Abstract
Objective
The aim of this study is to evaluate whether a parenchymal-sparing strategy provides similar results in terms of morbidity, mortality, and oncological outcome of non-PSH hepatectomies in a propensity score matched population (PSMP) in case of multiple (>3) bilobar colorectal liver metastases (CLM).
Background
The surgical treatment of bilobar liver metastasis is challenging due to the necessity to achieve complete resection margins and a sufficient future remnant liver. Two approaches are adaptable as follows: parenchymal-sparing hepatectomies (PSH) and extended hepatectomies (NON-PSH).
Methods
A total of 3036 hepatectomies were analyzed from a multicentric retrospective cohort of hepatectomies. Patients were matched in a 1:1 propensity score analysis in order to compare PSH versus NON-PSH resections.
Results
PSH was associated with a lower number of complications (≥1) (25% vs. 34%, p = 0.04) and a lower grade of Dindo-Clavien III and IV (10 vs. 16%, p = 0.03). Liver failure was less present in PSH (2 vs. 7%, p = 0.006), with a shorter ICU stay (0 day vs. 1 day, p = 0.004). No differences were demonstrated in overall and disease-free survival.
Conclusion
In conclusion, PSH resection for bilobar multiple CLMs represents a valid alternative to NON-PSH resection in selected patients with a reduced morbidity and comparable oncological results.
Introduction
The modern treatment of colorectal liver metastasis is based on a multidisciplinary approach and on negative resection margin resections in order to achieve the best long-term results for metastatic patients. Recently, the concept of parenchymal-sparing hepatectomies (PSH) has been even more considered1, 2, 3, 4 due to the possibility of reducing the risk of acute liver failure, by respecting the uninvolved liver parenchyma. Even if some critics focused on the higher rate of recurrence due to nearest resection margins5 and on an extended quantity of liver parenchyma which could host liver recurrence,6 recent series demonstrate the feasibility of a parenchymal-sparing strategy.1, 2, 3, 7, 8, 9 In case of bilateral liver metastases, strategies could be challenging due to the necessity to achieve a curative treatment as well as a sufficient future remnant liver. Most series comparing a parenchymal-sparing approach with extensive hepatectomies have not demonstrated any difference between these two approaches, even if heterogeneous populations were compared, with difficult interpretation of results.10, 11
The aim of this study is to evaluate the results of a parenchymal-sparing approach in terms of morbidity, mortality, and oncological outcome, in a propensity score matched population (PSMP) in case of multiple bilobar colorectal liver metastases.
Methods
Data were obtained from a questionnaire-based survey of patients who underwent surgery for colorectal liver metastases (CRLM) in 32 French centers from January 2006 to December 2013. This study was performed under the supervision of the French National Surgical Association (Association Française de Chirurgie – AFC) after institutional approval. Files were submitted by surgeons of each institution. Demographic data, preoperative, intraoperative, postoperative data and oncological results were collected and evaluated. Each site was responsible for data collection and entry. Files were then submitted to the AFC. Once anonymized, all questionnaires were merged to create a single database. To ensure completeness of data, questionnaires were sent back to the institutions in case of missing data (>10% per variable). Once this step was complete, patients without long-term follow-up information or with outlying values were excluded. The authors had complete access to the final dataset.
Study population
This study was designed to evaluate the short-term and long-term outcome of NON-PSH resections versus multiple PSH resections in patients who underwent first-time hepatectomies for CRLM with ≥3 bilobar nodules. Patients who underwent two-stage hepatectomy, re-hepatectomy, and macroscopic incomplete resection (R2) were excluded from the study. NON-PSH hepatectomy was defined as the resection of 3 and more consecutive liver segments. PSH hepatectomies were defined as the resection of a maximum of 1 segment. The number of resected segments was defined by the type of surgical resection according to the Brisbane classification.12 In case of multiple resections, the number of segments was added.
Postoperative morbidity was defined as the occurrence of any complication within 90 days after liver resection. It was categorized following the Dindo-Clavien classification.13 Patients were followed up using a serum tumor marker (carcinoembryonic antigen) (CEA) and thoraco-abdominal and pelvic computed tomography every 4 or 6 months depending on the center. Recurrence was defined as intra-hepatic or extra-hepatic biopsy-proven recurrent adenocarcinoma, or a lesion deemed suspicious on cross-sectional imaging. Overall survival (OS) was analyzed from the date of liver resection to the date of death, and disease-free survival (DFS) to the date of recurrence. The indication for adjuvant chemotherapy was discussed in a multidisciplinary meeting.
Statistical analysis
Quantitative variables were presented as medians. Qualitative variables were presented as numbers and percentages. Comparison of quantitative variables was performed using a Mann–Whitney test. Comparison of qualitative variables was performed using Pearson's chi-squared test2 or Fisher's exact test depending on numbers. A p value <0.05 was considered significant. Overall and disease-free survival probabilities were calculated using the Kaplan–Meier method.
A propensity score matching (PSM) was calculated to take into account and reduce selection biases as well as confusion between the two groups. This method allows to compare the effects of the two types of surgical procedure (NON-PSH vs. PSH) taking into account the variables which influence the choice of the procedure type. Patients were matched in a 1:1 analysis with the closest estimated PS within 0.2 of the PSM standard deviation. For PSM, we chose variables which are known to potentially affect the outcome of interest. The propensity score was assessed using logistic regression including the following variables: age, gender, co-morbidity, Body Mass Index (BMI), ASA score (American Society of Anesthesiologists), use of neoadjuvant therapy, primary tumor resection (yes vs. no), primary tumor localization (colon vs. rectum), primary tumor lymph node (N0 vs. N+), timing of metastasis assessment (synchronous vs. metachronous), neoadjuvant chemotherapy (>6 cycles vs. ≤6 cycles), total number of nodules, and type of resection. CEA was not included due to missing data. The choice of such variables was based on the results of the univariate analysis and/or on the known influence of specific factors on the selection of the intervention type. A 1:1 balance ratio was used for propensity score matching, based on the nearest matching PS method. After the matching process, both groups were compared regarding their initial characteristics in order to re-evaluate the comparability of both groups. Finally, matched groups could be compared regarding the different variables of interest in the study.
Analyses were performed using the 3.2.0 version R software (R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Results
Considering out data, a total of 3036 hepatectomies were performed for CRLMs. Among them, a total of 2086 hepatectomies (851 NON-PSH and 1235 PSH) were single-stage hepatectomies. Re-hepatectomies were excluded from the study. There were 691 hepatectomies for more than 3 bilobar nodules. In this population, there were 360 NON-PSH (52%) and 331 PSH (48%) with a median age of 61 years.
