Abstract
Purpose
Magnetocardiography (MCG) has been proposed as a noninvasive, diagnostic tool for risk-stratifying patients with acute myocardial infarction (AMI). This study evaluated whether MCG predicts long-term prognosis in AMI.
Materials and Methods
In 124 AMI patients (95 males, mean age 60±11 years), including 39 with ST-elevation myocardial infarction, a 64-channel MCG was performed within 2 days after AMI. During a mean follow-up period of 6.1 years, major adverse cardiac events (MACE) were evaluated.
Results
MACE occurred in 31 (25%) patients, including 20 revascularizations, 8 deaths, and 3 re-infarctions. Non-dipole patterns were observed at the end of the T wave in every patients. However, they were observed at T-peak in 77% (24/31) and 54% (50/93) of patients with and without MACE, respectively (p=0.03). Maximum current, field map angles, and distance dynamics were not different between groups. In the multivariate analysis, patients with non-dipole patterns at T-peak had increased age- and gender-adjusted hazard ratios for MACE (hazard ratio 2.89, 95% confidence interval 1.20–6.97, p=0.02) and lower cumulative MACE-free survival than those with dipole patterns (p=0.02).
Conclusion
Non-dipole patterns at T-peak were more frequently observed in patients with MACE and were related to poor long-term prognosis. Thus, repolarization heterogeneity measured by MCG may be a useful predictor for AMI prognosis.
Keywords: Acute myocardial infarction, prognosis, magnetocardiography
INTRODUCTION
Research into prognostic factors for acute myocardial infarction (AMI) is informative for optimizing therapeutic strategies. Traditional methods for stratifying risk for acute coronary syndrome are electrocardiography (ECG) and cardiac biomarkers. ECG is the most widely used test for evaluating patients with unstable angina and AMI. In the Thrombolysis in Myocardial Ischemia (TIMI) III Registry, independent predictors of 1-year death or myocardial infarction (MI) were left bundle branch block and new ST segment deviation >0.5 mm.1 Elevated cardiac biomarkers of myocardial necrosis [creatine kinase-MB (CK-MB), troponin] are associated with a worse long-term prognosis.2 Furthermore, elevated C-reactive protein correlates with an increased risk of mortality.3 Creatinine is another simple tool for AMI risk stratification. Creatinine or creatinine clearance is associated with increased mortality, independent of other standard risk factors.4 Other biomarkers related to increased cardiac risk include natriuretic peptides (brain natriuretic peptide or N-terminal pro-brain natriuretic peptide), white blood cell count, myeloperoxidase, and glucose or hemoglobin A1c. In addition, transthoracic echocardiography (TTE) is a popular method to assess patients with acute coronary syndrome. Left ventricular dysfunction, particularly left ventricular end-systolic volume, is known as a major prognostic factor for AMI.5 Although the above various methods can stratify risk for AMI, several promising new techniques have been studied to determine if they provide better prognostic prediction.
Magnetocardiography (MCG) is a noncontact, noninvasive, and radiation-free method for providing a complete investigation of a given patient's cardiac magnetic field (MF) within 10 min. Clinical research using MCG has been wide-ranging: it has been found to be more accurate than ECG for the evaluation of MI and ventricular repolarization abnormalities6,7 and is able to identify patients at risk for ventricular tachycardia.8 MCG has been proposed as a tool for risk-stratifying patients with AMI and ischemia.9,10,11 Increased intra-QRS fragmentation in MCG predicts arrhythmic events, especially ventricular tachycardia and mortality in post-MI patients with left ventricular dysfunction.12,13 Moreover, MCG has been reported to show higher non-dipolar structures on cardiac MF maps after ST-elevation and non-ST-elevation myocardial infarction.11,14 Also, temporal and spatial analysis of QT intervals in healthy subjects and in patients with coronary artery disease using MCG have revealed that the spatial distribution of QT intervals in patients differed from those in healthy subjects in three ways: they showed greater dispersion, greater local variability, and a change in overall MCG pattern.15 However, MCG patterns that link with AMI prognosis remain to be elucidated. Therefore, this study evaluated whether specific MCG findings could predict long-term prognosis in patients with AMI.
