Abstract
Objective
Frontotemporal dementia (FTD) is characterized by behavioral disturbances and language problems. Familial forms can be caused by genetic defects in microtubule-associated protein tau (MAPT), progranulin (GRN), and C9orf72. In light of upcoming clinical trials with potential disease-modifying agents, the development of sensitive biomarkers to evaluate such agents in the earliest stage of FTD is crucial. In the current longitudinal study we used arterial spin labeling MRI (ASL) in presymptomatic carriers of MAPT and GRN mutations to investigate early changes in cerebral blood flow (CBF).
Methods
Healthy first-degree relatives of patients with a MAPT or GRN mutation underwent ASL at baseline and follow-up after two years. We investigated cross-sectional and longitudinal differences in CBF between mutation carriers (n = 34) and controls without a mutation (n = 31).
Results
GRN mutation carriers showed significant frontoparietal hypoperfusion compared with controls at follow-up, whereas we found no cross-sectional group differences in the total study group or the MAPT subgroup. Longitudinal analyses revealed a significantly stronger decrease in CBF in frontal, temporal, parietal, and subcortical areas in the total group of mutation carriers and the GRN subgroup, with the strongest decrease in two mutation carriers who converted to clinical FTD during follow-up.
Interpretation
We demonstrated longitudinal alterations in CBF in presymptomatic FTD independent of grey matter atrophy, with the strongest decrease in individuals that developed symptoms during follow-up. Therefore, ASL could have the potential to serve as a sensitive biomarker of disease progression in the presymptomatic stage of FTD in future clinical trials.
Abbreviations: FTD, frontotemporal dementia; MAPT, microtubule-associated protein tau; GRN, progranulin; ASL, arterial spin labeling; CBF, cerebral blood flow; FDG-PET, positron emission tomography with 18F-fluorodeoxyglucose; MMSE, Mini-Mental State Examination; BDI-II, Beck Depression inventory II (BDI-II); RAVLT, Rey Auditory Verbal Learning Test; VAT, Visual Association Test; TMT, Trailmaking Test; WCST, Wisconsin Card Sorting Test; LDST, Letter Digit Substitution Test; BNT, Boston Naming Test; SAT, Semantic Association Test; AD, Alzheimer's disease
Keywords: Frontotemporal dementia, Arterial spin labeling, Cerebral blood flow, Presymptomatic
Highlights
-
•
Longitudinal alterations in cerebral blood flow in presymptomatic FTD
-
•
Larger decline in cerebral blood flow during conversion to symptomatic FTD
-
•
Arterial spin labeling might provide a useful biomarker for therapeutic trials.
1. Introduction
Frontotemporal dementia (FTD) is the second most common form of presenile dementia, characterized by behavioral disturbances and language disorders, which is caused by neurodegeneration of the frontal and temporal lobes (Rascovsky et al., 2011, Seelaar et al., 2011a). Mutations in microtubule-associated protein tau (MAPT), progranulin (GRN) and C9orf72, and, less frequently, CHMP2B and VCP can cause an autosomal dominant form of FTD (Renton et al., 2011, DeJesus-Hernandez et al., 2011, Baker et al., 2006, Hutton et al., 1998, Skibinski et al., 2005, Watts et al., 2004). There is currently no therapy available to prevent or cure the disease, but knowledge on the pathophysiological disease process is rapidly growing. As a result, clinical trials with disease-modifying agents are upcoming, urging the need for sensitive biomarkers to evaluate such therapies (Tsai and Boxer, 2014). We have previously shown changes in neuropsychological performance, white matter integrity and functional connectivity in the presymptomatic stage of FTD (Dopper et al., 2014).
Positron emission tomography with 18F-fluorodeoxyglucose (FDG-PET) is often suggested as a useful biomarker for the earliest stage of FTD. Hypometabolism in the frontal and anterior temporal lobes, and subcortical regions, is consistently seen in FTD patients (Ishii et al., 1998, Jeong et al., 2005, Diehl et al., 2004, Grimmer et al., 2004), as well as progression over time (Grimmer et al., 2004). Moreover, presymptomatic GRN mutation carriers already showed regional hypometabolism on FDG-PET, supporting its potential to detect functional brain changes in a very early stage (Jacova et al., 2013). However, FDG-PET has several serious disadvantages to be used as a biomarker for FTD, including the high costs, invasiveness, limited availability of PET-scanners and the need for exposure to a radioactive tracer (Wolk and Detre, 2012).
