens F.G., Van den Oord C.J., Veldman H., Vincent A. Development of innervation of skeletal muscle fibers in man: relation to acetylcholine receptors. Anat. Rec. 1993;236:553–562. doi: 10.1002/ar.1092360315. [DOI] [PubMed] [Google Scholar]
- 15.Hrabovska A., Bernard V., Krejci E. A novel system for the efficient generation of antibodies following immunization of unique knockout mouse strains. PLoS ONE. 2010;5:e12892. doi: 10.1371/journal.pone.0012892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrov K.A., Girard E., Nikitashina A.D., Colasante C., Bernard V., Nurullin L., Leroy J., Samigullin D., Colak O., Nikolsky E. Schwann cells sense and control acetylcholine spillover at the neuromuscular junction by α7 nicotinic receptors and butyrylcholinesterase. J. Neurosci. 2014;34:11870–11883. doi: 10.1523/JNEUROSCI.0329-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schara U., Christen H.J., Durmus H., Hietala M., Krabetz K., Rodolico C., Schreiber G., Topaloglu H., Talim B., Voss W. Long-term follow-up in patients with congenital myasthenic syndrome due to CHAT mutations. Eur. J. Paediatr. Neurol. 2010;14:326–333. doi: 10.1016/j.ejpn.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Shen X.M., Crawford T.O., Brengman J., Acsadi G., Iannaconne S., Karaca E., Khoury C., Mah J.K., Edvardson S., Bajzer Z. Functional consequences and structural interpretation of mutations of human choline acetyltransferase. Hum. Mutat. 2011;32:1259–1267. doi: 10.1002/humu.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berse B., Szczecinska W., Lopez-Coviella I., Madziar B., Zemelko V., Kaminski R., Kozar K., Lips K.S., Pfeil U., Blusztajn J.K. Expression of high affinity choline transporter during mouse development in vivo and its upregulation by NGF and BMP-4 in vitro. Brain Res. Dev. Brain Res. 2005;157:132–140. doi: 10.1016/j.devbrainres.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 20.Li B., Duysen E.G., Carlson M., Lockridge O. The butyrylcholinesterase knockout mouse as a model for human butyrylcholinesterase deficiency. J. Pharmacol. Exp. Ther. 2008;324:1146–1154. doi: 10.1124/jpet.107.133330. [DOI] [PubMed] [Google Scholar]
- 21.Manoharan I., Boopathy R., Darvesh S., Lockridge O. A medical health report on individuals with silent butyrylcholinesterase in the Vysya community of India. Clin. Chim. Acta. 2007;378:128–135. doi: 10.1016/j.cca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Minic J., Chatonnet A., Krejci E., Molgó J. Butyrylcholinesterase and acetylcholinesterase activity and quantal transmitter release at normal and acetylcholinesterase knockout mouse neuromuscular junctions. Br. J. Pharmacol. 2003;138:177–187. doi: 10.1038/sj.bjp.0705010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pridmore C., Baraitser M., Brett E.M., Harding A.E. Distal spinal muscular atrophy with vocal cord paralysis. J. Med. Genet. 1992;29:197–199. doi: 10.1136/jmg.29.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ingram G., Barwick K.E., Hartley L., McEntagart M., Crosby A.H., Llewelyn G., Morris H.R. Distal hereditary motor neuropathy with vocal cord paresis: from difficulty in choral singing to a molecular genetic diagnosis. Pract. Neurol. 2016;16:247–251. doi: 10.1136/practneurol-2015-001307. [DOI] [PubMed] [Google Scholar]
- 25.Ohno K., Engel A.G., Shen X.M., Selcen D., Brengman J., Harper C.M., Tsujino A., Milone M. Rapsyn mutations in humans cause endplate acetylcholine-receptor deficiency and myasthenic syndrome. Am. J. Hum. Genet. 2002;70:875–885. doi: 10.1086/339465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsujino A., Maertens C., Ohno K., Shen X.M., Fukuda T., Harper C.M., Cannon S.C., Engel A.G. Myasthenic syndrome caused by mutation of the SCN4A sodium channel. Proc. Natl. Acad. Sci. USA. 2003;100:7377–7382. doi: 10.1073/pnas.1230273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Habbout K., Poulin H., Rivier F., Giuliano S., Sternberg D., Fontaine B., Eymard B., Morales R.J., Echenne B., King L. A recessive Nav1.4 mutation underlies congenital myasthenic syndrome with periodic paralysis. Neurology. 2016;86:161–169. doi: 10.1212/WNL.0000000000002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palace J., Lashley D., Bailey S., Jayawant S., Carr A., McConville J., Robb S., Beeson D. Clinical features in a series of fast channel congenital myasthenia syndrome. Neuromuscul. Disord. 2012;22:112–117. doi: 10.1016/j.nmd.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Bazalakova M.H., Wright J., Schneble E.J., McDonald M.P., Heilman C.J., Levey A.I., Blakely R.D. Deficits in acetylcholine homeostasis, receptors and behaviors in choline transporter heterozygous mice. Genes Brain Behav. 2007;6:411–424. doi: 10.1111/j.1601-183X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 30.English B.A., Appalsamy M., Diedrich A., Ruggiero A.M., Lund D., Wright J., Keller N.R., Louderback K.M., Robertson D., Blakely R.D. Tachycardia, reduced vagal capacity, and age-dependent ventricular dysfunction arising from diminished expression of the presynaptic choline transporter. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H799–H810. doi: 10.1152/ajpheart.00170.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shao X.M., Feldman J.L. Cholinergic neurotransmission in the preBötzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neuroscience. 2005;130:1069–1081. doi: 10.1016/j.neuroscience.2004.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schueler F.W. A new group of respiratory paralyzants. I. The “hemicholiniums”. J. Pharmacol. Exp. Ther. 1955;115:127–143. [PubMed] [Google Scholar]
- 33.Haji A., Takeda R., Okazaki M. Neuropharmacology of control of respiratory rhythm and pattern in mature mammals. Pharmacol. Ther. 2000;86:277–304. doi: 10.1016/s0163-7258(00)00059-0. [DOI] [PubMed] [Google Scholar]
- 34.English B.A., Hahn M.K., Gizer I.R., Mazei-Robison M., Steele A., Kurnik D.M., Stein M.A., Waldman I.D., Blakely R.D. Choline transporter gene variation is associated with attention-deficit hyperactivity disorder. J. Neurodev. Disord. 2009;1:252–263. doi: 10.1007/s11689-009-9033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.