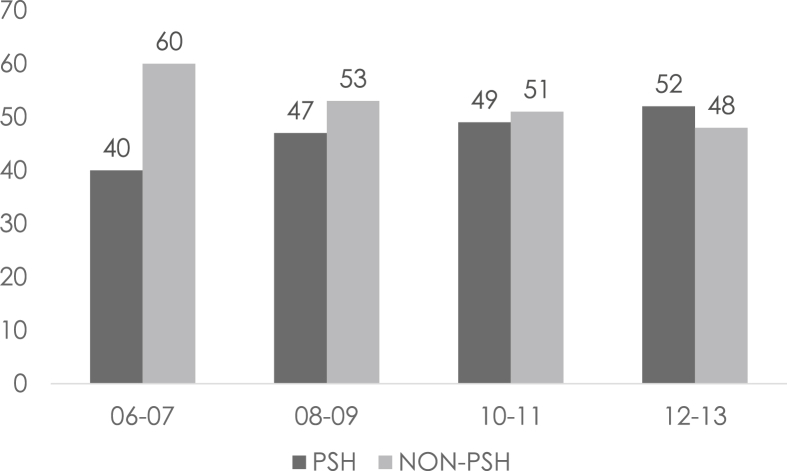
Considering the period analyzed in our study (2006–2013), we divided the timeframe into four periods (2006–2007, 2008–2009, 2010–2011, and 2012–2013) in order to evaluate if the number of patients and the kind of surgical approaches vary along the years. We determined a constant augmentation in surgical procedures, namely 157, 160, 176, and 198 respectively for each period. This regular augmentation is associated with an increase in the parenchymal-sparing approach with 67 (40%), 76 (47%), 87 (49%), and 104 (52%) respectively as shown in Fig. 1.
Figure 1.
Distribution over the years
Comparison of PSH vs. NON-PSH hepatectomies
No difference was demonstrated in demographic data between the two populations (Table 1, Table 2). In terms of primary tumors, no difference was demonstrated for primary tumor location, resection, and lymph node status. Both groups had similar rates of synchronous metastasis as well as the presence of extra-hepatic disease. Concerning neoadjuvant chemotherapy, more than 6 cycles were less frequent in PSH as compared to NON-PSH, 103 (31%) vs. 134 (37%) (p = 0.04) respectively. Concerning liver metastasis, there was a reduced number of lesions in PSH (4 vs. 5, p = 0.001) with a smaller median size (30 mm vs. 35 mm, p = 0.007). Fewer laparoscopic resections were performed in the PSH group (3 vs. 10%, p = 0.04). No differences were demonstrated in the surgical technique apart from less pedicle clamping in the PSH group (64 vs. 72%, p = 0.04). There was less postoperative liver failure in the PSH group (2 vs. 8%, p = 0.0001) and a shorter intensive care unit stay (0 (0–1) vs. 0 (0–21), p = 0.001).
Table 1.
Patients' preoperative and intraoperative data
| PSH (n = 331) | NON-PSH (n = 360) | P value | |
|---|---|---|---|
| Age, yr [median, (range)] | 61.7(40–81) | 61(27–82) | 0.35 |
| Gender, male, n(%) | 186(56) | 199(55) | 0.81 |
| ASA score 3–4, n(%) | 49(15) | 59(16) | 0.60 |
| Co-morbidity, yes, n(%) | 140(42) | 160(44) | 0.59 |
| Body mass index (kg/m2) [median, (range)] | 24.8(17.6–35) | 25.3(17.7–40.8) | 0.28 |
| Primary resected, yes, n(%) | 284(86) | 311(86) | 0.827 |
| Primary rectum, yes, n(%) | 80(24) | 87(24) | 1 |
| Primary nodes status positive, yes, n(%) | 97(29) | 134(37) | 0.05 |
| Liver metastasis synchronous, yes, n(%) | 149(45) | 145(40) | 0.58 |
| Liver metastasis ACE (μg/L) [median, (range)] | 28(1–3270) | 61(1–8091) | 0.29 |
| Liver metastasis neoadjuvant chemotherapy >6, yes, n(%) | 103(31) | 134(37) | 0.04 |
| Liver metastasis No. of lesion, [median, (range)] | 4(3–13) | 5(3–14) | 0.001 |
| Liver metastasis size of lesion, mm, [median, (range)] | 30(4–160) | 35(6–295) | 0.007 |
| Number of resected segments, [median, (range)] | 1(0–2) | 4(3–6) | 0.0001 |
| Liver resection + radiofrequency | 91(27) | 80(22) | 0.27 |
| Laparoscopy, yes, n(%) | 11(3) | 35(10) | 0.001 |
| Operative time, (min), [median, (range)] | 270(90–660) | 240(115–507) | 0.76 |
| Pedicle clamping, yes, n(%) | 214(65) | 259(72) | 0.04 |
| Pedicle clamping duration, min, [median, (range)] | 30(0–95) | 39(0–240) | 0.11 |
| Transfusion, yes, n(%) | 52(16) | 75(21) | 0.09 |
| Transfusion, [median, (range)] | 0(0–8) | 0(0–9) | 0.11 |
The bold values are considered statistically significant values.
Table 2.
Patients' intraoperative and postoperative data
| PSH (n = 331) | NON-PSH (n = 360) | P Value | |
|---|---|---|---|
| Morbidity≥1 | 90(27) | 117(32) | 0.13 |
| Morbidity > Dindo IIIA, n(%) | 42(13) | 74(21) | 0.07 |
| Non-surgical morbidity, n(%) | 55(16) | 57(15) | 0.83 |
| Pulmonary | 22(7) | 32(9) | 0.32 |
| Cardiac | 4(1) | 5(1) | 1 |
| Sepsis | 12(4) | 5(1) | 0.08 |
| Vascular | 11(3) | 5(1) | 0.12 |
| Acute renal failure | 1(0) | 2(1) | 1 |
| Surgical morbidity, n(%) | 59(18) | 81(23) | 0.13 |
| Deep collection | 48(15) | 72(20) | 0.20 |
| Wound infection | 7(2) | 5(1) | 0.56 |
| Liver failure | 5(2) | 28(8) | 0.0001 |
| Biliary fistula | 11(3) | 19(5) | 0.26 |
| Reoperation, n(%) | 17(5) | 16(4) | 0.72 |
| Mortality (90 days), n(%) | 2(1) | 1(1) | 0.68 |
| Intensive care unit stay, days, [median, (range)] | 0(0–1) | 0(0–21) | 0.002 |
| Total hospitalization, days, [median, (range)] | 11(3–53) | 11(6–47) | 0.922 |
| R1 liver metastasis resection, n(%) | 101(31) | 108(30) | 0.86 |
| Adjuvant chemotherapy | 211(64) | 179(50) | 0.001 |
| Recurrence, n(%) | 185(56) | 198(55) | 0.81 |
| Liver-only recurrence, n(%) | 81(24) | 74(21) | 0.23 |
| Liver recurrence treatment | |||
| Re-hepatectomy, n(%) | 40(12) | 32(9) | 0.56 |
| Radiofrequency, n(%) | 22(7) | 23(6) | 0.47 |
| Other, n(%) | 19(6) | 19(5) | 0.68 |
The bold values are considered statistically significant values.