Arterial spin labeling MRI (ASL) provides a non-invasive measure of brain perfusion by magnetically labeling water protons in arterial blood, thereby creating an endogenous tracer of cerebral blood flow (CBF), which is assumed to be tightly coupled to brain metabolism (Wolk and Detre, 2012). ASL studies in patients with FTD have provided highly similar results as FDG-PET studies with hypoperfusion in bilateral frontal lobes, the anterior cingulate cortex, insula, and thalamus compared with controls (Binnewijzend et al., 2014, Du et al., 2006, Hu et al., 2010, Shimizu et al., 2010, Zhang et al., 2011, Steketee et al., 2015). Besides one small study in presymptomatic CHMP2B mutation carriers showing widespread hypoperfusion in hippocampus, temporal, parietal, and occipital lobes compared with controls by means of spin echo contrast agent perfusion MRI (Lunau et al., 2012), CBF has not been investigated in the presymptomatic stage of FTD thus far.
In the current study we used longitudinal ASL to study early changes in CBF in presymptomatic carriers of MAPT and GRN mutations to investigate whether ASL has the potential to serve as a sensitive biomarker to detect FTD and track disease progression in the earliest disease stage.
2. Materials and methods
2.1. Participants
This study is part of a larger project investigating biomarkers in individuals at risk for FTD (Dopper et al., 2014). Healthy first-degree relatives (aged 20–70 years) of patients with FTD due to a GRN or MAPT mutation were included in this study. Subjects were excluded in case of MRI contraindication (n = 6), history of drug abuse (n = 2), or other neurologic (n = 1) or psychiatric (n = 1) disorders. The drug abuse or psychiatric disorders were not related to FTD, since it was either associated with non-carriership or there was no progression of symptoms over time. No subjects from families with the C9orf72 repeat expansion were included, since this gene defect was not yet identified at the start of this study (2010). DNA of all subjects was screened (Seelaar et al., 2008), resulting in a group of presymptomatic individuals with a MAPT or GRN mutation and a group of controls without a mutation. All participants were studied at baseline and two-years follow-up. In total, 73 participants underwent baseline and follow-up ASL-MRI scans, however, eight subjects had to be excluded from the analyses because of labeling errors or major artefacts at baseline (n = 3) or follow-up (n = 5), leaving in total 65 participants. We investigated cross-sectional differences in CBF between mutation carriers and controls at baseline and follow-up. Moreover, we investigated between-group differences in longitudinal CBF changes. All analyses were ran for the entire group of mutation carriers versus controls, and separately for subjects from MAPT (n = 18) and GRN (n = 47) families. Subsequently, we evaluated single-subject data. Conversion to symptomatic FTD was diagnosed according to established clinical criteria (Rascovsky et al., 2011, Gorno-Tempini et al., 2011). The local ethics committee approved the study and all participants provided written informed consent.
2.2. Neuropsychological assessment
All participants were screened using the Mini-Mental State Examination (MMSE) (Folstein et al., 1975) the Beck Depression inventory II (BDI-II) (Beck et al., 1996) and an extensive neuropsychological assessment including the Dutch version of the Rey Auditory Verbal Learning Test (RAVLT) (Rey, 1958), Visual Association Test (VAT) (Lindeboom et al., 2002), WAIS III subtests digit span, similarities, and block design (Wechsler, 1997, Wechsler, 2005), Trailmaking Test (TMT) (Battery, 1994), Stroop color-word test (Stroop, 1935), categorical and letter fluency (Thurstone and Thurstone, 1962), modified Wisconsin Card Sorting Test (WCST) (Nelson, 1976), Letter Digit Substitution Test (LDST) (Jolles et al., 1995), Boston Naming Test (BNT) (Kaplan et al., 1978), Semantic Association Test (SAT) (Visch-Brink et al., 2005), ScreeLing phonology (Doesborgh et al., 2003), clock drawing (Royall) (Royall et al., 1998), Ekman faces (Ekman and Friesen, 1976), and Happé Cartoons (Happe et al., 1999).
2.3. Image acquisition
MRI scans were acquired using a Philips 3.0 Tesla Achieve MRI scanner (Philips Medical Systems, Best, The Netherlands) using an eight-channel SENSE head coil. We obtained pseudo-continuous ASL scans using single-shot echo-planar imaging (EPI) with a background suppression scheme, consisting of a saturation pulse directly before labeling and inversion pulses at 1680 and 2830 ms after the saturation pulse. The following acquisition parameters were applied: repetition time = 4020 ms, echo time = 14 ms, label duration = 1650 ms, postlabeling delay = 1525 ms, 17 slices, voxel size = 3 × 3 × 7 mm, 40 pairs of label and control images, total scan duration = 5.5 min. The labeling plane was oriented perpendicular to the carotid arteries. Furthermore, we acquired whole brain T1-weighted images for registration purposes as described previously (Dopper et al., 2014).