MATERIALS AND METHODS
The study group
This study was conducted at the Cardiovascular Center of the Yonsei University Severance Hospital (Seoul, Korea) with the approval of the Institutional Review Board. Informed consent was obtained from all patients. MCGs were recorded from 140 consecutive AMI patients, aged 20–80 years, from March 2005 to July 2009. Among these patients, MCGs from 16 patients were unable to be evaluated due to the following: inverted T-wave (n=8); flat T-wave (n=5); and complete atrioventricular block (n=3). Ultimately, 124 patients (95 males, mean age 60±11 years), including 39 with ST-elevation myocardial infarction (STEMI), were evaluated. MCGs were not evaluated in any patient who met the following exclusion criteria: a prior diagnosis of MI, previous defibrillator implantation or coronary bypass surgery, and diagnosis of cancer.
MI was diagnosed by one of the following: typical chest pain with new Q wave or significant ST change on a 12-lead ECG; a significant increase in the plasma creatine kinase (CK) cardiac isoenzyme level; or an akinetic or dyskinetic ventricular wall motion abnormality in an area supplied by a stenosed coronary artery.16 Coronary angiography was performed and left ventricular ejection fraction (LVEF) was obtained in every patient. A significant coronary artery stenosis was defined by greater than 50% luminal narrowing of the vessel diameter. Data were collected and summarized using standardized abstraction forms by an abstractor with 3 years of training blinded to the outcome of interest.
Clinical parameters
Each patient was checked for a history of hypertension and diabetes mellitus. Two-dimensional TTE was performed within 48 hours of AMI to confirm LVEF. Coronary angiographic findings were converted to a coronary artery disease severity score, as follows: normal coronary=0, 1 vessel disease (VD)=1, 2VD=2, 3VD=3. Measured cardiac biomarkers were CK, CK-MB, and troponin-T.
MCG recording and interpretation
For all patients, 12-lead ECGs and MCGs were recorded within 30 min while the patient was at rest. High-resolution MCG recordings were obtained within 2 days after AMI using a KRISS 64-channel biomagnetometer (Bio-Signal Research Center, KRISS, Daejeon, Korea) in a magnetically shielded room at Cardiovascular Center of the Yonsei University Severance Hospital. The MCG system employs double relaxation oscillation superconducting quantum interference device sensors. The average noise spectral density of the entire system in the magnetically shielded room was 10 fT/Hz at 1 Hz and 5 fT/Hz over 100 Hz. The system is equipped with 64 planar first order superconducting quantum interference device gradiometers, which measure the tangential components of the cardiomagnetic fields. A high-pass filter of 0.5 Hz, a low-pass filter of 1.6 kHz, and a 60-Hz notch filter were used for recording.17,18 MCG recordings were carried out while the patient was at rest for 30 seconds, after resting for 2 min in a supine position on the bed. After the acquisition, MCG signals were baseline-corrected, digitally filtered, and averaged to increase the signal-to-noise ratio. Data were averaged, centering on the R wave peak.19
Analysis of MCG
Previous studies for diagnosis of ischemic heart disease using MCG suggests several MCG parameters, such as maximum current angle, distance of poles in current density vector (CDV), field map angle in MF maps and dipole or non-dipole patterns in T-peak, have significant correlation with myocardial ischemia.9,10,14
Maximum current angle, distance of poles in current density vector and field map angle in magnetic field maps
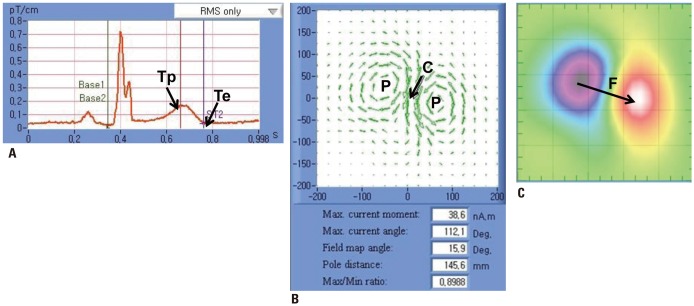
The sum of the raw signals from 64 recording sites was integrated as the MCG time tracing (Fig. 1A). The MCG time tracing analyzes the Q, R, and T waves and the QT interval. The end of the T wave (Te) is the visually determined vertex (maximum curvature) of the signal following the inflection point after the peak of the T wave (Tp).
Fig. 1. Measurement of MCG. (A) MCG tracing. (B) Current vector density map. (C) Magnetic field map. Maximum current angle (C), field map angle (F), and the number and distance of poles (P) were measured every 20 ms from T-end (Te) to T-peak (Tp). MCG, magnetocardiography.
