Abstract
Purpose
The objective of this study was to develop a new nomogram that can predict 28-day mortality in severe sepsis and/or septic shock patients using a combination of several biomarkers that are inexpensive and readily available in most emergency departments, with and without scoring systems.
Materials and Methods
We enrolled 561 patients who were admitted to an emergency department (ED) and received early goal-directed therapy for severe sepsis or septic shock. We collected demographic data, initial vital signs, and laboratory data sampled at the time of ED admission. Patients were randomly assigned to a training set or validation set. For the training set, we generated models using independent variables associated with 28-day mortality by multivariate analysis, and developed a new nomogram for the prediction of 28-day mortality. Thereafter, the diagnostic accuracy of the nomogram was tested using the validation set.
Results
The prediction model that included albumin, base excess, and respiratory rate demonstrated the largest area under the receiver operating characteristic curve (AUC) value of 0.8173 [95% confidence interval (CI), 0.7605–0.8741]. The logistic analysis revealed that a conventional scoring system was not associated with 28-day mortality. In the validation set, the discrimination of a newly developed nomogram was also good, with an AUC value of 0.7537 (95% CI, 0.6563–0.8512).
Conclusion
Our new nomogram is valuable in predicting the 28-day mortality of patients with severe sepsis and/or septic shock in the emergency department. Moreover, our readily available nomogram is superior to conventional scoring systems in predicting mortality.
Keywords: Severe sepsis, septic shock, mortality, nomograms
INTRODUCTION
Between 1988 and 2012, the United States in-hospital mortality rate of septic patients admitted to an intensive care unit (ICU) declined by 35%, despite an increase in illness severity.1 The most important factor in decreasing mortality among septic patients was the implementation of care bundle approaches (e.g., the Surviving Sepsis Campaign).2,3 However, sepsis is still the leading cause of death (18.4–29.0%),4,5 and the incidences of severe sepsis and septic shock are increasing.5,6 Therefore, much progress remains to be made in decreasing the mortality rate; one way to accomplish this is the early identification of patients likely to die. If we can predict the mortality of patients with severe sepsis and/or septic shock (hereafter referred to as "severe sepsis") in the emergency department (ED) and aggressively resuscitate them, the survival rate can be expected to increase.
Many studies have suggested prognostic factors for severe sepsis such as age, gender, ethnic origin, comorbidities, illness severity, and biomarkers associated with mortality.7,8 Conventional scoring systems such as the Acute Physiology and Chronic Health Evaluation II (APACHE II), Sepsis Organ Failure Assessment (SOFA), and National Early Warning Score (NEWS) have been used to predict mortality and multi-organ dysfunction.9 However, some studies considered that conventional scoring systems were more suitable for patients with early sepsis, and were unsuitable for those with severe sepsis or septic shock.10 Others have suggested a role for biomarkers in predicting mortality from severe sepsis.11,12 More than 170 different biomarkers have been evaluated, including coagulation, complement, contact system activation, inflammation, and apoptosis markers. Biomarkers can add accuracy and objectivity,13 but their effectiveness in mortality prediction is limited by a lack of specificity and sensitivity.12,14,15 It is difficult to precisely estimate prognosis from a single biomarker because the host immune response to infection is complex. Response to sepsis varies with time, and some specific biomarkers may be useful during specific time periods.12
Recently, some studies have reported a combinatory approach using scoring systems and biomarkers for predicting septic patient mortality.11,16 However, few studies suggested a combination of several biomarkers with and without scoring systems for mortality prediction in patients with severe sepsis.
Thus, the objective of this study was to determine a new nomogram (which is a user-friendly graphical representation of a statistical predictive model that generates a numerical probability of a clinical event)17 that can predict 28-day mortality in severe sepsis and/or septic shock patients using a combination of several biomarkers that are inexpensive and readily available in most EDs, with and without scoring systems.
