Abstract
Purpose
The aim of our study was to explore the relationships between the M2 isoform of pyruvate kinase (PKM2) and the sensitivity of human non-small cell lung cancer (NSCLC) cells to docetaxel in vitro.
Materials and Methods
With the method of plasmid transfection, we silenced the expression of PKM2 successfully in A549 and H460 cells. Western blotting and real-time PCR were applied to detect PKM2 expression at protein and gene levels. Cell viability was examined by CCK8 assay. Cell cycle distribution and apoptosis were examined by flow cytometry. P21 and Bax were detected.
Results
Expression of PKM2 mRNA and protein were significantly decreased by shRNA targeting PKM2. Silencing of PKM2 increased docetaxel sensitivity of human NSCLC A549 and H460 cells in a collaborative manner, resulting in strong suppression of cell viability. The results of flow cytometric assays suggested that knockdown of PKM2 or docetaxel treatment, whether used singly or in combination, blocked the cells in the G2/M phase, which is in consistent with the effect of the two on the expression of p21. Cells with PKM2 silencing were more likely to be induced into apoptosis by docetaxel although knockdown of PKM2 alone can't induce apoptosis significantly, which is in consistent with the effect of the two on Bax expression.
Conclusion
The results suggest that PKM2 knockdown could serve as a chemosensitizer to docetaxel in non-small lung cancer cells through targeting PKM2, leading to inhibition of cell viability, increase of cell arrest of G2/M phase and apoptosis.
Keywords: The M2 isoform of pyruvate kinase, shRNA, non-small cell lung cancer, chemotherapy, docetaxel, cell cycle, apoptosis
INTRODUCTION
Metabolism is the core of cell biology. Understanding how cancer cells cope with metabolic needs for their unique biology has been a focus of cancer research since the last century. In the 1920s, Warburg first discovered that active glycolytic metabolism was a significant biochemical characteristic of cancer cells. Even under aerobic conditions, glycolysis is still significantly active in malignant cells, and large amounts of glucose are converted to lactic acid via glycolysis. This effect, termed aerobic glycolysis or the Warburg effect, plays very important role in tumor metabolism and growth.1,2 Pyruvate kinase (PK) is one key enzyme in the process of glycolysis. There are four isoenzymes of PK expressed in mammalian cells: the L isoform of PK (PKL) in liver and kidney, the R isoform of PK (PKR) in red blood cells, the M1 isoform of PK (PKM1) in muscle and brain, and the M2 isoform of PK (PKM2) in embryonic, adult stem cells, and tumor cells.3,4 And with the embryonic development, PKM2 is gradually replaced by other three isozymes, but the expressions of PKM2 in tumor cells rise again and replace the original isozymes expressing in the tissues. For instance, PKL in hepatocellular carcinoma (HCC) can switch to PKM2, and PKM1 in brain tissue disappears and instead a lot of PKM2 emerge.5,6 PKM2 may be one of the key enzymes in the Warburg effect and very important for tumor metabolism and growth. In many tumor cells, PKM2 expression significantly increases, verified by pathological studies of tumor tissue.7,8,9 Meanwhile our preliminary research suggested that PKM2 expression in human non-small cell lung cancer (NSCLC) is negatively associated with clinical stages, differentiation.10
As one of the most common malignant tumors, lung cancer remains the most frequent cause of death from cancer in both men and women worldwide.11 NSCLC patients account for 85% of all lung cancer patients.12,13 At present, the therapeutic regimen for NSCLC is usually a combination therapy of surgery, radiotherapy, traditional chemotherapy and small molecule targeted drugs. Thirty to forty of NSCLC patients are diagnosed at locally advanced stage, and 40% of patients with distant metastases, therefore, surgery is not suitable for most of these patients. Chemotherapy is a systemic treatment, and has a therapeutic effect on controlling both primary lesions and metastatic lesions. Among numerous antitumor drugs, the taxanes are widely recognized. Docetaxel, a derivative of taxane, is a highly effective antineoplastic drug applied to treatment of many malignant tumors, including lung cancer.14,15 The existence of such antitumor drug alleviates the anxiety of clinical oncologists to a great extent. Nevertheless, not every cancer patients benefit satisfactory therapeutic effect because of the reasons that either the tumor is not sensitive to docetaxel, possess too powerful malignant proliferation ability, or patients can't tolerate the side effects. Therefore, it's essential to look for a method to increase the therapeutic effect of docetaxel or decrease the dosage under the premise of not affecting the effect.
At present, a few studies on the relationships between PKM2 and cell sensitivity to chemotherapy have been carried out. Sun concluded that the knockout of PKM2 significantly promotes the differentiation of acute promyelocytic leukemia (APL) drug-resistance cell lines.16 Yoo, et al.17 and Li, et al.18 showed that PKM2 expression is related to the resistance of tumor cells to cisplatin in gastric and ovarian cancer treatment, and inhibition of PKM2 expression decreases the resistance, and Martinez-Balibrea, et al.19 suggested similar results: in PKM2 expression in colon cancer cells resistant to cisplatin. Shin, et al.20 found that PKM2 expression increased significantly in 5-fluorouracil (5-FU)-resistant colon cancer cells; whereas Yoo, et al.17 suggested that there was no significant relevance between the expression of PKM2 and resistance to 5-FU.
In the present research, we explored whether knockdown of PKM2 with shRNA can influence the lung cancer cell lines A549 and H460 to chemotherapeutic agent docetaxel in vitro.
MATERIALS AND METHODS
Materials
Primary antibodies [PKM2 (#3198), p21 (#2947), Bax (#2772), and β-actin (#4970), rabbit anti-human] were purchased from Cell Signaling Technology (CST). Docetaxel, the reference drug, was purchased from Jiangsu Hengrui Medicine Co., Ltd.
Cell culture
The human NSCLC cell lines A549 and H460 were obtained from Shanghai Institutes for Biological Sciences and cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum. The cell lines were cultured in a humidified air, containing, 5% carbon dioxide at 37℃.
Plasmid construction, amplification and transfaction
To minimize the off-target effects of RNAi, a total of three shRNAs targeting the human PKM2 were planned according to references (Table 1).21,22 ShRNAs targeting both M1 and M2 isoforms (sh946) and specific shRNAs to M2 (sh1408 and sh1411) were designed and were obtained from GENECHEM. And a scrambled shRNA without the effect on PKM2 expression was applied as a control (5'-CCGGGAGGCTTCTTATAAGTGTTTACTCGAGTAAACACTTATAAGAAGCCTCTT TTTG-3'). The target sequence and the scrambled sequence (control shRNA) with no homology to any known human genes were synthesized, and connected to GV 248 (U6-MCS-Ubi-EGFP). All constructs were identified by sequence analysis. The shRNA plasmids or control plasmid vectors were transfected into A549 and H460 cells with Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's protocol.