2.4. Image analyses
We used in-house developed MATLAB (Matrix laboratory http://www.mathworks.nl/products/matlab/) scripts to convert the raw scanner data to NIfTI files. The remaining analyses were carried out in FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk) (Smith et al., 2004). First the raw ASL images were motion corrected and brain-extracted. Then the perfusion weighted maps, i.e. CBF images, were calculated by pairwise subtraction of control from label images and averaging these images across time. These CBF maps were then registered to the anatomical scans and to MNI-152 (T1 standard brain averaged over 152 subjects; Montreal Neurological Institute, Montreal, QC, Canada) standard space, and smoothed with an isotropic Gaussian kernel of 3.4 mm. The derived CBF images were divided by the mean perfusion in the occipital pole, a region typically spared in FTD (Ishii et al., 1998, Du et al., 2006), to account for global variations in CBF independent of the disease process. We subtracted follow-up CBF images from baseline maps per subject in order to obtain maps of changes in perfusion over time for repeated measures analyses as implemented in FSL (see http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/GLM#Experimental_Designs_-_Repeated_measures).
We investigated cross-sectional differences between mutation carriers and controls at baseline and follow-up, and longitudinal group differences in change over time by means of permutation-based testing using 5000 permutations, applying a 2-sample t-test with age, gender and voxel-wise grey matter volume (baseline grey matter volume for baseline analyses and follow-up grey matter volume for follow-up and longitudinal analyses) as confound regressors, to compare regional CBF between mutation carriers and controls independent of grey matter atrophy. We thresholded the statistical images at p < 0.05, corrected for multiple comparisons using threshold-free cluster enhancement (Smith and Nichols, 2009). The analyses were restricted to grey matter voxels that were covered by the ASL scan in all participants.
2.5. Statistical analyses
We analyzed demographic features using independent samples t-tests and Pearson's χ (Seelaar et al., 2011a) tests in SPSS 21.0 for Windows (SPSS, Chicago, IL), applying a significance level of p < 0.05. We investigated correlations between neuropsychological performance and cerebral blood flow by means of linear regression analyses.
3. Results
3.1. Demographic features
DNA screening revealed a GRN mutation in 23 at-risk individuals, a MAPT mutation in 11 and no mutation in 31 subjects (control group). The total groups of mutation carriers and controls did not differ in age, time between MRI scans, gender, level of education, and scores on MMSE and BDI (Table 1). GRN and MAPT mutation carriers were respectively 7.9 ± 7.5 and 6.4 ± 10.5 years younger than the mean onset age in their affected family members (p = 0.630). Two mutation carriers, one with a MAPT mutation and one with a GRN mutation, converted to the clinical stage of behavioral variant FTD during the follow-up period as demonstrated by behavioral disturbances reported by their relatives and a significant cognitive decline at neuropsychological examination.
Table 1.
Demographic features.
| Controls (n = 31) | Carriers (n = 34) | p-value | |
|---|---|---|---|
| Age t1, y | 51.0 (10.3) | 50.1 (9.7) | 0.696 |
| Years till mean onset age onset in affected family members, y | –7.4 (8.9) | –7.4 (8.4) | 0.988 |
| Interval t1–t2, y | 2.3 (0.1) | 2.2 (0.1) | 0.460 |
| Females | 52% | 62% | 0.409 |
| GRN mutationa | – | 68% | – |
| Level of educationb | 5.3 (1.0) | 5.7 (0.8) | 0.064 |
| MMSE score t2 | 29.2 (1.3) | 28.7 (1.9) | 0.231 |
| MMSE Δt1–t2 | 0.0 (1.3) | 0.6 (1.6) | 0.109 |
| BDI score t2 | 3.5 (3.7) | 2.9 (4.0) | 0.592 |
| BDI Δt1-t2 | 0.1 (2.8) | 0.1 (3.2) | 0.943 |
GRN = progranulin; MMSE = Mini-Mental State Examination; BDI = Beck Depression Inventory.
Values denote mean (SD) or percentage of subjects.
Remaining mutation carriers have a microtubule-associated protein tau (MAPT) mutation.
Level of education was determined on a Dutch 7-point scale ranging from 1 (less than elementary school) to 7 (university or technical college) (Verhage, 1964).
