Abstract
Background
99mTc-mebrofenin-hepatobiliary-scintigraphy (HBS) enables measurement of future remnant liver (FRL)-function and was implemented in our preoperative routine after calculation of the cut-off value for prediction of postoperative liver failure (LF). This study evaluates our results since the implementation of HBS. Additionally, CT-volumetric methods of FRL-assessment, standardized liver volumetry and FRL/body-weight ratio (FRL-BWR), were evaluated.
Methods
163 patients who underwent major liver resection were included. Insufficient FRL-volume and/or FRL-function <2.7%/min/m2 were indications for portal vein embolization (PVE). Non-PVE patients were compared with a historical cohort (n = 55). Primary endpoints were postoperative LF and LF related mortality. Secondary endpoint was preoperative identification of patients at risk for LF using the CT-volumetric methods.
Results
29/163 patients underwent PVE; 8/29 patients because of insufficient FRL-function despite sufficient FRL-volume. According to FRL-BWR and standardized liver volumetry, 16/29 and 11/29 patients, respectively, would not have undergone PVE. LF and LF related mortality were significantly reduced compared to the historical cohort. HBS appeared superior in the identification of patients with increased surgical risk compared to the CT-volumetric methods.
Discussion
Implementation of HBS in the preoperative work-up led to a function oriented use of PVE and was associated with a significant decrease in postoperative LF and LF related mortality.
Introduction
As the management of postoperative liver failure after major liver resection is difficult and remains mainly supportive,1 more emphasis should be placed on the preoperative assessment of the future remnant liver (FRL) in order to prevent this potentially lethal complication. Traditionally, FRL assessment is performed using Computed Tomography (CT) volumetry. However, volumetric measurement of the FRL does not provide any information on the FRL-function. This may lead to an under-detection of patients at risk for postoperative liver failure, especially patients with compromised liver parenchyma as the quality of the parenchyma is usually unknown pre-operatively.2 Other mathematical methods of preoperative FRL assessment such as FRL/body-weight ratio (FRL-BWR) and standardized liver volumetry, claim to overcome this limitation through individualizing CT volumetry of FRL by calculating the minimally required FRL-volume relative to patients' weight and body surface area (BSA), respectively.3, 4
99mTc-mebrofenin hepatobiliary scintigraphy (HBS) with SPECT-CT is a quantitative method of liver functional assessment,5 which allows measurement of the FRL-function depending on quality of the liver parenchyma rather than volume alone.6 HBS can therefore be applied in both patients with compromised and non-compromised liver, using the previously calculated cut-off value of 2.7%/min/m2.7 Patients with FRL uptake function below the cut-off value have a risk of postoperative liver failure of 2.4% with negative predictive value of 97.6% and a likelihood ratio for negative test result of 0.12. The positive predictive value of HBS is 57.1% with a likelihood ratio for positive test result of 6.8. Since the identification of the cut-off value, HBS is being used at our center as standard preoperative assessment of FRL together with CT volumetry, in patients scheduled for major liver resection.
The impact of HBS on preoperative management and the postoperative outcomes has not been assessed since its implementation. The aim of this study was to evaluate the value of preoperative HBS in a subsequent series of patients eligible for major liver resection. Data were compared with FRL-BWR and standardized liver volumetry as alternative, mathematical methods of FRL assessment for identification of patients at risk for developing postoperative liver failure.
Methods
Patients
All patients who had undergone preoperative HBS and CT-volumetry followed by major liver resection (≥3 Couinaud liver segments) at the Academic Medical Center in Amsterdam, The Netherlands, from November 2006 to June 2014, were included. HBS was performed in patients with increased surgical risk, i.e. patients suspected of perihilar cholangiocarcinoma (PHC) and patients scheduled for extended hemihepatectomy or right hemihepatectomy, except when an evident surplus of FRL-volume was determined on CT imaging. A historical cohort (May 2000–November 2006) was used for the comparison of the postoperative outcomes before and after the implementation of HBS in our preoperative routine. This historical cohort consisted of 55 patients from a previous series in whom HBS wasn't part of the standard preoperative work-up but was performed for the calculation of the HBS cut-off value only.7 The study has been approved by the institutional review board, and the need for written informed consent was waived.