Table 1. The Sequences of Designed Control shRNA and shRNA Targeting Human PKM2 Gene.
| Intensive therapy | |
|---|---|
| Control shRNA | 5'-CCGGGAGGCTTCTTATAAGTGTTTACTCGAGTAAACACTTATAAGAAGCCTCTTTTTG-3' |
| PKM2 shRNA-946 | 5'-CCGGGCTGTGGCTCTAGACACTAAACTCGAGTTTAGTGTCTAGA-GCCACAGCTTTTTG-3' |
| PKM2 shRNA-1408 | 5'-CCGGCTACCACTTGCAATTATTTGACTCGAGTCAAATAATTGCAAG-TGGTAGTTTTTG-3' |
| PKM2 shRNA-1411 | 5'-CCGGCCACTTGCAATTATTTGAGGACTCGAGTCCTCAAATAATTGCAAGTGGTTTTTG-3' |
PKM2, the M2 isoform of pyruvate kinase.
The underlines indicate the target sequences for PKM2, and the bold letters show the loop sequences.
Rescue constructs and retroviral production
Flag-tagged mouse PKM2 was cloned into the GV 248 (U6-MCS-Ubi-EGFP) and were co-transfected into 293T cells. Plamid was harvested 36 h after transfection, and 5 mg of m21 poly-brene was added. A549 and H460 cells were infected with harvested plasmid.
Western blotting
The levels of PKM2 protein expression in A549 and H460 cells were examined with Western blotting (WB) analysis using a PKM2-specific antibody at 48 h after transfection. Briefly, all adhesive cells were washed twice with cold PBS and subsequently lysed in the cold lysis buffer with 1 mM phenylmethanesulfonyl fluoride for 30 min on ice. A BCA Protein Assay Kit (Beyotime) was used to detect the protein concentration. Eight% SDS-PAGE was applied to fractionate protein extracts (about 30 µg) and then the gel was transferred to polyvinylidene fluoride (PVDF) membrane (Millipore, Boston, MA, USA). Membranes were incubated in a blocking buffer (containing 5% nonfat dry milk and 0.05% Tween-20) for 2 h, followed by 3 h of incubation with the primary antibody (PKM2, p21, Bax or β-actin with 1:1000 dilution) in the blocking buffer as described above at room temperature. The membrane was then washed three times with TBS-T and incubated with secondary antibody (1:10000) for 1 h. The membrane was finally washed three times with TBS-T and protein expression was determined with enhanced chemiluminescence (ECL) (Millipore). β-actin was used as an endogenous reference for quantication.
RT-PCR
Following the manufacturer's instructions, the total RNA was isolated from the cells using the Trizol reagent (Invitrogen), quantified, and applied for synthesizing cDNA. The PCR for the amplification of PKM2 was carried out as described previously. 23 β-actin was used as internal control. The following primers were used: PKM2: 5'-CCCAGCTTCCCGATCAGTG-3' (sense); and 5'-GATGAGCCC AGTTCGGATCTC-3' (antisense). β-actin: 5'-GATCATTGCTCCTCCTGAGC-3' (sense), and 5'-ACTCCTGCTTGCTGATCCAC-3' (antisense). Relative amounts of mRNA were calculated according to the comparative cycle threshold method.
Cell viability assay
Cell proliferation was detected using the Cell Counting Kit-8 (Dojindo, Shanghai, China) as per manufacturer's instructions. In brief, each well in 96-well plates was seeded with cells at a density of 5000. After 12 h, following cell adherence to the plate, cells were disposed with different dose of docetaxel. Ten µL of Cell Counting Kit-8 solution was then added to each well 24 h, 48 h or 72 h later and incubated at 37℃ for 2 h in an incubator. Subsequently, absorbance at 450 nm was measured using a DTX-880 multimode microplate reader (Beckman, Brea, CA, USA). Cell viability was calculated as follows: cell viability (%)=(mean absorbency in test wells)/(mean absorbency in control wells)×100. Each experiment was performed in triplicate.
Cell cycle analysis by flow cytometry
Cellular DNA content was measured by flow cytometry. Cells grown in six-well plates were collected. After being washed twice with ice-cold PBS, the cells were fixed and permeated overnight by adding 1 mL of 70% ethanol to every tube at 40℃. After centrifugation, the supernatant was removed carefully and the deposits were re-suspended in 0.5 mL of staining solution containing 200 µL each of DNAse-free RNAse (Sigma, San Francisco, CA, USA) and propidium iodide (PI), followed by 30 min of incubation at room temperature in complete darkness. The cells were analyzed immediately by flow cytometry with FACScan (Becton Dickinson, Franklin Lakes, NJ, USA) by applying the CELL Quest program.
Apoptosis detections by flow cytometry
Cells were stained using Annexin V-FITC and PI. At 24 and 48 h after drug administration, all cells, containing adherent and non-adherent cells, were collected, and apoptosis was detected using the Annexin V-FITC Apoptosis Detection Kit (BD Biosciences Pharmingen, Franklin Lakes, NJ, USA). Briefly, cells were washed twice with ice-cold PBS and re-suspended in 100 µL of Annexin-V binding buffer, followed by incubation with FITC-conjugated Annexin-V and PI for 15 min at room temperature in complete darkness. The cells were re-suspended in 500 µL of binding buffer and analyzed using Cytomics™ FC500 flow cytometer (Beckman).
Statistical analysis
Statistical analysis was done with SPSS 20.0 (Novell, Austin, TX, USA). Data were presented as mean±standard deviation (x̄±s). Significant differences in the results were determined by Student's t-test. p<0.05 was considered as statistically significant.
RESULTS
Effect of targeting PKM2 with shRNA on expression of PKM2 in A549 and H460 cell lines
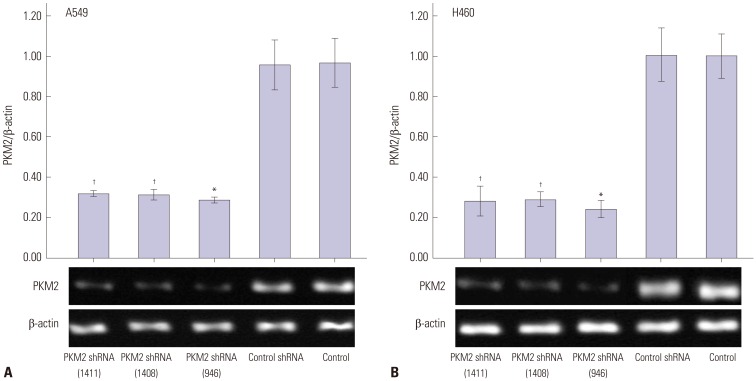
To silence PKM2 gene with shRNA, GFP-containing vectors were applied. After transfection, more than 70% of cells in each vector-transfected group were GFP positive, indicating high transfection efficiency. Moreover, both Western blot and RT-PCR analyses revealed lower PKM2 expression levels in PKM2 shRNA-transfected cells. As shown in Fig. 1, 48 h after transfection, compared with that in control cells, PKM2 protein expressions were markedly reduced in both A549 and H460 cells transfected with three PKM2 shRNAs respectively. Densitometric analysis showed 70.079% inhibition (p<0.001 vs. control) and 73.07% inhibition (p<0.001 vs. control) of PKM2 protein expression in PKM2 shRNA-946 group, respectively. Moreover, compared with PKM2 shRNA-1408 and PKM2 shRNA-1411 group, the protein expressions of PKM2 were significantly lower in PKM2 shRNA-946 group (p<0.01 vs. PKM2 shRNA-946). RT-PCR analysis showed that PKM2 mRNA expression decreased by 70.83% (p<0.001 vs. control) and 76.04% (p<0.001 vs. control) with PKM2 shRNA-946 48 h after transfection in A549 and H460 cells, respectively (Fig. 2). Likewise, compared with PKM2 shRNA-1408 and PKM2 shRNA-1411 group, the mRNA expressions of PKM2 were significantly lower in PKM2 shRNA-946 group (p<0.01 vs. PKM2 shRNA-946). Thus, PKM2 shRNA-946 was selected as the optimal shRNA; the above results showed no significant difference between control and shRNA-control groups (p<0.05 vs. control), obviously demonstrating that PKM2 shRNA-946 was highly efficient for PKM2 gene knockdown in A549 and H460 cells in vitro.
