Dear Editor,
Multidrug resistance of Pseudomonas aeruginosa has been attributed to both intrinsic and acquired antibiotic-resistance mechanisms. Multidrug-resistant (MDR) P. aeruginosa isolates have become a serious healthcare problem worldwide because they are resistant to almost all β-lactams, aminoglycosides, and quinolones. Production of zinc-dependent metallo-β-lactamases (MBLs) has been identified as the most significant mechanism among carbapenem-resistant P. aeruginosa isolates [1]. MBLs are of particular clinical concern because of their broad-spectrum activities, and Imipenemase (IMP)-, Verona Integron-Encoded Metallo-β-lactamase (VIM)-, Sao Paulo metallo-β-lactamase (SPM)-, Germany imipenemase (GIM)-, and New Delhi Metallo-β-lactamase (NDM)-type MBLs have been identified in P. aeruginosa worldwide [2]. Forty-six variants of VIM enzymes have been identified to date (http://www.lahey.org/Studies/other.asp). VIM-38 was recently identified in P. aeruginosa isolates in Turkey and was shown to differ from VIM-5 by a single substitution (Ala316Val) [3]. In P. aeruginosa, VIM-type MBLs have been reported within mobile genetic elements such as integrons, which contribute to the dissemination of antibiotic resistance [3].
We here report a new clinical P. aeruginosa strain isolated from a blood sample on January 2015 at Rize State Hospital in Turkey and identified by using the API 32GN system (bioMerieux, Marcy-l'Etoile, France). Minimal inhibitory concentrations were determined on a VITEK system for the following antibiotics: piperacillin/tazobactam, ceftazidime, cefepime, amikacin, netilmicin, ciprofloxacin, levofloxacin, imipenem, meropenem, cefoperazone-sulbactam, and inducible β-lactamase. 16S rDNA sequencing was used for molecular identification, performed according to Cicek et al [4].
The P. aeruginosa isolate was screened for β-lactamase-encoding genes and the class 1–class 2 integrases conserved region by PCR. The primers used for detection of β-lactamase-encoding genes and class 1 and class 2 integron gene cassettes are listed in Table 1 [4,5,6,7,8].
Table 1. Primers used in the amplification of selected genes.
| Primers | 5'→3' | Amplicon size | Tm (℃) |
|---|---|---|---|
| blaTEM | F: AGTATTCAACATTTYCGTGT | 847 | 56 |
| R: TAATCAGTGAGGCACCTATCTC | |||
| blaSHV | F: ATGCGTTATATTCGCCTGTG | 843 | 55 |
| R: TTAGCGTTGCCAGTGCTC | |||
| blaCTX-M1 | F: GCGTGATACCACTTCACCTC | 260 | |
| R: TGAAGTAAGTGACCAGAATC | |||
| blaCTX-M2 | F: TGATACCACCACGCCGCTC | 341 | |
| R: TATTGCATCAGAAACCGTGGG | |||
| blaGES | F: ATGCGCTTCATTCACGCAC | 863 | 056 |
| R: CTATTTGTCCGTGCTCAGGA | |||
| blaVEB | F: ATTTCCCGATGCAAAGCGT | 542 | 55 |
| R: TTATTCCGGAAGTCCCTGT | |||
| blaPER-2 | F: ATGAATGTCATCACAAAATG | 860 | 45 |
| R: ATAATAGCTTCATTGGTTC | |||
| blaKPC | F: ATGTCACTGTATCGCCGTCT | 893 | 55 |
| R: TTTTCAGAGCCTTACTGCCC | |||
| blaIMP | F: CATGGTTTGGTGGTTCTTGT | 488 | 56 |
| R: ATAATTTGGCGGACTTTGGC | |||
| blaVIM | F: ATTGGTCTATTTGACCGCGTC | 780 | 58 |
| R: TGCTACTCAACGACTGAGCG | |||
