Abstract
Introduction
During the World Trade Center (WTC) attacks, responders who helped in search, rescue, and recovery endured multiple traumatic and toxic exposures. One-fifth subsequently developed post-traumatic stress disorder (PTSD). PTSD has been linked to dementia in veterans. This study examined the association between WTC-related PTSD and cognitive impairment (CI) in WTC responders.
Methods
A one-third sample of responders (N = 818) reporting for annual monitoring visits were screened for cognitive impairment and dementia using the Montreal Cognitive Assessment from January 2014–April 2015. Concurrent diagnoses of PTSD and major depressive disorder (MDD), as well as serial PTSD and depressive symptom inventories, collected since 2002, were examined in relation to current CI.
Results
Approximately 12.8% and 1.2% of responders in this sample respectively had scores indicative of CI and possible dementia. Current PTSD and MDD were associated with CI. Longitudinal results revealed that re-experiencing symptoms were consistently associated with CI (aRR = 2.88, 95% confidence interval = 1.35–6.22), whereas longitudinal increases in other PTSD and depressive symptoms in the years before screening were evident only among those with CI.
Conclusions
Analyses replicated results from Veterans studies and further highlighted the importance of re-experiencing symptoms, a major component of PTSD that was consistently predictive of CI 14 years later. Clinicians should monitor CI when treating individuals with chronic PTSD.
Keywords: Epidemiology, World Trade Center, Disasters, Posttraumatic stress disorder, Cognitive impairment, Psychiatry
Dementia is a degenerative disease of the brain that is expected to affect as many as 40% of the U.S. population [1], [2]. Although dementia is an uncommonly devastating disease, milder prodromal forms of cognitive impairment (CI) are more common and also implicate substantial losses in cognitive functioning [3]. Risk factors for CI and dementia include age, smoking, alcohol intake, cardiovascular disease, education, diabetes, and depression [4]. Research suggests that post-traumatic stress disorder (PTSD) may also be associated with reduced cognitive functioning and increased risk of dementia [5], [6]. PTSD involves complex memory, emotional, and behavioral processes [7], and encompasses distinct domains including re-experiencing, effortful avoidance, emotional numbing, and hyperarousal resulting from a traumatic event [8]. Exact mechanisms for this association remain understudied [9]. Theories suggest, on the one hand, that PTSD may be a unique part of the causal pathway leading to CI [10]. However, the association may be confounded by co-morbid features that are independently associated with PTSD and dementia, such as traumatic brain injury [11]. Finally, symptoms indicative of PTSD, which is commonly comorbid with major depressive disorder (MDD) [12], may be manifestations of CI and dementia [13]. It is thus unclear whether reported associations between PTSD and cognitive impairments and dementia are due to reverse causation.
1.1. Setting
Thousands of responders who helped in search, rescue, and cleanup efforts after the World Trade Center (WTC) were exposed to an extraordinary array of psychological traumas and toxic exposures. Although few were physically injured by their efforts, many responders witnessed the disaster or death and dismemberment of others, helped civilians flee, lost colleagues in the tower collapse, and dug through debris to search for survivors [14]. Since then, researchers examining WTC responders have found high rates of chronic PTSD. Yet, the potential effects of persistent mental health symptoms on CI have not been examined [15].
2. Hypotheses
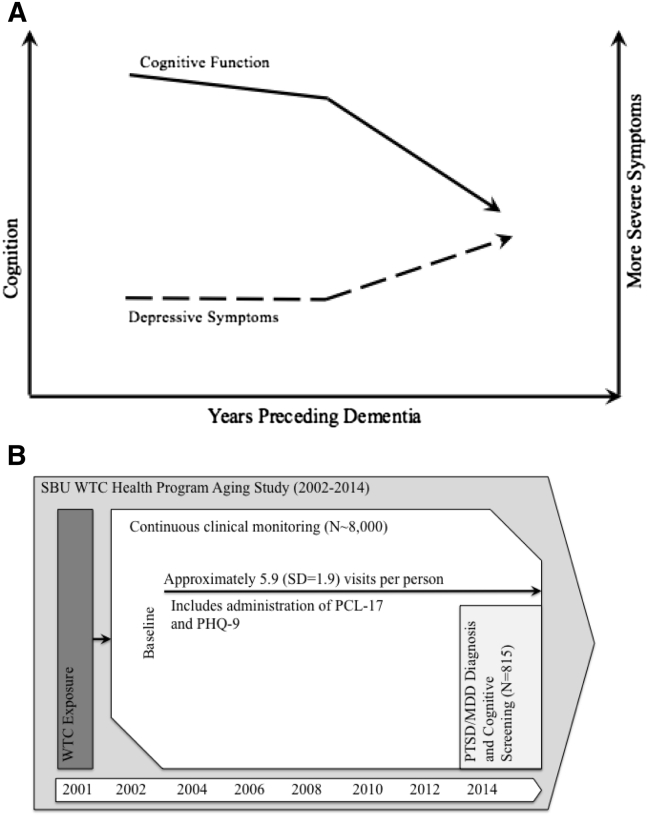
Based on existing studies, we hypothesized that current WTC-PTSD will be associated with CI. Dementia researchers largely agree that CI is generally characterized as undergoing a period of accelerated decline before becoming diagnostic (Fig. 1A) [16], [17]. We posited that such change might also be observed in noncognitive outcomes, such as via increasing depressive symptoms (dashed line), in a way similar to cognitive outcomes. Thus, longitudinal analyses of depressive and PTSD symptoms before cognitive screening may help interpret concurrent associations. Specifically, changes in related noncognitive factors could be used to help mark the course of the disease. If so, then reverse causation would be evident by significantly increasing symptoms before screening positive for CI. In contrast, risk factors may be those that, alternatively, precede the course of the disease and/or are not subject to such reverse causal effects.