MATERIALS AND METHODS
Study design and populations
We performed a retrospective, observational, registry-based study using the early goal-directed therapy (EGDT) registry data from Yonsei University College of Medicine, Severance Hospital, Seoul, Korea between January 1, 2012 and December 31, 2014. Since November 2007, the protocol-based EGDT has been implemented as a critical pathway for patients presenting to the ED of our institution with severe sepsis. If a patient meeting two or more systemic inflammatory response syndrome (SIRS) criteria along with having suspicious signs of organ dysfunction or hypoperfusion with infection presented to the ED, the patient's eligibility for EGDT was assessed. Patients were enrolled in the EGDT registry if one or both of the following conditions existed: 1) an initial systolic blood pressure (SBP) <90 mm Hg, despite a 20 mL/kg intravenous crystalloid fluid challenge; or 2) an initial serum lactate level ≥4 mmol/L. The exclusion criteria were as follows: 1) those aged <19 years, 2) pregnancy, 3) an acute cerebrovascular or coronary syndrome, 4) active gastrointestinal bleeding, 5) a contraindication to a central venous catheter, 6) trauma, 7) a requirement for immediate surgical intervention, 8) a transfer to another hospital within 6 hours after ED admission, and 9) those on do-not-resuscitate status. After reviewing the final diagnosis, we also excluded patients with non-infectious conditions leading to SIRS. The final study population was then allocated to either the training or the validation set at a 7:3 ratio by simple random sampling. The study was reviewed and approved by the Institutional Review Board of Yonsei University College of Medicine, Severance Hospital. The requirement of informed consent was waived by the ethics committee because of the retrospective nature of the study.
Data collection
One investigator (M.H.S.) collected data through a retrospective review of medical records. We attempted to use a standardized data abstraction form to minimize bias in the present retrospective study.
We collected demographic data, pre-existing chronic comorbidities, and initial vital signs including respiratory rate (RR). Initial laboratory data sampled at the time of ED admission that were known to be relevant to the diagnosis or prognosis of sepsis, as well as laboratory data collected routinely in the ED, were recorded. Parameters included white blood cell count, hematocrit, platelet count, red cell distribution width (RDW), delta neutrophil index (DNI), international normalized ratio, activated partial thromboplastin time (aPTT), pH, pCO2, pO2, base excess (BE), HCO3-, lactate, blood urea nitrogen (BUN), creatinine, albumin, creatine kinase-MB (CK-MB), troponin T, N-terminal brain natriuretic peptide, C-reactive protein (CRP), and procalcitonin. APACHE II score, SOFA score, and NEWS were also obtained using the worst values during the initial 24 hours after ED admission. The primary outcome was 28-day overall mortality.
Statistical analysis
Statistical analysis was performed using SAS version 9.2 (SAS Inc., Cary, NC, USA). The categorical variables are described as frequencies (%) and continuous variables as mean±standard deviation.
We used independent two-sample t tests for comparisons of continuous variables and the chi-squared test or Fisher's exact test for categorical variables. For the training set, multicollinearity between the variables was taken into consideration. First, we created various types of combinations composed of variables with p<0.05 on univariate binary logistic regression, including individual scoring systems such as APACHE II, and then generated models using variables that were identified as independently associated with 28-day mortality by a stepwise multivariate analysis method. Subsequently, we selected the model with the largest area under the receiver operating characteristic curve (AUC). The Delong method was used to compare the AUC for the predictive value of the new nomogram with those of APACHE II, NEWS, and SOFA regarding 28-day survival of patients with severe sepsis. Finally, a simplified nomogram was developed for the prediction of 28-day mortality with the training set using variables included in the selected model. The performances of the nomograms in predicting outcomes were evaluated with respect to discrimination and calibration.17 The Hosmer-Lemeshow goodness-of-fit test was used to assess the suitability of the models, because it showed how well the nomogram was calibrated; namely, a close approximation between the observed and predicted probabilities shows good calibration and confirms the exportability of the model. Thereafter, the diagnostic accuracy of the new nomogram generated with the training set was subsequently tested in the validation set. p<0.05 was considered statistically significant.