| blaNDM | F: GAGATTGCCGAGCGACTTG | 497 | 57 |
| R: CGAATGTCTGGCAGCACACTT | |||
| blaOXA-51 | F: TAATGCTTTGATCGGCCTTG | 353 | 52 |
| R: TGGATTGCACTTCATCTTGG | |||
| blaOXA-23 | F: GATCGGATTGGAGAACCAGA | 501 | |
| R: ATTTCTGACCGCATTTCCAT | |||
| blaOXA-40 | F: GGTTAGTTGGCCCCCTTAAA | 246 | |
| R: AGTTGAGCGAAAAGGGGATT | |||
| blaOXA-58 | F: AAGTAT TGGGGCTTGTGCTG | 599 | |
| R: CCCCTCTGCGCTCTACATAC | |||
| blaGIM-1 | F: TCGACACACCTTGGTCTG AA | 477 | |
| R: AACTTCCAACTTTGCCATGC | |||
| blaSPM-1 | F: AAAATCTGGGTACGCAAACG | 271 | |
| R: ACATTATCCGCTGGAACAGG | |||
| blaSIM-1 | F: TACAAGGGATTCGGCATC G | 570 | |
| R: TAATGGCCT GTTCCCATGTG | |||
| blaCMY | F: GACAGCCTCTTTCTCCACA | 1,000 | 50 |
| R: TGGAACGAAGGCTA CGTA | |||
| 5'-CS | F: GGCATCCAAGCAGCAAG | 56 | |
| 3'-CS | R: AAGCAGACTTGACCTGA | ||
| hep51 | F: GATGCCATCGCAAGTACGAG | 55 | |
| hep74 | R: CGGGATCCCGGACGGATGCACGATTTGTA |
Abbreviations: Tm, melting temperature; CS, conserved segment of class-I integron; hep51, forward primer of of class-II integron; hep75, reverse primer of of class-II integron; CMY cephalomycinase coding gene.
The positive PCR product of the class 1 integron was cloned into pGEM-T easy vector (Promega, Madison, WI, USA) and then sequenced by Macrogen (Amsterdam, The Netherlands). Sequencing results were analyzed by using the BLAST alignment search tool (http://www.ncbi.nlm.nih.gov/BLAST) and the multiple sequence alignment program CLUSTALW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/).
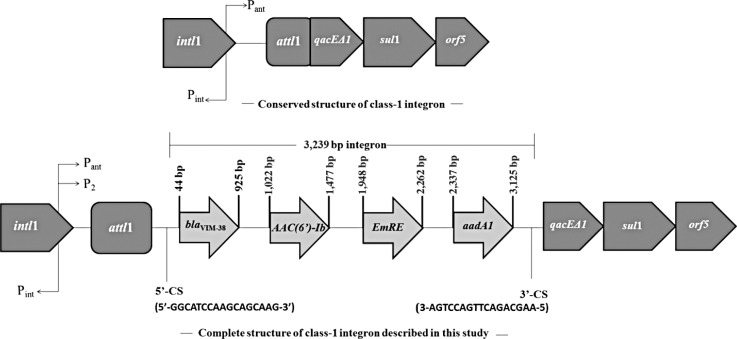
Transferability of antibiotic resistance was tested according to the previously defined protocol [9], by using the rifampin-resistant Escherichia coli K-12 strain J53-2 as a recipient [4]. Susceptibility testing of the MBL-producing integron-positive P. aeruginosa isolate showed that it was resistant to imipenem, meropenem, piperacillin/tazobactam, ceftazidime, cefepime, and cefoperazone-sulbactam. PCR analysis showed that the isolate did not harbor any of the antibiotic resistance genes listed in Table 1, except for blaVIM-type MBL. Sequence analysis of the blaVIM-variant identified it as blaVIM-38. The P. aeruginosa isolate contained a class 1 integron gene cassette, but not a class 2 integron gene cassette. The blaVIM-38-harboring class 1 integron gene cassette was sequenced and was found to be 3,239 bp long. DNA sequence analysis revealed that blaVIM-38 MBL was located on the class 1 integron gene cassette together with AAC(6´)-Ib/EmrE/aadA1 (Fig. 1). The conjugation assay revealed that the class 1 integron cassette is not transferable.
Fig. 1. Structure of the blaVIM-38-carrying class 1 integron gene cassette in Pseudomonas aeruginosa.
Abbreviations: Pint, integrase promoter; Pant, promoter of inserted gene(s); intI1, class 1 integrase; attI1, integron-associated recombination site; qacEΔ1, quaternary ammonium compound resistance gene cassette; ™, sulfonamide resistance gene; blaVIM-38, Verona Integron-Encoded Metallo-β-lactamase 38; CS, conserved segment.