Volumetric measurements
Volumetric measurements were performed using CT images in portal-venous phase. Three-dimensional reconstructions of the liver were made using 5 mm thick axial slices. Portal and hepatic veins were used as landmarks for segmental division. The total liver, tumor and FRL were outlined manually. Integrated software (Mx-View 3.52, Philips Medical Systems) was used to calculate total liver volume, tumor volume and FRL volume. FRL volume is expressed as a percentage of total liver volume. The volumetric cut-off value for safe resection was set at FRL-volume of at least 25% for patients in whom healthy liver parenchyma was expected. In patients diagnosed with PHC typically associated with (post)cholestatic livers, 35% of the total liver volume was considered as minimum for resection.
For the purpose of this study, the required FRL was additionally calculated using the FRL-BWR method3 and standardized liver volumetry as proposed by Vauthey.4 According to the FRL-BWR, the minimal FRL-volume should be at least 0.5% of patient's weight.3 The standardized liver volumetry uses a validated mathematical formula in order to estimate the total liver volume (estimated total liver volume). The ratio of the FRL-volume, measured with CT-volumetry, and the estimated total liver volume represents the percentage of liver tissue that will remain after the resection and is called standardized FRL-volume.4
Liver function assessment
HBS was performed after a 4 h fast, as food consumption stimulates hepatic function and bile flow, which might influence test results. When patients presented with jaundice, HBS was performed after biliary drainage. Patients were positioned supine on the imaging table of a large-field-of-view (FOV) SPECT/CT camera (Symbia T16; Siemens) positioned over the liver and heart region. The SPECT/CT camera was equipped with low-energy high-resolution collimators. After intravenous administration of 200 MBq freshly prepared 99mTc-mebrofenin (Bridatec; GE-Amersham Health), dynamic acquisition was obtained (36 frames of 10 s/frame, 128 matrix), which was used for calculation of the hepatic mebrofenin uptake rate (MUR). Subsequently, a fast SPECT acquisition was performed (60 projections of 8 s/projection, 128 matrix), centered on the peak of the hepatic time–activity curve, which was used for the 3-dimensional assessment of liver function and calculation of functional liver volume. Immediately after SPECT, a low-dose, non-contrast-enhanced CT scan was obtained for attenuation correction and anatomical mapping. In order to evaluate biliary excretion a second dynamic acquisition (15 frames of 60 s/frame, 128 matrix) was obtained. Data were processed on a Hermes workstation (Hermes Medical Solutions, Sweden).
The HBS parameters related to MUR in the total liver and FRL were calculated as described before.5, 8, 9 A cut-off value of 2.7%/min/m2 was used to discriminate normal from decreased FRL uptake rate as was described in a previous study.7
Preoperative portal vein embolization
Patients with insufficient FRL-volume, i.e. <25% in patients with presumed healthy liver parenchyma and <35% in patients diagnosed with PHC, and/or FRL-function below the selected cut-off value of 2.7%/min/m2 were considered for preoperative portal vein embolization (PVE).
Data collection
Demographic, clinical, intraoperative and postoperative data were extracted from electronic patient records. In case of discrepancies between the actual resection and the liver segments that were anticipated as the FRL during preoperative assessment, the FRL-function and/or the FRL-volume were recalculated using reprocessed data, thereby ensuring comparability. For patients who underwent PVE, the FRL-volume and function prior to PVE were not recalculated; the required FRL-volume and FRL-function that were anticipated prior to PVE were used for the analyses as the indication for PVE was based on these findings. Final diagnosis was extracted from the histology reports. Postoperative complications were graded according to the international complication classification by Clavien-Dindo.10
Study endpoints
The primary endpoint of this study was the impact of assessment of preoperative FRL-function using HBS on the preoperative management and the postoperative outcomes of patients who underwent major liver resection. The impact on preoperative management was defined as interventions during the preoperative course that were based on the results of HBS. Postoperative outcomes were defined as the occurrence of clinically relevant postoperative liver failure (i.e. including admission to the intensive care unit; grade C according to the definition for postoperative liver failure by the International Study Group of Liver Surgery11) and the occurrence of liver failure related mortality. The postoperative outcome of patients who underwent HBS as part of their preoperative work-up after implementation of HBS was compared with the postoperative outcome in patients of the historical cohort.7 In the latter analysis, patients who underwent preoperative PVE were excluded as the historical cohort did not include patients who had undergone PVE.
Secondary endpoints were the differences in outcome between the use of HBS, FRL-BWR and standardized liver volumetry in the same patients. We examined if the latter two methods could play an additional role in the identification of patients who are at risk for postoperative liver failure.
Statistical analysis
Continuous data are presented as mean ± standard deviation (SD) and in case of non-normally distributed data, as median and interquartile range (IQR). Univariate analysis was performed with independent t-test or Mann–Whitney U-test for continuous parameters and by chi-square or Fisher's exact test for categorical data. All statistical tests were two-tailed, and differences were considered significant at a P value of ≤0.05. Analyses were performed in Statistical Package for Social Sciences (SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp.)
