Abstract
Background
The Comprehensive Complication Index (CCI) is a new tool to evaluate the postoperative condition by calculating the sum of all complications weighted by their severity. The aim of this study was to identify independent risk factors for a high CCI score (≥40) in 229 patients after major hepatectomies with biliary reconstruction for biliary cancers.
Methods
The CCI was calculated online via www.assessurgery.com. Independent risk factors were identified by multivariable analysis.
Results
57 (25%) patients were classified as having CCI ≥ 40. On multivariable analysis, volume of intraoperative blood loss (≥2.5 L) (p = 0.004) and combined pancreatoduodenectomy (PD) (p = 0.006) were independent risk factors for CCI ≥ 40. A high level of maximum serum total bilirubin was identified as independent risk factors for a high volume of intraoperative blood loss. Liver failure (p = 0.046) was more frequent in patients with combined PD than in those without.
Discussion
Patients who undergo preoperative external biliary drainage for severe jaundice might have impaired production of coagulation factors. When blood loss during liver transection becomes difficult to control, surgeons should consider various strategies, such as second-stage biliary or pancreatic reconstruction. In patients planned to undergo major hepatectomy with combined PD, preoperative portal vein embolization is mandatory to prevent postoperative liver failure.
Introduction
For patients with biliary cancers, including perihilar cholangiocarcinoma or gallbladder carcinoma, surgical resection offers the only possibility of cure.1 However, hepatectomy for these diseases is more difficult than for hepatocellular carcinoma or metastatic disease to the liver. The increased complexity comes from the need for biliary tract reconstruction and, on occasion, the need to resect and reconstruct the portal vein or hepatic artery, or add pancreatoduodenectomy (PD).1, 2, 3 Therefore, it is possible that risk factors for postoperative complications in patients who undergo such complex procedures are different from those who undergo simple hepatectomy. Few reports have examined independent risk factors for morbidity or mortality in patients who have undergone hepatectomy for cholangiocarcinoma (Table 1).1, 4, 5, 6, 7, 8, 9
Table 1.
Independent risk factors for morbidity or mortality of patients who underwent resection for perihilar cholangiocarcinoma or tumor in previous reports
| Risk factor for endpoints | Endpoint |
|
|---|---|---|
| Morbidity |
Mortality |
|
| No. Reference | ||
| Variable | ||
| Preoperative cholangitis | 4 | 1, 5, 9 |
| Intraoperative blood loss or blood transfusion | 8(>900 ml) | 1(≥2500 ml),6 (blood transfusion) |
| Low liver function or %FLRa | 1, 9 | |
| Preoperative bilirubin (>3 mg/dL) | 7 | |
Plasma disappearance rate of indocyanine green (ICGK) < 0.14(1), FLR < 30%.9
In recent years, the Clavien–Dindo classification (CDC) grade has become the standard for reporting postoperative complications. This classification grades complications according to the most severe complication or events judged to be relevant.10 By this system, complications of lesser magnitude, as well as the total number of complications, are not accounted for. To address this issue, in 2013, Slankamenac et al. presented a new tool for scoring, the so-called “Comprehensive Complication Index (CCI)”.11, 12 The CCI is calculated as the sum of all complications weighted by severity (available at www.assessurgery.com).10, 13 The formula for the CCI yields a continuous scale to rank the severity of any combination of complications from 0 (no complications) to 100 (death) in a single patient. For example, a patient with a CCI of 8.7 would have a single CDC grade I complication, one with a CCI of 20.6 would have a grade II complication, one with a CCI of 26.2 would have a grade IIIa complication, one with a CCI of 33.7 would have a grade IIIb complication, one with a CCI of 44.2 would have a grade IVa complication, and one with a CCI of 46.2 would have a grade IVb complication. In addition, the formula for the CCI can calculate the summative severity of several complications in a single patient. For example, the CCI index for the sum of one CDC grade I and two CDC grade II complications in a single patient is 30.8. There have been no reports that have identified risk factors for a severe postoperative CCI score (CCI > 40) in a large cohort of patients undergoing hepatectomy for malignant biliary disease.
The aim of this study was to identify predictive factors for patients who developed a severe postoperative CCI score following major hepatectomy with biliary reconstruction for biliary cancer.