3.2. Cerebral blood flow – cross-sectional
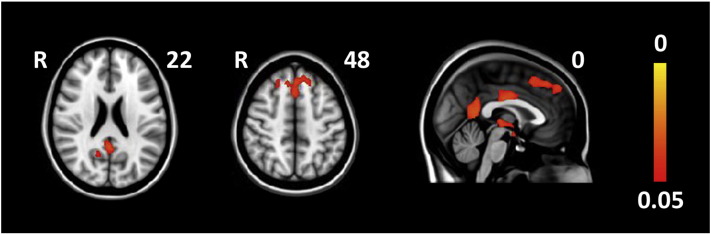
There were no significant group differences at baseline for the total study group or the GRN and MAPT subgroups. However, GRN mutation carriers showed significantly lower CBF in the frontal pole, superior frontal gyrus, paracingulate gyrus, posterior (mid)cingulate gyrus, precuneus, and thalamus compared with controls at follow-up (Fig. 1, Supplementary Table 1). We found no significant group differences in the total group or the MAPT group at follow-up.
Fig. 1.
Significant cross-sectional CBF differences between GRN mutation carriers and controls at follow-up. Maps illustrate clusters of significantly lower CBF in mutation carriers compared with controls. Color bar represents p-values corrected for multiple comparisons.
3.3. Cerebral blood flow - longitudinal
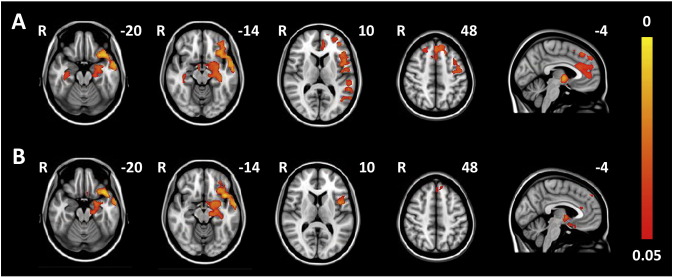
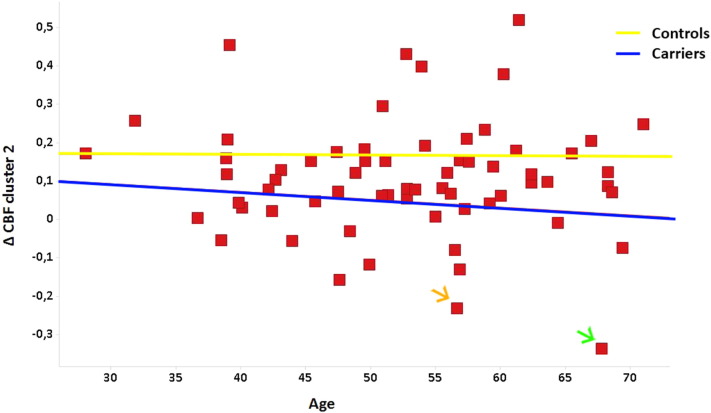
In longitudinal analyses the total group of mutation carriers showed a significantly stronger decrease in CBF over time compared with controls in widespread frontal, temporal, parietal, and subcortical regions (Fig. 2a, Supplementary Table 2). The GRN carrier who converted to clinical FTD showed the largest decrease in CBF in all significant clusters, with the second strongest decline in the converted MAPT carrier in three out of five significant clusters including frontal and right medial temporal regions (clusters 2, 3, and 5 in Supplementary Table 2) (Fig. 3). However, the abovementioned findings were not solely driven by the converters, as excluding them from the analyses still resulted in similar, albeit smaller, significant differences between carriers and controls.
Fig. 2.
Significant longitudinal CBF differences between mutation carriers and controls in the total study group (A) and the GRN subgroup (B). Maps illustrate clusters of significantly stronger decline in CBF over time in mutation carriers compared with controls. Color bar represents p-values corrected for multiple comparisons.
Fig. 3.
Individual values of decline in CBF over time in cluster 2 (superior frontal gyrus, frontal pole, anterior cingulate cortex, paracingulate gyrus (Supplementary Table 2)). The blue line represents mutation carriers and the yellow line control subjects. For reasons of anonymity individual mutation status is not shown. The orange arrow points to the converted subject with a MAPT mutation and the green arrow to the converter with a GRN mutation. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
GRN mutation carriers showed largely similar longitudinal differences as the total group of mutation carriers, although less significant (Fig. 2b, Supplementary Table 3), whereas we found no significant longitudinal differences for the MAPT group.
Restricting the cross-sectional analyses for the total group to the significant clusters in the longitudinal analyses revealed lower CBF in mutation carriers compared with controls in the superior frontal gyrus, left temporal pole, frontal orbital cortex, middle and superior temporal gyrus, right temporal fusiform cortex, parahippocampal gyrus, hippocampus, and thalamus at follow-up (Supplementary Table 4), whereas we still found no group differences at baseline. Again, without the two converters, these differences were smaller, but still significant.