Results
Patients
From November 2006 to June 2014, 246 major liver resections were performed among a total of 583 liver resections in adult patients. Preoperative HBS and CT-volumetry were performed in 169/246 patients. The remaining 77 patients did not undergo preoperative HBS as preoperative CT imaging showed an evident surplus of the FRL-volume, defined as >50% of total liver volume. Six patients were excluded from the study as HBS was not used in the decision making process. Finally, 163 patients were included in the study. The majority of patients was male, 92/163 (56.4%), with a median age of 63.0 years (IQR 54.0–71.0). The most frequent final diagnoses were perihilar cholangiocarcinoma in 71/163 (43.6%) patients and liver metastases in 50/163 (30.7%) patients. Table 1 shows the baseline characteristics of the 163 patients included in this study. Four out of the 163 (2.5%) patients underwent a major liver resection as part of a two-stage approach.
Table 1.
Baseline characteristics of the 163 patients who underwent preoperative HBS and CT-volumetry as preoperative assessment for major liver surgery
| Patient characteristics | |
| Age in years, median (IQR) | 63.0 (54.0–71.0) |
| Male sex, n (%) | 92 (56.4) |
| ASA classification, n (%) | |
| Grade I | 41 (25.2) |
| Grade II | 92 (56.4) |
| Grade III | 30 (18.4) |
| Type of resection, n (%) | |
| Right hemihepatectomy | 67 (41.1) |
| Left hemihepatectomy | 56 (34.4) |
| Extended right hemihepatectomy | 30 (18.4) |
| Extended left hemihepatectomy | 5 (3.1) |
| Central resection | 2 (1.2) |
| Segmentectomy of ≥3 liver segments | 3 (1.8) |
| Type of tumor, n (%) | |
| PHC | 71 (43.6) |
| Hepatic metastases | 50 (30.7) |
| Benign tumor | 20 (12.3) |
| HCC | 12 (7.4) |
| Intrahepatic cholangiocarcinoma | 4 (2.5) |
| Gallbladder carcinoma | 2 (1.2) |
| Other, malignant tumors | 4 (2.5) |
| Compromised liver parenchyma, n (%) | 103 (63.2) |
IQR, interquartile range; ASA-classification, physical status classification according to American Society of Anaesthesiologists; PHC, perihilar cholangiocarcinoma; HCC, hepatocellular carcinoma.
Preoperative management
Overall, the preoperative management was adjusted in 38/163 (23.3%) patients based on the results of FRL assessment. Twenty-nine (17.8%) patients underwent preoperative PVE due to insufficient FRL, while 9 (5.5%) other patients underwent additional procedures due to cholestasis or the effects of long-term cholestasis that were observed using HBS. The results of HBS are described separately for patients with presumed non-compromised liver parenchyma and patients with (post)cholestatic livers.
Patients with non-compromised liver parenchyma
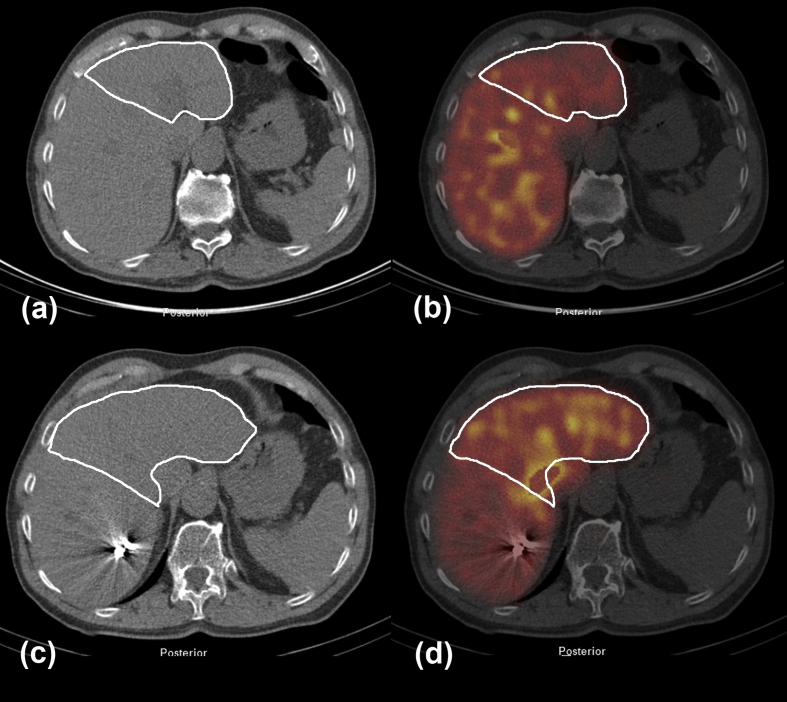
In 91/163 (55.8%) patients non-compromised liver parenchyma was expected. The median FRL-volume and median FRL-function in these patients were 39.9% (IQR 29.7–58.8) and 3.6%/min/m2 (IQR 2.76–5.53), respectively. Based on the preoperative assessment of FRL-volume and FRL-function, preoperative PVE was performed in 22/91 (24.2%) patients. In these patients, the median FRL-volume prior to PVE was 23.7% (IQR 18.2–27.8) while the median FRL-function was 1.92%/min/m2 (IQR 1.48–2.23). In 7 out of the 22 patients, PVE was performed because of insufficient FRL-function, in spite of sufficient FRL-volume. The remaining 69/91 patients had both FRL-volume ≥25% and FRL-function ≥2.7%/min/m2. Fig. 1 illustrates functional changes after PVE.