Patients and methods
Patients
Between March 1999 and March 2013, 255 patients underwent hepatectomy with biliary reconstruction for biliary cancer (perihilar cholangiocarcinoma or gallbladder carcinoma) with curative intent at the Department of Gastroenterological Surgery II, Hokkaido University Hospital. Twenty-six (10%) patients were excluded from the present study due to a lack of clinical records (n = 10), having a history of surgery including biliary reconstruction (n = 11), or undergoing minor hepatectomy (n = 5). Thus, 229 (90%) patients were included for the further study. This study was approved by the Institutional Review Board of Hokkaido University Hospital (No. 014-0374).
Preoperative preparation
The patients were treated in accordance with departmental guidelines, which were established in 1999 for patients with hilar cholangiocarcinoma.14, 15 Pre-operative biliary decompression was performed to reduce serum bilirubin concentrations below 34 μmol/L (2 mg/dL) for all patients with jaundice and to control segmental cholangitis. Previously, percutaneous transhepatic biliary drainage (PTBD) was used for drainage. Beginning in 2005, endoscopic nasobiliary drainage (ENBD) of the future remnant liver was adopted for initial drainage.
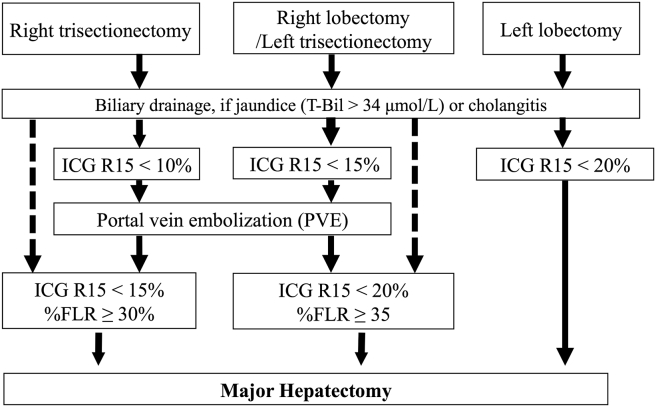
Preoperative portal vein embolization (PVE) of the liver to be resected was considered when right hepatectomy or right- or left-trisectionectomy was planned.16 More than 2 weeks after PVE, the patients' liver volumes were semi-automatically measured using contrast-enhanced computed tomography imaging data (volume data or 5-mm-thick axial imaging data).16 The basic decision criteria for the ratio of the future liver remnant volume/total liver volume (%FLR) limit for each type of hepatectomy are shown in Fig. 1.
Figure 1.
Decision criteria for the strategy for preoperative management of major hepatectomy in biliary cancer. Preoperative biliary decompression is performed to reduce the serum bilirubin concentration to below 2 mg/dL. When the value of the indocyanine green retention rate at 15 min (ICG R15) after relief of jaundice meets the requirements, limited PE is performed. At 2 weeks after PVE, patients with ICG R15 and the appropriate ratio of the future liver remnant volume/total liver volume (%FLR) are considered candidates for major hepatectomy with biliary reconstruction
Surgical technique
In patients with gallbladder carcinoma and intrahepatic cholangiocarcinoma, before radical resection, para-aortic lymphadenectomy and pathological examination by frozen sections were performed immediately after laparotomy to decide whether radical resection should be performed.17, 18, 19 For patients with extrahepatic cholangiocarcinoma, para-aortic lymphadenectomy was performed for nodes with suspected metastases on preoperative imaging findings. Systematic lymphadenectomy of the hepatoduodenal ligament, including the nodes around the head of the pancreas, was routinely performed in all three types of biliary carcinoma. Portal vein resection and reconstruction with macroscopic infiltration by the tumor were completed before hepatic parenchymal dissection and hepatic ductal division.20, 21 Hepatic arteries that were determined to have cancer invasion were resected en bloc with the bile duct and reconstructed by direct end-to-end anastomosis of the hepatic arteries or in situ grafting of the right epigastric artery or the gastroduodenal artery using a microscopic technique. When infiltration of tumor proceeded too peripherally to allow reconstruction, arterio-portal shunting was performed.22 Liver transection was performed using the forceps clamp crushing method during both hepatic artery and portal vein clamping for 15 min with 5-min intervals. Biliary tract reconstruction was performed by bilio-enterostomy using a Roux-en-Y jejunal limb.23
Evaluation of postoperative complications
The information about complications was acquired from medical records in all patients. Each postoperative event during the hospital stay in each patient was assessed and graded according to the CDC.10 The CCI was then calculated as the sum of all complications online by free access at www.assessurgery.com. In this study, a severe postoperative condition was defined as CCI ≥ 40, because it was thought that severe postoperative conditions corresponded to one or more CDC grade IV (life-threatening) complications.