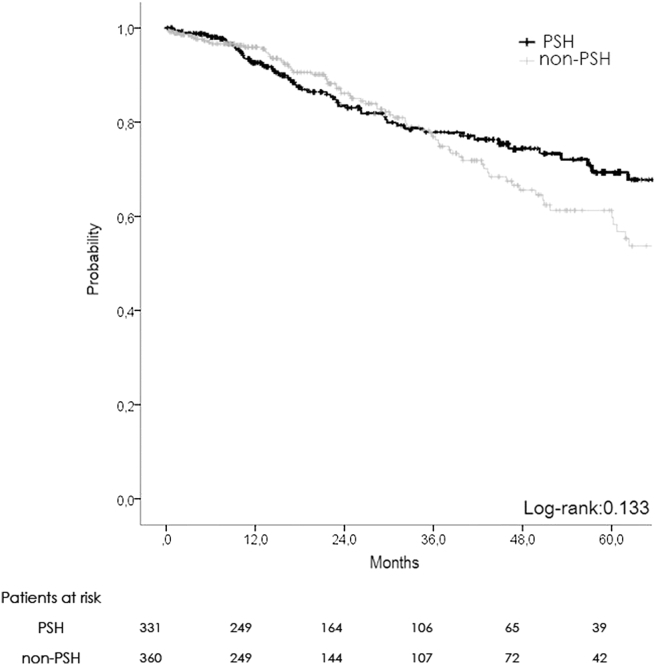
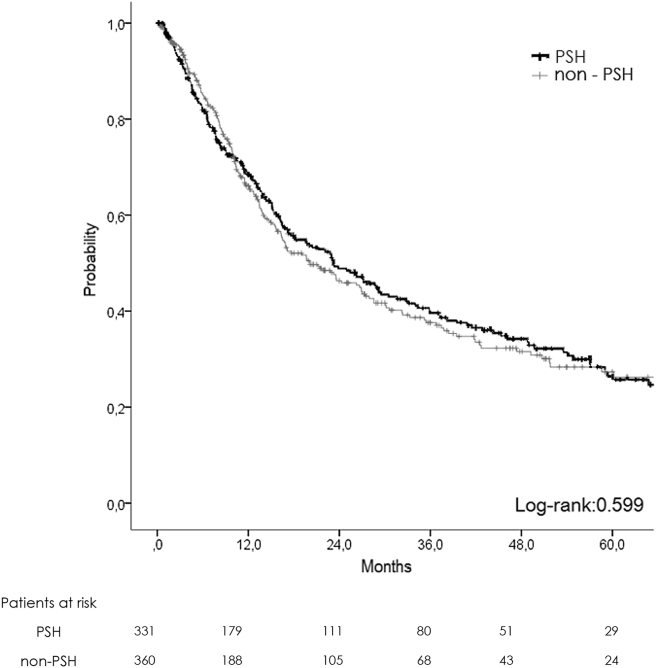
There was no difference in terms of oncological results, with 1-, 3-, 5-year OS at 92, 77, and 67% in PSH and 95, 74, and 59% in NON-PSH respectively (Fig. 2). DFS was similar with 1-, 3-, 5-year survival at 68, 39, and 25% in PSH vs. 63, 37, and 26% in NON-PSH (Fig. 3).
Figure 2.
Overall survival plot (non-matched)
Figure 3.
Disease-free survival plot (non-matched)
Comparison of PSH vs NON-PSH after PSM
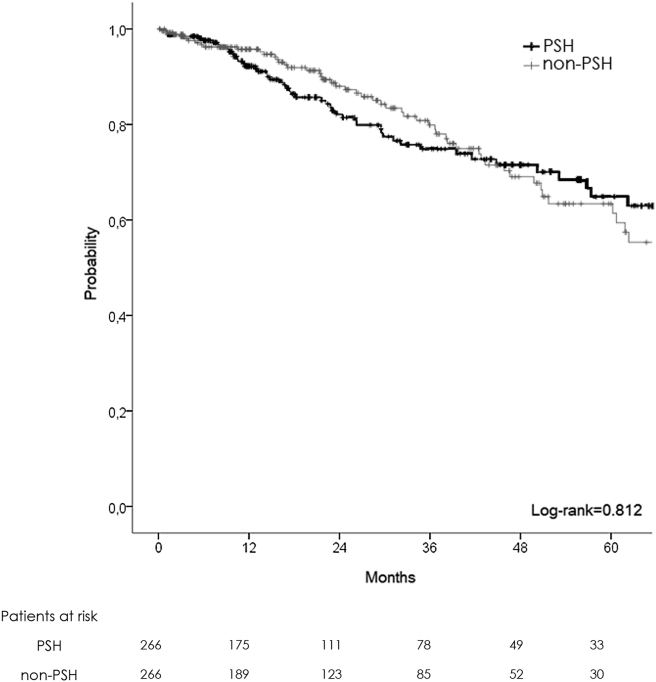
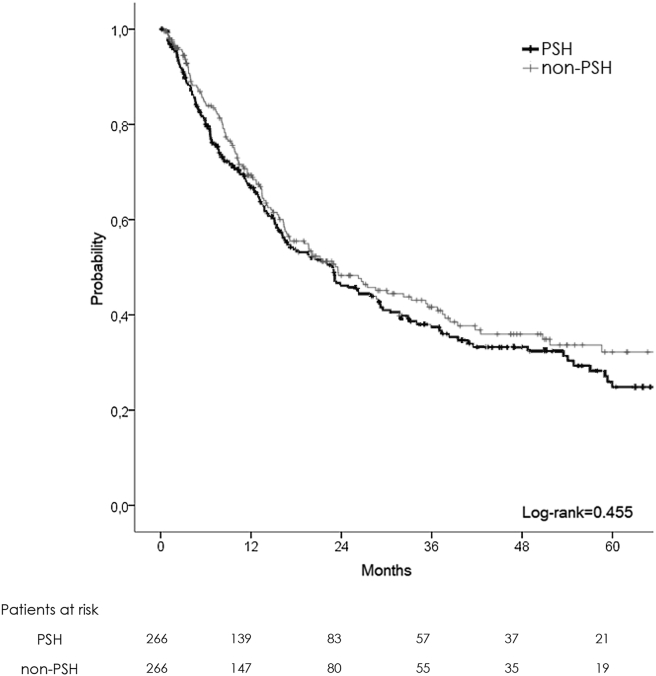
After 1:1 PSM of the two populations, two homogeneous groups were obtained (PSH: 266, NON-PSH: 266) (Table 3, Table 4). No difference was demonstrated in demographical and intraoperative data, except for an augmented number of laparoscopic hepatectomies in the NON-PSH group (12 vs. 4%, p = 0.001). PSH was associated with a lower morbidity rate (25 vs. 34%, p = 0.04) and fewer complications graded Dindo-Clavien III and IV (10 vs. 16%, p = 0.03). Liver failure was less present in PSH (2% vs. 7%, p = 0.006), with a shorter intensive care unit stay (0 day vs. 1 day, p = 0.004). No differences were demonstrated for oncological results, with 1-, 3-, 5-year OS at 92, 74, and 64% in PSH and 95, 79, and 63 in NON-PSH respectively (Fig. 4). DFS was similar with 1-, 3-, 5-year survival at 66, 37 and 24% in PSH vs. 68, 40 and 32% in NON-PSH respectively (Fig. 5). More adjuvant therapy was present in the PSH group (66 vs. 50%, p = 0.004).
Table 3.