3.4. Correlation between cerebral blood flow and neuropsychological performance
Exploratory correlation analyses revealed that worse performance on the RAVLT recognition subtask was associated with decreased CBF in the left (medial) temporal and frontal cortex, TMT B with frontal, anterior cingulate, and right medial temporal hypoperfusion, categorical fluency and WCST with right and left medial temporal lobe respectively, and BNT and Ekman faces with decreased CBF in the frontal cortex (Supplementary Table 5). However, these correlations are no longer significant after correction for multiple testing.
4. Discussion
The present study is the first to show that significant decrease in CBF in frontal, temporal, parietal and subcortical regions during two years of follow-up can be detected by ASL in presymptomatic carriers of GRN and MAPT mutations compared with controls independent of grey matter atrophy. Interestingly, the two subjects that have converted to clinically manifest FTD showed the strongest decline in perfusion in these regions. We found no cross-sectional group differences at baseline, but mutation carriers demonstrated significantly lower CBF in these regions at follow-up. These findings underline the value of ASL in detecting early changes in brain perfusion in FTD, which could be used to evaluate future therapies in clinical trials.
Our findings of a longitudinal CBF decline in frontal and subcortical regions in mutation carriers is in line with previous cross-sectional ASL studies in patients with FTD (Binnewijzend et al., 2014, Du et al., 2006, Hu et al., 2010, Shimizu et al., 2010, Zhang et al., 2011, Steketee et al., 2015). Frontal hypoperfusion has been correlated with behavioral disturbances (Du et al., 2006). In contrast to most previous studies, we also found a longitudinal decrease in temporal and parietal CBF in the current presymptomatic mutation carriers. The discrepancy regarding temporal hypoperfusion between the present and previous studies, might be explained by incomplete brain coverage in previous ASL studies (Du et al., 2006, Shimizu et al., 2010), since our finding is supported by FDG-PET studies in patients with FTD, demonstrating hypometabolism in the anterior temporal lobes (Ishii et al., 1998, Jeong et al., 2005). The longitudinal decrease in posterior temporal and parietal perfusion is probably mostly driven by the GRN mutation carriers, as GRN mutations have consistently been shown to affect more posteriorly located brain regions as well (Le Ber et al., 2008, Rohrer et al., 2008, Seelaar et al., 2011b), which is supported by our findings in the separate GRN analyses. Not surprisingly, the GRN converter only, and not the converted subject with a MAPT mutation, showed the strongest decline in these posteriorly located areas. On the other hand, parietal hypometabolism was not found in a previous FDG-PET study in presymptomatic GRN mutation carriers, and parietal damage was suggested to be a later phenomenon in the disease process than frontotemporal involvement (Jacova et al., 2013). However, our finding of hypoperfusion in the precuneus and posterior cingulate cortex in GRN carriers at follow-up underlines that parietal alterations can occur at an early disease stage in GRN carriers.
The finding that the two mutation carriers that developed symptoms of FTD during follow-up showed the strongest decline in CBF suggest that the hypoperfusion is related to disease severity. This is further supported by the correlations between decreased CBF and worse performance on several neuropsychological tests that are typically affected in early FTD, although these results were not significant after correction for multiple testing. Therefore, further studies are needed to confirm this correlation between CBF and neuropsychological performance.
Although we found no significant group differences for the smaller MAPT group, the strong decline in CBF in frontal and subcortical areas in the MAPT converter together with the less significant results in the longitudinal analyses of GRN mutation carriers only compared to the total study group differences, suggest similar CBF alterations over time in presymptomatic MAPT carriers. The lack of significant differences in the MAPT subgroup could be due to a lack of power. Therefore, larger study groups are needed to define the exact distribution of hypoperfusion in MAPT carriers.
In contrast to the present study, a more widespread hypoperfusion pattern including temporal, parietal, and occipital lobes was found using spin-echo contrast agent perfusion MRI in presymptomatic CHMP2B mutation carriers (Lunau et al., 2012). However, this likely reflects grey matter atrophy in this genetic group, as correction for grey matter volume was not applied in these subjects, who were previously shown to have generalized atrophy in the presymptomatic stage (Rohrer et al., 2009).