Figure 1.
Low dose CT and 99mTc-mebrofenin SPECT before (a, b) and 3 weeks after (c, d) right sided PVE in a patient with a primary liver tumor (tumor not visible on low dose CT, white line indicates the FRL). Both volume (c) and function (d) of left liver segments show increase after PVE. There is a decrease in function in the right sided embolized liver lobes (d)
Patients with (post)cholestatic liver parenchyma
Seventy-two out of the 163 (44.2%) patients were suspected of PHC (one was finally diagnosed with a benign lesion). The median FRL-volume and FRL-function were 57.6% (IQR 36.2–75.6) and 5.5%/min/m2 (IQR 3.23–7.38), respectively. PVE was performed in 7/72 (9.7%) patients. Among these patients, the median FRL-volume was 25.0% (IQR 20.1–33.4) and the FRL-function was 2.0%/min/m2 (IQR 1.53–3.2). In one patient PVE was performed due to insufficient FRL-function while the FRL-volume was >35% of the total liver volume.
In another 9/72 (12.5%) patients with suspicion on perihilar cholangiocarcinoma, preoperative PVE was not performed although the FRL-function was below the cut-off value; i.e. 2.19%/min/m2 (IQR 1.19–2.42). The FRL-volume was above the cut-off value for safe resection in 5/9 patients (37.1%; IQR 30.9–47.1). In this subpopulation, HBS was used as a diagnostic tool in order to determine whether decreased FRL-function was based on inadequate biliary drainage or compromised liver parenchyma (unpublished data). Consequently, we performed additional biliary drainage or extended the waiting time to the resection until the effects of longstanding cholestasis had resolved (n = 5) and/or performed a modified type of resection, i.e. parenchyma sparing resection (n = 5).
Among the remaining 56/72 (77.8%) patients, 51/56 (91.1%) underwent resection with sufficient FRL-volume and FRL-function while 5/56 (8.9%) patients had sufficient FRL-function but insufficient FRL-volume. These 5 patients did not undergo PVE. Table 2 summarizes the functional and volumetric measurements of the PVE and non-PVE patients.
Table 2.
Results of the (first) preoperative FRL assessment using CT-volumetry and 99mTc-mebrofenin hepatobiliary scintigraphy with SPECT-CT
| FRL-volume, % (IQR 25–75) | FRL-function, %/min/m2 (IQR 25–75) | Standardized FRL-volume, % (IQR 25–75) | FRL-BWR ≥0.5% weight, n (%) | |
|---|---|---|---|---|
| All patients, (n = 163) | 49.3 (37.3–70.1) | 4.6 (3.3–6.39) | 54.3 (38.9–77.9) | 161 (98.8) |
| PVE patients, (n = 29) | 23.7 (18.7–27.8) | 1.93 (1.5–2.36) | 24.5 (18.3–29.4) | 16 (55.2) |
| Non-PVE patients, (n = 134) | 53.9 (37.4–73.5) | 5.00 (3.49–7.10) | 57.2 (41.6–79.7) | 133 (99.3) |
FRL, future remnant liver; FRL-volume, percentage FRL of the total liver volume; FRL-BWR, future remnant liver/body weight ratio; PVE, portal vein embolization; IQR, interquartile range.