PSM patients' preoperative and intraoperative data
| PSH (n = 266) | NON-PSH (n = 266) | P Value | |
|---|---|---|---|
| Age, yr [median, (range)] | 62.4(40–80) | 61(29–82) | 0.98 |
| Gender, male, n(%) | 146(55) | 145(55) | 1 |
| ASA score 3–4, n(%) | 45(17) | 46(17) | 1 |
| Co-morbidity, yes, n(%) | 125(47) | 131(49) | 0.68 |
| Body mass index (kg/m2) [median, (range)] | 25(17–33) | 25(17–36) | 0.20 |
| Primary resected, yes, n(%) | 255(96) | 256(99) | 1 |
| Primary rectum, yes, n(%) | 72(27) | 77(29) | 0.70 |
| Primary nodes status positive, yes, n(%) | 120(45) | 125(47) | 0.77 |
| Liver metastasis synchronous, yes, n(%) | 134(50) | 127(48) | 0.62 |
| Liver metastasis ACE (μg/L) [median, (range)] | 29(1–3727) | 61(1–8091) | 0.39 |
| Liver metastasis neoadjuvant chemotherapy >6, yes, n(%) | 92(35) | 84(32) | 0.51 |
| Liver metastasis number of lesion, [median, (range)] | 4(3–9) | 4(3–15) | 0.53 |
| Liver metastasis size of lesion, mm, [median, (range)] | 35(6–110) | 33(6–200) | 0.15 |
| Number of resected segments, [median, (range)] | 1(0–2) | 4(3–6) | 0.0001 |
| Liver resection + radiofrequency | 71(27) | 59(22) | 0.27 |
| Laparoscopy, yes, n(%) | 11(4) | 32(12) | 0.001 |
| Operative time, (min), [median, (range)] | 250(120–660) | 240(120–420) | 0.96 |
| Pedicle clamping, yes, n(%) | 139(73) | 210(79) | 0.16 |
| Pedicle clamping duration, min, [median, (range)] | 30(2–95) | 38(2–240) | 0.39 |
| Transfusion, yes, n(%) | 48(18) | 61(23) | 0.20 |
| Transfusion, [median, (range)] | 0(0–8) | 0(0–7) | 0.37 |
Table 4.
PSM patients' intraoperative and postoperative data
| PSH (n = 266) | NON-PSH (n = 266) | P Value | |
|---|---|---|---|
| Morbidity≥1 | 68(25) | 91(34) | 0.04 |
| Morbidity >Dindo IIIA, n(%) | 27(10) | 45(16) | 0.03 |
| Non-surgical morbidity, n(%) | 37(13) | 42(15) | 0.62 |
| Pulmonary | 26(10) | 37(13) | 0.17 |
| Cardiac | 4(2) | 5(2) | 1 |
| Sepsis | 9(3) | 3(1) | 0.11 |
| Vascular | 9(3) | 4(2) | 0.26 |
| Acute renal failure | 0 | 2(1) | 0.49 |
| Surgical morbidity, n(%) | 49(18) | 59(22) | 0.33 |
| Deep collection | 47(17) | 57(21) | 0.33 |
| Wound infection | 7(3) | 3(1) | 0.34 |
| Liver failure | 5(2) | 19(7) | 0.006 |
| Biliary fistula | 10(4) | 17(6) | 0.23 |
| Reoperation, n(%) | 16(6) | 13(5) | 0.74 |
| Mortality (90 days), n(%) | 2(1) | 3(1) | 1 |
| Intensive care unit stay, days, [median, (range)] | 0(0–1) | 0(0–8) | 0.004 |
| Total hospitalization, days, [median, (range)] | 11(7–36) | 11(7–47) | 0.85 |
| R1 liver metastasis resection, n(%) | 85(32) | 75(28) | 0.57 |
| Adjuvant chemotherapy | 175(66) | 33(50) | 0.001 |
| Recurrence, n(%) | 166(62) | 160(60) | 0.68 |
| Liver-only recurrence, n(%) | 79(30) | 59(22) | 0.06 |
| Liver recurrence treatment | |||
| Re-hepatectomy, n(%) | 25(22) | 17(29) | 0.86 |
| Radiofrequency, n(%) | 7(9) | 3(5) | 0.51 |
| Other, n(%) | 47(59) | 39(66) | 0.74 |
The bold values are considered statistically significant values.
Figure 4.
Overall survival plot (matched patient)
Figure 5.
Disease-free survival plot (matched patient)
Subanalysis in patients with liver recurrence only
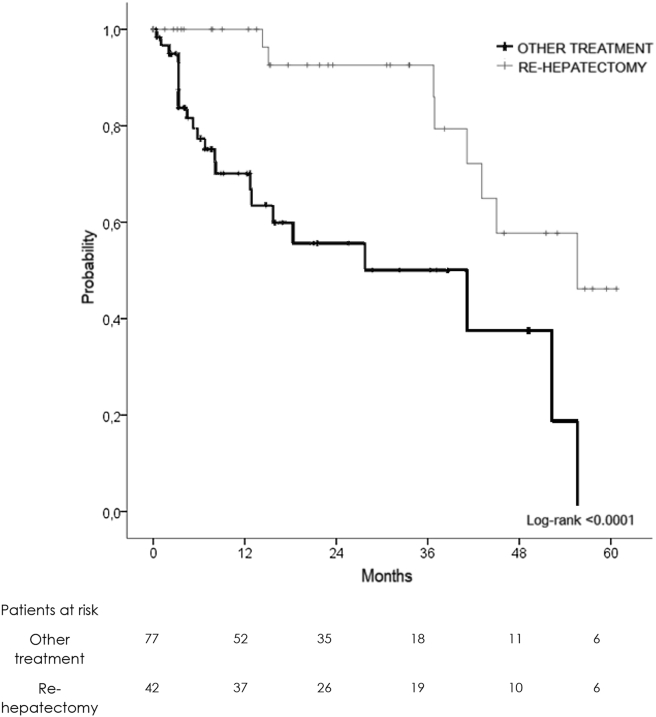
Among PSM patients, there was no difference in terms of liver-only recurrence among the groups and their treatment. Repeat hepatectomy, when it was feasible, obtained the best 3-year OS (94 versus 62%) as compared to other treatments (Fig. 6).
Figure 6.
Overall survival curve of patients underwent recurrence treatment
Prognostic factors for DFS and OS
Multivariate analysis demonstrated that primary tumor nodes positive after resection (RR = 1.47, p = 0.03), R1 liver resection (RR = 1.68, p = 0.0007) and more than 6 cycles of neoadjuvant chemotherapy (RR = 1.93, p = 0.007) had the worst significant impact on recurrence in this study population. No data were found to have an impact on overall survival.
Discussion
To our knowledge, this study represents the first PSM-based comparison for patients with bilobar multiple (>3) lesions. This study demonstrated benefits in terms of morbidity and liver failure related to PSH as compared to NON-PSH, without changing oncological outcomes.
Initially, the PSH approach for liver malignancies was considered less efficient due to the possibility to have reduced resection margin with an augmented rate of local recurrence, due to the major amount of unresected liver parenchyma. This theory, initially applied for primary liver tumor and extended to all liver malignancies, was recently called into question due to the increasing number of papers who demonstrate that the most important goal was to obtain an R0 resection, without impact of the width of resection margin. This new conception has been diffused in recent era as demonstrated in our multicentric series in last 7 years (Fig. 1), prioritizing the importance of the FRL, compared to the necessity to obtain a wide resection margin. This attitude could be justified also with the necessity to spare the majority of parenchyma and especially hepatic vein, in order to allow rehepatectomies, who actually represent the best treatment for this high recurrence rates pathology.