RESULTS
Study population
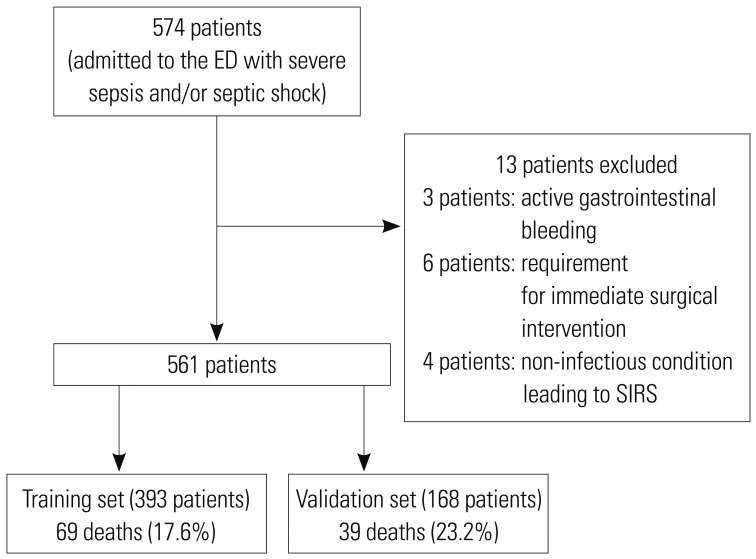
A total of 574 patients with suspected severe sepsis underwent EGDT in the ED during the study period and were initially enrolled in the present study. Upon chart review, we excluded 13 patients for the following reasons: active gastrointestinal bleeding (n=3), a requirement for immediate surgical intervention (n=6), and a non-infectious condition leading to SIRS (n=4) (Fig. 1). We included 561 patients in the final analysis, of whom 393 were randomly allocated to the training set and 168 to the validation set. The baseline characteristics in the training and validation sets that were related to 28-day mortality are summarized in Table 1. In the training set of 393 patients, 69 (17.6%) did not survive 28 days. Moreover, there were 39 (23.2%) deaths during the follow-up period in the validation set.
Fig. 1. Flow diagram of the study subjects. ED, emergency department; SIRS, systemic inflammatory response syndrome.
Table 1. Demographic and Other Features of the Study Population.
| Training set | Validation set | |||||
|---|---|---|---|---|---|---|
| Survivor (n=324) | Deceased (n=69) | p value | Survivor (n=129) | Deceased (n=39) | p value | |
| Age | 66.5±15.0 | 68.0±13.7 | 0.454 | 67.9±14.4 | 70.6±11.4 | 0.278 |
| Sex | 0.591 | 0.132 | ||||
| Male | 157 (48.6) | 36 (52.2) | 65 (50.4) | 25 (64.1) | ||
| Female | 166 (51.4) | 33 (47.8) | 64 (49.6) | 14 (35.9) | ||
| SBP (mm Hg) | 91.9±28.0 | 87.1±29.8 | 0.201 | 88.3±20.3 | 84.7±25.4 | 0.363 |
| DBP (mm Hg) | 57.3±15.0 | 54.5±17.9 | 0.240 | 55.5±11.5 | 54.2±16.9 | 0.663 |
| HR (beats/min) | 102.9±24.7 | 106.0±24.5 | 0.337 | 100.0±21.7 | 102.3±25.3 | 0.580 |
| RR (respirations/min) | 19.4±4.0 | 21.9±5.8 | <0.001 | 19.5±4.1 | 21.7±6.2 | 0.046 |
| BT (℃) | 37.7±1.4 | 36.9±1.4 | <0.001 | 37.8±1.6 | 36.9±1.5 | 0.003 |
| O2 saturation (%) | 94.0±8.3 | 91.3±11.0 | 0.051 | 95.0±6.3 | 88.9±10.7 | 0.001 |
| WBC (/µL) | 13934.4±8533.1 | 15733.3±13324.9 | 0.285 | 13952.0±9990.8 | 13023.3±11906.5 | 0.628 |
| HCt (%) | 35.7±7.3 | 34.6±7.4 | 0.259 | 35.9±6.7 | 33.1±6.6 | 0.023 |
| RDW (%) | 14.6±1.9 | 15.7±2.1 | <0.001 | 14.4±2.3 | 15.6±1.7 | 0.001 |
| PLT (103/µL) | 206.0±120.7 | 188.4±134.2 | 0.280 | 223.0±202.8 | 151.3±108.7 | 0.005 |
| DNI (%) | 9.0±9.8 | 13.3±14.4 | 0.021 | 8.9±10.3 | 17.1±20.1 | 0.018 |
| INR | 1.4±1.1 | 1.9±2.5 | 0.103 | 1.2±0.8 | 1.7±1.8 | 0.147 |
| aPTT (s) | 33.4±9.2 | 39.4±19.3 | 0.013 | 32.5±7.0 | 38.9±17.0 | 0.026 |
| CRP (mg/L) | 135.5±109.5 | 164.9±121.4 | 0.049 | 133.7±104.8 | 165.6±89.4 | 0.094 |
| PCT (ng/mL) | 21.8±41.8 | 38.1±72.4 | 0.267 | 32.6±59.9 | 41.3±54.3 | 0.610 |