Resection and postoperative course
As mentioned above, all patients underwent a major liver resection of which right and left hemihepatectomy were the most frequently performed procedures, see Table 1. Overall, clinically relevant complications (Clavien-Dindo grade ≥3a) were seen in 63/163 (38.7%) patients and postoperative mortality occurred in 12/163 (7.4%) of the patients. Postoperative liver failure grade C was seen in 3/163 patients (1.8%) while liver failure related mortality occurred in 2 out of these 3 patients. One of these patients, diagnosed with CRLM, had undergone preoperative PVE due to insufficient FRL-volume and FRL-function. Despite increased function and volume after PVE, the patient died because of postoperative liver failure in combination with portal vein thrombosis. The remaining 2 patients were diagnosed with PHC. In both patients, FRL-function was above the cut-off value (4.10 and 4.62%/min/m2 respectively), while the FRL-volume was 26.4% and 33.8% respectively.
There were no differences in postoperative outcomes between PVE and non-PVE patients in terms of clinically relevant, postoperative complications including postoperative liver failure and liver failure related mortality, Table 3.
Table 3.
Postoperative outcome after major liver resection in PVE and non-PVE patients
| PVE-patients, (n = 29) | Non-PVE patients, (n = 134) | p= | |
|---|---|---|---|
| Clinically relevant complicationsa, n (%) | 10 (34.5) | 53 (39.6) | 0.678 |
| Postoperative liver failureb, n (%) | 1 (3.4) | 2 (1.5) | 0.447 |
| Mortality due to liver failure, n (%) | 1 (3.4) | 1 (0.7) | 0.325 |
PVE, portal vein embolization.
Complications grade ≥3a according to Clavien-Dindo classifications.
Grade C liver failure according to the definition of International Study Group of Liver Surgery.
Reduction of postoperative liver failure
Both, the historical cohort (n = 55) and the cohort after the implementation of HBS (n = 134, non-PVE patients), were comparable in terms of demographic, intraoperative, histological and parenchymal characteristics (Table 4). The majority of the patients in both groups was diagnosed with perihilar cholangiocarcinoma. The median, preoperative FRL-volume and FRL-function were significantly lower in the historical group (Table 4).
Table 4.
Baseline characteristics and postoperative outcome of the 134 patients who underwent HBS as part of the preoperative work-up (and no preoperative PVE) and the 55 patients who underwent major liver resection before implementation of HBS in our routine
| After implementation of HBS, (n = 134) | Before implementation of HBS, (n = 55) | p= | |
|---|---|---|---|
| Patient characteristics | |||
| Age in years, median (IQR 25–75) | 63.0 (54.0–71.0) | 62.4 (53.3–67.9) | 0.553 |
| Male sex, n (%) | 74 (55.2) | 26 (47.3) | 0.340 |
| ASA classification, n (%) | 0.188 | ||
| Grade I | 34 (25.4) | 8 (14.5) | |
| Grade II | 72 (53.7) | 37 (67.3) | |
| Grade III | 28 (20.9) | 10 (18.2) | |
| Type of resection, n (%) | 0.055 | ||
| Right hemihepatectomy | 53 (39.6) | 26 (47.3) | |
| Left hemihepatectomy | 56 (41.8) | 14 (25.5) | |
| Extended right hemihepatectomy | 15 (11.2) | 14 (25.5) | |
| Extended left hemihepatectomy | 5 (3.7) | 1 (1.8) | |
| Central resection | 2 (1.5) | 0 | |
| Segmentectomy of ≥3 segments | 3 (2.2) | 0 | |
| Type of tumor, n (%) | 0.392 | ||
| PHC | 64 (47.8) | 19 (34.5) | |
| Hepatic metastases | 33 (24.6) | 14 (25.5) | |
| Benign tumor | 17 (12.7) | 13 (23.6) | |
| HCC | 11 (8.2) | 6 (10.9) | |
| Intrahepatic cholangiocarcinoma | 3 (2.2) | 3 (5.5) | |
| Gallbladder carcinoma | 2 (1.5) | ||
| Other, malignant tumors | 4 (3.0) | ||
| FRL measurements | |||
| FRL-volume, % (IQR 25–75) | 53.9 (37.4–73.5) | 41.7 (35.3–61.6) | 0.028 |
| FRL-function, %/min/m2 (IQR 25–75) | 5.00 (3.49–7.10) | 3.94 (2.69–4.85) | 0.005 |
| Standardized FRL-volume, % (IQR 25–75) | 57.2 (41.6–79.7) | 46.4 (36.3–73.3) | 0.120 |
| FRL-BWR, (IQR 25–75) ≥0.5% weight, n (%) |
1.25 (0.89–1.72) 133 (93.3) |
0.99 (0.79–1.57) 55 (100) |
0.120 1.000 |
| Postoperative outcome | |||
| Clinically relevant complicationsa, n (%) | 53 (39.6) | 28 (50.9) | 0.195 |
| Postoperative liver failureb, n (%) | 2 (1.5) | 9 (16.4) | <0.001 |
| Mortality due to liver failure, n (%) | 1 (0.7) | 8 (14.5) | <0.001 |
PVE, portal vein embolization.