Starting from all this consideration, the management of colorectal liver metastasis has significantly changed over recent years7 and integrated with the evolution in oncosurgical strategy. Initially contraindicated for surgery, bilateral, synchronous and extra-hepatic lesions are now eligible for surgery due to the evolution of chemotherapy and patient management options. Currently, even initially non-resectable bilobar lesions could be eligible for an aggressive combined onco-surgical management achieving an acceptable 5-year OS of 36% as demonstrated by Faitot et al.14 As previously mentioned, margins have always9 represented a matter of debate, and historically parenchymal-sparing approaches were considered less effective due to the theoretical impossibility to achieve satisfying resection margins. Based on this idea, many authors have preferred to use a NON-PSH strategy to have wider margins to leave less “at risk liver” in place.9, 15, 16 Based on this idea, a two-stage hepatectomy has largely gained consensus, achieving a satisfying 30–35% 5-year OS in these patients.17, 18, 19, 20, 21, 22 This strategy has some NON-PSH limitations due to the high risk of dropouts between the first and second stage19, 20 (30% of cases) due to tumor progression, re-discussing the role of resection margins as compared to a high dropout risk. In a recent study including 3000 CRLMs, Hamady et al.23 demonstrated that a 1 mm margin was sufficient to be considered curative and more extended margins did not lead to oncological benefits in terms of recurrence. This demonstrates that margins did not represent a priority in bilobar liver metastasis resection. Based on this concept, even more PSHs for CRLMs were performed in recent years.24, 25, 26 This change of attitude, based on the use of multiple simultaneous PSH hepatic resections associated with radiofrequency of deep lesions, is also related to a lower postoperative mortality, preventing the resection of uninvolved parenchyma.7, 27, 28 Kokudo et al.6 demonstrated that NON-PSH hepatectomies did not decrease the risk of intra-hepatic recurrence and in case of non-anatomical liver resections for CRLM, the risk of ipsilateral recurrence was only 20%.
This change of approach has been initially described by Gold et al.7 who demonstrated that simultaneous liver resections (wedge resections) of bilateral liver resection are justified with no negative impact on oncological outcome. Similar results were recently evidenced by Mise et al.9 who demonstrated that, in case of single CRLM of 3 cm, the parenchymal-sparing technique improved survival in case of liver recurrence, allowing for salvage re-hepatectomy as compared to a NON-PSH approach.
To our knowledge, there was no report in the literature establishing a comparison between NON-PSH and PSH for multiple bilobar resections for more than 3 lesions which analyzed short-term and long-term results. We chose to exclude re-hepatectomy and two-staged hepatectomy to obtain a population who could theoretically undergo both treatments. A propensity score analysis was performed to reduce the differences between the two populations to a minimum. One of the main goals of our study was to verify if one of the two strategies had an impact on disease-free and overall survival. We verified that no differences were present in terms of oncological results. Similar rates of R1 resection were achieved in both groups (32 vs. 28%, p = 0.57), demonstrating the non-inferiority of the PSH resection group. This equality has no impact on the recurrence rate which seems comparable, with 62 vs. 60% in PSH vs. NON-PSH respectively. The only difference concerned adjuvant chemotherapy, which was more present in the PSH group, (66 vs. 50%, 0.001), probably due to the idea of treating micro-metastasis, which could be considered more present in a larger quantity of parenchyma.
Laparoscopic liver resection, even more frequent for benign29 and malign30 lesion, remains adapted only in selected CLM cases. Unsurprisingly, it still remains limited to 9% of the entire population in our study, confirming the necessity to be performed by expert surgeons in high-volume centers. In our series, most laparoscopic hepatectomies were performed in the NON-PSH group, probably due to the fact that major laparoscopic liver resections are still practiced in a reduced number of expert centers. In high-volume centers, laparoscopic NON-PSH could be performed without impacting postoperative morbidity, as demonstrated by Allard et al.31 Other reasons concern technical difficulties due to tumor location and to the necessity to associate multiple resections with laparoscopic radiofrequency ablation, with a higher risk of uncomplete treatment.
Regarding postoperative morbidity and mortality, important results demonstrated a lower rate of complications in patients in the PSH group (25 vs. 34%; p = 0.03) with a lower rate of Dindo-Clavien III and IV (10 vs. 16%, p = 0.04). Even if these results did not impact length of stay and postoperative mortality in our study, reports in the literature have demonstrated how postoperative complications were related to recurrence-free survival32 (with a reduction of survival (69 vs. 23 months, p < 0.001) and overall survival (74 vs. 28 months, p < 0.001) in patients with postoperative complications. The mechanism which explains how morbidity impacts long-term results is still unclear, even if it has been demonstrated that infectious complications promote metastatic diffusion33, 34 in animal models.
Liver failure was a reduced event in the PSH group (2 vs. 7%, p = 0.006), in relation to the future remnant liver due to a parenchymal-sparing approach. As known, it remains the most serious liver-specific complication still reported in up to 16% of patients with CRLM in high-volume centers,35 with a postoperative mortality rate of 5%. The main causes of liver failure remain chemotherapy-induced liver injury36 and a small future remnant liver.37, 38, 39, 40 Considering progress made in chemotherapy, the reduction in the number of chemotherapy cycles could well represent one of the solutions to reduce liver failure in the future. On the other hand, a parenchymal-sparing approach seems to be the best solution to preserve a sufficient quantity of functional parenchyma, to reduce postoperative risks of liver failure to a minimum as demonstrated by Narita.35 One factor which has influenced the augmented use of parenchymal-sparing hepatectomies is the use of an ablative technique for CRLM. Even if it is identified as one of the predicting factors of early local recurrence,41, 42 in a recent multicentric study from Evrard et al.43 among 288 patients treated with combined ablation and resection, only 49 (17%) had local recurrence, demonstrating that combined strategies could be considered effective and safe in selected cases.
All these encouraging data consolidate the tendency over the last few years to stay away from large resections and switch to PSH. These data are confirmed by our studies, with a reduction in NON-PSH from 40 to 52% from 2006 to 2013. This approach was anticipated by Gold et al. who demonstrated a significant reduction from 92 to 72% associated with a reduction of resected segments for bilateral liver metastases in a relatively old period comprised between 1992 and 2003. New data on this subject have been introduced by Kingham et al.32 who showed a reduction from 65.5 to 35.8% over the past 19 years and by Mise et al.9 who showed a reduction from 60 to 22% of NON-PSH resections in case of single 3 cm lesions.
Recurrence still represents the Achilles' heel of CRLM surgical treatment. As recently described in a meta-analysis,44 it arises up to 75% at 18 months in resected patients. However, the role of adjuvant chemotherapy is still debated and controversial, even if chemotherapy should be recommended44 after metastasectomy. Adjuvant chemotherapy was performed in 66% of patients treated with PSH resections, 15% more as compared to the NON-PSH group. This is probably due to the augmented postoperative morbidity of the NON-PSH group, which could have a negative impact on the performance status of patients who could be less adapted to receive adjuvant chemotherapy.