Our longitudinal analyses suggest that ASL is a promising measure of disease progression around the time of symptom onset, possibly even at the individual level. However, based on current findings CBF does not provide an accurate predictor for disease conversion at baseline, since values of the two converted subjects were still within the normal range at that time. This contrasts findings in Alzheimer's disease (AD) showing an association between baseline perfusion and subsequent cognitive deterioration in healthy elderly and in patients with mild cognitive impairment (Chao et al., 2010, Xekardaki et al., 2015). The low number of converters in the current study might have hampered the identification of baseline predictors. However, it is also possible that changes in CBF occur earlier in AD compared to FTD, as suggested in a previous ASL study in AD and FTD (Du et al., 2006).
Several major advantages of ASL over FDG-PET for the use in future clinical trials were already mentioned, including the lower costs, its non-invasiveness, the absence of radiation exposure, and the fact that MRI scanners are more widely available compared to PET scanners. Moreover, ASL can be easily combined with other MRI techniques in a single session. We previously demonstrated presymptomatic alterations in white matter integrity and functional connectivity using diffusion tensor imaging and resting-state fMRI in the same cohort (Dopper et al., 2014). Perhaps a combination of these three MRI techniques could further improve their sensitivity for measuring therapy effects in future clinical trials. It would be interesting to compare sensitivity and specificity of the different MRI techniques in a larger cohort such as the GENFI initiative. A drawback of ASL is the huge diversity in acquisition methods. Recently, an international consortium has published consensus recommendations for ASL (Alsop et al., 2014), which will hopefully result in a more standardized use of ASL, which is crucial for its use in clinical trials.
Limitations to our study include the small number of converted subjects and the lack of participants with C9orf72 repeat expansions. Another important issue in both ASL and FDG-PET is the choice of a reference region to correct for normal global variations in cerebral perfusion. The use of mean global perfusion for normalization has resulted in regions of artifactual hyperperfusion (Hu et al., 2010, Dukart et al., 2013, Yakushev et al., 2008). As the use of a non-affected region for normalization seems to be more appropriate, we have used the occipital pole as a reference region in the present study (Ishii et al., 1998, Du et al., 2006). The cerebellum is also often used as a reference region (Dukart et al., 2013), but was not always completely covered by the field-of-view of our ASL protocol.
To conclude, we demonstrated longitudinal alterations in CBF in presymptomatic carriers of MAPT and GRN mutations over two years of time, which appear to be related to approaching symptom onset. These findings suggest that ASL could provide a sensitive biomarker of disease progression in the presymptomatic stage of FTD, which can be useful for future clinical trials.
Acknowledgements
This work was supported by Dioraphte Foundation grant 09-02-03-00, the Association for Frontemporal Dementias Research Grant 2009, The Netherlands Organization for Scientific Research (NWO) grant HCMI 056-13-018 and Netherlands Alzheimer Foundation. S.A. Rombouts is sponsored by a grant from The Netherlands Organization for Scientific Research (NWO), grant number 016130677. A. Hafkemeijer is sponsored by a grant from The Netherlands Organization for Scientific research (NWO), grant number 05613010.
Footnotes
Funding: This work was supported by Dioraphte Foundation [grant number 09-02-03-00], the Association for Frontemporal Dementias Research Grant 2009, The Netherlands Organization for Scientific Research (NWO) [grant number HCMI 056-13-018] and Netherlands Alzheimer Foundation. S.A. Rombouts is sponsored by a grant from The Netherlands Organization for Scientific Research (NWO) [grant number 016130677]. A. Hafkemeijer is sponsored by a grant from The Netherlands Organization for Scientific research (NWO) [grant number 05613010].
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.nicl.2016.08.001.
Appendix A. Supplementary data
Supplementary tables.