Fig. 1. PKM2 protein expression in A549 and H460 cells. Both A549 cells (A) and H460 cells (B) were untransfected (Control), or transfected by the method of plasmid transfection, including PKM2 shRNA-transfection (PKM2 shRNA) or control shRNA-transfection (Control shRNA). 48 hrs after transfection, Western blot analyses were performed to examine the inhibition effect. The graph depicts mean±SEM for three independent determinations of optical density of the PKM2 Western blot bands. *Compared to control, p<0.05, †Compared to PKM2 shRNA (946), p<0.05. PKM2, the M2 isoform of pyruvate kinase.
Fig. 2. PKM2 mRNA expression in A549 and H460 cells. Both A549 cells (A) and H460 cells (B) were untransfected (Control), or transfected by the method of plasmid transfection, including PKM2 shRNA-transfection (PKM2 shRNA) or control shRNA-transfection (Control shRNA). 48 hrs after transfection, RT-PCR were performed to examine the inhibition effect. The graph depicts mean±SEM for three independent determinations of PKM2 mRNA relative to β-actin mRNA value. *Compared to control, p<0.05, †Compared to PKM2 shRNA (946), p<0.05. PKM2, the M2 isoform of pyruvate kinase.
To address whether it was the M2 isoform that was specifically critical for cell proliferation, we successfully reconstituted PKM2 expression in the knockdown cells, including A549 and H460 cells, with mouse PKM2-expressing vector. However, there was no dramatic difference in the PKM2 protein or mRNA expression level, between the PKM2 rescue cells and untransfected cells, and the PKM2 rescue cells had significantly less PKM2 than the PKM2-knockdown cells.
Silencing of PKM2 in combination with docetaxel produced synergistic inhibition of cell viability in A549 and H460 cells
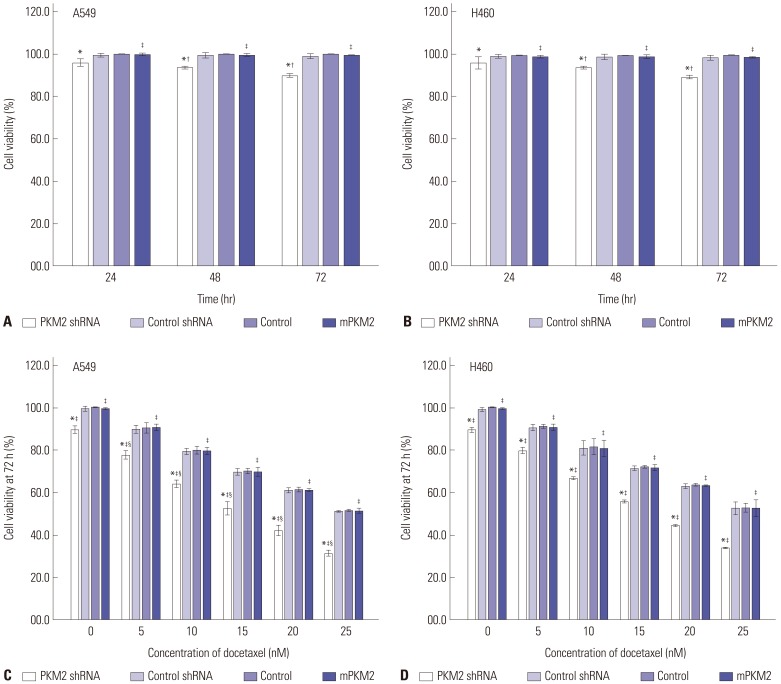
The effect of silencing of PKM2, docetaxel and the combined treatment of both on the survival of A549 and H460 cells was studied by CCK8 assay. Cells were incubated with different concentration of docetaxel, ranging from 0 to 25 nM, for up to 72 hrs. Results in Fig. 3A and B show that knockdown of PKM2 suppressed the cell viability of A549 and H460 cells in a time-dependent manner. At three different time points, 24 h, 48 h and 72 h after incubation, cell viability in the PKM2 shRNA group were dramatically lower than that in control group. PKM2 shRNA alone significantly inhibited the viability of A549 and H460 cells not only as early as 24 hrs for incubation, but also more dramatically at 48 or 72 hrs after incubation. Compared with that of control groups, the cell viability of A549 and H460 cells were significantly lower in the PKM2-shRNA group at 24, 48 and 72 hrs (p<0.05). The inhibition rate of PKM2-shRNA on A549 and H460 cells were 4.40% and 3.93%, 6.13% and 5.2%, 10.73% and 10.43% at 24, 48 and 72 hours, respectively. Docetaxel has been shown to suppress the cell viability of A549 and H460 cells both in time- and dose-dependent manners.24 As shown in Fig. 3C and D, our results indicate that docetaxel, ranging from 0 to 25 nM, suppressed the viability of A549 and H460 cells in a dose-dependent manner at 72 hrs after incubation. Moreover, compared with either knockdown of PKM2 or docetaxel, PKM2 shRNA in combination with docetaxel at concentrations ranging from 0 to 25 nM resulted in more dramatic inhibition of survival of A549 and H460 cells during 24–72 hrs. For instance, at 72 hrs, compared to 20 nM docetaxel treatment, the combination therapy reduced the viability of A549 and H460 cells by 31.5% and 30.43%, respectively, whereas, shRNA-control-transfection had no obvious effect on the viability of A549 and H460 cells at all time. These data indicate that shRNA-PKM2 and docetaxel brought about synergistic inhibitory effect on the viability of both A549 and H460 cells.