Complications grade ≥3a according to Clavien-Dindo classifications.
Grade C liver failure according to the definition of International Study Group of Liver Surgery; ASA-classification, physical status classification according to American Society of Anaesthesiologists; PHC, perihilar cholangiocarcinoma; HCC, hepatocellular carcinoma; FRL-volume, percentage FRL of the total liver volume; FRL-BWR, future remnant liver/body weight ratio; IQR, interquartile range.
The postoperative course in both groups was comparable, except for postoperative liver failure which occurred more often in the time period before the implementation of HBS, i.e. 9/55 (16.4%) and 2/134 (1.5%) in the historical group and present series, respectively (p < 0.001). The same accounts for liver failure related mortality that was seen in 8/55 (14.5%) and 1/134 (0.7%) of the patients in the historical cohort and present series, respectively (p < 0.001).
FRL-BWR and standardized FRL-volume
The preoperative FRL-BWR and standardized FRL-volume values in the 163 patients who underwent liver resection after implementation of HBS are presented in Table 2. Regarding the 29 patients who underwent preoperative PVE, 16/29 and 11/29 patients had sufficient FRL-volume according to FRL-BWR and standardized FRL-volume, respectively, and therefore, would not have undergone PVE if the decision was based on these methods. The same accounts for the 9/163 (5.5%) patients suspected of PHC with insufficient FRL-function in whom additional procedures were performed, as in all 9 patients the FRL-BWR was ≥0.5% of patient's weight and the standardized FRL-volume was ≥35% in 7/9 patients. None of the three patients who had developed postoperative liver failure would have been identified using FRL-BWR while 1/3 patients would have been identified using standardized CT volumetry.
Furthermore, the standardized FRL-volume and FRL-BWR in both the present cohort after implementation of HBS and the historical cohort were comparable, as opposed to the significant differences found in the measured FRL-volume and FRL-function.
Discussion
99mTc-mebrofenin hepatobiliary scintigraphy (HBS) is one of the most advanced quantitative liver function tests available in Western countries as it offers evaluation of the FRL using one single cut-off value and enables segmental measurement of FRL-function.12 Since the identification of the cut-off value for safe resection, HBS is part of our preoperative work-up in patients with increased surgical risk who are scheduled for major liver surgery. We sought to assess the value and the impact of additional HBS on preoperative management and postoperative outcomes in patients undergoing resection in a period following a previous study.
Results of this study show that HBS can be used as a guideline in the preoperative work-up. Twenty-nine patients in this cohort underwent preoperative PVE based on the outcome of functional FRL assessment. If the decision to perform PVE had been based on FRL-volumetric measurements only, eight out of the 29 patients would not have undergone PVE. Although we cannot ascertain what the postoperative results would have been if these patients would have undergone resection without preoperative PVE, we know from our previous studies that FRL-function rather than FRL-volume is predictive for the occurrence of postoperative liver failure.7, 13 There were no significant differences in the occurrence of postoperative liver failure or liver failure related mortality between the PVE and the non-PVE patients, suggesting that unilateral embolization of the portal vein in these patients has contributed to the prevention of postoperative liver failure. Furthermore, in nine patients suspected of PHC, HBS was used as a diagnostic tool to identify patients who might benefit from additional procedures prior to the operation such as additional biliary drainage. It is well known that cholestasis has a negative impact on postoperative outcome in PHC patients.14 Again, the postoperative outcomes in these patients were not different from patients who did not undergo any additional procedures or modified type of resection. Important to note is the high number of patients suspected/diagnosed with PHC (44.2%) in the entire cohort. These patients are especially prone to developing postoperative liver failure with unfavourable postoperative outcome. HBS can offer an additional benefit in this group of patients as next to the hepatic uptake of mebrofenin, biliary excretion of mebrofenin can be assessed using HBS providing additional information on the quality of biliary drainage, which is an important factor in secondary segmental liver dysfunction. The competition between mebrofenin and bilirubin has often been a point of discussion, however by evaluating the excretion phase, cholestatic patients are readily differentiated from the non-cholestatic patients which makes a validated cut-off value for maximum serum bilirubin of less importance.