Post-hepatectomy liver failure, intra-abdominal hemorrhage, and bile leakage that occurred during a patient's hospital stay were evaluated according to the definitions and grading of the International Study Group of Liver Surgery (ISGLS).24, 25, 26 Grade B or C of the ISGLS of the 3 above complications was defined as positive in this study. Similarly, a pancreatic fistula that occurred during a patient's hospital stay was evaluated according to the International Study Group of Pancreatic Fistula (ISGPF),27 and grade B or C was defined as positive. For liver failure, bile leakage, and pancreatic fistula, evaluation was performed at or after postoperative day (POD) −5,24 on POD 3,26 and on POD 5,27 respectively.
Statistics
Univariable and multivariable analyses were performed using logistic regression. On multivariable analysis, factors identified as p < 0.30 on univariable analyses were examined. P < 0.05 was considered significant. Analyses were performed using JMP software (version 8.0.2, SAS Institute, Inc., Cary, NC, USA).
Results
Patients
The median age was 67 years (range, 40–86 years), and 151 (66%) patients were men. The primary lesion involved the extrahepatic bile duct in 150 (66%) patients, the intrahepatic bile duct in 41 (18%) patients, and the gallbladder, including the cystic duct, in 38 (17%) patients. A total of 128 (56%) patients, including 5 (2%) patients whose portal veins were occluded by tumor, underwent preoperative PVE. The data for %FLR before PVE in 105 (82%) of 128 patients who underwent PVE were obtained. In those 105 patients, the mean ± SD increase rate of %FLR after PVE compared with that before PVE was 10.8% ± 5.5%. The mean ± standard deviation (SD) final %FLR in each type of major hepatectomy and the combinations of vascular resection and PD with each type of major hepatectomy are shown in Table 2.
Table 2.
Surgical procedures performed
| Type of major hepatectomy |
||||
|---|---|---|---|---|
| Right hepatectomy (S 1, 5, 6, 7, 8) |
Right trisectionectomy (S1, 4, 5, 6, 7, 8) |
Left hepatectomy (S1, 2, 3, 4) |
Left trisectionectomy (S1, 2, 3, 4, 5, 8) |
|
| %FLR (mean ± SD, %) | 47.9 ± 8.6 | 36.3 ± 8.2 | 70.4 ± 10.1 | 47.6 ± 11.3 |
| Number of patients | 122 | 10 | 82 | 15 |
| Combined procedure | ||||
| PVR | 86 | 4 | 28 | 8 |
| AR | 1 | 0 | 17 | 7 |
| PD | 29 | 2 | 8 | 2 |
S, Couinaud's hepatic segment; %FLR, ratio of the future liver remnant/total liver volume; SD, standard deviation; PVR, portal vein reconstruction; AR, artery reconstruction; PD, pancreatoduodenectomy.
The highest CDC grade complication during the hospital stay was no complication in 14 (6%), grade I in 20 (9%), grade II in 89 (39%), grade III in 81 (35%), grade IV in 11 (5%), and grade V (hospital death) in 14 (6%) patients. The median hospital stay after surgery of 14 patients with CDC grade V was 76 days (range, 2–147 days).
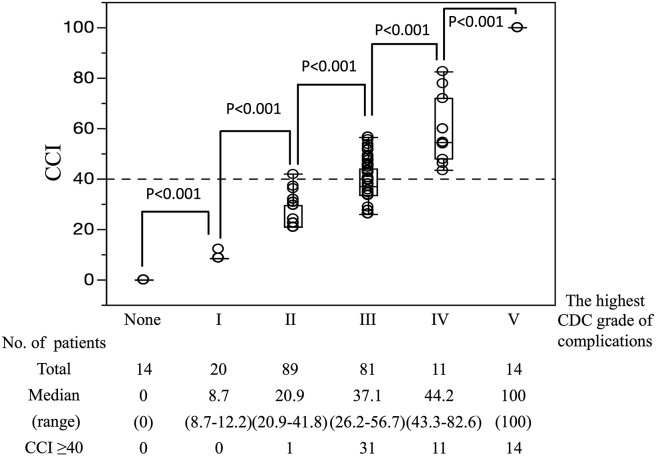
The median CCI of the 229 patients was 28 (range, 0–100). The number of patients with CCI ≥ 40 was 57 (25%). The associations between CCI and the highest CDC grade of individual patients' complications are shown in Fig. 2.