Fig. 1.
Hypotheses and data structure for the Stony Brook University World Trade Center Aging Study 2002–2015. (A) Hypothesized progression of cognitive and non-cognitive changes. Solid lines, charted on the left y-axis, reflect changes in cognition whereas dashed lines, charted on the right y-axis, reflect changes in non-cognitive mental health symptoms. (B) Structure of the longitudinal PTSD sample and cross-sectional cognitive sample. Enrollment is open, and some patients have left. WTC exposures precede data collection, but mental health symptoms have been gathered since 2002, and diagnoses and cognitive screening were done concurrently in 2014–2015.
3. Methods
3.1. Parent monitoring study
In July 2002, the Centers for Disease Control and Prevention initiated a monitoring and treatment program for WTC responders, spanning five clinical centers. Since then, more than 33,000 responders have enrolled in the WTC Health Program and form the WTC general responders cohort [14]. Stony Brook University (SBU) runs the second largest clinical center, monitoring >8000 responders residing on Long Island, NY. SBU's population had similar exposures to the general responder cohort and was similar in age on 9/11/2001 (38.7 in the WTC general responder cohort versus 38.4 at SBU) [14]. However, the SBU population includes relatively more law enforcement personnel and men and fewer individuals without a high-school degree. The SBU Institutional Review Board approved this study, and responders provided written informed consent; >95% of responders consented for data to be used for research purposes.
3.2. Sample
Trained clinicians screened responders for CI during monitoring visits at the SBU clinics starting in 2014. Of those approached, 89.8% completed the screening, 0.6% did not complete due to extenuating circumstances, 0.4% only finished part of the screening, and 8.7% refused to participate in this cognitive screening. Those who refused testing did not differ in terms of PTSD (P = .165), WTC exposures (P = .940), educational attainment (P = .580), or law enforcement status (P = .105) from those who completed testing. On average, SBU responders were aged 52.8 years when this sample was taken. A total of 818 Responders with valid diagnostic information were eligible for this study. To assess exclusion bias, 128 responders screened using a different PTSD diagnostic tool were also screened for CI. Data were linked to existing monitoring records that included PTSD and depressive symptoms measured obtained serially since baseline (Fig. 1B). Five responders who reported any head injury obtained on-site were excluded, leaving an analytic sample of 813 responders. Clinic visit date was recorded.
3.3. Assessments
3.3.1. Cognitive impairment
Trained clinicians administered the Montreal Cognitive Assessment (MoCA) starting February 2014. The MoCA consists of multiple short-form neuropsychological tests that are scored in a standard way. A relatively conservative cutoff score of <23 was used to indicate CI, and a score of <18 was used to suggest possible dementia. These cutoffs have been shown to have >95% sensitivity and specificity in community-dwelling older adults [18]. Presence of the apolipoprotein-ε4 (APOE) has been strongly associated with the risk for Alzheimer's disease [19]. APOE status was measured using polymerase chain reaction on blood banked in a subsample of these responders (n = 593).
3.3.2. Diagnoses of PTSD and MDD
Trained psychologists administered the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders-IV to diagnose both PTSD and MDD [20]. Inter-rater agreement was high (κ = 0.82) among 55 independent ratings. To facilitate rapport and interpretation, interviewers were tasked with reviewing participant's histories before assessments. The PTSD module used WTC exposures as the index trauma. Both current (i.e., active in the past month) and remitted (i.e., not active in the past month) diagnoses were analyzed. Treatment for PTSD is freely available in the clinic to responders in need; 70.8% of those categorized as having current PTSD and 43.3% of those with remitted PTSD received treatment for PTSD.
3.3.3. Longitudinal PTSD and depressive symptoms measures
PTSD symptoms were assessed at each monitoring visit using the PTSD checklist, specific trauma version tailored to the WTC disaster (PCL-17 trauma specific version) [21]. Individuals rated the extent to which they were bothered by 17 DSM-IV WTC-related PTSD symptoms in the past month on a scale from 1 (not at all) to 5 (extremely). Items were summed within four PTSD symptom dimensions consistent with four-factor models of PTSD dimensionality [8]: re-experiencing the event (e.g., flashbacks/nightmares), effortful avoidance (e.g., actively avoiding reminders), emotional numbing (e.g., emotionally distancing from life), and hyperarousal (e.g., being ever aware and on edge). Depressive symptoms were measured using the Patient Health Questionnaire (PHQ-9) [22]. PHQ-9 items, rated on a scale from 0–3 over the past 2 weeks, were summed in a standard way to provide a total score. For comparative purposes, both scales were transformed to range from 0 (no symptomatology) to 1 (maximal observed symptomatology). Baseline symptomatology refers to symptomatology collected during a responder's first clinic visit.