For the purpose of this study, the preoperative measurements were recalculated according to the actual FRL after resection in case of discrepancies with the preoperatively anticipated FRL, which is necessary to objectively correlate preoperative volumetry and HBS with postoperative outcomes. Not unusually, the resection is modified into a parenchyma sparing type of resection in case of borderline insufficient FRL (as alternative to PVE) or needs to be extended because of new findings during the operation.15 The volume and function parameters of preoperatively anticipated FRL do not provide an accurate estimation of the diagnostic value of the tests in these cases, which is often a major limitation of similar diagnostic studies.
Three patients in our current series developed severe liver failure (grade C according to the International Study Group of Liver Surgery11), which proved lethal in two of them. Based on assessment of function and volume, preoperative PVE had been performed in one of the three patients who developed liver failure. Although PVE in this patient was followed by a sufficient hypertrophy response, the patient died due to liver failure in combination with thrombosis of the portal vein. The remaining two patients in whom liver failure occurred were diagnosed with PHC. One of these two patients did not experience cholestasis prior to the operation and had sufficient FRL-function. In the other PHC patient, ongoing biliary obstruction was established using the excretion phase of HBS, although FRL-function was not yet impaired and action was taken.
FRL-BWR would not have been able to help us identify any of these 3 patients, while standardized liver volumetry showed insufficient FRL-volume in 1/3 patients. Furthermore, both the FRL-BWR and standardized liver volumetry tended to overestimate the FRL in this series in comparison to our preoperative assessment using HBS and CT-volumetry. Of the 29 patients who underwent preoperative portal vein embolization, PVE would have been omitted in 11 and 16 patients, respectively, if the decision for PVE was based on these methods. Given these findings, we see no additional advantage in implementation of other volumetric methods of FRL assessment next to, or instead of regular CT-volumetry for the identification of high-risk patients who need to undergo major liver resection. It should however, be noted that in a recent publication the use of FRL-BWR at a cut-off of 0.5% was restricted to patients without malignant obstructive jaundice.16
Comparison with the historical cohort shows an improvement in the postoperative outcomes in the period after HBS was implemented in our preoperative work-up. The difference found in the occurrence of mortality due to postoperative liver failure between the current series and the historical cohort should be interpreted with some caution, as we need to be aware of the improvements in the supportive treatment of patients who suffer from postoperative liver failure that have evolved in the meantime. As this study deals with a retrospective analysis of consecutive patients, the results cannot be compared to a similar control group of patients who had undergone major liver resection without preoperative HBS, during the same period of time at our center. Such study design would have eliminated the possible bias caused by these improvements. Notwithstanding this limitation, we observed a dramatic and significant improvement in the occurrence of clinically relevant postoperative liver failure.7 The occurrence of postoperative liver failure is primarily caused by an insufficient FRL-function. Furthermore, it is important to note that there were no differences in the occurrence of other postoperative complications (Clavien-Dindo grade ≥3a) between both groups. In the latter analysis, patients who had undergone PVE were excluded in order to provide a fair comparison between the current and historical cohort as preoperative PVE was not often used at our center before 2005. However, the observed differences in postoperative liver failure and liver failure related mortality between the historical and current cohort remain significant even when the PVE patients are included in the current cohort (p < 0.001 for both postoperative liver failure and liver failure related mortality). In addition, the extent of the liver resections performed in both cohorts remains not significantly different when the PVE patients are included in the analysis (p = 0.632). Therefore, although there are time-dependent differences between both cohorts, we believe that the observed decrease in the occurrence of liver failure can be explained by improved preoperative patient selection and a function oriented use of PVE since the implementation of HBS in our preoperative work-up of patients eligible for major liver resection.
Conclusion
Preoperative HBS provides useful functional information on the FRL in patients requiring major liver resection. Patients with sufficient FRL-volume but decreased FRL-function may benefit from additional procedures (PVE, ALPPS) or a modified, parenchyma sparing type of resection. Preoperative assessment of the FRL by volumetric methods or FRL-BWR only may lead to an overestimation of the FRL. Implementation of HBS in the preoperative work-up for major liver resection led to a function oriented use of PVE and was associated with a decreased incidence of postoperative liver failure in non-PVE patients.
Funding sources
None.
Conflicts of interest
None declared.
Contributor Information
Kasia P. Cieslak, Email: k.p.cieslak@amc.uva.nl.
Thomas M. van Gulik, Email: t.m.vangulik@amc.uva.nl.