Figure 2.
Associations between the highest CDC grade and CCI. CCI according to the highest CDC grade of complications that occurred in each patient. Box-and-whisker plots display median, interquartile range, and extreme values. p-Values were calculated using the Mann–Whitney U-test
The results of comparisons of risk factors between CCI < 40 and CCI ≥ 40 on univariable and multivariable analyses are shown in Table 3.
Table 3.
Risk factors for high CCI in 229 patients who underwent major hepatectomy for biliary cancer
| Variable | CCI |
Univariable, p | Multivariable |
|||
|---|---|---|---|---|---|---|
| <40 (n = 172, 100%) |
≥40 (n = 57, 100%) |
Odds ratio | 95% CI | p | ||
| Age (≥70 years) | 78 (45%) | 28 (49%) | 0.621 | |||
| Male | 110 (64%) | 41 (72%) | 0.266 | 1.26 | 0.63–2.61 | 0.516 |
| Past history of upper abdominal laparotomy | 17 (10%) | 8 (14%) | 0.400 | |||
| Diabetes mellitus | 35 (20%) | 11 (19%) | 0.740 | |||
| Preoperative cholangitis | 64 (37%) | 32 (56%) | 0.013 | 1.65 | 0.85–3.24 | 0.141 |
| Body mass index (≥25 kg/m2) | 28 (16%) | 10 (18%) | 0.893 | |||
| Blood platelet count (≤15 × 104/mL) | 14 (8%) | 4 (7%) | 0.783 | |||
| PT-INR (≥1.2) | 24 (14%) | 6 (11%) | 0.498 | |||
| Preoperative serum albumin (<3.5 g/dL) | 33 (19%) | 14 (25%) | 0.391 | |||
| Preoperative maximum serum T.Bil (≥100 μmol/L) | 54 (31%) | 21 (37%) | 0.451 | |||
| ENBD or PTCD performed | 134 (78%) | 52 (91%) | 0.017 | 2.36 | 0.86–7.75 | 0.100 |
| ICG R15 (≥15%) | 32 (19%) | 6 (11%) | 0.139 | 2.08 | 0.80–6.22 | 0.136 |
| %FLR (<50%) | 72 (42%) | 34 (60%) | 0.020 | 1.65 | 0.84–3.26 | 0.145 |
| Combined gastrointestinal tract resection | 11 (6%) | 5 (9%) | 0.551 | |||
| Combined portal vein resection and reconstruction | 91 (53%) | 35 (61%) | 0.262 | 1.10 | 0.55–2.23 | 0.785 |
| Combined hepatic artery resection and reconstruction | 19 (11%) | 6 (11%) | 0.913 | |||
| Combined PD | 22 (13%) | 19 (33%) | <0.001 | 2.98 | 1.38–6.43 | 0.006 |
| Volume of intraoperative blood loss (≥2.5 L) | 38 (22%) | 24 (42.1%) | 0.004 | 2.82 | 1.39–5.78 | 0.004 |
CI, confidence interval; PT-INR, prothrombin-international ratio; ENBD, endoscopic nasobiliary drainage; PTBD, percutaneous transhepatic biliary drainage; ICG R15, indocyanine green retention rate at 15 min; %FLR, ratio of the future liver remnant/the total liver volume.
Numerical values connected with significant factors in multivariable analysis shows bold.
Associations between large volume of intraoperative blood loss (≥2.5 L) and various pre- or intra-operative factors were examined. The results are shown in Table 4.
Table 4.