Fig. 3. The effect of shRNA-PKM2 and docetaxel or the combined treatment of both on the viability of A549 and H460 cells. 48 hrs after transfection, all A549 and H460 cells, untransfected (Control), PKM2 shRNA-transfected (PKM2 shRNA) or control shRNA-transfected (Control shRNA), were all cultured with different concentrations of docetaxel, ranging from 0 to 25 nM, for up to 72 hrs. (A and B) Cells were incubated with docetaxel at 0 nm for 72 hrs. The cell viability was detected at 24 hrs, 48 hrs and 72 hrs after incubation. (C and D) Cells were incubated with docetaxel at concentrations ranging from 0 nm to 25 nm for 72 hrs. Cell viability was quantified using Cell Counting Kit-8 and expressed as the percentage of the viability of control cells (0 h). Results are presented as mean±SEM of three separate experiments conducted in duplicate. *Compared to control cells with the same dose of docetaxel incubation at the same time point, p<0.05, †Compared to PKM2 shRNA-transfected cells with 24 hrs incubation of 0 nM docetaxel, p<0.05, ‡Compared to PKM2 shRNA-transfected cells with the same dose of docetaxel incubation in the same time point, p<0.05, §Compared to control cells with 72 hrs incubation of 0 nM docetaxel, p<0.05. PKM2, the M2 isoform of pyruvate kinase.
Results in Fig. 3 also show that, after the PKM2 expression was reconstituted in the knockdown cells, the cell viability rebound to almost the level of untransfected cells, and was markedly stronger than that of knockdown cells at any time point. When treated with docetaxel (ranging from 0 to 25 nM for up to 72 hrs), the viability of PKM2 rescue cells was dramatically higher than that of knockdown cells, and was not also statistically different from that of untrasfected cells at any time point. Therefore, the above results suggested that it was PKM2 that influenced the viability of cancer cells and chemosensitivity to the chemotherapy drug docetaxel. Therefore, although not specific only to M2 isoform but also both M1 and M2 isoforms PKM2 shRNA-946, was selected to silence PKM2 and applied in the subsequent study because of its strongest inhibition.
Knockdown of PKM2 affected the cell cycle distribution
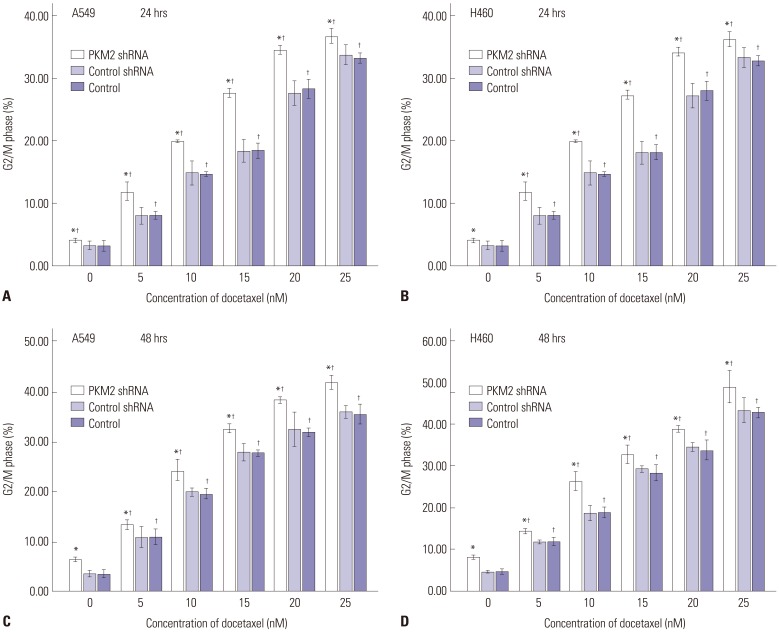
Docetaxel can arrest the cells at the G2/M phase and consequently result in apoptosis in many human cancers, apoptotic cell death appearing to come about after mitotic arrest.24 In order to investigate the mechanisms by which silencing of PKM2 in combination with docetaxel produced synergistic inhibition of cell viability, we applied flow cytometry to analyse the cell cycle distribution and apoptosis. Thus, the cells were treated with different concentrations of docetaxel, ranging from 0 to 25 nM, for up to 48 hrs. The results depicted in Fig. 4A and B indicated that knockdown of PKM2 with shRNA brought about a dramatical arrest at G2/M phase, accompanied by the decrease of G0/G1 cells. After 24 hours of incubation, in a dose-dependent manner, docetaxel alone induced a dramatically typical delay at G2/M transition in untransfected A549 and H460 cells. Moreover, compared with untransfected cells groups, G2/M phase cells of both A549 and H460 cells with different concentrations of docetaxel were significantly more in the shRNA-PKM2 group, accompanied by lower percentage of G0/G1 phase cells. After 48 hrs of incubation, the above phenomenon continued in every group (Fig. 4C and D). When the cells were transfected with shRNA PKM2, the percentage at the G2/M phase escalated from 3.66±0.31% and 4.60±0.30% to 6.53±0.23% and 8.10±0.20% in A549 and H460 cells, respectively. In untransfected cells, docetaxel brought about a remarkable accumulation of cells at G2/M phase; 3.66±0.31% and 4.60±0.30% in the 0 nM group and 35.50±1.01% and 42.80±0.47% in the 25 nM group, in A549 and H460 cells, respectively. Furthermore, compared with untransfected cells, PKM2 shRNA-transfected cells dealt with different concentrations of docetaxel showed higher percentage of cells at G2/M phase and lower percentage at G0/G1 phase. Control-shRNA-transfection alone didn't evidently change the cell cycle distribution.
Fig. 4. Effects of PKM2 shRNA and docetaxel on cell cycle distribution. 48 hrs after transfection, all A549 and H460 cells, PKM2 shRNA-transfected (PKM2 shRNA), control-shRNA transfected (Control shRNA) or non-transfected (Control), were incubated with docetaxel at concentrations ranging from 0 to 25 nM for up to 48 hrs, and the cell cycle distribution was evaluated using PI staining and flow cytometry analysis. In both A549 and H460 cells, cell cycle distribution was measured at two different time points after incubation, 24 hrs (A and B) and 48 hrs (C and D). Results are presented as mean±SEM of three separate experiments conducted in duplicate. *Compared to control cells, p<0.05, †Compared to control cells with incubation of 0 nM docetaxel, p<0.05. PKM2, the M2 isoform of pyruvate kinase; PI, propidium oidide.
Knockdown of PKM2 increased the expression of p21
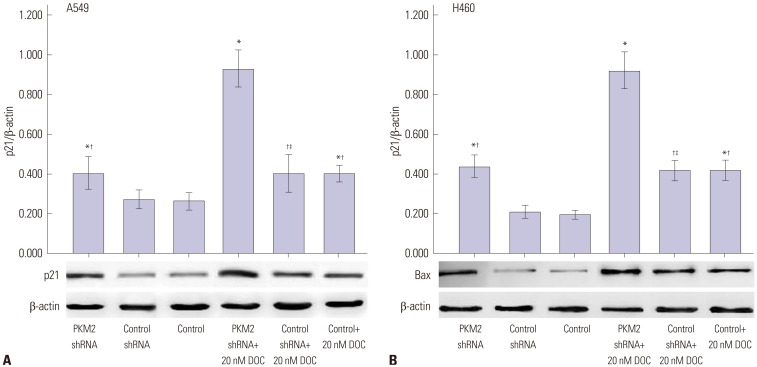
In order to investigate the mechanism of how silencing PKM2 markedly arrested cell cycle at G2/M phase, the expression of p21 protein was examined by WB analysis. As shown in Fig. 5, the expression levels of p21 in both A549 and H460 cells were enhanced by PKM2 shRNA, and 20 nM docetaxel also enhanced the expression of p21 in untransfected A549 and H460 cells. Therefore, compared with either treatment alone, the combined treatment of silencing PKM2 and docetaxel significantly increased more the expression of p21. On the other hand, control-shRNA-transfection alone didn't affect the levels of p21 statistically.
Fig. 5. p21 protein expression induced by 0 nM and 20 nM docetaxel in PKM2-shRNA transfected, shRNA-control transfected or non-transfected cells at 48 hrs. 48 hrs after transfection, all A549 (A) and H460 (B) cells, PKM2 shRNA-transfected (PKM2 shRNA), control-shRNA transfected (Control shRNA) or non-transfected (Control), were incubated with 20 nM docetaxel for up to 48 hrs, Western blot analyses were performed to detect the expression of p21. The graph depicts mean±SEM for three independent determinations of optical density of the p21 Western blot bands. *Compared to control cells with incubation of 0 nM docetaxel, p<0.05, †Compared to PKM2 shRNA-transfected cells with incubation of 20 nM docetaxel, p<0.05, ‡Compared to control shRNA-transfected cells with incubation of 0 nM docetaxel, p<0.05. PKM2, the M2 isoform of pyruvate kinase; DOC, docetaxel.
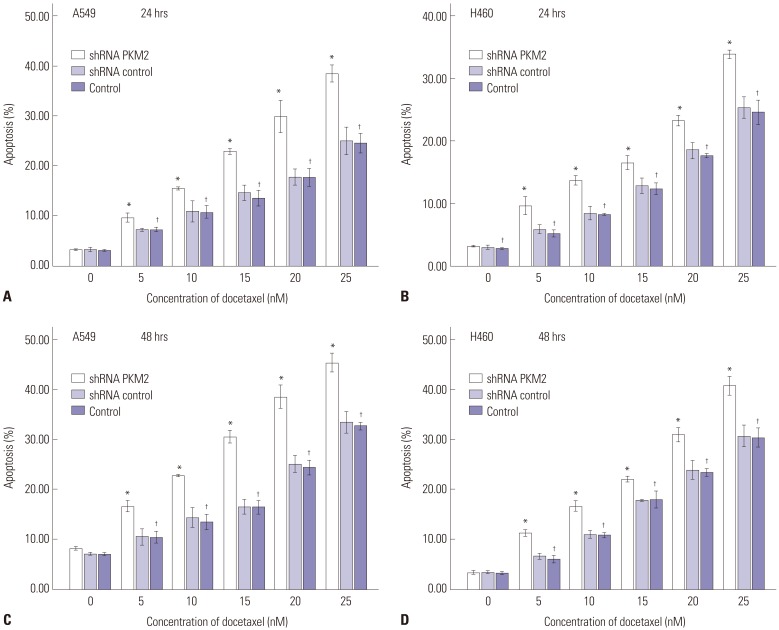
Knockdown of PKM2 increased apoptosis induced by docetaxel
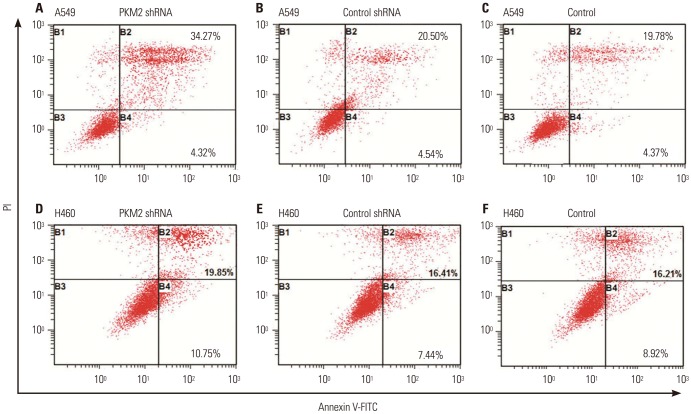
Next, we performed flow cytometric assays to analyze the apoptotic cell death. As shown in Fig. 6, silencing of PKM2 with shRNA could not induce more apoptosis at 24 h and 48 h. However, apoptosis was induced in untransfected A549 and H460 cells in dose and time-dependent manners, following treatment with 0 to 25 nM docetaxel, and the higher percentage of apoptotic cells was observed in the cells treated with PKM2 shRNA transfection. Thus, it is quite likely that knockdown of PKM2 increased apoptosis induction effect of docetaxel on A549 and H460 cells. Fig. 7 shows a representative analysis of apoptosis induced by 20 nM docetaxel, PKM2 shRNA+20 nM docetaxel and control-shRNA+20 nM docetaxel at 48 hrs.
Fig. 6. Effects of shRNA PKM2 and docetaxel on apoptosis. 48 hrs after transfection, all A549 and H460 cells, PKM2 shRNA-transfected (PKM2 shRNA), control-shRNA transfected (Control shRNA) or non-transfected (Control), were incubated with docetaxel at concentrations ranging from 0 to 25 nM for up to 48 hrs, and apoptosis was evaluated using Annexin V-FITC/PI staining and flow cytometry analysis. In both A549 and H460 cells, apoptosis was detected at two different time points after incubation, 24 hrs (A and B) and 48 hrs (C and D). Results are presented as mean±SEM of three separate experiments conducted in duplicate. *Compared to control cells, p<0.05, †Compared to control cells with incubation of 0 nM docetaxel, p<0.05. PKM2, the M2 isoform of pyruvate kinase; PI, propidium iodide.
Fig. 7. Representative analysis of apoptosis induced by 20 nM docetaxel in PKM2-shRNA transfected, shRNA-control transfected or non-transfected cells at 48 hrs. 48 hrs after transfection, all A549 and H460 cells, PKM2 shRNA-transfected (PKM2 shRNA), control-shRNA transfected (Control shRNA) or non-transfected (Control), were incubated with 20 nM docetaxel for up to 48 hrs, and apoptosis was evaluated using Annexin V-FITC/PI staining and flow cytometry analysis. (A) PKM2-shRNA transfected A549 cells treated with 20 nM DOC. (B) shRNA-control transfected A549 cells treated with 20 nM DOC. (C) Non-transfected A549 cells treated with 20 nM DOC. (D) PKM2-shRNA transfected H460 cells treated with 20 nM DOC. (E) shRNA-control transfected H460 cells treated with 20 nM DOC. (F) Non-transfected H460 cells treated with 20 nM DOC. Bottom right quadrant; cells stained mainly by Annexin V (early apoptotic cells); top right quadrant, cells stained by both PI and Annexin V (late apopototic cells); top left quadrant, cells stained mainly by PI (necrotic cells); bottom left quadrant, cells negative for both Annexin V and PI. PKM2, the M2 isoform of pyruvate kinase; PI, propidium iodide.
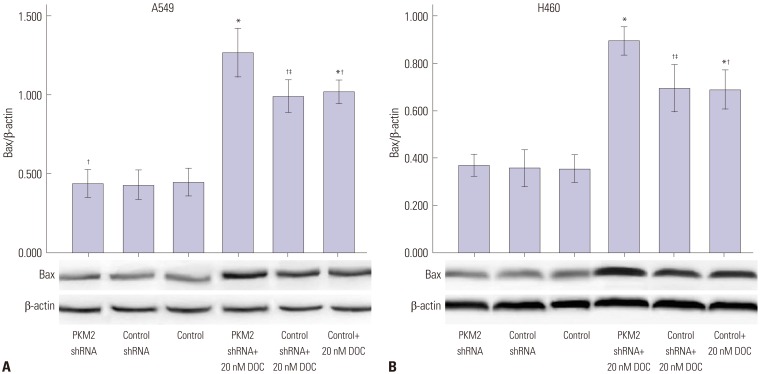
Knockdown of PKM2 increased the expression of Bax induced by docetaxel
Based on the above results, we also examined whether the promotion of docetaxed-induced apoptosis by silencing PKM2 was accompanied by the activation of Bax. As shown in Fig. 8, knockdown of PKM2 didn't increase the expression of Bax; 20 nM docetaxel treatment enhanced the expression of Bax in untransfected A549 and H460 cells, and this effect was further increased by PKM2 shRNA transfection. The difference in the expression of Bax was not observed between shRNA-control-transfected cells and untransfected cells.
Fig. 8. Bax protein expression induced by 0 nM and 20 nM docetaxel in PKM2-shRNA transfected, shRNA-control transfected or non-transfected cells at 48 hrs. 48 hrs after transfection, all A549 (A) and H460 (B) cells, PKM2 shRNA-transfected (PKM2 shRNA), control-shRNA transfected (Control shRNA) or non-transfected (Control), were incubated with 20 nM docetaxel for up to 48 hrs, Western blot analyses were performed to detect the expression of Bax. The graph depicts mean±SEM for three independent determinations of optical density of the Bax Western blot bands. *Compared to control cells with incubation of 0 nM docetaxel, p<0.05, †Compared to PKM2 shRNA-transfected cells with incubation of 20 nM docetaxel, p<0.05, ‡Compared to control shRNA-transfected cells with incubation of 0 nM docetaxel, p<0.05. PKM2, the M2 isoform of pyruvate kinase; DOC, docetaxel.
The results of flow cytometric assays together suggest that knockdown of PKM2 with shRNA first arrested cells at G2/M phase and docetaxel then made more cells to be blocked at G2/M phase followed by apoptosis.
DISCUSSION
Although cancer death rate resulting from lung cancer has currently declined regionally, such as in the USA, lung cancer is still one of the most common malignant tumors and the first cause of cancer-related death worldwide, which had become a serious threat to human health.12 Its morbidity and mortality have remained stubbornly high. According to the World Health Organization (WHO), the number of lung cancer patients was 1.825 million and deaths caused by lung cancer accounts for 23% of all cancer deaths worldwide in 2012.25 The latest surveys show that lung cancer has dominated the incidence of malignant tumors in males and malignant tumor mortality in both males and females in China.12 Current therapeutic approaches are not optimally effective. Therefore, searching for novel and effective therapy for lung cancer is imperative in terms of clinical oncologists. Especially for patients at advanced stage, who have lost the chance of surgery, effective chemotherapy is particularly important and urgently needed.
Docetaxel, which inhibits micro-tubule-disassembly and impairs mitosis, consequently interfering mitotic progress through blocking the cells at G2/M phase and promoting apoptosis, is applied widely to treat various cancers, including breast cancer, NSCLC and other solid tumors, and has achieved significant therapeutic results. Despite the doubtless clinical efficacy, however, docetaxel did not achieve a totally satisfactory therapeutic effect yet. Therefore, oncologists urgently need a method to enhance the chemotherapy effect of docetaxel.
Though the mechanism has not yet been clear, Christofk, et al.26 found that PKM2 is of crucial importance in the Warburg effect. PKM2 regulate efficient aerobic glycolysis, causing cancer cells to grow in the hypoxia environment for their advantage. Several studies have demonstrated that PKM2 is a multifunctional protein and concerned with metabolic regulation, cell growth, apoptotic cell death and immunological reactions. 27,28 These findings rapidly made PKM2 a potential target for the treatment of cancer. To carry out the PKM2-targeting tumor treatment is a research direction of highly potential value. Nevertheless, a question of whether PKM2 affects the chemotherapy sensitivity has not yet been deeply researched. Based on these reports described above, we planned to study the potential of PKM2 in influencing antitumor activity of docetaxel in vitro in NSCLC cell lines A549 and H460. In the current work, therefore, we investigated the effects of silencing of PKM2, combined with docetaxel, on cell viability, cell cycle distribution and apoptosis.
In our current study, with the method of plasmid transfection, we successfully silenced the expression of PKM2 at the level of gene and protein in A549 and H460 cells. ShRNA-control-transfection had no influence on the expression of PKM2, thus we are certain that plasmid vector and liposome had no impact on PKM2 expression.
After A549 and H460 cells were treated with different concentrations of docetaxel, a dose-dependent suppressive effect on cell viability was observed, in consistent with previous reports. Via regulating the Warburg effect, PKM2 promotes tumorigenesis and contributes to cell growth and proliferation.29,30 In our present study, knockdown of PKM2 with shRNA dramatically inhibited the viability of A549 and H460 cells. Further, combination treatments with shRNA PKM2 and chemotherapeutic agent docetaxel showed stronger inhibition of the cell viability than either single treatment in NSCLC cell lines A549 and H460 in vitro. These results demonstrated that silencing of PKM2 with shRNA improved the sensitivity of human NSCLC cell lines A549 and H460 to docetaxel in a synergistic manner, causing strong inhibition of cell viability.
In order to verify whether it was the M2 isoform that was specifically critical for cell proliferation, we made stable cell lines expressing flag-tagged mouse PKM2 (mPKM2) and then induced stable knockdown of endogenous PKM2 using shRNA expression. The PKM2 rescue cells exhibited the same active cell viability as the normal A549 and H460 cells. Therefore, we assumed that it was just the M2 isoform that was indispensable for cell proliferation. Among the four PK isoforms existing in mammals, the M1 isoform expressed in most adult tissues, and the M2 isoform which was a splice variant of M1, expressed during embryonic development and in tumour tissues exclusively.31 Therefore, although PKM2 shRNA-946 targeted both M1 and M2, the knockdown of PKM1 has no effect on the biological character of tumor cells, because PKM1 either was extremely low in tumor cells or itself had little effect on cell proliferation. Hence, PKM2 shRNA-946, though targeting both M1 and M2 isoform, was selected to silence PKM2 in the study.
In order to further exlpore the potential mechanism by which shRNA PKM2 affected the lethal effect of docetaxel on tumor cells, flow cytometry was carried out to analyze the cell cycle distribution and apoptosis. Our results demonstrated that PKM2 shRNA increased the cytotoxic effects of docetaxel on not only mitotic arrest but also apoptosis. Docetaxel plays its role in stabilizing microtubule formation through promoting tubulin assembly and suppressing microtubule depolymerisation at G2/M phase of cell cycle. By this means, it suppresses mitotic progress, blocking cells at the metaphase to anaphase transition due to activation of spindle assembly checkpoint, subsequently resulting in apoptosis. In our study, docetaxel led to cell cycle arrest at the G2/M phase and resulted in apoptosis in dose-dependent manner. Furthermore, it induced cell cycle arrest at the G2/M phase more dramatically in shRNA-PKM2-transfected cells than in untransfected cells, because shRNA PKM2 has already brought about a dramatic arrest at the G2/M phase before docetaxel function. However, knockdown of PKM2 alone could't significantly induce apoptosis, and docetaxel induced apoptosis more dramatically in shRNA-PKM2-transfected cells than in untransfected cells. It is well known that tumor cells with docetaxel treatment are arrested at the G2/M phase and undergo apoptosis next, and apoptosis is closely related to the block of G2/M phase, suggesting that docetaxel might be cell cycle specific.24,32 Docetaxel treatment increases the expression of Bax and Bcl-2 phosphorylation in cells arrested at G2/M phase, down-regulates the expression of Bcl-xL protein, induces p53, thereby bringing about apoptosis.33 Thus, we can assume that decreased expression of PKM2 in human NSCLC cells didn't start the tumor cell apoptosis process directly, although it can inhibit the cell viability and block the cells at G2/M phase.
In order to verify the present results that PKM2 knockdown combined with docetaxel treatment synergistically increased the G2/M phase cell cycle arrest, and that PKM2 knockdown enhanced the effect of docetaxel on cell apoptosis, the relevant expression levels of G2/M phase and apoptotic marker proteins, p21, one member of cyclin dependent kinase inhibitor (CKI) family, and Bax were detected, respectively. Cyclin, cyclin dependent kinase (CDK) and CKI together regulate the cell cycle.34 CDK1 regulates the G2/M phase with specificity, and p21 may cause G2/M phase arrest by inhibiting the functions of CDK1.35 And, p21 may induce apoptosis under certain conditions, but sometimes does not. As an upstream gene of pro-apoptotic genes, p21 might be involved in the apoptosis of cancer cells induced by the taxol anticancerogen.36 To date, however, a question of whether PKM2 knockdown is related to p21 has seldomly been studied. Bax and Bcl-2 are the most representative pro-apoptotic gene and apoptosis suppressor gene in Bcl-2 family, respectively. And, Bax is the main regulator of Bcl-2 and plays an important role in modulating tumor cells.37,38
Our present findings indicated that both 20 nM docetaxel, and PKM2 gene knockdown significantly promoted the expression of p21, which was supported by the result that either treatment could arrest cell cycle at G2/M phase, however, it was not exactly consistent with the data about apoptosis; the expression of p21 in the combined group rose more dramatically than that in either therapy given alone group. All these can be explained as follows; the increase of p21 induced by PKM2 gene knockdown didn't result in the activation of apoptotic pathways, and the increase of p21 induced by docetaxel might contribute to the apoptosis. 20 nM docetaxel dramatically increased the expression of Bax, but PKM2 knockdown didn't, and the combined treatment promoted the expression of Bax more significantly than any treatment alone. Again, these findings indicated that PKM2 knockdown hadn't directly contributed to apoptosis, but significantly enhanced the docetaxel-induced apoptosis of human NSCLC cells in vitro.
In summary, the present results showed that shRNA PKM2 could serve as a collaborative treatment to docetaxel in nonsmall lung cancer cells, through aiming at PKM2, resulting in inhibition of cell viability, increase of cell arrest of G2/M phase and apoptosis. The results further suggest that application of targeting the PKM2 has a potential to be a therapeutic strategy for NSCLC and provides one possible way to improve the chemotherapy effect of docetaxel meantime.
ACKNOWLEDGEMENTS
This study is supported by a grant from outstanding young talent training plan of Jinshan district health system, Shanghai, China (No. JWKJ-RCYQ-201206).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Mazurek S. Pyruvate kinase type M2: a key regulator of the metabolic budget system in tumor cells. Int J Biochem Cell Biol. 2011;43:969–980. doi: 10.1016/j.biocel.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Yeh CS, Wang JY, Chung FY, Lee SC, Huang MY, Kuo CW, et al. Significance of the glycolytic pathway and glycolysis related-genes in tumorigenesis of human colorectal cancers. Oncol Rep. 2008;19:81–91. [PubMed] [Google Scholar]
- 4.Gumińska M, Ignacak J, Kedryna T, Stachurska MB. Tumor-specific pyruvate kinase isoenzyme M2 involved in biochemical strategy of energy generation in neoplastic cells. Acta Biochim Pol. 1997;44:711–724. [PubMed] [Google Scholar]
- 5.Hacker HJ, Steinberg P, Bannasch P. Pyruvate kinase isoenzyme shift from L-type to M2-type is a late event in hepatocarcinogenesis induced in rats by a choline-deficient/DL-ethionine-supplemented diet. Carcinogenesis. 1998;19:99–107. doi: 10.1093/carcin/19.1.99. [DOI] [PubMed] [Google Scholar]
- 6.Steinberg P, Klingelhöffer A, Schäfer A, Wüst G, Weisse G, Oesch F, et al. Expression of pyruvate kinase M2 in preneoplastic hepatic foci of N-nitrosomorpholine-treated rats. Virchows Arch. 1999;434:213–220. doi: 10.1007/s004280050330. [DOI] [PubMed] [Google Scholar]
- 7.Mazurek S. Pyruvate kinase type M2: a key regulator within the tumour metabolome and a tool for metabolic profiling of tumours. Ernst Schering Found Symp Proc. 2007:99–124. doi: 10.1007/2789_2008_091. [DOI] [PubMed] [Google Scholar]
- 8.Bluemlein K, Grüning NM, Feichtinger RG, Lehrach H, Kofler B, Ralser M. No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis. Oncotarget. 2011;2:393–400. doi: 10.18632/oncotarget.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Z, Feng JG, Tuersun A, Liu T, Liu H, Liu Q, et al. Proteomic identification of differentially-expressed proteins in esophageal cancer in three ethnic groups in Xinjiang. Mol Biol Rep. 2011;38:3261–3269. doi: 10.1007/s11033-010-0586-0. [DOI] [PubMed] [Google Scholar]
- 10.Yuan SJ, Qiao TK, Chen W, Zhuang XB. The expression of PKM2 in early stage human non-small cell lung cancer and its clinical significance. J Clin Intern Med. 2015;32:524–527. [Google Scholar]
- 11.Huang BT, Lu JY, Lin PX, Chen JZ, Kuang Y, Chen CZ. Comparison of Two RapidArc Delivery Strategies in Stereotactic Body Radiotherapy of Peripheral Lung Cancer with Flattening Filter Free Beams. PLoS One. 2015;10:e0127501. doi: 10.1371/journal.pone.0127501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Han S, Zheng MJ, Xue Y, Liu WC. Clinical characteristics of 274 non-small cell lung cancer patients in China. Onkologie. 2013;36:248–254. doi: 10.1159/000350301. [DOI] [PubMed] [Google Scholar]
- 13.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 14.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 15.Hsiao JR, Leu SF, Huang BM. Apoptotic mechanism of paclitaxel-induced cell death in human head and neck tumor cell lines. J Oral Pathol Med. 2009;38:188–197. doi: 10.1111/j.1600-0714.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 16.Sun ML, Wang GJ, Li J, Cui JW, Zhang AL, Wang ZN, et al. [Construction of shRNA eukaryotic expression vectors of pkm2 gene and their effect on drug resistant cell line of acute promyelocytic leukemia] Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2010;18:85–89. [PubMed] [Google Scholar]
- 17.Yoo BC, Ku JL, Hong SH, Shin YK, Park SY, Kim HK, et al. Decreased pyruvate kinase M2 activity linked to cisplatin resistance in human gastric carcinoma cell lines. Int J Cancer. 2004;108:532–539. doi: 10.1002/ijc.11604. [DOI] [PubMed] [Google Scholar]
- 18.Li SL, Ye F, Cai WJ, Hu HD, Hu P, Ren H, et al. Quantitative proteome analysis of multidrug resistance in human ovarian cancer cell line. J Cell Biochem. 2010;109:625–633. doi: 10.1002/jcb.22413. [DOI] [PubMed] [Google Scholar]
- 19.Martinez-Balibrea E, Plasencia C, Ginés A, Martinez-Cardús A, Musulén E, Aguilera R, et al. A proteomic approach links decreased pyruvate kinase M2 expression to oxaliplatin resistance in patients with colorectal cancer and in human cell lines. Mol Cancer Ther. 2009;8:771–778. doi: 10.1158/1535-7163.MCT-08-0882. [DOI] [PubMed] [Google Scholar]
- 20.Shin YK, Yoo BC, Hong YS, Chang HJ, Jung KH, Jeong SY, et al. Upregulation of glycolytic enzymes in proteins secreted from human colon cancer cells with 5-fluorouracil resistance. Electrophoresis. 2009;30:2182–2192. doi: 10.1002/elps.200800806. [DOI] [PubMed] [Google Scholar]
- 21.Cortés-Cros M, Hemmerlin C, Ferretti S, Zhang J, Gounarides JS, Yin H, et al. M2 isoform of pyruvate kinase is dispensable for tumor maintenance and growth. Proc Natl Acad Sci U S A. 2013;110:489–494. doi: 10.1073/pnas.1212780110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 23.Zha L, Qiao T, Yuan S, Lei L. Enhancement of radiosensitivity by CpG-oligodeoxyribonucleotide-7909 in human non-small cell lung cancer A549 cells. Cancer Biother Radiopharm. 2010;25:165–170. doi: 10.1089/cbr.2009.0686. [DOI] [PubMed] [Google Scholar]
- 24.Zeng S, Chen YZ, Fu L, Johnson KR, Fan W. In vitro evaluation of schedule-dependent interactions between docetaxel and doxorubicin against human breast and ovarian cancer cells. Clin Cancer Res. 2000;6:3766–3773. [PubMed] [Google Scholar]
- 25.International Agency for Research on Cancer. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. [accessed on]. Available at: http://globocan.iarc.fr/Default.aspx.
- 26.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 27.Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, et al. PKM2 phosphorylates histone H3 and promotes gene transcription and tumorigenesis. Cell. 2012;150:685–696. doi: 10.1016/j.cell.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo W, Semenza GL. Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol Metab. 2012;23:560–566. doi: 10.1016/j.tem.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta V, Bamezai RN. Human pyruvate kinase M2: a multifunctional protein. Protein Sci. 2010;19:2031–2044. doi: 10.1002/pro.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bissery MC, Guénard D, Guéritte-Voegelein F, Lavelle F. Experimental antitumor activity of taxotere (RP 56976, NSC 628503), a taxol analogue. Cancer Res. 1991;51:4845–4852. [PubMed] [Google Scholar]
- 31.Jurica MS, Mesecar A, Heath PJ, Shi W, Nowak T, Stoddard BL. The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure. 1998;6:195–210. doi: 10.1016/s0969-2126(98)00021-5. [DOI] [PubMed] [Google Scholar]
- 32.Martinez VG, O’Connor R, Liang Y, Clynes M. CYP1B1 expression is induced by docetaxel: effect on cell viability and drug resistance. Br J Cancer. 2008;98:564–570. doi: 10.1038/sj.bjc.6604195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kucukzeybek Y, Gul MK, Cengiz E, Erten C, Karaca B, Gorumlu G, et al. Enhancement of docetaxel-induced cytotoxicity and apoptosis by all-trans retinoic acid (ATRA) through downregulation of survivin (BIRC5), MCL-1 and LTbeta-R in hormone- and drug resistant prostate cancer cell line, DU-145. J Exp Clin Cancer Res. 2008;27:37. doi: 10.1186/1756-9966-27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stark GR, Taylor WR. Control of the G2/M transition. Mol Biotechnol. 2006;32:227–248. doi: 10.1385/MB:32:3:227. [DOI] [PubMed] [Google Scholar]
- 35.Torricelli C, Salvadori S, Valacchi G, Souček K, Slabáková E, Muscettola M, et al. Alternative pathways of cancer cell death by rottlerin: apoptosis versus autophagy. Evid Based Complement Alternat Med. 2012;2012:980658. doi: 10.1155/2012/980658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barboule N, Chadebech P, Baldin V, Vidal S, Valette A. Involvement of p21 in mitotic exit after paclitaxel treatment in MCF-7 breast adenocarcinoma cell line. Oncogene. 1997;15:2867–2875. doi: 10.1038/sj.onc.1201469. [DOI] [PubMed] [Google Scholar]
- 37.Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, et al. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 38.Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]