References
- Alsop D.C., Detre J.A., Golay X. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: a consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2014 doi: 10.1002/mrm.25197. (Apr 8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M., Mackenzie I.R., Pickering-Brown S.M. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442(7105):916–919. doi: 10.1038/nature05016. Aug 24. [DOI] [PubMed] [Google Scholar]
- Battery A.I.T. War Department, Adjutant General's office; Washington DC: 1994. Manual of Directions and Scoring. [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio, TX: 1996. Manual for the Beck Depression Inventory-II. [Google Scholar]
- Binnewijzend M.A., Kuijer J.P., van der Flier W.M. Distinct perfusion patterns in Alzheimer's disease, frontotemporal dementia and dementia with Lewy bodies. Eur. Radiol. 2014;24(9):2326–2333. doi: 10.1007/s00330-014-3172-3. Sep. [DOI] [PubMed] [Google Scholar]
- Chao L.L., Buckley S.T., Kornak J. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis. Assoc. Disord. 2010;24(1):19–27. doi: 10.1097/WAD.0b013e3181b4f736. Jan–Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72(2):245–256. doi: 10.1016/j.neuron.2011.09.011. Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl J., Grimmer T., Drzezga A. Cerebral metabolic patterns at early stages of frontotemporal dementia and semantic dementia. A PET study. Neurobiol. Aging. 2004;25(8):1051–1056. doi: 10.1016/j.neurobiolaging.2003.10.007. Sep. [DOI] [PubMed] [Google Scholar]
- Doesborgh S.J., van de Sandt-Koenderman W.M., Dippel D.W. Linguistic deficits in the acute phase of stroke. J. Neurol. 2003;250(8):977–982. doi: 10.1007/s00415-003-1134-9. Aug. [DOI] [PubMed] [Google Scholar]
- Dopper E.G., Rombouts S.A., Jiskoot L.C. Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology. 2014;83(2):e19–e26. doi: 10.1212/WNL.0000000000000583. Jul 8. [DOI] [PubMed] [Google Scholar]
- Du A.T., Jahng G.H., Hayasaka S. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology. 2006;67(7):1215–1220. doi: 10.1212/01.wnl.0000238163.71349.78. Oct 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukart J., Perneczky R., Forster S. Reference cluster normalization improves detection of frontotemporal lobar degeneration by means of FDG-PET. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P., Friesen W.V. Consulting psychologists press; Palo Alto, CA: 1976. Pictures of Facial Affect. [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. Nov. [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M.L., Hillis A.E., Weintraub S. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006–1014. doi: 10.1212/WNL.0b013e31821103e6. Mar 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmer T., Diehl J., Drzezga A. Region-specific decline of cerebral glucose metabolism in patients with frontotemporal dementia: a prospective 18F-FDG-PET study. Dement. Geriatr. Cogn. Disord. 2004;18(1):32–36. doi: 10.1159/000077732. [DOI] [PubMed] [Google Scholar]
- Happe F., Brownell H., Winner E. Acquired ‘theory of mind’ impairments following stroke. Cognition. 1999;70(3):211–240. doi: 10.1016/s0010-0277(99)00005-0. Apr 1. [DOI] [PubMed] [Google Scholar]
- Hu W.T., Wang Z., Lee V.M. Distinct cerebral perfusion patterns in FTLD and AD. Neurology. 2010;75(10):881–888. doi: 10.1212/WNL.0b013e3181f11e35. Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M., Lendon C.L., Rizzu P. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. Jun 18. [DOI] [PubMed] [Google Scholar]
- Ishii K., Sakamoto S., Sasaki M. Cerebral glucose metabolism in patients with frontotemporal dementia. J. Nucl. Med. 1998;39(11):1875–1878. Nov. [PubMed] [Google Scholar]
- Jacova C., Hsiung G.Y., Tawankanjanachot I. Anterior brain glucose hypometabolism predates dementia in progranulin mutation carriers. Neurology. 2013;81(15):1322–1331. doi: 10.1212/WNL.0b013e3182a8237e. Oct 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong Y., Cho S.S., Park J.M. 18F-FDG PET findings in frontotemporal dementia: an SPM analysis of 29 patients. J. Nucl. Med. 2005;46(2):233–239. Feb. [PubMed] [Google Scholar]
- Jolles J., Houx P.J., van Boxtel M.P.J. Neuropsych Publishers; Maastricht, The Netherlands: 1995. Maastricht Aging Study: Determinants of Cognitive Aging. [Google Scholar]
- Kaplan E., Goodglass H., Weintraub S. Lea & Febiger; Philadelphia: 1978. The Boston Naming Test. [Google Scholar]
- Le Ber I., Camuzat A., Hannequin D. Phenotype variability in progranulin mutation carriers: a clinical, neuropsychological, imaging and genetic study. Brain. 2008;131(Pt 3):732–746. doi: 10.1093/brain/awn012. Mar. [DOI] [PubMed] [Google Scholar]