CDV maps represent electrical activation signals of the heart. The maximum current angle is the angle of maximum electrical current and dynamic distance of poles is the distance between magnetic poles in the heart (Fig. 1B). MF maps express the MF derived from electrical activation signals with colorcoded images (Fig. 1C). In the MF map, the red and blue poles display outgoing and inward MFs with respect to the plane of the thorax, respectively. The field map angle is the angle of direction from the center of the negative blue pole to the center of the positive red pole. MF and CDV maps were analyzed from Te back to Tp.
Dipole and non-dipole patterns
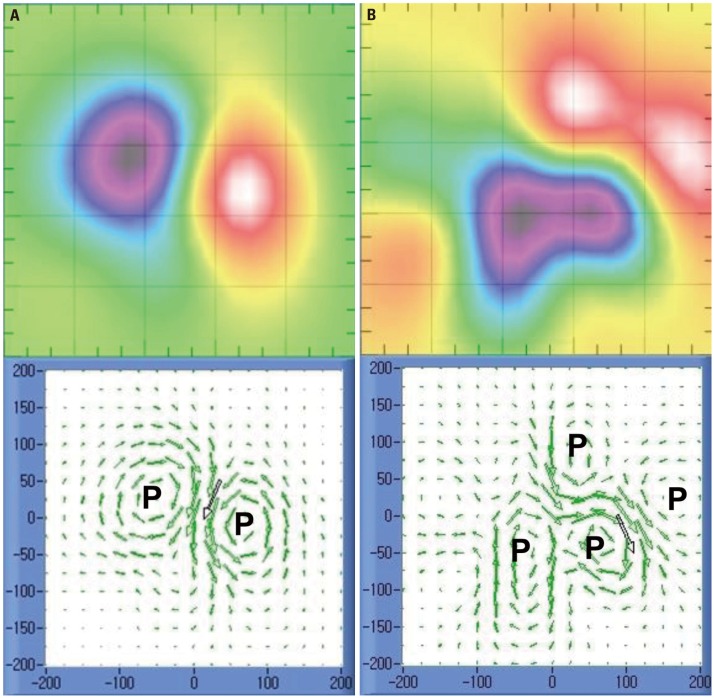
A dipole pattern was defined as a MF containing two poles. If there were more than 2 poles, it was defined as a non-dipole pattern (Fig. 2). Fig. 3A is a typical example of normal repolarization showing a dipole pattern; there was a single electrical current from the right inferior direction. Fig. 3B shows an abnormal repolarization with a non-dipole pattern; the electrical current was deconcentrated from the left inferior direction.
Fig. 2. Examples of dipole (A) and non-dipole pattern (B). The number of poles (P) is 2 and 4 in dipole and non-dipole patterns, respectively.
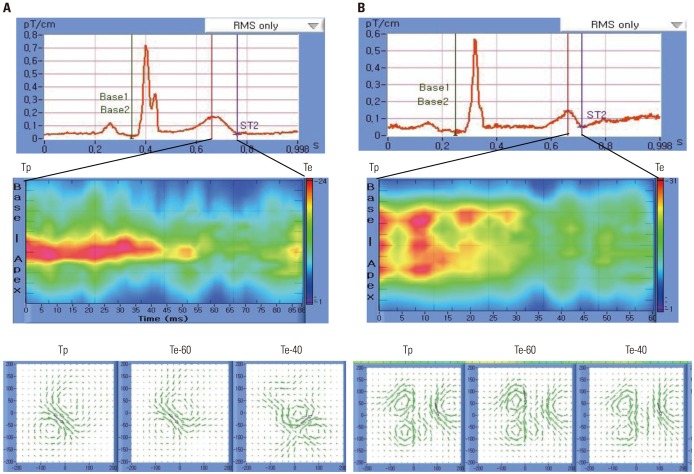
Fig. 3. Typical MCG finding from a 79-year-old female patient without MACE (A) and a 66-year-old female patient with MACE (B). MCG tracing (upper panels), spatiotemporal activation graph (middle panels), and magnetic field and current vector density maps (lower panels). While the dipole pattern was observed at Tp in the patient without MACE, the non-dipole pattern can be seen in the patient with MACE. Note continuous change and dispersion of the magnetic field from Tp to Te. MACE, major adverse cardiac events; MCG, magnetocardiography; Te, T-end; Tp, T-peak.
Spatiotemporal activation graph
A spatiotemporal activation graph (STAG) expresses the time-dependent activation of an electromagnetic field from the base to the apex (Fig. 3, middle panels). The Fig. 3 STAG images represent time-dependent tracing of the MF in a dipole pattern and a non-dipole pattern, respectively.