As confirmed in our series, recurrence was present in 61% of patients, with 26% of exclusive intrahepatic recurrence. No difference was demonstrated between NON-PSH and PSH in terms of disease-free and overall survival after recurrence. Mise et al.9 demonstrated that a PSH had a better overall survival after intra-hepatic recurrence due to the possibility of sparing a NON-PSH amount of hepatic parenchyma, allowing for repeat hepatectomy, which is the treatment guaranteeing the best overall survival after recurrence although no difference was demonstrated in our series between NON-PSH and PSH in terms of OS and DFS after recurrence. Repeat hepatectomy was the best treatment for exclusive intra-hepatic recurrence of CRLM, with a 3-year OS of 82% as compared to 51% for all other treatments.
The main limitation of this study is its retrospective and multicentric characteristics which increase the disparities of chosen strategies among surgeons. Theoretically, not all patients could benefit of PSH due to the localization and size of the lesions. We tried to reduce this bias to a minimum with the application of a propensity score, in order to reduce differences among groups.
In conclusion, PSH resection for bilobar multiple CRLMs represents a valid alternative to NON-PSH resection in selected patients associated with a reduced morbidity and comparable oncological results. A randomized studies who compare PSH and non-PSH strategies could consolidate our theory, even if the increasing use of PSH in most center could represent a limit in the feasibility of the study, reserving the non-PSH approach to cases who couldn't benefit of PSH.
Contributors
Amiens, CHU Amiens Picardie: Cyril Cosse, Delphine Lignier, Jean Marc Regimbeau; Angers, CHU Angers: Julien Barbieux, Emilie Lermite, Antoine Hamy; Beauvais, CH Beauvais: François Mauvais; Bordeaux, Groupe Hospitalier Saint André: Christophe Laurent; Chambéry, CH Chambéry: Irchid Al Naasan; Créteil, CHU Henri Mondor: Alexis Laurent, Philippe Compagnon; Eaubonne, Hôpital Simone Veil: Mohammed Sbai Idrissi; Epinal, Polyclinique de la Ligne Bleue: Frédéric Martin; Gap, CH des Alpes du Sud: Jérôme Atger; Lyon, Hôpital de la Croix Rousse: Jacques Baulieux, Benjamin Darnis, Jean Yves Mabrut; Lyon, Hôpital Edouard Herriot: Vahan Kepenekian, Julie Perinel, Mustapha Adham; Lyon, CH Lyon Sud: Olivier Glehen; Lyon, Centre Léon Bérard: Michel Rivoire; Marseille, Hôpital de la Conception: Jean Hardwigsen, Anaïs Palen, Yves Patrice Le Treut; Marseille, Institut Paoli-Calmettes: Jean Robert Delpero, Olivier Turrini; Montpellier, Hôpital Saint Eloi: Astrid Herrero, Fabrizio Panaro; Nancy, CHU Brabois: Laurent Bresler; Nancy, Institut de Cancérologie de Lorraine Alexis-Vautrin: Philippe Rauch, François Guillemin, Frédéric Marchal; Nice, Hôpital de l’Archet: Jean Gugenheim, Antonio Iannelli; Kremlin-Bicêtre, CHU Kremlin-Bicêtre: Stéphane Benoist, Antoine Brouquet; Paris, Hôpital Lariboisière: Marc Pocard, Rea Lo Dico; Paris, Institut Mutualiste Montsouris: David Fuks; Paris, Hôpital Saint Antoine: Olivier Scatton, Olivier Soubrane; Paris, Hôpital de la Pitié Salpétrière: Jean-Christophe Vaillant; Reims, Hôpital Robert Debré: Tullio Piardi, Daniel Sommacale, Reza Kianmanesh; La Roche-sur-Yon, Centre Départemental de Vendée: Michel Comy; Strasbourg, Hôpital de Hautepierre: Philippe Bachellier, Elie Oussoultzoglou, Pietro Addeo; Strasbourg, Nouvel Hôpital Civil: Dimitrios Ntourakis, Didier Mutter, Jacques Marescaux; Toulouse, Hôpital Rangueil: Loïc Raoux, Bertrand Suc, Fabrice Muscari; Troyes, Hôpital des Hauts-Clos: Georges ELHOMSY; Villejuif, Hôpital Paul Brousse: Maximiliano Gelli, Denis Castaing, Daniel Cherqui; Gabriella PIttau, Oriana Ciacio, Eric Vibert; Villejuif, Gustave Roussy: Dominique Elias, Fabrizio Vittadello.
Authors would also like to thank Christopher Burel and Guy Temporal, professional medical proofreaders, for their assistance in revising the manuscript.
Conflicts of interest
None declared.
Acknowledgments
The authors would like to thank all participating centers for their contribution to this study.
Contributor Information
Patrick Pessaux, Email: patrick.pessaux@chru-strasbourg.fr.
French Colorectal Liver Metastases Working Group, Association Française de Chirurgie (AFC):
Cyril Cosse, Delphine Lignier, Jean Marc Regimbeau, Julien Barbieux, Emilie Lermite, Antoine Hamy, François Mauvais, Christophe Laurent, Irchid Al Naasan, Alexis Laurent, Philippe Compagnon, Mohammed Sbai Idrissi, Frédéric Martin, Jérôme Atger, Jacques Baulieux, Benjamin Darnis, Jean Yves Mabrut, Vahan Kepenekian, Julie Perinel, Mustapha Adham, Olivier Glehen, Michel Rivoire, Jean Hardwigsen, Anaïs Palen, Yves Patrice Le Treut, Jean Robert Delpero, Olivier Turrini, Astrid Herrero, Fabrizio Panaro, Laurent Bresler, Philippe Rauch, François Guillemin, Frédéric Marchal, Jean Gugenheim, Antonio Iannelli, Stéphane Benoist, Antoine Brouquet, Marc Pocard, Rea Lo Dico, David Fuks, Olivier Scatton, Olivier Soubrane, Jean-Christophe Vaillant, Tullio Piardi, Daniel Sommacale, Reza Kianmanesh, Michel Comy, Philippe Bachellier, Elie Oussoultzoglou, Pietro Addeo, Dimitrios Ntourakis, Didier Mutter, Jacques Marescaux, Loïc Raoux, Bertrand Suc, Fabrice Muscari, Georges Elhomsy, Maximiliano Gelli, Denis Castaing, Daniel Cherqui, Gabriella PIttau, Oriana Ciacio, Eric Vibert, Dominique Elias, and Fabrizio Vittadello
References
- 1.Stewart G.D., O'Súilleabháin C.B., Madhavan K.K., Wigmore S.J., Parks R.W., Garden O.J. The extent of resection influences outcome following hepatectomy for colorectal liver metastases. Eur J Surg Oncol. 2004;30:370–376. doi: 10.1016/j.ejso.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 2.von Heesen M., Schuld J., Sperling J., Grünhage F., Lammert F., Richter S. Parenchyma-preserving hepatic resection for colorectal liver metastases. Langenbeck's Arch Surg/Dtsch Gesellschaft für Chir. 2012 Mar;397:383–395. doi: 10.1007/s00423-011-0872-x. http://www.ncbi.nlm.nih.gov/pubmed/22089696 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 3.Karanjia N.D., Lordan J.T., Quiney N., Fawcett W.J., Worthington T.R., Remington J. A comparison of right and extended right hepatectomy with all other hepatic resections for colorectal liver metastases: a ten-year study. Eur J Surg Oncol. 2009 Jan;35:65–70. doi: 10.1016/j.ejso.2007.12.002. http://www.ncbi.nlm.nih.gov/pubmed/18222623 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K., Shimada H., Matsumoto C., Matsuo K., Takeda K., Nagano Y. Impact of the degree of liver resection on survival for patients with multiple liver metastases from colorectal cancer. World J Surg. 2008 Sep;32:2057–2069. doi: 10.1007/s00268-008-9610-0. http://www.ncbi.nlm.nih.gov/pubmed/18454272 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 5.Adams R.B., Aloia T.A., Loyer E., Pawlik T.M., Taouli B., Vauthey J.