The blaVIM-38 gene was identified in P. aeruginosa isolates in Turkey in 2014, and found to be located in a class 1 integron containing only two gene cassettes (blaVIM-38/orfD) [3]. This genetic structure has also been associated with the blaVIM-5 gene in a clinical isolate of Enterobacter cloaceae from Turkey [9]. Moreover, steady-state kinetic analyses in a study on the enzymatic properties of VIM-38 showed that VIM-38 hydrolyzed all of the tested penicillins, cephalosporins, and carbapenems [10].
In the present study, the class 1 integron included four gene cassettes with blaVIM-38 followed by AAC(6´)-Ib, EmrE (multi-drug transporter), and aadA1. This is the first report in Turkey of the blaVIM-38/AAC(6´)-Ib/EmrE/aadA1 gene cassette array. Therefore, we report a novel gene cassette array with an MBL gene in a P. aeruginosa clinical isolate, and this is the second report for the detection of VIM-38 in a P. aeruginosa isolate in Turkey with different hospitalization and isolation times.
In conclusion, the presence of class 1 integrons in P. aeruginosa leads to increased resistance to antibiotics. The present study demonstrates the emergence of VIM-producing MDR P. aeruginosa strains harboring class 1 integrons and a gene cassette in Turkey. In particular, the blaVIM-38 MBL gene appears to be spreading among P. aeruginosa isolates in Turkey.
Acknowledgments
This work was partially supported by Recep Tayyip Erdogan University Research Fund Grants (BAP-2014.102.03.03. and BAP-2014.102.03.02).
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Cornaglia G, Giamarellou H, Rossolini GM. Metallo-β-lactamases: a last frontier for β-lactams? Lancet Infect Dis. 2011;11:381–393. doi: 10.1016/S1473-3099(11)70056-1. [DOI] [PubMed] [Google Scholar]
- 2.Bebrone C. Metallo-β-lactamases (classification, activity, genetic organization, structure, zinc coordination) and their superfamily. Biochem Pharmacol. 2007;74:1686–1701. doi: 10.1016/j.bcp.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Iraz M, Duzgun AO, Cicek AC, Bonnin RA, Ceylan A, Saral A, et al. Characterization of novel VIM carbapenemase, VIM-38, and first detection of GES-5 carbapenem-hydrolyzing β-lactamases in Pseudomonas aeruginosa in Turkey. Diagn Microbiol Infect Dis. 2014;78:292–294. doi: 10.1016/j.diagmicrobio.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Cicek AC, Duzgun AO, Saral A, Sandalli C. Determination of a novel integron-located variant (blaOXA-320) of Class D β-lactamase in Proteus mirabilis. J Basic Microbiol. 2014;54:1030–1035. doi: 10.1002/jobm.201300264. [DOI] [PubMed] [Google Scholar]
- 5.Iraz M, Özad Düzgün A, Sandallı C, Doymaz MZ, Akkoyunlu Y, Saral A, et al. Distribution of β-lactamase genes among carbapenem-resistant Klebsiella pneumoniae strains isolated from patients in Turkey. Ann Lab Med. 2015;35:595–601. doi: 10.3343/alm.2015.35.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellington MJ, Kistler J, Livermore DM, Woodford N. Multiplex PCR for rapid detection of genes encoding acquired metallo-beta-lactamases. J Antimicrob Chemother. 2007;59:321–322. doi: 10.1093/jac/dkl481. [DOI] [PubMed] [Google Scholar]
- 7.Woodford N, Ellington MJ, Coelho JM, Turton JF, Warda ME, Brown S, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006;27:351–353. doi: 10.1016/j.ijantimicag.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Zhao S, Qaiyumi S, Friedman S, Singh R, Foley SL, White DG, et al. Characterization of Salmonella enterica serotype newport isolated from humans and food animals. J Clin Microbiol. 2003;41:5366–5371. doi: 10.1128/JCM.41.12.5366-5371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gacar GG, Midilli K, Kolayli F, Ergen K, Gundes S, Hosoglu S, et al. Genetic and enzymatic properties of metallo-β-lactamase VIM-5 from a clinical isolate of Enterobacter cloacae. Antimicrob Agents Chemother. 2005;49:4400–4403. doi: 10.1128/AAC.49.10.4400-4403.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makena A, Düzgün AÖ, Brem J, McDonough MA, Rydzik AM, Abboud MI, et al. Comparison of Verona Integron-Borne Metallo-β-lactamase (VIM) variants reveals differences in stability and inhibition profiles. Antimicrob Agents Chemother. 2015;60:1377–1384. doi: 10.1128/AAC.01768-15. [DOI] [PMC free article] [PubMed] [Google Scholar]