Risk factors for high volume of intraoperative blood loss in 229 patients who underwent major hepatectomy for biliary cancer
| Variable | Volume of intraoperative blood loss |
Univariable, p | Multivariable |
|||
|---|---|---|---|---|---|---|
| <2.5 L |
≥2.5 L |
Odds ratio | 95% CI | p | ||
| (n = 167, 100%) | (n = 62, 100%) | |||||
| Age (≥70 years) | 82 (49%) | 24 (39%) | 0.162 | 1.47 | 0.79–2.79 | 0.225 |
| Male | 103 (62%) | 48 (77%) | 0.027 | 2.19 | 1.11–4.53 | 0.029 |
| Past history of upper abdominal laparotomy | 17 (10%) | 8 (13%) | 0.558 | |||
| Diabetes mellitus | 33 (20%) | 13 (21%) | 0.840 | |||
| Preoperative cholangitis | 68 (41%) | 28 (45%) | 0.545 | |||
| Body mass index (≥25 kg/m2) | 26 (16%) | 12 (19%) | 0.495 | |||
| Blood platelets (≤15 × 104/mL) | 8 (5%) | 10 (16%) | 0.007 | 3.63 | 1.26–10.87 | 0.018 |
| PT-INR (≥1.2) | 23 (14%) | 7 (11%) | 0.621 | |||
| Preoperative serum albumin (<3.5 g/dL) | 32 (19%) | 15 (24%) | 0.323 | |||
| Preoperative maximum serum T.Bil (≥100 μmol/L) | 47 (28%) | 28 (45%) | 0.016 | 2.08 | 1.09–3.99 | 0.027 |
| ENBD or PTCD performed | 136 (81%) | 50 (81%) | 0.892 | |||
| ICG R15 (≥15%) | 23 (14%) | 15 (24%) | 0.063 | 1.5 | 0.66–3.29 | 0.327 |
| %FLR (<50%) | 76 (46%) | 30 (48%) | 0.698 | |||
| Combined gastrointestinal tract resection | 11 (7%) | 5 (8%) | 0.697 | |||
| Combined portal vein resection and reconstruction | 88 (53%) | 38 (61%) | 0.246 | 1.24 | 0.66–2.38 | 0.497 |
| Combined hepatic artery resection and reconstruction | 17 (10%) | 8 (13%) | 0.558 | |||
| Combined PD | 29 (17%) | 12 (19%) | 0.727 | |||
CI, confidence interval; PT-INR, prothrombin-international ratio; ENBD, endoscopic nasobiliary drainage; PTBD, percutaneous transhepatic biliary drainage; ICG R15, indocyanine green retention rate at 15 min; %FLR, ratio of the future liver remnant/the total liver volume.
Postoperative complications of patients with combined PD
Frequencies of 4 representative in-hospital complications (ISGLS grade B or C of postoperative liver failure, bile leakage, intra-abdominal hemorrhage, and ISGPF grade B or C of pancreatic fistula) were compared between patients who underwent major hepatectomy with and without PD. The results are shown in Table 5.
Table 5.
Associations between representative postoperative complications and combined PD
| Representative postoperative complication | Combined PD |
p | |
|---|---|---|---|
| Without |
With |
||
| n = 187a (100%) | n = 41 | ||
| Liver failureb | 60 (32%) | 20 | 0.046 |
| Bile leakageb | 28 (15%) | 6 | 0.956 |
| Intra-abdominal hemorrhageb | 14 (8%) | 7 | 0.074 |
| Pancreatic fistulac | 12 (6%) | 28 | <0.001 |
PD, pancreatoduodenectomy.
One patient was excluded, because he died on postoperative day 2.
Grade B/C of ISGLS definitions.
Grade B/C of ISGPF definition.
Discussion
Recently, postoperative courses have come to be evaluated based on a single complication that required the most invasive treatment using the CDC.10 However, patients' conditions during the postoperative course include various complications. For example, it is possible that several CDC III or less grade complications provide patients more discomfort than a single Grade IV complication. Since CCI is a grading system that assesses the severity of postoperative conditions by the sum of all complications graded according to the CDC, the CCI enables comprehensive evaluations of patients' postoperative conditions more accurately and objectively than the conventional approach.12
The previous studies of independent risk factors for postoperative morbidity or mortality in cholangiocarcinoma from 6 institutions are shown in Table 1. Preoperative cholangitis was identified as an independent risk factor for postoperative morbidity or mortality.1, 4, 5 In the present study, preoperative cholangitis was associated with CCI ≥ 40 on the univariable analysis, but not on the multivariable analysis. The reason was thought to be that all patients in the current study with preoperative cholangitis were treated by biliary drainage, as shown in Fig. 1. Sakata et al. reported that the mortality rate was higher in patients who suffered from cholangitis until the date of definitive surgery than in those who were cured of cholangitis by the date of surgery.5 Kanai et al. reported that, in patients who suffered from cholangitis, the morbidity rate after hepatectomy was significantly lower in patients who were treated with biliary drainage than in those not treated with biliary drainage.28 As mentioned above, in patients who have preoperative cholangitis, the bile duct obstructed by tumor should be decompressed by biliary drainage immediately, and surgery should be delayed until the cholangitis is cured.