References
- 1.van den Broek M.A., Olde Damink S.W., Dejong C.H., Lang H., Malago M., Jalan R. Liver failure after partial hepatic resection: definition, pathophysiology, risk factors and treatment. Liver Int. 2008;28:767–780. doi: 10.1111/j.1478-3231.2008.01777.x. [DOI] [PubMed] [Google Scholar]
- 2.Cieslak K.P., Runge J.H., Heger M., Stoker J., Bennink R.J., van Gulik T.M. New perspectives in the assessment of future remnant liver. Dig Surg 2014. 2014;31:255–268. doi: 10.1159/000364836. [DOI] [PubMed] [Google Scholar]
- 3.Truant S., Oberlin O., Sergent G., Lebuffe G., Gambiez L., Ernst O. Remnant liver volume to body weight ratio > or =0.5%: a new cut-off to estimate postoperative risks after extended resection in noncirrhotic liver. J Am Coll Surg. 2007;204:22–33. doi: 10.1016/j.jamcollsurg.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Vauthey J.N., Abdalla E.K., Doherty D.A., Gertsch P., Fenstermacher M.J., Loyer E.M. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 5.Erdogan D., Heijnen B.H., Bennink R.J., Kok M., Dinant S., Straatsburg I.H. Preoperative assessment of liver function: a comparison of 99mTc-Mebrofenin scintigraphy with indocyanine green clearance test. Liver Int. 2004;24:117–123. doi: 10.1111/j.1478-3231.2004.00901.x. [DOI] [PubMed] [Google Scholar]
- 6.de Graaf W., van Lienden K.P., van Gulik T.M., Bennink R.J. (99m)Tc-mebrofenin hepatobiliary scintigraphy with SPECT for the assessment of hepatic function and liver functional volume before partial hepatectomy. J Nucl Med. 2010;51:229–236. doi: 10.2967/jnumed.109.069724. [DOI] [PubMed] [Google Scholar]
- 7.de Graaf W., van Lienden K.P., Dinant S., Roelofs J.J., Busch O.R., Gouma D.J. Assessment of future remnant liver function using hepatobiliary scintigraphy in patients undergoing major liver resection. J Gastrointest Surg. 2010;14:369–378. doi: 10.1007/s11605-009-1085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekman M., Fjalling M., Friman S., Carlson S., Volkmann R. Liver uptake function measured by IODIDA clearance rate in liver transplant patients and healthy volunteers. Nucl Med Commun. 1996;17:235–242. doi: 10.1097/00006231-199603000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Bennink R.J., Dinant S., Erdogan D., Heijnen B.H., Straatsburg I.H., van Vliet A.K. Preoperative assessment of postoperative remnant liver function using hepatobiliary scintigraphy. J Nucl Med. 2004;45:965–971. [PubMed] [Google Scholar]
- 10.Clavien P.A., Barkun J., de Oliveira M.L., Vauthey J.N., Dindo D., Schulick R.D. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 11.Rahbari N.N., Garden O.J., Padbury R., Brooke-Smith M., Crawford M., Adam R. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Bennink R.J., Tulchinsky M., de Graaf W., Kadry Z., van Gulik T.M. Liver function testing with nuclear medicine techniques is coming of age. Semin Nucl Med. 2012;42:124–137. doi: 10.1053/j.semnuclmed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Dinant S., de Graaf W., Verwer B.J., Bennink R.J., van Lienden K.P., Gouma D.J. Risk assessment of posthepatectomy liver failure using hepatobiliary scintigraphy and CT volumetry. J Nucl Med. 2007;48:685–692. doi: 10.2967/jnumed.106.038430. [DOI] [PubMed] [Google Scholar]
- 14.Farges O., Regimbeau J.M., Fuks D., Le Treut Y.P., Cherqui D., Bachellier P. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013;100:274–283. doi: 10.1002/bjs.8950. [DOI] [PubMed] [Google Scholar]
- 15.van Gulik T.M., Ruys A.T., Busch O.R., Rauws E.A., Gouma D.J. Extent of liver resection for hilar cholangiocarcinoma (Klatskin tumor): how much is enough? Dig Surg. 2011;28:141–147. doi: 10.1159/000323825. [DOI] [PubMed] [Google Scholar]
- 16.Truant S., Boleslawski E., Sergent G., Leteurtre E., Duhamel A., Hebbar M. Liver function following extended hepatectomy can be accurately predicted using remnant liver volume to body weight ratio. World J Surg. 2015;39:1193–1201. doi: 10.1007/s00268-014-2929-9. [DOI] [PubMed] [Google Scholar]