3.4. Covariates
Predisposing characteristics were included. Education improves cognitive reserve [23]; because >98% of responders had at least a high-school degree, education were categorized into those with some college, those completing a bachelor's degree, versus those with less education. Occupation was dichotomized into law enforcement (the majority at SBU) versus nontraditional responders (e.g., construction or utility workers). Pre-WTC PTSD was assessed using the SCID. Pre-WTC history of head injury was coded as none, previous loss of consciousness, concussion, or multiple head injuries.
Trauma severity was assessed at enrollment using a structured history. Two measures of exposure were included: early arrival (arrived on 9/11 and were caught in the dust cloud or saw human remains) and chronic exposure (responders who worked at least 7 days in September 2001 digging through debris).
PTSD might impact cognition through impaired health and health behaviors [24]. Five indicators were included: smoking status; hazardous drinking (Alcohol Use Disorder Identification Test ≥8) [25]; obesity, operationalized as objectively measured body mass index >30; and diagnosed hypertension or diabetes [26]. Analyses also include WTC-related conditions including upper respiratory disease, lower respiratory disease, and gastroesophageal reflux disease.
3.5. Statistical analysis
3.5.1. Descriptive analyses
Descriptive sample statistics provide means and standard deviations, as well as percentages. Sample characteristics were also reported separately for those with and without CI. T tests were used to compare continuous variables between groups; χ2 tests were used to provide P values for dichotomous predictors.
PTSD/MDD symptom growth could be interpreted as indicative of reverse causation resulting from early noncognitive changes in mental health accompanying CI and may thus help us to chart the course of the disease and identify whether PTSD preceded or may have resulted from CI. Associations that are consistent and predate such reverse causation may then be interpretable as risk factors for CI. Descriptive analyses thus examined trajectories of symptom change for responders with and without current CI. Longitudinal multivariate models were used to examine symptom change leading up to cognitive assessments [27]. Models integrated individual-level random intercepts to account for unobserved heterogeneity in baseline symptomatology. Random slopes estimate individual-specific growth and account for heteroskedasticity common in growth models [28]. Enrollment date was used to model temporal change in sample selection; temporal metrics were centered in the observational window. Using an unstructured covariance, matrix further accounted for associations between baseline capability and change over time. To facilitate comprehension, graphical trajectories were provided. The 813 responders provided 7.24 (SD = 2.95) person-years of data collected during 3995 clinic visits from 2002–2014. Analyses were implemented in Stata 14.1/IC.
3.5.2. Multivariable analyses
Multivariable logistic regression was used to examine the potential for diagnoses of PTSD and MDD to predict CI, and subdomains of PTSD identified as robust to reverse causation in predicting CI. The risk of CI was not rare in this sample so odds ratios will overestimate the relative risk. Multivariable-adjusted risk ratios (aRRs) were derived from logistic regression using the Muller and MacLehose [29] risk ratio.
3.5.3. Sensitivity analyses
Law enforcement officers differ from other responders with respect to exposures, extent of disaster training, and burden of PTSD. Similarly, those with pre-WTC head injuries may differ from those without. Analyses were stratified to examine whether results differed across these subpopulations; Chow test was used to test whether differences were significant. For clarity, analyses further examined whether excluding individuals enrolled in the study after 2010 modified results. Longitudinal analyses also examined any changes to conclusions when modifying distributional assumptions, including examining negative binomial, ordinal, and logistic regression specifications. Similarly, analyses examined whether conclusions drawn from trajectory analyses changed when limited to individuals with at least three observations. Analyses examined whether excluding those screening positive for possible dementia (MoCA<18) changed conclusions, and further multinomial logistic regression was used to jointly examine conclusions predicting both CI and dementia. Because treatment may provide a mechanism through which PTSD affects CI, analyses examined whether treatment for PTSD-mediated results.
4. Results
4.1. Sample characteristics
As shown in Table 1, 104 of 813 (12.8%) responders had any CI, of whom 10 (1.2% of 813) had probable dementia. Bivariable analyses (Table 1) showed that responders with CI had lower education, nontraditional occupations, older age and were more likely to be current smokers than those without CI. Current WTC-PTSD and MDD were both associated with CI, as was receiving treatment for PTSD.
Table 1.