Follow-up
Patients were followed at 1-month, 3-month, and 6-month intervals after discharge from the clinic. The end-point was a major adverse cardiac event (MACE), including composite of death from any cause, reinfarction, and percutaneous coronary intervention (PCI) during the follow-up period. Any patients who had symptoms and signs of angina pectoris or reinfarction during the follow-up period underwent a coronary work-up to confirm coronary lesion and treatment. If a patient had typical chest pain with elevated cardiac enzymes, the patient was categorized as having non-fatal reinfarction.
Statistical analysis
The data were analyzed using SPSS 20.0 for windows (IBM Corp., Armonk, NY, USA). All continuous variables are expressed as means±standard deviations, and categorical data are reported as an absolute number or percentage. Baseline data were compared using two-sided t-tests for continuous data or chi-square tests for categorical data. The hazard ratios (HRs) and 95% confidence intervals (CIs) for MACE were calculated with the Cox proportional-hazards model. The multivariate model included age, gender, CK-MB, serum creatinine, and non-dipole pattern at Tp. Kaplan-Meier survival curves were plotted for dipole and non-dipole patterns at Tp and were compared by means of the log-rank test. Significance was set at p<0.05.
RESULTS
Baseline characteristics
During the mean follow-up duration of median (25–75% percentile) and 6.1 years (2.8–8.4 years), MACE occurred in 31 (25%) patients, including 20 PCIs, 8 deaths, and 3 reinfarctions. Clinical characteristics of patients are presented in Table 1. More females than males had a MACE (p=0.01), and patients with MACE had higher levels of serum creatinine (p=0.04) at the time of symptom presentation. Patients managed with PCI had more MACEs (p=0.02) and patients in the coronary artery bypass graft group had no MACEs (p=0.02). Other clinical parameters and medication use show no differences between the 2 groups.
Table 1. Baseline Characteristics of Study Patients.
| Parameters | Total (n=124) | MACE (−) (n=93) | MACE (+) (n=31) | p value |
|---|---|---|---|---|
| Age, yrs | 59.8±11.3 | 59.0±11.3 | 62.1±11.4 | 0.20 |
| Sex (female), n (%) | 29 (23) | 17 (18) | 12 (39) | 0.02 |
| STEMI, n (%) | 39 (32) | 27 (29) | 12 (39) | 0.27 |
| Hypertension, n (%) | 64 (52) | 49 (53) | 15 (48) | 0.69 |
| Diabetes, n (%) | 37 (30) | 31 (33) | 6 (19) | 0.25 |
| Serum creatinine, mg/dL | 1.2±1.3 | 1.1±0.53 | 1.6±2.4 | 0.04 |
| CK, IU/L | 813.8±1199.7 | 765.5±1071.6 | 958.7±1533.4 | 0.44 |
| CK-MB, ng/mL | 206.3±156.1 | 92.4±117.3 | 147.9±235.1 | 0.09 |
| Troponin-T, ng/mL | 2.0±3.1 | 1.8±2.7 | 2.8±3.9 | 0.11 |
| LVEF, % | 52.1±13.4 | 52.4±12.8 | 51.0±15.0 | 0.59 |
| Multivessel CAD, n (%) | 79 (64) | 55 (59) | 24 (77) | 0.07 |
| Management, n (%) | ||||
| PCI | 90 (73) | 62 (67) | 28 (90) | 0.02 |
| CABG | 14 (11) | 14 (15) | 0 (0) | 0.02 |
| Medication only | 20 (16) | 17 (18) | 3 (10) | 0.40 |
| Medication, n (%) | ||||
| Antiplatelet | 121 (98) | 91 (98) | 30 (97) | 1.00 |
| Beta blocker | 92 (74) | 79 (85) | 22 (71) | 0.19 |
| ACEI or ARB | 101 (82) | 72 (77) | 20 (65) | 0.34 |
| Statin | 110 (89) | 81 (87) | 29 (94) | 0.18 |
MACE, major adverse cardiac events; STEMI, ST-elevation myocardial infarction; CK, creatine kinase; CK-MB, creatine kinase MB; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor antagonist.
Comparison of MCG parameters
Non-dipole patterns were observed at 40 ms before Te (Te40) in 89 (72%) patients, whereas non-dipole patterns were observed at T-peak in 77% (24/31) and 54% (50/93) of patients with and without MACE, respectively. Non-dipole patterns at Tp were more frequently observed in patients with than without MACE (p=0.02) (Table 2). There were no differences in the maximum current angles at the Tp (42.5±91.5° vs. 53.4±92.0°, p=0.57), MF map angles (-11.3±83.6° vs. -9.64±94.0°, p=0.93), or pole distance (144.0±24.5 mm vs. 143.7±32.3 mm, p=0.97) between patients with and without MACE.