-N. Selection for hepatic resection of colorectal liver metastases: expert consensus statement. HPB. 2013 Feb;15:91–103. doi: 10.1111/j.1477-2574.2012.00557.x. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3719914&tool=pmcentrez&rendertype=abstract [Internet]. Available from: [cited 2016 Feb 27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokudo N., Tada K., Seki M., Ohta H., Azekura K., Ueno M. Anatomical major resection versus nonanatomical limited resection for liver metastases from colorectal carcinoma. Am J Surg. 2001 Feb;181:153–159. doi: 10.1016/s0002-9610(00)00560-2. http://www.ncbi.nlm.nih.gov/pubmed/11425058 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 7.Gold J.S., Are C., Kornprat P., Jarnagin W.R., Gönen M., Fong Y. Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome. Ann Surg. 2008;247:109–117. doi: 10.1097/SLA.0b013e3181557e47. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00000658-200801000-00017 [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 8.Conrad C., Ogiso S., Inoue Y., Shivathirthan N., Gayet B. Laparoscopic parenchymal-sparing liver resection of lesions in the central segments: feasible, safe, and effective. Surg Endosc – Springer US. 2015;29:2410–2417. doi: 10.1007/s00464-014-3924-9. http://link.springer.com/10.1007/s00464-014-3924-9 [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 9.Mise Y., Aloia T.A., Brudvik K.W., Schwarz L., Vauthey J.N., Conrad C. Parenchymal-sparing hepatectomy in colorectal liver metastasis improves salvageability and survival. Ann Surg. 2016 Jan;263:146–152. doi: 10.1097/SLA.0000000000001194. [DOI] [PubMed] [Google Scholar]
- 10.Taylor A., Langeberg W., Mowat F., Alexander D., Choti M., Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012 Nov;4:283. doi: 10.2147/CLEP.S34285. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3496330&tool=pmcentrez&rendertype=abstract [Internet]. Available from: [cited 2016 Jan 10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang X., Hershman D.L., Abrams J.A., Feingold D., Grann V.R., Jacobson J.S. Predictors of survival after hepatic resection among patients with colorectal liver metastasis. Br J Cancer. 2007 Dec 17;97:1606–1612. doi: 10.1038/sj.bjc.6604093. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2360280&tool=pmcentrez&rendertype=abstract [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strasberg S.M., Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg. 2012 Oct 10;257:377–382. doi: 10.1097/SLA.0b013e31825a01f6. http://www.ncbi.nlm.nih.gov/pubmed/22895397 [Internet]. Available from: [cited 2013 Feb 6] [DOI] [PubMed] [Google Scholar]
- 13.Dindo D., Demartines N., Clavien P.-A. Classification of surgical complications. Ann Surg. 2004 Aug;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00000658-200408000-00003 [Internet]. Available from: [cited 2013 Jan 28] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faitot F., Faron M., Adam R., Elias D., Cimino M., Cherqui D. Two-stage hepatectomy versus 1-stage resection combined with radiofrequency for bilobar colorectal metastases. Ann Surg. 2014 Nov;260:822–828. doi: 10.1097/SLA.0000000000000976. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00000658-201411000-00015 [Internet]. Available from: [cited 2014 Oct 8] [DOI] [PubMed] [Google Scholar]
- 15.DeMatteo R.P., Palese C., Jarnagin W.R., Sun R.L., Blumgart L.H., Fong Y. Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg. Jan 2000;4:178–184. doi: 10.1016/s1091-255x(00)80054-2. http://www.ncbi.nlm.nih.gov/pubmed/10675241 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 16.Hughes K.S., Simon R., Songhorabodi S., Adson M.A., Ilstrup D.M., Fortner J.G. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986 Aug;100:278–284. http://www.ncbi.nlm.nih.gov/pubmed/3526605 [Internet]. Available from: [cited 2016 Mar 12] [PubMed] [Google Scholar]
- 17.Zorzi D., Mullen J.T., Abdalla E.K., Pawlik T.M., Andres A., Muratore A. Comparison between hepatic wedge resection and anatomic resection for colorectal liver metastases. J Gastrointest Surg. 2006 Jan;10:86–94. doi: 10.1016/j.gassur.2005.07.022. http://www.ncbi.nlm.nih.gov/pubmed/16368496 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 18.Chun Y.S., Vauthey J.-N., Ribero D., Donadon M., Mullen J.T., Eng C. Systemic chemotherapy and two-stage hepatectomy for extensive bilateral colorectal liver metastases: perioperative safety and survival. J Gastrointest Surg. 2007 Nov;11:1498–1504. doi: 10.1007/s11605-007-0272-2. http://www.ncbi.nlm.nih.gov/pubmed/17849166 discussion 1504–5. [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 19.Tsai S., Marques H.P., de Jong M.C., Mira P., Ribeiro V., Choti M.A. Two-stage strategy for patients with extensive bilateral colorectal liver metastases. HPB. 2010 May;12:262–269. doi: 10.1111/j.1477-2574.2010.00161.x. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2873649&tool=pmcentrez&rendertype=abstract [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homayounfar K., Liersch T., Schuetze G., Niessner M., Goralczyk A., Meller J. Two-stage hepatectomy (R0) with portal vein ligation–towards curing patients with extended bilobular colorectal liver metastases. Int J Colorectal Dis. 2009 Apr;24:409–418. doi: 10.1007/s00384-008-0620-z. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2829132&tool=pmcentrez&rendertype=abstract [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pamecha V., Nedjat-Shokouhi B., Gurusamy K., Glantzounis G.K., Sharma D., Davidson B.R. Prospective evaluation of two-stage hepatectomy combined with selective portal vein embolisation and systemic chemotherapy for patients with unresectable bilobar colorectal liver metastases. Dig Surg. 2008 Jan;25:387–393. doi: 10.1159/000176063. http://www.ncbi.nlm.nih.gov/pubmed/19033722 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 22.Wicherts D.A., Miller R., de Haas R.J., Bitsakou G., Vibert E., Veilhan L.-A. Long-term results of two-stage hepatectomy for irresectable colorectal cancer liver metastases. Ann Surg. 2008 Dec;248:994–1005. doi: 10.1097/SLA.0b013e3181907fd9. http://www.ncbi.nlm.nih.gov/pubmed/19092344 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 23.Hamady Z.Z.R., Lodge J.P.A., Welsh F.K., Toogood G.J., White A., John T. One-millimeter cancer-free margin is curative for colorectal liver metastases. Ann Surg. 2014 Mar;259:543–548. doi: 10.1097/SLA.0b013e3182902b6e. http://www.ncbi.nlm.nih.gov/pubmed/23732261 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 24.Torzilli G. Advances in the surgical treatment of colorectal cancer liver metastases through ultrasound. Surg Today. 