Volume of intraoperative blood loss (≥2.5 L) was found to be the most important risk factor for a severe postoperative condition after major hepatectomy with biliary reconstruction in the present study. Previous reports also have found that large intra-operative blood loss was associated with increased risk of complications, as shown in Table 1.1, 6, 8 In the present study, preoperative risk factors for large volume of intraoperative blood loss (≥2.5 L) were identified as male sex, low blood platelet count, and high level of preoperative maximum serum total bilirubin, as shown in Table 4. The reason why a high level of preoperative maximum serum total bilirubin was identified as an independent risk factor for large volume of intraoperative blood loss was unclear. However, patients with severe obstructive jaundice are likely to have impaired production of coagulation factors because of loss of vitamin K. In addition to bile replacement,29 administration of vitamin K might be useful for patients with obstructive severe jaundice to prevent a potential coagulation disorder.30 On the other hand, an attempt to identify intraoperative factors that are most closely associated with a large volume of intraoperative blood loss failed. However, blood loss usually occurs during the course of liver transection. Therefore, surgeons should make sufficient preparations prior to liver transection, such as optimization of hemodynamics, including the Pringle maneuver, clamping of the inferior vena cava,31 and consideration of administration of tranexamic acid.32 In patients in whom hepatopancreatoduodenectomy (HPD) is planned, liver transection might be done prior to pancreatic resection. When the amount of intraoperative blood loss is large at the completion of liver transection, one should consider delayed reconstruction, especially of the pancreatic anastomosis, in patients who undergo HPD.33 In addition, preoperative consideration of ways to optimize %FLR is critical.34, 35 In a review of HPD by Ebata et al., it was reported that the incidence of liver failure has decreased gradually in patients undergoing HPD since 2000. It was thought that this was due to the fact that PVE has been widely used in preoperative management.36 It was also reported that in several departments in which PVE was aggressively used, lower mortality was seen in patients who underwent HPD than in those in whom PVE was not performed.3
In conclusion, to prevent the development of high CCI conditions in patients after major hepatectomy with biliary reconstruction for biliary cancer, surgeons should make every effort to optimize %FLR preoperatively and optimize surgical decision-making to minimize the risk of massive blood loss, including considering delayed reconstruction.
Funding
This study had no outside financial support.
Conflicts of interest
None declared.
References
- 1.Nagino M., Ebata T., Yokoyama Y., Igami T., Sugawara G., Takahashi Y. Evolution of surgical treatment for perihilar cholangiocarcinoma: a single-center 34-year review of 574 consecutive resections. Ann Surg. 2013;258:129–140. doi: 10.1097/SLA.0b013e3182708b57. [DOI] [PubMed] [Google Scholar]
- 2.Nagino M., Nimura Y., Nishio H., Ebata T., Igami T., Matsushita M. Hepatectomy with simultaneous resection of the portal vein and hepatic artery for advanced perihilar cholangiocarcinoma: an audit of 50 consecutive cases. Ann Surg. 2010;252:115–123. doi: 10.1097/SLA.0b013e3181e463a7. [DOI] [PubMed] [Google Scholar]
- 3.Ebata T., Yokoyama Y., Igami T., Sugawara G., Takahashi Y., Nimura Y. Hepatopancreatoduodenectomy for cholangiocarcinoma: a single-center review of 85 consecutive patients. Ann Surg. 2012;256:297–305. doi: 10.1097/SLA.0b013e31826029ca. [DOI] [PubMed] [Google Scholar]