Table 2. Non-Dipole Patterns in Patients with or without Major Adverse Cardiac Events (MACE).
| Parameters | Total (n=124) | No MACE (n=93) | MACE (n=31) | p value |
|---|---|---|---|---|
| Non-dipole pattern, n (%) | ||||
| Te* | 124 (100) | 93 (100) | 31 (100) | - |
| Te-20 ms | 115 (93) | 85 (91) | 30 (97) | 0.45 |
| Te-40 ms | 89 (72) | 62 (67) | 27 (87) | 0.04 |
| Te-60 ms | 78 (63) | 53 (57) | 25 (81) | 0.02 |
| T-peak | 74 (60) | 50 (54) | 24 (77) | 0.02 |
| Maximum current angle (°) | 45.2±19.4 | 42.5±91.5 | 53.4±92.0 | 0.57 |
| Field map angle (°) | −10.9±85.9 | −11.3±83.6 | −9.64±94.0 | 0.93 |
| Pole distance, mm | 143.9±26.5 | 144.0±24.5 | 143.7±32.3 | 0.97 |
*End of T-wave.
In the univariate analysis, the predictors of MACE were female gender (HR 2.67, 95% CI 1.31–5.56, p=0.01), and non-dipole pattern at Tp (HR 2.61, 95% CI 1.12–6.06, p=0.03). In the multivariate analysis, the predictors of MACE were female gender (HR 2.29, 95% CI 1.01–5.17, p=0.05), and non-dipole pattern at Tp (HR 2.89, 95% CI 1.20–6.97, p=0.02) (Table 3).
Table 3. Non-Dipole Patterns in Patients with or without Major Adverse Cardiac Events (MACE).
| Parameters | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Age, >65 yrs | 1.78 (0.88–3.62) | 0.11 | 1.22 (0.55–2.69) | 0.63 |
| Female sex | 2.67 (1.31–5.56) | 0.01 | 2.29 (1.01–5.17) | 0.05 |
| Hypertension | 0.80 (0.40–1.62) | 0.54 | 1.01 (0.46–2.22) | 0.98 |
| Diabetes | 0.53 (0.22–1.29) | 0.16 | 0.61 (0.23–1.61) | 0.32 |
| LVEF <40% | 1.19 (0.48–2.91) | 0.71 | 1.09 (0.43–2.74) | 0.85 |
| Multi-vessel CAD | 2.23 (0.94–5.18) | 0.06 | 2.19 (0.89–5.46) | 0.94 |
| STEMI | 1.39 (0.67–2.86) | 0.37 | 1.80 (0.85–3.81) | 0.12 |
| Non-dipole pattern | 2.61 (1.12–6.06) | 0.03 | 2.89 (1.20–6.97) | 0.02 |
*End of T-wave.
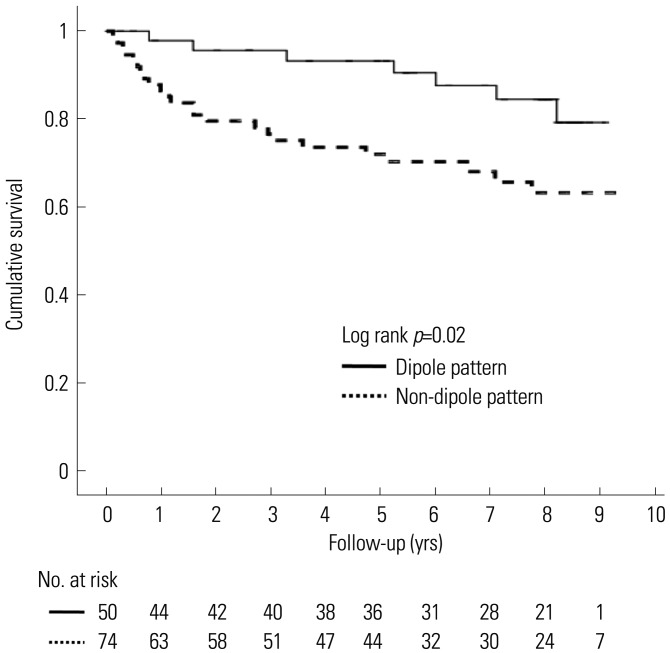
Fig. 4 shows the Kaplan-Meier survival curves for MACE in patients with dipole and non-dipole patterns. Patients with non-dipole patterns had lower cumulative MACE-free survival than did patients with a dipole pattern (p=0.02).