2011 Sep;41:1184–1189. doi: 10.1007/s00595-010-4527-2. http://www.ncbi.nlm.nih.gov/pubmed/21874412 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 25.Torzilli G., Procopio F., Botea F., Marconi M., Del Fabbro D., Donadon M. One-stage ultrasonographically guided hepatectomy for multiple bilobar colorectal metastases: a feasible and effective alternative to the 2-stage approach. Surgery. 2009 Jul;146:60–71. doi: 10.1016/j.surg.2009.02.017. http://www.ncbi.nlm.nih.gov/pubmed/19541011 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 26.Elias D., Goéré D., Leroux G., Dromain C., Leboulleux S., de Baere T. Combined liver surgery and RFA for patients with gastroenteropancreatic endocrine tumors presenting with more than 15 metastases to the liver. Eur J Surg Oncol. 2009 Oct;35:1092–1097. doi: 10.1016/j.ejso.2009.02.017. http://www.ncbi.nlm.nih.gov/pubmed/19464140 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 27.Fan S.T., Lo C.M., Liu C.L., Lam C.M., Yuen W.K., Yeung C. Hepatectomy for hepatocellular carcinoma: toward zero hospital deaths. Ann Surg. 1999 Mar;229:322–330. doi: 10.1097/00000658-199903000-00004. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1191696&tool=pmcentrez&rendertype=abstract [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Billingsley K.G., Jarnagin W.R., Fong Y., Blumgart L.H. Segment-oriented hepatic resection in the management of malignant neoplasms of the liver. J Am Coll Surg. 1998 Nov;187:471–481. doi: 10.1016/s1072-7515(98)00231-2. http://www.ncbi.nlm.nih.gov/pubmed/9809562 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 29.de'Angelis N., Memeo R., Calderaro J., Felli E., Salloum C., Compagnon P. Open and laparoscopic resection of hepatocellular adenoma: trends over 23 years at a specialist hepatobiliary unit. HPB. 2014 Sep;16:783–788. doi: 10.1111/hpb.12257. http://www.ncbi.nlm.nih.gov/pubmed/24852081 [Internet]. Available from: [cited 2014 Oct 17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Memeo R., de'Angelis N., Compagnon P., Salloum C., Cherqui D., Laurent A. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case-control study. World J Surg. 2014 Nov;38:2919–2926. doi: 10.1007/s00268-014-2659-z. http://www.ncbi.nlm.nih.gov/pubmed/24912628 [Internet]. Available from: [cited 2014 Oct 17] [DOI] [PubMed] [Google Scholar]
- 31.Allard M.-A., Cunha A.S., Gayet B., Adam R., Goere D., Bachellier P. Early and long-term oncological outcomes after laparoscopic resection for colorectal liver metastases. Ann Surg. 2015;262:794–802. doi: 10.1097/SLA.0000000000001475. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=fulltext&D=ovft&MODE=ovid&NEWS=N&SEARCH=“0003-4932”.is and “262”.vo and “5”.ip and “794”.pg&CSC=Y&FT_format=PDF [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 32.Kingham T.P., Correa-Gallego C., D'Angelica M.I., Gönen M., Dematteo R.P., Fong Y. Hepatic parenchymal preservation surgery: decreasing morbidity and mortality rates in 4,152 resections for malignancy. J Am Coll Surg. 2015;220:471–479. doi: 10.1016/j.jamcollsurg.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu R.Y.C., Chan C.H.F., Spicer J.D., Rousseau M.C., Giannias B., Rousseau S. LPS-induced TLR4 signaling in human colorectal cancer cells increases beta1 integrin-mediated cell adhesion and liver metastasis. Cancer Res. 2011 Mar 1;71:1989–1998. doi: 10.1158/0008-5472.CAN-10-2833. http://www.ncbi.nlm.nih.gov/pubmed/21363926 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 34.Spicer J.D., McDonald B., Cools-Lartigue J.J., Chow S.C., Giannias B., Kubes P. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res. 2012 Aug 15;72:3919–3927. doi: 10.1158/0008-5472.CAN-11-2393. http://www.ncbi.nlm.nih.gov/pubmed/22751466 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 35.Narita M., Oussoultzoglou E., Bachellier P., Jaeck D., Uemoto S. Post-hepatectomy liver failure in patients with colorectal liver metastases. Surg Today – Springer Japan. 2015;45:1218–1226. doi: 10.1007/s00595-015-1113-7. http://dx.doi.org/10.1007/s00595-015-1113-7 [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 36.Aloia T., Sebagh M., Plasse M., Karam V., Lévi F., Giacchetti S. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006 Nov 1;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. http://www.ncbi.nlm.nih.gov/pubmed/17075116 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 37.Ferrero A., Viganò L., Polastri R., Muratore A., Eminefendic H., Regge D. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007 Aug;31:1643–1651. doi: 10.1007/s00268-007-9123-2. http://www.ncbi.nlm.nih.gov/pubmed/17551779 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 38.Schindl M.J., Redhead D.N., Fearon K.C.H., Garden O.J., Wigmore S.J. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005 Feb;54:289–296. doi: 10.1136/gut.2004.046524. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1774834&tool=pmcentrez&rendertype=abstract [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yigitler C., Farges O., Kianmanesh R., Regimbeau J.-M., Abdalla E.K., Belghiti J. The small remnant liver after major liver resection: how common and how relevant? Liver Transpl. 2003 Sep;9:S18–S25. doi: 10.1053/jlts.2003.50194. http://www.ncbi.nlm.nih.gov/pubmed/12942474 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 40.Kishi Y., Abdalla E.K., Chun Y.S., Zorzi D., Madoff D.C., Wallace M.J. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009 Oct;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. http://www.ncbi.nlm.nih.gov/pubmed/19730239 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 41.Tanis E., Nordlinger B., Mauer M., Sorbye H., van Coevorden F., Gruenberger T. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur J Cancer. 2014 Mar;50:912–919. doi: 10.1016/j.ejca.2013.12.008. http://www.ncbi.nlm.nih.gov/pubmed/24411080 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 42.Viganò L., Capussotti L., Lapointe R., Barroso E., Hubert C., Giuliante F. Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol. 2014 Apr;21:1276–1286. doi: 10.1245/s10434-013-3421-8. http://www.ncbi.nlm.nih.gov/pubmed/24346766 [Internet]. Available from: [cited 2016 Mar 12] [DOI] [PubMed] [Google Scholar]
- 43.Evrard S., Poston G., Kissmeyer-Nielsen P., Diallo A., Desolneux G., Brouste V. Combined ablation and resection (CARe) as an effective parenchymal sparing treatment for extensive colorectal liver metastases. PLoS One. 2014;9:e114404. doi: 10.1371/journal.pone.0114404. http://dx.plos.org/10.1371/journal.pone.0114404 [Internet]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brandi G., De Lorenzo S., Nannini M., Curti S., Ottone M., Dall'Olio F.G. Adjuvant chemotherapy for resected colorectal cancer metastases: literature review and meta-analysis. World J Gastroenterol. 2016 Jan;22:519–533. doi: 10.3748/wjg.v22.i2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]