| pH | 7.4±0.1 | 7.4±0.1 | 0.001 | 7.4±0.1 | 7.4±0.2 | 0.034 |
| pCO2 (mm Hg) | 25.5±6.0 | 24.2±7.5 | 0.189 | 26.0±6.0 | 24.9±10.0 | 0.490 |
| pO2 (mm Hg) | 89.5±37.1 | 91.0±52.4 | 0.815 | 82.5±27.7 | 96.8±59.2 | 0.150 |
| BE (mmol/L) | −6.9±5.3 | −10.5±6.0 | <0.001 | −6.9±4.6 | −10.8±7.6 | 0.004 |
| HCO3- (mmol/L) | 17.6±4.5 | 14.8±4.6 | <0.001 | 17.6±3.7 | 14.8±5.6 | 0.004 |
| Lactate (mmol/L) | 4.1±3.3 | 6.0±4.0 | <0.001 | 3.4±2.6 | 6.6±4.8 | <0.001 |
| BUN (mg/dL) | 33.9±27.1 | 47.4±34.0 | 0.003 | 32.5±24.9 | 46.7±25.5 | 0.002 |
| Cr (mg/dL) | 2.1±2.1 | 2.4±1.9 | 0.192 | 1.9±1.9 | 2.9±2.2 | 0.005 |
| Albumin (g/dL) | 3.2±0.7 | 2.6±0.6 | <0.001 | 3.2±0.7 | 2.7±0.6 | <0.001 |
| CK-MB (ng/mL) | 3.9±10.7 | 8.9±17.0 | 0.027 | 4.2±8.4 | 10.0±14.4 | 0.029 |
| Troponin T (ng/mL) | 0.1±0.7 | 0.1±0.2 | 0.763 | 0.1±0.2 | 0.5±2.3 | 0.254 |
| BNP (pg/mL) | 5319.1±9100.0 | 8285.2±11049.1 | 0.059 | 4005.8±6617.8 | 9148.4±11225.1 | 0.041 |
| Comorbidities | ||||||
| DM | 123 (38.0) | 25 (36.2) | 0.788 | 45 (34.9) | 17 (43.6) | 0.324 |
| CRF | 34 (10.5) | 12 (17.4) | 0.106 | 13 (10.1) | 10 (25.6) | 0.013 |
| CHF | 18 (5.6) | 5 (7.3) | 0.574 | 12 (9.3) | 1 (2.6) | 0.303 |
| COPD | 16 (5.0) | 6 (8.7) | 0.246 | 6 (4.7) | 3 (7.7) | 0.435 |
| LC | 10 (3.1) | 3 (4.4) | 0.708 | 3 (2.3) | 4 (10.3) | 0.052 |
| Infection site | <0.001 | 0.051 | ||||
| Lung | 62 (19.1) | 31 (44.9) | <0.001 | 25 (19.4) | 14 (35.9) | 0.032 |
| Intra-abdominal | 88 (27.2) | 11 (15.9) | 0.051 | 40 (31.0) | 8 (20.5) | 0.049 |
| Urinary tract | 101 (31.2) | 7 (10.1) | <0.001 | 41 (31.8) | 6 (15.4) | 0.046 |
| Multiple | 16 (4.9) | 4 (5.8) | 0.764 | 6 (4.7) | 2 (5.1) | >0.999 |
| Others | 57 (17.6) | 16 (23.2) | 0.278 | 17 (13.2) | 9 (23.1) | 0.134 |
| APACHE II score | 16.0±5.3 | 18.5±6.1 | <0.001 | 16.0±5.1 | 21.1±6.5 | <0.001 |
| NEWS | 8.5±3.0 | 9.7±3.7 | 0.011 | 8.7±2.8 | 10.7±2.7 | <0.001 |
| SOFA | 6.4±2.5 | 7.2±2.7 | 0.015 | 6.7±2.1 | 8.5±2.3 | <0.001 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; RR, respiratory rate; BT, body temperature; WBC, white blood cell; HCt, hematocrit; RDW, red cell distribution width; PLT, platelet count; INR, international normalized ratio; aPTT, activated partial thromboplastin time; DNI, delta neutrophil index; CRP, C-reactive protein; PCT, procalcitonin; BE, base excess; BUN, blood urea nitrogen; Cr, creatinine; CK-MB, creatine kinase-MB; BNP, N-terminal brain natriuretic peptide; DM, diabetes mellitus; CRF, chronic renal failure; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; LC, liver cirrhosis; APACHE II, Acute Physiology and Chronic Health Evaluation II; NEWS, National Early Warning Score; SOFA, Sepsis Organ Failure Assessment.