Fig. 4. MACE-free survival. Kaplan-Meier survival curves for cardiac events in patients with different repolarization patterns at Tp. Patients with non-dipole patterns at Tp had lower cumulative cardiac event-free survival than did the dipole pattern group (p=0.02). MACE, major adverse cardiac events; Tp, T-peak.
DISCUSSION
Major findings
The primary finding in this study is that a heterogeneous repolarization pattern was observed in post-MI patients. Interestingly, a non-dipole pattern at T-peak was more frequently observed in patients with MACE and was associated with poor long-term prognosis. This finding suggests that a repolarization heterogeneity measured by MCG might be used to predict the prognosis of AMI.
MCG patterns in post-MI patients
ECG is the most popular non-invasive diagnostic tool for diagnosing AMI, and it can also reflect disease severity and prognosis. MCG, another non-invasive diagnostic modality, may provide a more precise approach to ischemic heart disease than ECG.6,7 In the present study, MCG facilitated the detection of non-dipoles because of its superior spatial resolution and also because it shows the differences in physical properties between magnetic and electrical fields. It is, therefore, useful for detecting cardiac changes at early stages that are currently undetectable by ECG.20
Previous studies found that abnormal heterogenous repolarization patterns in T-peak have significant correlation with diagnosis of myocardial ischemia. They classified abnormal MF map patterns as compressed, stretched, broken, or rotated poles.10 However, all of these patterns commonly appear in ischemic heart disease patients, and there are no different clinical findings according to these four patterns. Therefore, in the present study, we categorized all types of abnormal MF map patterns as having a non-dipole pattern. Through analysis of MCG findings from AMI patients, we confirmed these two types of MF map patterns (dipole and non-dipole) in the repolarization phase. Moreover, in the current density map, healthy people showed dipole patterns, whereas non-dipole patterns were found in post-MI patients.20 These finding support the idea that abnormal cardiac conductivity is caused by ischemia:21 In this study, most patients showed a non-dipole pattern at Te and 60 ms prior to Te. However, only 60% of patients showed non-dipole patterns at Tp. This magnetic dispersion at the T wave suggests a heterogeneous repolarization abnormality due to ischemia.22
Heterogeneous repolarization in MCG patterns and long-term prognosis
MCG is a novel method for studying AMI, however, more studies are needed to confirm the clinical importance of variable MCG findings. Previous studies have confirmed specific MCG findings in ischemic heart disease patients, and these investigations focused on the diagnostic value of MCG modality.9,10,11 However, whether specific MCG findings could predict prognosis had not yet been elucidated. Typical parameters useful for diagnosis of ischemic heart disease were maximum current angle, field map angle, pole distance, and abnormal repolarization patterns in the MF map. In the present study, a non-dipole pattern at the Tp was found to have significant prognostic value in post-MI patients. On the other hand, other parameters, including maximum current angle, field map angle, and pole distance, had no prognostic value in post-MI patients. Some previous studies suggested that the T peak-end interval positively correlated with the prognosis of MI: the patients with T peak-end interval more than 140 ms have significantly higher clinical events than those with T peak-end interval less than 140 ms.23 Furthermore, successful primary PCI was found to significantly reduce T peak-end interval in patients with STEMI.24 Consistent with these observations, our present study supported the idea that repolarization heterogeneity has a significant correlation with poor prognosis in AMI patients.
LVEF is one of the most important prognostic factors for total mortality, sudden cardiac death, and heart failure in post-MI patients. In our present study, however, LVEF was not different between the patients with MACE or without MACE. This discrepancy might be explained by the fact that many patients in our study had relatively preserved LVEF. Moreover, LVEF could be influenced by myocardial stunning and segmental hyperkinesia outside the infarction area at an early stage of MI.25
Limitations
This study has several limitations. Ten percent of our post-MI patients had T-wave inversion or non-specific ST segment change on ECG. Because it was difficult to select the T-peak, these patients were excluded. Display characteristics, added noise, and different analysts might affect manual repolarization interval measurements in MCG.26 Undefined or unclear clinical meaning and pathophysiologic backgrounds of MCG findings are major limitations of this study.