- 4.Sano T., Shimada K., Sakamoto Y., Yamamoto J., Yamasaki S., Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240–247. doi: 10.1097/01.sla.0000217605.66519.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakata J., Shirai Y., Tsuchiya Y., Wakai T., Nomura T., Hatakeyama K. Preoperative cholangitis independently increases in-hospital mortality after combined major hepatic and bile duct resection for hilar cholangiocarcinoma. Langenbeck's Arch Surg/Deutsche Gesellschaft fur Chir. 2009;394:1065–1072. doi: 10.1007/s00423-009-0464-1. [DOI] [PubMed] [Google Scholar]
- 6.Nuzzo G., Giuliante F., Ardito F., Giovannini I., Aldrighetti L., Belli G. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147:26–34. doi: 10.1001/archsurg.2011.771. [DOI] [PubMed] [Google Scholar]
- 7.Loehrer A.P., House M.G., Nakeeb A., Kilbane E.M., Pitt H.A. Cholangiocarcinoma: are North American surgical outcomes optimal? J Am Coll Surg. 2013;216:192–200. doi: 10.1016/j.jamcollsurg.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Furusawa N., Kobayashi A., Yokoyama T., Shimizu A., Motoyama H., Miyagawa S. Surgical treatment of 144 cases of hilar cholangiocarcinoma without liver-related mortality. World J Surg. 2014;38:1164–1176. doi: 10.1007/s00268-013-2394-x. [DOI] [PubMed] [Google Scholar]
- 9.Ribero D., Zimmitti G., Aloia T.A., Shindoh J., Forchino F., Amisano M. Preoperative cholangitis and future liver remnant volume determine the risk of liver failure in patients undergoing resection for hilar cholangiocarcinoma. J Am Coll Surg. 2016;223:87–97. doi: 10.1016/j.jamcollsurg.2016.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Slankamenac K., Graf R., Barkun J., Puhan M.A., Clavien P.A. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258:1–7. doi: 10.1097/SLA.0b013e318296c732. [DOI] [PubMed] [Google Scholar]
- 12.Slankamenac K., Nederlof N., Pessaux P., de Jonge J., Wijnhoven B.P., Breitenstein S. The comprehensive complication index: a novel and more sensitive endpoint for assessing outcome and reducing sample size in randomized controlled trials. Ann Surg. 2014;260:757–762. doi: 10.1097/SLA.0000000000000948. discussion 62–3. [DOI] [PubMed] [Google Scholar]
- 13.Clavien P.A., Barkun J., de Oliveira M.L., Vauthey J.N., Dindo D., Schulick R.D. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 14.Kondo S., Hirano S., Ambo Y., Tanaka E., Okushiba S., Morikawa T. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240:95–101. doi: 10.1097/01.sla.0000129491.43855.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirano S., Kondo S., Tanaka E., Shichinohe T., Tsuchikawa T., Kato K. Outcome of surgical treatment of hilar cholangiocarcinoma: a special reference to postoperative morbidity and mortality. J Hepatobiliary Pancreat Sci. 2010;17:455–462. doi: 10.1007/s00534-009-0208-1. [DOI] [PubMed] [Google Scholar]
- 16.Sakuhara Y., Abo D., Hasegawa Y., Shimizu T., Kamiyama T., Hirano S. Preoperative percutaneous transhepatic portal vein embolization with ethanol injection. Am J Roentgenol. 2012;198:914–922. doi: 10.2214/AJR.11.6515. [DOI] [PubMed] [Google Scholar]
- 17.Kondo S., Nimura Y., Hayakawa N., Kamiya J., Nagino M., Uesaka K. Regional and para-aortic lymphadenectomy in radical surgery for advanced gallbladder carcinoma. Br J Surg. 2000;87:418–422. doi: 10.1046/j.1365-2168.2000.01384.x. [DOI] [PubMed] [Google Scholar]
- 18.Nasu Y., Tanaka E., Hirano S., Tsuchikawa T., Kato K., Matsumoto J. The prognosis after curative resection of gallbladder cancer with hilar invasion is similar to that of hilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2012;19:274–280. doi: 10.1007/s00534-011-0439-9. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama T., Tsuchikawa T., Shichinohe T., Nakamura T., Ebihara Y., Hirano S. Pathological confirmation of para-aortic lymph node status as a potential criterion for the selection of intrahepatic cholangiocarcinoma patients for radical resection with regional lymph node dissection. World J Surg. 2014;38:1763–1768. doi: 10.1007/s00268-013-2433-7. [DOI] [PubMed] [Google Scholar]