Data are expressed as the mean±SD or n (%). There were no differences between the training set and the validation set except for SOFA.
Albumin, base excess, and respiratory rate are independent prognostic factors in the training set
Among training set patients, the RR of those who died was higher than that of survivors (21.9±5.8 vs. 19.4±4.0; p<0.001), and body temperature was also significantly different between the groups (p<0.001). There were several differing laboratory findings between the surviving and deceased groups. High RDW, DNI, aPTT, and CRP values were correlated with increased 28-day mortality in severely septic patients (p<0.001, p=0.021, p=0.013, and p=0.049, respectively). Low pH, HCO3-, and BE values as well as a high lactate value were also significantly associated with increased 28-day mortality (p=0.001, p<0.001, p<0.001, and p<0.001, respectively). Low albumin, high BUN, and high CK-MB values were predictive of increased mortality (p<0.001, p=0.003, and p=0.027, respectively). Procalcitonin values were available for only 150 patients (38.2%) because this test was not performed during the earlier stage of the study period, and did not show statistical significance predicting 28-day mortality in our study (p=0.267). In the training set, the APACHE II, NEWS, and SOFA scores of deceased patients were higher than those of survivors (18.5±6.1 vs. 16.0±5.3; p<0.001, 9.7±3.7 vs. 8.5±3.0; p=0.011, and 7.2±2.7 vs. 6.4±2.5; p=0.015, respectively) (Table 1).
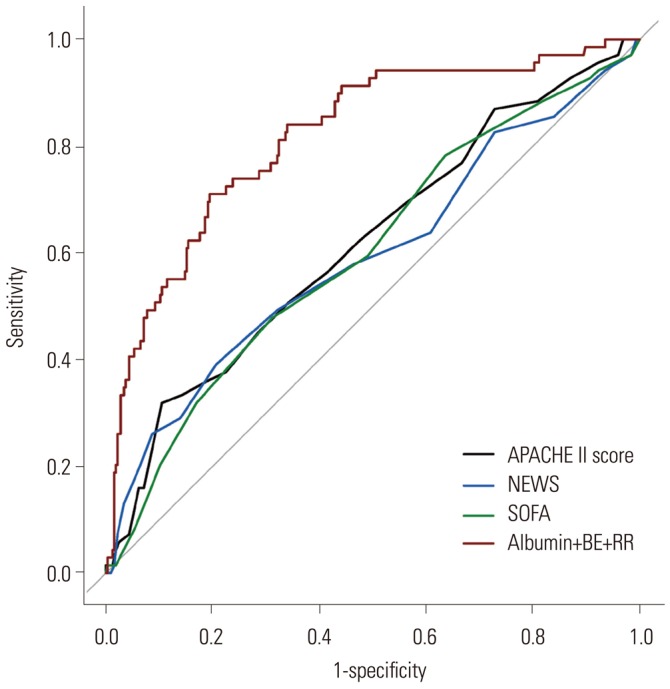
All significant variables in the univariate binary logistic regression were considered when generating models to predict 28-day mortality in patients with severe sepsis. Each scoring system was entered individually into the multivariate logistic regression analysis with clinical variables and laboratory variables among the training set (models 1–3). We also created a model that did not include a scoring system (model 4). Consequently, four models were generated by a stepwise multivariate analysis method in our study. Because pH is highly correlated with HCO3-, this variable was dropped from subsequent models. Table 2 summarizes the results of the multivariate logistic regression analysis. In the models that included scoring systems, the multivariate logistic analysis revealed that no scoring system was associated with 28-day mortality in patients with severe sepsis. Model 4, which did not include a scoring system, demonstrated the largest AUC value of 0.8173 (95% CI, 0.7605–0.8741) among the models. This model was composed of albumin, BE, and RR as predictive factors and showed a significantly higher AUC value than those of conventional scoring systems (p<0.001) (Fig. 2).
Table 2. A Multivariate Analysis Predicting 28-Day Mortality in Septic Patients from the Training Set.
| Model 1 (AUC: 0.7901) | Model 2 (AUC: 0.7901) | Model 3 (AUC: 0.8173) | Model 4 (AUC: 0.8173) | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Albumin | 0.276 (0.178–0.427) | <0.001 | 0.276 (0.178–0.427) | <0.001 | 0.254 (0.161–0.403) | <0.001 | 0.254 (0.161–0.403) | <0.001 |
| BE | 0.899 (0.856–0.944) | <0.001 | 0.899 (0.856–0.944) | <0.001 | 0.901 (0.856–0.948) | <0.001 | 0.901 (0.856–0.948) | <0.001 |
| RR | 1.119 (1.057–1.183) | <0.001 | 1.119 (1.057–1.183) | <0.001 | ||||
AUC, area under the receiver operating characteristic curve; OR, odds ratio; CI, confidence interval; BE, base excess; RR, respiratory rate; APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sepsis Organ Failure Assessment; NEWS, National Early Warning Score.
Each scoring system was entered individually into the multivariate logistic regression analysis with clinical variables and laboratory variables in the training set. A total of four models were generated by a stepwise multivariate analysis method. Model 1 includes clinical variables, laboratory variables, and APACHE II; Model 2 includes clinical variables, laboratory variables, and NEWS; Model 3 includes clinical variables, laboratory variables, and SOFA; and Model 4 includes clinical variables and laboratory variables without a scoring system.
Fig. 2. Comparisons of APACHE II, NEWS, and SOFA scores versus model 4 in predicting 28-day mortality. Model 4 was composed of albumin, BE, and RR as predictive factors, and showed an AUC value of 0.8173 (95% CI, 0.7605–0.8741). The AUCs of the APACHE II, NEWS, and SOFA scores were 0.6177 (95% CI, 0.5423–0.6931), 0.5940 (95% CI, 0.5137–0.6743), and 0.6005 (95% CI, 0.5256–0.6754), respectively. Model 4 demonstrated a significantly higher AUC value than those of conventional scoring systems (p<0.001) by the Delong test for comparisons of receiver operating characteristic curves. APACHE II, Acute Physiology and Chronic Health Evaluation II; NEWS, National Early Warning Score; SOFA, Sepsis Organ Failure Assessment; BE, base excess; RR, respiratory rate; AUC, area under the curve; CI, confidence interval.
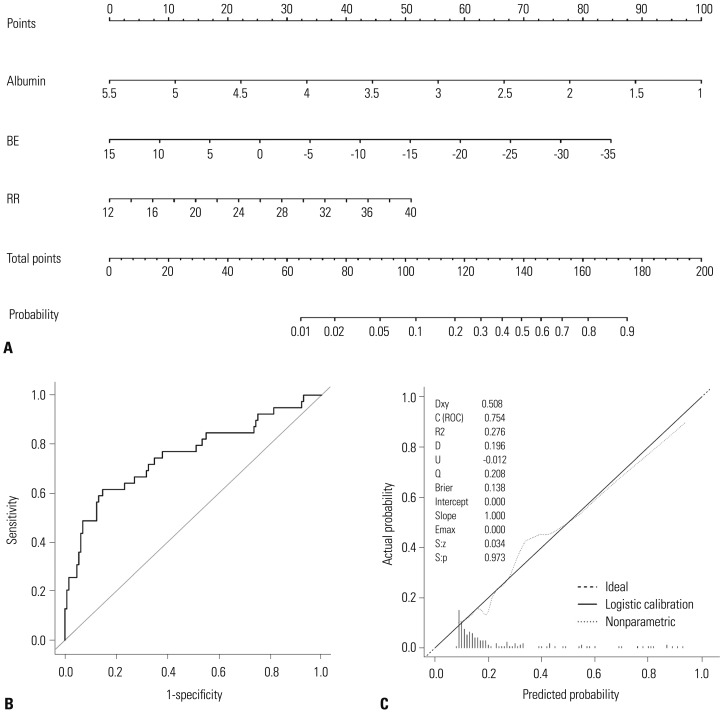
Nomogram shows that hypoalbuminemia, low base excess values, and tachypnea predict 28-day mortality
A nomogram incorporating three prognostic factors was established from the final model (Fig. 3). The nomogram illustrated that albumin was the largest contributor to prognosis, followed by BE and RR. By calculating the total number of points and locating it on the total point scale, we were easily able to draw a straight line down to estimate the predicted probability of 28-day mortality. For example, a 55-year-old man presented with complaints of a 3-day history of fever associated with pneumonia. On admission, he presented with tachypnea [20/min (15 points)] and his blood test results were BE=-10 mmol/L (43 points) and albumin=2 g/dL (78 points). The sum of the nomogram values was 136, and the predicted probability for 28-day mortality was 43%.