- Lindeboom J., Schmand B., Tulner L. Visual association test to detect early dementia of the Alzheimer type. J. Neurol. Neurosurg. Psychiatry. 2002;73(2):126–133. doi: 10.1136/jnnp.73.2.126. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunau L., Mouridsen K., Rodell A. Presymptomatic cerebral blood flow changes in CHMP2B mutation carriers of familial frontotemporal dementia (FTD-3), measured with MRI. BMJ Open. 2012;2(2) doi: 10.1136/bmjopen-2011-000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H.E. A modified card sorting test sensitive to frontal lobe defects. Cortex. 1976;12(4):313–324. doi: 10.1016/s0010-9452(76)80035-4. Dec. [DOI] [PubMed] [Google Scholar]
- Rascovsky K., Hodges J.R., Knopman D. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(Pt 9):2456–2477. doi: 10.1093/brain/awr179. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton A.E., Majounie E., Waite A. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72(2):257–268. doi: 10.1016/j.neuron.2011.09.010. Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. Presses Universitaires de France; Paris, France: 1958. L'examen Clinique en Psychologie. [Google Scholar]
- Rohrer J.D., Ahsan R.L., Isaacs A.M. Presymptomatic generalized brain atrophy in frontotemporal dementia caused by CHMP2B mutation. Dement. Geriatr. Cogn. Disord. 2009;27(2):182–186. doi: 10.1159/000200466. [DOI] [PubMed] [Google Scholar]
- Rohrer J.D., Warren J.D., Omar R. Parietal lobe deficits in frontotemporal lobar degeneration caused by a mutation in the progranulin gene. Arch. Neurol. 2008;65(4):506–513. doi: 10.1001/archneur.65.4.506. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall D.R., Cordes J.A., Polk M. CLOX: an executive clock drawing task. J. Neurol. Neurosurg. Psychiatry. 1998;64(5):588–594. doi: 10.1136/jnnp.64.5.588. May. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelaar H., Papma J.M., Garraux G. Brain perfusion patterns in familial frontotemporal lobar degeneration. Neurology. 2011;77(4):384–392. doi: 10.1212/WNL.0b013e3182270456. Jul 26. [DOI] [PubMed] [Google Scholar]
- Seelaar H., Rohrer J.D., Pijnenburg Y.A. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J. Neurol. Neurosurg. Psychiatry. 2011;82(5):476–486. doi: 10.1136/jnnp.2010.212225. May. [DOI] [PubMed] [Google Scholar]
- Seelaar H., Kamphorst W., Rosso S.M. Distinct genetic forms of frontotemporal dementia. Neurology. 2008;71(16):1220–1226. doi: 10.1212/01.wnl.0000319702.37497.72. Oct 14. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Zhang Y., Laxamana J. Concordance and discordance between brain perfusion and atrophy in frontotemporal dementia. Brain Imaging Behav. 2010;4(1):46–54. doi: 10.1007/s11682-009-9084-1. Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibinski G., Parkinson N.J., Brown J.M. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet. 2005;37(8):806–808. doi: 10.1038/ng1609. Aug. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. Jan 1. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Steketee R.M., Bron E.E., Meijboom R. Early-stage differentiation between presenile Alzheimer's disease and frontotemporal dementia using arterial spin labeling MRI. Eur. Radiol. 2015 doi: 10.1007/s00330-015-3789-x. (May 31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- Thurstone L.L., Thurstone T.G. Science Research Associates; Chicago: 1962. Primary Mental Abilities. [Google Scholar]
- Tsai R.M., Boxer A.L. Treatment of frontotemporal dementia. Curr. Treat. Options Neurol. 2014;16(11):319. doi: 10.1007/s11940-014-0319-0. Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage F. van Gorcum; Assen: 1964. Intelligence and Age: Research on Dutch People Aged Twelve to Seventy-Seven Years Old. [Google Scholar]
- Visch-Brink E., Stronks D., Denes G. Swets & Zeitlinger; Lisse: 2005. SAT: Semantische Associatie Test. [Google Scholar]
- Watts G.D., Wymer J., Kovach M.J. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat. Genet. 2004;36(4):377–381. doi: 10.1038/ng1332. Apr. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Psychological Corporation; San Antonio, TX: 1997. WAIS-III Administration and Scoring Manual. [Google Scholar]
- Wechsler D. Harcourt Test Publishers; Lisse: 2005. WAIS-III Nederlandse Bewerking. Technische handleiding. [Google Scholar]
- Wolk D.A., Detre J.A. Arterial spin labeling MRI: an emerging biomarker for Alzheimer's disease and other neurodegenerative conditions. Curr. Opin. Neurol. 2012;25(4):421–428. doi: 10.1097/WCO.0b013e328354ff0a. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xekardaki A., Rodriguez C., Montandon M.L. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology. 2015;274(2):490–499. doi: 10.1148/radiol.14140680. Feb. [DOI] [PubMed] [Google Scholar]
- Yakushev I., Landvogt C., Buchholz H.G. Choice of reference area in studies of Alzheimer's disease using positron emission tomography with fluorodeoxyglucose-F18. Psychiatry Res. 2008;164(2):143–153. doi: 10.1016/j.pscychresns.2007.11.004. Nov 30. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Schuff N., Ching C. Joint assessment of structural, perfusion, and diffusion MRI in Alzheimer's disease and frontotemporal dementia. Int. J. Alzheimers Dis. 2011;2011:546871. doi: 10.4061/2011/546871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables.