Introduction
Hedgehog pathway inhibitors (HPIs) are indicated for locally advanced basal cell carcinoma (laBCC) and metastatic basal cell carcinoma with reported treatment length of 5.5 months to more than 5 years.1, 2, 3 The definition of laBCC is historically ill described and only recently has a consensus definition been proposed.1, 4 Treatment is indicated until tumor resistance or adverse events cause interruption. Therefore, we do not know the length of treatment necessary for tumor clearance. We describe a patient who received a single 2-week pulse treatment of HPI therapy and subsequently experienced sustained clinical and histologic clearance of his tumor.
Case report
A medium-complected Hispanic man in his 50s presented with a 6-month history of a laBCC on the left eye. Fourteen months previously, the patient received radiation therapy (RT), but the tumor recurred and was now obstructing his vision. His history was significant for smoking, working as handyman, and family history of melanoma. Physical examination found a 3.0- × 2.7-cm vascular, friable, tumor on his left lower eyelid with surrounding erythema, ectropion, and injected conjunctiva (Fig 1). Two biopsies found nodular basal cell carcinoma (BCC). Consultation with the ophthalmic plastic surgery department recommended orbital exenteration. Imaging studies could not be performed because of the patient's uninsured and low-income status.
Fig 1.
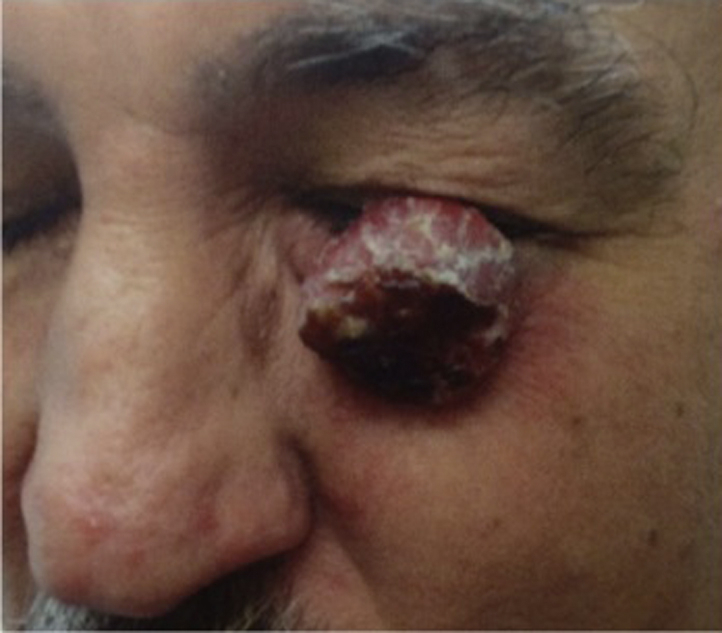
A Hispanic man presented with a large BCC, which was previously treated with radiation. He was started on vismodegib, but treatment abruptly ended after only 2 weeks.
The patient was started on 150 mg vismodegib daily. Treatment abruptly ended after 15 days when the patient was incarcerated. Six weeks later, the patient noted involution of the tumor. Twelve weeks after treatment discontinuation, the patient had complete clinical and histologic clearance confirmed by biopsy. Sixteen months after his 2-week treatment with vismodegib, he showed clinical and histologic resolution as confirmed by repeat punch biopsy (Fig 2).
Fig 2.
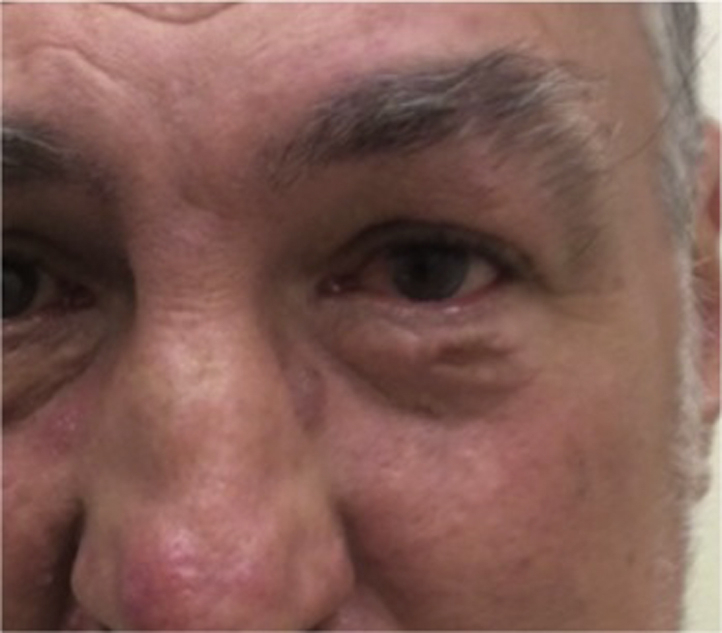
Clearance of BCC after pulse vismodegib treatment. The patient experienced persistent complete clinical and histologic clearance of BCC 16 months after a 2-week pulse with vismodegib.
Discussion
To our knowledge, no previously published study has reported resolution of BCC with vismodegib after such short treatment length. In the 3 largest studies of vismodegib efficacy and safety to date,2, 5, 6 median duration of treatment has ranged from 6.5 to 12.9 months with the median time to response approximately 2.5 months (Table I).
Table I.
Median duration of vismodegib exposure and median time to response in the 3 largest studies to date
It appears that pharmacologic response to HPI therapy is rapid. In a study of vismodegib for basal cell nevus syndrome, patients had reduced hedgehog target gene expression and diminished tumor cell proliferation after 1 month of treatment.7 Our patient had complete sustained tumor resolution for at least 14 months after 2 weeks of therapy. It is, therefore, likely that physiologic change also occurs within a short timeframe. Response of BCC tumors to HPI therapy is varied. It is possible that this variability depends both on tumor and stromal characteristics.8
Significantly, our patient had received RT therapy 1 year earlier. The late changes of RT include alterations of the dermis, and one possibility is that stromal alteration rendered the tumor more susceptible to the short course of HPI therapy. However, scarring from RT may also make tumor elimination more difficult. Several recently published articles explore the use of HPI as an adjuvant to surgery or radiation,1 although further research is needed to determine the relative response rates for laBCC after these combined modalities.
The utility of vismodegib in treating basal cell carcinoma has not been clearly defined. This case highlights the need for further surveillance of unusual clinical scenarios to help determine appropriate uses.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Jacobsen A.A., Aldahan A.S., Hughes O.B., Shah V.V., Strasswimmer J. Hedgehog Pathway Inhibitor Therapy for Locally Advanced and Metastatic Basal Cell Carcinoma: A Systematic Review and Pooled Analysis of Interventional Studies. JAMA Dermatol. 2016;152:816–824. doi: 10.1001/jamadermatol.2016.0780. [DOI] [PubMed] [Google Scholar]
- 2.Sekulic A., Migden M.R., Lewis K. Pivotal ERIVANCE basal cell carcinoma (BCC) study: 12-month update of efficacy and safety of vismodegib in advanced BCC. J Am Acad Dermatol. 2015;72(6):1021–1026.e8. doi: 10.1016/j.jaad.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Simone P.S.J., Strasswimmer J. Four year experience of vismodegib hedgehog inhibitor therapy. JAAD. 2016;74:1264–1265. doi: 10.1016/j.jaad.2015.12.035. [DOI] [PubMed] [Google Scholar]
- 4.Peris K., Licitra L., Ascierto P.A. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Future Oncol. 2015;11(4):703–712. doi: 10.2217/fon.14.281. [DOI] [PubMed] [Google Scholar]
- 5.Chang A.L., Solomon J.A., Hainsworth J.D. Expanded access study of patients with advanced basal cell carcinoma treated with the Hedgehog pathway inhibitor, vismodegib. J Am Acad Dermatol. 2014;70(1):60–69. doi: 10.1016/j.jaad.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Basset-Seguin N., Hauschild A., Grob J.J. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-planned interim analysis of an international, open-label trial. Lancet Oncol. 2015;16(6):729–736. doi: 10.1016/S1470-2045(15)70198-1. [DOI] [PubMed] [Google Scholar]
- 7.Tang J.Y., Mackay-Wiggan J.M., Aszterbaum M. Inhibiting the hedgehog pathway in patients with the basal-cell nevus syndrome. N Engl J Med. 2012;366(23):2180–2188. doi: 10.1056/NEJMoa1113538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lesack K., Naugler C. Morphometric characteristics of basal cell carcinoma peritumoral stroma varies among basal cell carcinoma subtypes. BMC Dermatol. 2012;12:1. doi: 10.1186/1471-5945-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