- 20.Kondo S., Katoh H., Hirano S., Ambo Y., Tanaka E., Kawarada Y. Wedge resection of the portal bifurcation concomitant with left hepatectomy plus biliary reconstruction for hepatobiliary cancer. J Hepatobiliary Pancreat Surg. 2002;9:603–606. doi: 10.1007/s005340200081. [DOI] [PubMed] [Google Scholar]
- 21.Kondo S., Katoh H., Hirano S., Ambo Y., Tanaka E., Okushiba S. Portal vein resection and reconstruction prior to hepatic dissection during right hepatectomy and caudate lobectomy for hepatobiliary cancer. Br J Surg. 2003;90:694–697. doi: 10.1002/bjs.4084. [DOI] [PubMed] [Google Scholar]
- 22.Kondo S., Hirano S., Ambo Y., Tanaka E., Kubota T., Katoh H. Arterioportal shunting as an alternative to microvascular reconstruction after hepatic artery resection. Br J Surg. 2004;91:248–251. doi: 10.1002/bjs.4428. [DOI] [PubMed] [Google Scholar]
- 23.Hirano S., Tanaka E., Tsuchikawa T., Matsumoto J., Shichinohe T., Kato K. Techniques of biliary reconstruction following bile duct resection (with video) J Hepatobiliary Pancreat Sci. 2012;19:203–209. doi: 10.1007/s00534-011-0475-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahbari N.N., Garden O.J., Padbury R., Brooke-Smith M., Crawford M., Adam R. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Rahbari N.N., Garden O.J., Padbury R., Maddern G., Koch M., Hugh T.J. Post-hepatectomy haemorrhage: a definition and grading by the International Study Group of Liver Surgery (ISGLS) HPB (Oxford) 2011;13:528–535. doi: 10.1111/j.1477-2574.2011.00319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch M., Garden O.J., Padbury R., Rahbari N.N., Adam R., Capussotti L. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Bassi C., Dervenis C., Butturini G., Fingerhut A., Yeo C., Izbicki J. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Kanai M., Nimura Y., Kamiya J., Kondo S., Nagino M., Miyachi M. Preoperative intrahepatic segmental cholangitis in patients with advanced carcinoma involving the hepatic hilus. Surgery. 1996;119:498–504. doi: 10.1016/s0039-6060(96)80257-1. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida Y., Ajiki T., Ueno K., Shinozaki K., Murakami S., Okazaki T. Preoperative bile replacement improves immune function for jaundiced patients treated with external biliary drainage. J Gastrointest Surg Off J Soc Surg Aliment Tract. 2014;18:2095–2104. doi: 10.1007/s11605-014-2674-2. [DOI] [PubMed] [Google Scholar]
- 30.Oussoultzoglou E., Jaeck D. Patient preparation before surgery for cholangiocarcinoma. HPB (Oxford) 2008;10:150–153. doi: 10.1080/13651820801992559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huntington J.T., Royall N.A., Schmidt C.R. Minimizing blood loss during hepatectomy: a literature review. J Surg Oncol. 2014;109:81–88. doi: 10.1002/jso.23455. [DOI] [PubMed] [Google Scholar]
- 32.Wu C.C., Ho W.M., Cheng S.B., Yeh D.C., Wen M.C., Liu T.J. Perioperative parenteral tranexamic acid in liver tumor resection: a prospective randomized trial toward a “blood transfusion”-free hepatectomy. Ann Surg. 2006;243:173–180. doi: 10.1097/01.sla.0000197561.70972.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyagawa S., Makuuchi M., Kawasaki S., Hayashi K., Harada H., Kitamura H. Outcome of major hepatectomy with pancreatoduodenectomy for advanced biliary malignancies. World J Surg. 1996;20:77–80. doi: 10.1007/s002689900014. [DOI] [PubMed] [Google Scholar]
- 34.Seyama Y., Kubota K., Sano K., Noie T., Takayama T., Kosuge T. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giraudo G., Greget M., Oussoultzoglou E., Rosso E., Bachellier P., Jaeck D. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: a large single institution experience. Surgery. 2008;143:476–482. doi: 10.1016/j.surg.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Ebata T., Yokoyama Y., Igami T., Sugawara G., Mizuno T., Nagino M. Review of hepatopancreatoduodenectomy for biliary cancer: an extended radical approach of Japanese origin. J Hepatobiliary Pancreat Sci. 2014;21:550–555. doi: 10.1002/jhbp.80. [DOI] [PubMed] [Google Scholar]