Sample characteristics, Stony Brook University World Trade Center Aging Study 2002–2015
| Variable | No CI (n = 709) | CI (n = 104) | P |
|---|---|---|---|
| Age in years | 51.58 (8.41) | 54.41 (8.64) | .002 |
| Enrollment year | 2007.88 (3.39) | 2008.08 (3.47) | .576 |
| PTSD | |||
| Remitted | 58 (8.2%) | 9 (8.7%) | .013 |
| Current | 69 (9.7%) | 20 (19.2%) | |
| MDD | |||
| Remitted | 75 (10.6%) | 13 (12.5%) | .007 |
| Current | 53 (7.5%) | 17 (16.3%) | |
| Female Sex | 51 (7.2%) | 6 (5.8%) | .595 |
| Law Enforcement | 510 (71.9%) | 63 (60.6%) | .018 |
| Education | |||
| Some College | 342 (48.2%) | 52 (50.0%) | .025 |
| University degree | 208 (29.3%) | 19 (18.3%) | |
| Head injury | |||
| Lost consciousness | 33 (4.6%) | 9 (8.5%) | .645 |
| Concussion | 61 (8.6%) | 9 (8.4%) | |
| Multiple | 126 (17.7%) | 15 (14.5%) | |
| Pre-WTC PTSD | 96 (13.5%) | 16 (15.4%) | .301 |
| Early Arrival | 330 (46.5%) | 41 (39.4%) | .173 |
| Chronic exposure | 413 (58.3%) | 63 (60.6%) | .653 |
| Obese | 348 (49.1%) | 46 (44.2%) | .355 |
| Current smoker | 48 (6.8%) | 12 (11.5%) | .016 |
| Hazardous Drinker | 35 (5.0%) | 5 (5.1%) | .989 |
| Hypertensive | 201 (28.3%) | 27 (26.0%) | .613 |
| Diabetic | 36 (5.1%) | 5 (4.8%) | .907 |
| URD | 527 (64.9%) | 477 (58.7%) | .216 |
| LRD | 328 (40.3%) | 328 (40.4%) | .993 |
| GERD | 182 (22.4%) | 164 (20.2%) | .608 |
| PTSD Treatment | 116 (14.2%) | 180 (22.1%) | .037 |
Abbreviations: CI, cognitive impairment; GERD, gastro-esophageal reflux disease; URD, upper respiratory disease; LRD, lower respiratory disease; MDD, major depressive disorder; PTSD, posttraumatic stress disorder.
NOTE. Data presented as mean (SD) or n (%).
4.2. Diagnostic results
Diagnoses were associated with CI after accounting for covariates. Indeed, Table 2 revealed that current PTSD remained significantly associated with CI after adjusting for predisposing, WTC exposures, and for health and behavioral mediators (model 1). Adjusted analyses also found that current MDD remained associated with CI (model 2).
Table 2.
Association between CI and PTSD or MDD, Stony Brook University World Trade Center Aging Study 2002–2015
| Model 1 | Model 2 | |
|---|---|---|
| PTSD | ||
| None | 1.00 | |
| Remitted | 1.27 (0.63–2.27) | |
| Current | 1.93 (1.2–2.78) | |
| MDD | ||
| None | 1.00 | |
| Remitted | 1.35 (0.76–2.19) | |
| Current | 2.16 (1.31–3.13) | |
Abbreviations: CI, cognitive impairment; MDD, major depressive disorder; PTSD, posttraumatic stress disorder.
NOTE. Results presented as RR (95% CI). Reference category is shown using 1.00. Model 1 incorporates PTSD but not MDD, whereas model 2 incorporates PTSD but not MDD. Both models adjust for all other covariates.
4.3. Longitudinal symptomatology
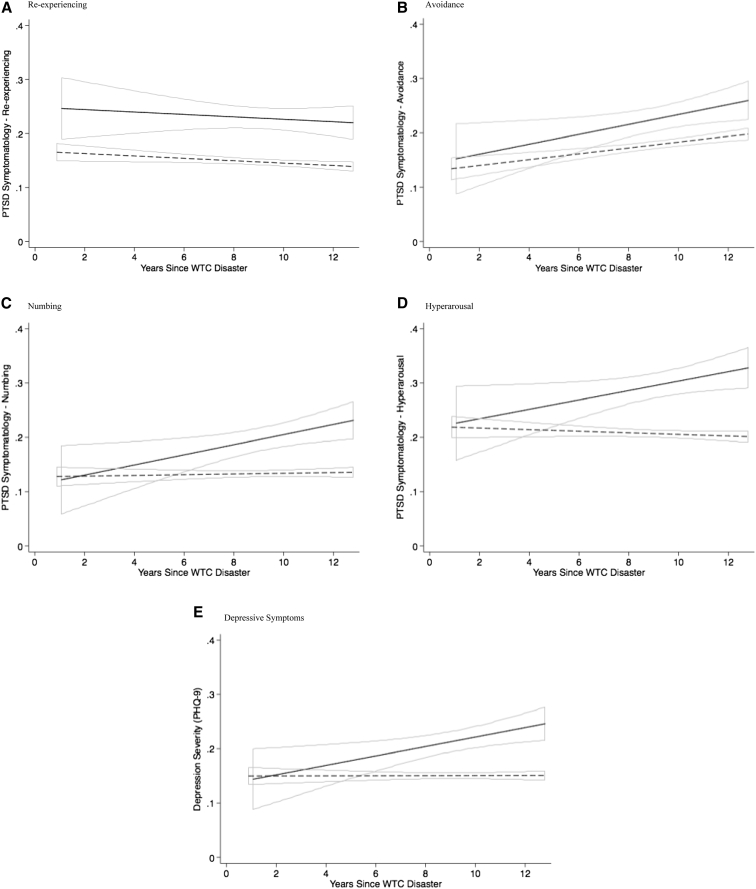
The lack of association between current, but not remitted, diagnoses might be indicative of a potential for longitudinal symptom growth. Indeed, longitudinal analyses (Fig. 2B–E) suggested that baseline levels of avoidance, hyperarousal, numbing, and depressive symptoms were not associated with CI. Instead, longitudinal analyses revealed that symptoms increased more rapidly (by ∼0.9%/year) among responders with current CI. In contrast, for re-experiencing symptoms, longitudinal analyses suggest consistent associations with CI beginning as early as 2002 that did not change with time (Fig. 2A).
Fig. 2.
Longitudinal trajectories of change in PTSD and depressive symptoms during the years since the WTC disaster, separated by PTSD subdomain, Stony Brook University World Trade Center Aging Study 2002–2015. (A) Re-experiencing; (B) avoidance; (C) numbing; (D) hyperarousal; (E) depressive symptoms.
4.4. Re-experiencing symptoms
Because longitudinal analyses indicated that re-experiencing symptom severity may be a risk factor for CI, adjusted analyses were used to examine the robustness of this domain in predicting CI (Table 3). Re-experiencing symptom severity ranged from 0 to 1 and average 0.17 (SD = 0.20) in general and ranged from 0.16 (SD = 0.19) in those not screening positive for CI to 0.23 (SD = 0.23) among those who screened positive for CI. Adjusting for predisposing factors (model 1) showed a significant association between age and CI. Accounting for WTC exposures (model 2) and for health factors including treatment for PTSD and baseline depressive symptoms (Model 3) did not improve model fit (model 2, P = .654; model 3, P = .406). Notably, baseline re-experiencing symptom severity predicted diagnoses of remitted and current PTSD and MDD (eTable 2).
Table 3.
Associations between cognitive impairment and re-experiencing symptoms for World Trade Center–related post-traumatic stress disorder, Stony Brook University World Trade Center Aging Study 2002–2015
| Model 1 |
Model 2 |
Model 3 |
|
|---|---|---|---|
| aRR (95% CI) | aRR (95% CI) | aRR (95% CI) | |
| Re-experiencing symptoms | 2.88 (1.35–6.11) | 2.91 (1.32–6.43) | 3.82 (1.24–11.74) |
| Year of first clinic visit | 1.03 (0.97–1.09) | 1.03 (0.98–1.09) | 1.04 (0.99–1.11) |
| Age in years | 1.03 (1.01–1.05) | 1.03 (1.01–1.06) | 1.04 (1.01–1.06) |
Abbreviations: 95% CI, 95% confidence interval; aRR: adjusted risk ratio.
NOTE. Results presented as RR (95% CI). Model 1 accounts for variables shown along with predisposing factors including sex, law enforcement, educational attainment, head injury, and pre-WTC PTSD. Model 2 further accounts for trauma severity. Model 3 additionally accounts for contemporary health and health behaviors, baseline depressive symptoms, and having ever received treatment for PTSD.
4.5. Sensitivity analyses
Bivariate analyses showed no association between enrollment year and the odds of CI, and longitudinal analyses show no association between enrollment year and PTSD. Stratifying analyses by enrollment year, law enforcement status, head injury status, and omitting those with possible dementia did not alter conclusions. Further stratifying by age of responder provided similar results. Multinomial logistic regression results adjusting for age rely on small numbers (eTable 3) but revealed strong independent associations between re-experiencing and both CI (RR = 3.94 [1.53–10.17]) and dementia (RR = 17.45 [1.10–275.69]). Longitudinal results were robust using different distributional assumptions. Examining exclusion bias, prevalence in a sample of responders who were ineligible for this study (n = 128) showed similar prevalence of CI (12.5% vs. 12.8% above, difference = 0.3%, P = .925). APOE ε4/ε4 was associated with increased risk of possible dementia (RR = 9.68 [1.13–83.11]). Adjusting for medications did not decrease the size of the association between diagnosed PTSD and CI (to 1.83 from 1.92) but attenuated its significance.
5. Discussion
This study examined the extent of CI among WTC responders and associations between CI and WTC-PTSD/MDD in a sample of 813 WTC responders without WTC-related head injury. Approximately one in seven (12.8%) of sampled SBU WTC responders screened positive with CI, whereas 1.2% had possible dementia. If representative of actual prevalence of CI in the general responder cohort (N∼33,000) [14], results may translate into 3740–5300 and 240–810 with CI and dementia respectively in that patient population. These numbers are staggering, considering that the average age of responders was 53 during this study. Future research is needed to diagnose, and clarify determinants of, CI in this population.
Current, but not remitted, WTC-PTSD and MDD were associated with a two-fold increase in CI. Longitudinal analyses highlight growth in a number of symptom domains, including avoidance, numbing, hyperarousal, and depressive symptoms among those who later screen positive for CI. Such growth may indicate early manifestations of CI. Strikingly, analyses suggested that re-experiencing symptoms were consistently associated with CI (aRR = 2.88, 95% CI = 1.35–6.22), irrespective of observational period. To our knowledge, this is the first study to examine the association of PTSD and MDD with CI and to do so in a civilian sample of WTC responders without concurrent head trauma.
5.1. Limitations
Results require validation using comprehensive batteries of cognition and diagnostic evaluations by a trained clinician to diagnose the cause of CI found here to ensure that WTC-CI is not a unique disease and that discussed are not spurious. Such analyses should start by examining domain-specific factors and longitudinal declines in cognitive functioning. Although CI and dementia were associated with APOE status, replicating earlier analyses [19], APOE was not associated with risk of PTSD in these data nor did APOE status modify the association between exposure and PTSD. Results are limited to participants who were selected for screening, and this study does not inform us about the relative risk of WTC responders to other individuals with similar occupational exposures. Although a separate sample of SBU responders who were ineligible for this study revealed similar prevalence (12.5% with CI), efforts may seek to improve generalizability by gathering prospective data from cognitively normal WTC responders and from nonexposed controls to determine incidence of CI. Finally, although four WTC responders with WTC-related head injury were excluded, one-third of the sample reported some history of head trauma including loss of consciousness or concussion often due to sports injuries or car accidents. Although no significant association was found linking head injuries and CI, future research should explore whether specific aspects of prior head injury (e.g., age of first occurrence, number of injuries) may modify the relationship between PTSD and CI.
5.2. Aging
Traumatic stress can arise from various sources ranging from natural and human-made disasters to interpersonal traumas. Approximately 5%–10% of individuals exposed to trauma develop chronic PTSD [30]. In a primary care sample aged 65 years and older, 24% reported some lifetime trauma, and one third of them (8% of patients) had symptoms consistent with PTSD [31]. Few studies of survivors of, or responders to, disasters have considered the potential impact of PTSD on CI and the reverse [15]. Indeed, a lone longitudinal study of PTSD symptom change in relation to memory (n = 28) found inconsistent results [32], although a large study of veterans found that PTSD was associated with a two-fold increase in the risk of incident dementia [33]. The present study revealed that experiencing trauma resulting in PTSD is associated with increased risk of CI, thereby extending results from Veterans studies to a civilian sample while highlighting the importance of monitoring cognitive functioning in traumatized populations.
5.3. Neurodegeneration
Dementia results from several causes including neurodegeneration resulting in CI [34]. The prodromal period is usually characterized by accelerated cognitive aging, which can last a decade [35], and increasing levels of CI in the years before becoming diagnostic for dementia [16]. Milder forms of CI are the largest risk factor for incident dementia and are thus often called prodromal [34], [36]. The prodromal period can be accompanied by noncognitive behavioral and emotional changes [37]. For example, one prospective study of incident dementia found that the risk of depression increased among previously nondepressed respondents during the prodromal period suggesting that depression has the potential to act as an early manifestation or risk indicator of dementia [38]. Our study analyzed changes in PTSD and depressive symptoms measured routinely across over a decade before cognitive screening and found that depressive and many PTSD symptoms were no different in 2002 between those with and without CI and increased only in those who later screened positive for CI. Yet, whereas results for most symptom domains suggested that changes in PTSD and depressive symptoms may be part of the prodrome of CI, associations between re-experiencing symptoms and CI uniquely lead to a different set of conclusions, namely that they preceded the onset of CI and were not subject to reverse causation.
5.4. Post-traumatic stress disorder
Common treatments for PTSD include cognitive behavioral therapy and pharmacological treatments, notably selective serotonin reuptake inhibitors [39]. Currently, 70.8% of responders with current PTSD are being treated, making this study underpowered to examine the potential role of medications versus diagnoses. Nevertheless, studies suggest that cognitive deficits are not evident with common PTSD treatments [40]. Moreover, in sensitivity analyses, the association between re-experiencing symptoms, which are insufficient for diagnosis alone, and CI remained even after adjusting for medications, suggesting that re-experiencing symptoms and not medications were attributable for lowered cognitive performance.
Results support research noting the importance of re-experiencing symptoms as an early marker of mental pathology [7]. Intrusive re-experiencing symptoms are the embodiment of a neurological flash bulb memory processes, which can be triggered by stressful events and tend to remain consistent for many individuals [41] that are a foundational element of chronic PTSD. Re-experiencing symptoms occur when individuals react physically and emotionally to memories of past trauma that intrude during daily activities and while asleep. Sleep disturbances are fundamental to PTSD [42], may exacerbate allostatic load [43], and have been linked to incident dementia [44]. However, intrusive stress while awake has also been previously associated with poorer episodic memory [45] and poorer fluid cognition [46], potentially highlighting the central importance of cognitive interference to CI.
Evidence linking such complex memory and behavioral processes to CI could be interpreted in several ways. Re-experiencing traumatic events could cause CI, possibly through a number of plausible biological mechanisms including increased allostatic load [10]. If so, efforts to prevent or treat PTSD may have long-term benefits. Re-experiencing symptoms may be part of the PTSD syndrome, with chronic PTSD linked to risk of CI, in which case diagnostic efforts may benefit from cognitive screening. Alternatively, those at risk of re-experiencing the event may be similarly vulnerable to CI in some unobserved fashion. Further research is needed to examine whether broken sleep cycles are a mechanism linking PTSD to CI.
5.5. Clinical implications
Clinicians should be aware of the potential for CI among individuals with PTSD, and may want to monitor patients for complications due to CI. For example, CI may compound the course of PTSD and depression, impairing the patient beyond the impact of PTSD itself. Second, as found here, CI may result in increased PTSD symptom severity. Furthermore, CI implies reduced ability to comply with therapeutic regimes and self-manage chronic disease. Thus, clinicians should be alert to CI when caring for traumatized patients and when making decisions about treatment schedules.
Research in context.
-
1.
Systematic review: Prior systematic reviews have noted a relationship between post-traumatic stress disorder (PTSD) and lowered cognition as well as increased risk of dementia and in traumatized populations.
-
2.
Interpretation: Associations between cognitive impairment (CI) and PTSD were replicated in a non-military WTC-exposed population without any 9/11-related head injury. Strikingly, re-experiencing symptoms as early as 2002 were associated with CI in 2014. In contrast, emotional and behavioral domains of PTSD have increased drastically since 2002 among those with CI versus those without CI.
-
3.
Future directions: The article proposes a guide for clarifying temporality of noncognitive symptoms of CI. Insofar as results replicate, clinicians should be aware of CI when caring for traumatized populations. Future research should seek to diagnose the cause of CI in this sample, identify whether prevalence of CI is higher in this sample than non-WTC-exposed controls, and further delineate impacted domains of cognitive functioning.
Acknowledgements
The authors would like to acknowledge the support from the National Institute of Occupational Safety and Health (NIOSH) who support World Trade Center clinical and monitoring efforts at Stony Brook University (CDC/NIOSH 200-2011-39361: PI: Luft). Research efforts were supported by funding to incorporate the SCID (CDC/NIOSH 200-2011-39410; PI: Bromet) and to examine cognitive impairment in this population (NIH/NIA R01 049953; PI: Clouston). We gratefully acknowledge the support of World Trade Center responders for generously contributing their time and energy. We also wish to thank the research staff at the Stony Brook World Trade Center Health Program for their support.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.dadm.2016.08.001.
Supplementary data
References
- 1.McKhann G.M., Knopman D.S., Chertkow H., Hymna B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer's Association Alzheimer's disease: facts and figures. Alzheimers Dement. 2014;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Petersen R.C. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 4.Plassman B.L., Williams J.W., Burke J.R., Holsinger T., Benjamin S. Systematic Review: Factors Associated With Risk for and Possible Prevention of Cognitive Decline in Later Life. Ann Intern Med. 2010;153:182–193. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- 5.Schuitevoerder S., Rosen J.W., Twamley E.W., Ayers C.R., Sones H., Lohr J.B. A meta-analysis of cognitive functioning in older adults with PTSD. J Anxiety Disord. 2013;27:550–558. doi: 10.1016/j.janxdis.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Veitch D.P., Friedl K.E., Weiner M.W. Military risk factors for cognitive decline, dementia and Alzheimer's disease. Curr Alzheimer Res. 2013;10:907–930. doi: 10.2174/15672050113109990142. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence-Wood E., Van Hooff M., Baur J., McFarlane A.C. Re-experiencing phenomena following a disaster: The long-term predictive role of intrusion symptoms in the development of post-trauma depression and anxiety. J Affect Disord. 2016;190:278–281. doi: 10.1016/j.jad.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 8.King D.W., Leskin G.A., King L.A., Weathers F.W. Confirmatory factor analysis of the clinician-administered PTSD Scale: Evidence for the dimensionality of posttraumatic stress disorder. Psychol Assess. 1998;10:90. [Google Scholar]
- 9.Pitman R.K. Posttraumatic stress disorder and dementia posttraumatic stress disorder and dementia. JAMA. 2010;303:2287–2288. doi: 10.1001/jama.2010.767. [DOI] [PubMed] [Google Scholar]
- 10.Greenberg M.S., Tanev K., Marin M.-F., Pitman R.K. Stress, PTSD, and dementia. Alzheimers Dement. 2014;10:S155–S165. doi: 10.1016/j.jalz.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 11.McKee A.C., Robinson M.E. Military-related traumatic brain injury and neurodegeneration. Alzheimers Dement. 2014;10:S242–S253. doi: 10.1016/j.jalz.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shalev A.Y., Freedman S., Peri T., Brandes D., Sahar T., Orr S.P. Prospective study of posttraumatic stress disorder and depression following trauma. Am J Psychiatry. 1998;155(5):630–637. doi: 10.1176/ajp.155.5.630. [DOI] [PubMed] [Google Scholar]
- 13.van Achterberg M.E., Southwick S.M. Emergence of PTSD in trauma survivors with dementia. J Clin Psychiatry. 2001;62:206–207. doi: 10.4088/jcp.v62n0312c. [DOI] [PubMed] [Google Scholar]
- 14.Dasaro C.R., Holden W.L., Berman K.D., Crane M.A., Kaplan J.R., Lucchini R.G. Cohort Profile: World Trade Center Health Program General Responder Cohort. Int J Epidemiol. 2015 doi: 10.1093/ije/dyv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neria Y., DiGrande L., Adams B.G. Posttraumatic stress disorder following the September 11, 2001, terrorist attacks: A review of the literature among highly exposed populations. Am Psychol. 2011;66:429. doi: 10.1037/a0024791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amieva H., Jacqmin-Gadda H., Orgogozo J.-M., Nicolas Le Carret, Catherine Helmer, Luc Letenneur. The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain. 2005;128:1093–1101. doi: 10.1093/brain/awh451. [DOI] [PubMed] [Google Scholar]
- 18.Luis C.A., Keegan A.P., Mullan M. Cross validation of the Montreal Cognitive Assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry. 2009;24:197–201. doi: 10.1002/gps.2101. [DOI] [PubMed] [Google Scholar]
- 19.Strittmatter W.J., Roses A.D. Apolipoprotein E and Alzheimer disease. Proc Natl Acad Sci U S A. 1995;92:4725–4727. doi: 10.1073/pnas.92.11.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.First M., Spitzer R., Gibbon M., Williams J.B.W. New York Psychiatric Institute, Biometrics Research Department; New York, NY: 1996. Structured Clinical Interview for DSM-IV Axis I Disorders–Clinician version (SCID-I/P, Version 2.0) [Google Scholar]
- 21.Blanchard E.B., Jones-Alexander J., Buckley T.C., Forneris C.A. Psychometric properties of the PTSD Checklist (PCL) Behav Res Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K., Spitzer R.L., Williams J.B. The Phq-9. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clouston S., Kuh D., Herd P., Elliott J., Richards M., Hofer S.M. Benefits of educational attainment on adult fluid cognition: International evidence from three birth cohorts. Int J Epidemiol. 2012;41:1729–1736. doi: 10.1093/ije/dys148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackin R.S., Lesselyong J.A., Yaffe K. Pattern of cognitive impairment in older veterans with posttraumatic stress disorder evaluated at a memory disorders clinic. Int J Geriatr Psychiatry. 2012;27:637–642. doi: 10.1002/gps.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bohn M.J., Babor T.F., Kranzler H.R. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56:423–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- 26.Barnes D.E., Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gibbons R., Hedeker D., Waternaux C., Davis J.M. Random regression models: a comprehensive approach to the analysis of longitudinal psychiatric data. Psychopharmacol Bull. 1988;24:438. [PubMed] [Google Scholar]
- 28.Bliese P.D., Ployhart R.E. Growth modeling using random coefficient models: Model building, testing, and illustrations. Organizational Res Methods. 2002;5:362–387. [Google Scholar]
- 29.Muller C.J., MacLehose R.F. Estimating predicted probabilities from logistic regression: different methods correspond to different target populations. Int J Epidemiol. 2014;43:962–970. doi: 10.1093/ije/dyu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 31.Rauch S.A., Morales K.H., Zubritsky C., Knott K., Oslin D. Posttraumatic stress, depression, and health among older adults in primary care. Am J Geriatr Psychiatry. 2006;14:316–324. doi: 10.1097/01.JGP.0000199382.96115.86. [DOI] [PubMed] [Google Scholar]
- 32.Yehuda R., Tischler L., Golier J.A., Grossman R., Brand S.R., Kaufman S. Longitudinal assessment of cognitive performance in Holocaust survivors with and without PTSD. Biol Psychiatry. 2006;60:714–721. doi: 10.1016/j.biopsych.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 33.Yaffe K., Vittinghoff E., Lindquist K., Barnes D., Covinsky K.E., Neylan T. Posttraumatic stress disorder and risk of dementia among us veterans. Arch Gen Psychiatry. 2010;67:608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Insel P.S., Mattsson N., Mackin R.S., Schöll M., Nosheny R.L., Tosun D. Accelerating rates of cognitive decline and imaging markers associated with β-amyloid pathology. Neurology. 2016;86:1887–1896. doi: 10.1212/WNL.0000000000002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clouston S., Glymour M., Muñiz-Terrera G. Educational inequalities in aging-related declines in fluid cognition and the onset of cognitive pathology. Alzheimer's & Dementia: Diagnosis, Assessment, and Disease Monitoring. 2015;1:303–310. doi: 10.1016/j.dadm.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jessen F., Wolfsgruber S., Wiese B., Bickel H., Mösch E., Kaduskiewicz H. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement. 2014;10:76–83. doi: 10.1016/j.jalz.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Jack C.R., Knopman D.S., Jagust W.J., Shaw L.M., Aisen P.S., Weiner M.W. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li G., Wang L.Y., Shofer J.B., Thompson M.L., Peskind E.R., McCormick W. Temporal relationship between depression and dementia: Findings from a large community-based 15-year follow-up study. Arch Gen Psychiatry. 2011;68:970–977. doi: 10.1001/archgenpsychiatry.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Foa E.B., Keane T.M., Friedman M.J., Cohen J.A. Guilford Press; New York, NY: 2008. Effective treatments for PTSD: practice guidelines from the International Society for Traumatic Stress Studies. [Google Scholar]
- 40.Biringer E., Rongve A., Lund A. A review of modern antidepressants' effects on neurocognitive function. Curr Psychiatry Rev. 2009;5:164–174. [Google Scholar]
- 41.Hirst W., Phelps E.A., Buckner R.L., Budson A.E., Cuc A., Gabrieli J.D. Long-term memory for the terrorist attack of September 11: flashbulb memories, event memories, and the factors that influence their retention. J Exp Psychol Gen. 2009;138:161. doi: 10.1037/a0015527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Germain A. Sleep disturbances as the hallmark of PTSD: where are we now? Am J Psychiatry. 2013;170:372–382. doi: 10.1176/appi.ajp.2012.12040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McEwen B.S., Karatsoreos I.N. Sleep deprivation and circadian disruption: stress, allostasis, and allostatic load. Sleep Med Clin. 2015;10:1–10. doi: 10.1016/j.jsmc.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sterniczuk R., Theou O., Rusak B., Rockwood K. Sleep disturbance is associated with incident dementia and mortality. Curr Alzheimer Res. 2013;10:767–775. doi: 10.2174/15672050113109990134. [DOI] [PubMed] [Google Scholar]
- 45.Stawski R.S., Sliwinski M.J., Smyth J.M. The effects of an acute psychosocial stressor on episodic memory. Eur J Cogn Psychol. 2009;21:897–918. doi: 10.1080/09541440802333042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stawski R.S., Sliwinski M.J., Smyth J.M. Stress-related cognitive interference predicts cognitive function in old age. Psychol Aging. 2006;21:535. doi: 10.1037/0882-7974.21.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.