Conclusion
The magnetic dispersion was observed as non-dipole pattern in MCG. And, most of the AMI patients with MACE showed magnetic dispersion at the T-peak. This finding suggests that MCG may be used to diagnose the repolarization dispersion produced by ischemia, thus predicting the prognosis for AMI.
ACKNOWLEDGEMENTS
This study was supported by research grants from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (NRF-2012R1A2A2A02045367), and a grant from the Korean Healthcare technology R&D project funded by the Ministry of Health & Welfare (HI16C0058, HI15C1200).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Cannon CP, McCabe CH, Stone PH, Rogers WJ, Schactman M, Thompson BW, et al. The electrocardiogram predicts one-year outcome of patients with unstable angina and non-Q wave myocardial infarction: results of the TIMI III Registry ECG Ancillary Study. Thrombolysis in Myocardial Ischemia. J Am Coll Cardiol. 1997;30:133–140. doi: 10.1016/s0735-1097(97)00160-5. [DOI] [PubMed] [Google Scholar]
- 2.Kleiman NS, Lakkis N, Cannon CP, Murphy SA, DiBattiste PM, Demopoulos LA, et al. Prospective analysis of creatine kinase muscle-brain fraction and comparison with troponin T to predict cardiac risk and benefit of an invasive strategy in patients with non-ST-elevation acute coronary syndromes. J Am Coll Cardiol. 2002;40:1044–1050. doi: 10.1016/s0735-1097(02)02119-8. [DOI] [PubMed] [Google Scholar]
- 3.Morrow DA, Rifai N, Antman EM, Weiner DL, McCabe CH, Cannon CP, et al. C-reactive protein is a potent predictor of mortality independently of and in combination with troponin T in acute coronary syndromes: a TIMI 11A substudy. Thrombolysis in Myocardial Infarction. J Am Coll Cardiol. 1998;31:1460–1465. doi: 10.1016/s0735-1097(98)00136-3. [DOI] [PubMed] [Google Scholar]
- 4.Gibson CM, Pinto DS, Murphy SA, Morrow DA, Hobbach HP, Wiviott SD, et al. Association of creatinine and creatinine clearance on presentation in acute myocardial infarction with subsequent mortality. J Am Coll Cardiol. 2003;42:1535–1543. doi: 10.1016/j.jacc.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Breithardt G, Borggrefe M, Fetsch T, Böcker D, Mäkijärvi M, Reinhardt L. Prognosis and risk stratification after myocardial infarction. Eur Heart J. 1995;16(Suppl G):10–19. doi: 10.1093/eurheartj/16.suppl_g.10. [DOI] [PubMed] [Google Scholar]
- 6.Fenici R, Brisinda D, Meloni AM. Clinical application of magnetocardiography. Expert Rev Mol Diagn. 2005;5:291–313. doi: 10.1586/14737159.5.3.291. [DOI] [PubMed] [Google Scholar]
- 7.Kwong JS, Leithäuser B, Park JW, Yu CM. Diagnostic value of magnetocardiography in coronary artery disease and cardiac arrhythmias: a review of clinical data. Int J Cardiol. 2013;167:1835–1842. doi: 10.1016/j.ijcard.2012.12.056. [DOI] [PubMed] [Google Scholar]
- 8.Endt P, Montonen J, Mäkijärvi M, Nenonen J, Steinhoff U, Trahms L, et al. Identification of post-myocardial infarction patients with ventricular tachycardia by time-domain intra-QRS analysis of signal-averaged electrocardiogram and magnetocardiogram. Med Biol Eng Comput. 2000;38:659–665. doi: 10.1007/BF02344872. [DOI] [PubMed] [Google Scholar]
- 9.Lim HK, Chung N, Kim K, Ko YG, Kwon H, Lee YH, et al. Can magnetocardiography detect patients with non-ST-segment elevation myocardial infarction? Ann Med. 2007;39:617–627. doi: 10.1080/07853890701538040. [DOI] [PubMed] [Google Scholar]
- 10.Lim HK, Kwon H, Chung N, Ko YG, Kim JM, Kim IS, et al. Usefulness of magnetocardiogram to detect unstable angina pectoris and non-ST elevation myocardial infarction. Am J Cardiol. 2009;103:448–454. doi: 10.1016/j.amjcard.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Van Leeuwen P, Hailer B, Beck A, Eiling G, Grönemeyer D. Changes in dipolar structure of cardiac magnetic field maps after ST elevation myocardial infarction. Ann Noninvasive Electrocardiol. 2011;16:379–387. doi: 10.1111/j.1542-474X.2011.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korhonen P, Husa T, Tierala I, Väänänen H, Mäkijärvi M, Katila T, et al. Increased intra-QRS fragmentation in magnetocardiography as a predictor of arrhythmic events and mortality in patients with cardiac dysfunction after myocardial infarction. J Cardiovasc Electrophysiol. 2006;17:396–401. doi: 10.1111/j.1540-8167.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 13.Korhonen P, Pesola K, Järvinen A, Mäkijärvi M, Katila T, Toivonen L. Relation of magnetocardiographic arrhythmia risk parameters to delayed ventricular conduction in postinfarction ventricular tachycardia. Pacing Clin Electrophysiol. 2002;25:1339–1345. doi: 10.1046/j.1460-9592.2002.01339.x. [DOI] [PubMed] [Google Scholar]
- 14.Kyoon Lim H, Kim K, Lee YH, Chung N. Detection of non-ST-elevation myocardial infarction using magnetocardiogram: new information from spatiotemporal electrical activation map. Ann Med. 2009;41:533–546. doi: 10.1080/07853890903107883. [DOI] [PubMed] [Google Scholar]
- 15.Van Leeuwen P, Hailer B, Lange S, Grönemeyer D. Spatial distribution of repolarization times in patients with coronary artery disease. Pacing Clin Electrophysiol. 2003;26:1706–1714. doi: 10.1046/j.1460-9592.2003.t01-1-00256.x. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, White HD Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 17.Lee YH, Kim JM, Kim K, Kwon H, Yu KK, Kim IS. 64-channel magnetocardiogram system based on double relaxation oscillation SQUID planar gradiometers. Supercond Sci Technol. 2006;19:S284–S288. [Google Scholar]
- 18.Kim K, Kwon H, Lee YH, Kim TE, Kim JM, Park YK, et al. Clinical parameter assessment in magnetocardiography by using the support vector machine. Int J Bioelectromagn. 2005;7:224–227. [Google Scholar]
- 19.Kim K, Lee YH, Kwon H, Kim JM, Kim IS, Park YK. Optimal sensor distribution for measuring the tangential field components in MCG. Neurol Clin Neurophysiol. 2004;2004:60. [PubMed] [Google Scholar]
- 20.Ikefuji H, Nomura M, Nakaya Y, Mori T, Kondo N, Ieishi K, et al. Visualization of cardiac dipole using a current density map: detection of cardiac current undetectable by electrocardiography using magnetocardiography. J Med Invest. 2007;54:116–123. doi: 10.2152/jmi.54.116. [DOI] [PubMed] [Google Scholar]
- 21.Stinstra JG, Shome S, Hopenfeld B, MacLeod RS. Modelling passive cardiac conductivity during ischaemia. Med Biol Eng Comput. 2005;43:776–782. doi: 10.1007/BF02430957. [DOI] [PubMed] [Google Scholar]
- 22.Takala P, Hänninen H, Montonen J, Korhonen P, Mäkijärvi M, Nenonen J, et al. Heart rate adjustment of magnetic field map rotation in detection of myocardial ischemia in exercise magnetocardiography. Basic Res Cardiol. 2002;97:88–96. doi: 10.1007/s395-002-8391-y. [DOI] [PubMed] [Google Scholar]
- 23.Lin XM, Yang XL, Liu HL, Lai YQ. [Clinical assessment of Tpeak-end interval for prediction of myocardial infarction] Nan Fang Yi Ke Da Xue Xue Bao. 2010;30:2169–2170. [PubMed] [Google Scholar]
- 24.Eslami V, Safi M, Taherkhani M, Adibi A, Movahed MR. Evaluation of QT, QT dispersion, and T-wave peak to end time changes after primary percutaneous coronary intervention in patients presenting with acute ST-elevation myocardial infarction. J Invasive Cardiol. 2013;25:232–234. [PubMed] [Google Scholar]
- 25.Wita K, Filipecki A, Szydło K, Turski M, Tabor Z, Wróbel W, et al. Prediction of long-term outcome after primary percutaneous coronary intervention for acute anterior myocardial infarction. Kardiol Pol. 2010;68:393–400. [PubMed] [Google Scholar]
- 26.Smith FE, Langley P, Trahms L, Steinhoff U, Bourke JP, Murray A. Errors in repolarization measurement using magnetocardiography. Pacing Clin Electrophysiol. 2002;25:1223–1229. doi: 10.1046/j.1460-9592.2002.01223.x. [DOI] [PubMed] [Google Scholar]