Fig. 3. The newly developed nomogram and external validation. (A) A nomogram for predicting 28-day mortality among patients with severe sepsis and/or septic shock using the training set. (B and C) External validation of the nomograms using the validation set. The discriminative ability of the nomogram was good, with an AUC value of 0.7537 (95% CI, 0.6563–0.8512) (B). Calibration plots (dotted line) show close approximations to the logistic calibration (solid line), indicating good agreement between the predicted and observed probabilities of 28-day mortality (C). BE, base excess; RR, respiratory rate; AUC, area under the curve; CI, confidence interval.
The p value from the Hosmer-Lemeshow test was 0.61 for model 4, indicating that the model was suitable. In the validation set, discrimination was good with an AUC value of 0.7537 (95% CI, 0.6563–0.8512). The calibration plot of the nomogram presented excellent agreement between predicted and observed probabilities of 28-day mortality, and exhibited a close approximation between the probabilities.
DISCUSSION
In this study, we developed and validated a new nomogram using three independent variables to identify 28-day mortality in patients with severe sepsis in the ED. This nomogram is solely based on hypoalbuminemia, a low BE value, and tachypnea, which are easily and readily obtainable during the patient's course in the ED. Therefore, it can be used to identify patients who are at high risk of severe sepsis and require more aggressive treatment. Our nomogram demonstrated a significantly higher AUC value than those of conventional scoring systems, despite it being simpler than such systems.
We found that the initial albumin value sampled at the time of ED admission was associated with 28-day mortality in severe sepsis patients and was the largest contributor to prognosis. Albumin, which is considered a negative acute-phase protein, is frequently decreased in the acute phase of several diseases such as sepsis or trauma, and its levels are reduced by approximately 10–15 g/L within 1 week of the event.18 Hypoalbuminemia can be caused by reduced hepatic synthesis, a decrease in the supply of amino acids, increased leakage into the interstitial space, and tissue catabolism or distributional issues.19 The reduction in albumin synthesis during inflammation is likely associated with the effect of monocytic products such as interleukin-6 and tumor necrosis factor-α.20 Although the precise mechanisms have not been fully described, serum albumin has protective effects such as maintaining physiologic homeostasis, antioxidant activity, anti-inflammatory effects, and prevention of apoptosis.21,22 Therefore, these protective biologic functions may be impaired in hypoalbuminemic conditions, and increased morbidity and mortality can consequently develop in severely septic patients.23,24 In patients with community-acquired bloodstream infection who require intensive care, hypoalbuminemia was an independent risk factor associated with global mortality (odds ratio, 0.34; 95% CI, 0.15–0.76).23 A previous study by Vincent, et al.24 also reported that each 10 g/L decline in serum albumin concentration significantly raised the odds of mortality by 137%, morbidity by 89%, prolonged ICU stay by 28%, prolonged hospital stay by 71%, and increased resource utilization by 66%.
BE was defined as the concentration of titratable hydrogen ion required to return the pH to 7.4 while maintaining pCO2 at 40 mm Hg by equilibration.25 BE can be correlated with the level of deterioration secondary to severe sepsis. The decrease in BE during septic shock is associated with critical reductions in oxygen use, resulting in tissue hypoperfusion, anaerobic metabolism, and lactic acidosis.26 Moreover, these were related to mortality in patients with severe sepsis.27 In this study, the initial BE was -6.9±5.3 and -6.9±4.6 mmol/L in survivors vs. -10.5±6.0 and -10.8±7.6 mmol/L in non-survivors in the training and validation sets (p<0.001 and p=0.004), respectively. This result is consistent with a previous study.28 Some scoring systems used pH and lactate values to evaluate metabolic acidosis with tissue hypoperfusion.29,30 However, BE has been shown to be superior to pH for evaluating metabolic acidosis. Davis, et al.31 reported that BE was superior to pH as a marker of metabolic acidosis clearance after shock in a retrospective study of 674 patients. Similarly, BE is superior to lactate, because lactate does not entirely account for metabolic acidosis development from systemic hypoperfusion.27 Accordingly, our scoring system more accurately estimated metabolic acidosis in patients with severe sepsis by using the BE value.
Tachypnea is a compensatory mechanism for metabolic acidosis secondary to sepsis. Respiratory difficulty (defined as tachypnea, low oxygen saturation, or high oxygen requirement) was significantly predictive of mortality in septic patients; therefore, recent scoring systems such as the Mortality in Emergency Department Sepsis (MEDS) and Predisposition Insult Response and Organ Failure (PIRO) incorporated tachypnea as a key criterion.30,32 In our study, the respiratory rates of deceased patients were 21.9±5.8 in the training set and 21.7±6.2 in the validation set. These results met the tachypneic criteria of the MEDS and PIRO systems.
There are several scoring systems to predict outcomes of severe sepsis patients, with APACHE II and SOFA being the most popular. However, the predictive ability of these conventional scoring systems was somewhat suboptimal; the AUC for APACHE II was 0.71,33 and that for SOFA was 0.708,34 in previous reports. In our study, the AUC values of APACHE II, NEWS, and SOFA were 0.618, 0.594, and 0.601, respectively; these values were significantly lower than those of our new nomogram. One explanation for this outcome may be that the conventional scoring systems were designed for patients in the ICU, not for those in the ED as our nomogram was.
Our new scoring system carries the virtues of simplicity and promptitude to predict 28-day mortality in patients with severe sepsis in the ED. In this study, the variables selected in models 3 and 4 were the same; hence, the AUCs of these two models were the same as well. For the simplicity and promptitude of our new scoring system, we ultimately selected model 4, as it does not include the conventional, complex scoring system. Furthermore, by building a user-friendly nomogram, our system is more readily available, and predicting mortality in severely septic patients is easier to calculate than with other conventional scoring systems. Our nomogram uses the initial values of variables rather than the worst values; therefore, we are able to predict mortality earlier, which can be helpful for selecting high-risk patients who require more aggressive interventions. Several studies reported that early, intensive, and effective treatment could decrease the mortality rate by 8–16%.35 Therefore, our nomogram could be applied to triage decisions, and could be used to select severely ill patients for closer monitoring and aggressive treatment. Additionally, our nomogram includes only three independent variables, while others include a greater number of variables and are thus much more complex to calculate. Our nomogram could be performed significantly more predictably than other scoring systems, with AUCs of 0.8173 from the training set and 0.7537 from the validation set.
This study has several limitations. The main limitation is that it is a retrospective study of data collected from a single center; therefore, it is possible that the results could differ from those of other centers, and the predictive probability could be overestimated compared to a prospective study. Although we performed internal validation to reduce bias, a multi-center study is required for further validation. Another source of bias was the population enrollment criteria, in which organ dysfunction and hypoperfusion were regarded as an initial SBP of <90 mm Hg, despite a 20 mL/kg intravenous crystalloid fluid challenge; or an initial serum lactate level ≥4 mmol/L. These criteria did not completely encompass severe sepsis; however, lactate is a commonly used marker of hypoxic tissue.36 Accordingly, these patients could be regarded as severely septic in our study. Our nomogram did not include information such as the cause of infection or the type of cultured pathogen. However, our goal was to construct a system to predict mortality among patients with severe sepsis, but not to identify a specific infection or pathogen. We performed comparisons with conventional scoring systems, such as APACHE II, NEWS, and SOFA, but these scoring systems are not intended for use in ED environments. However, MEDS, Mortality In Severe Sepsis in the Emergency Department, and PIRO have been developed for use in the ED to risk-stratify septic cases.30,32,37 In a future study, we aim to compare these scoring systems to ours, and to extend the validation to a multi-center study. An increase in treatment bundle compliance improves the outcome of severe sepsis patients.38,39 However, we could not accurately measure the compliance rate of EGDT at our institution because of the retrospective nature of this study. The overall mortality rate of our study population was similar to that of a recently published randomized controlled study,40 indicating that our compliance rate was not necessarily lower than that observed in a developed country.
In conclusion, our new nomogram is valuable in predicting the 28-day mortality of patients with severe sepsis and/or septic shock at an early stage after ED admission, and it is superior to the APACHE II, NEWS, and SOFA scoring systems in predicting mortality, and is readily available.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Zimmerman JE, Kramer AA, Knaus WA. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care. 2013;17:R81. doi: 10.1186/cc12695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 3.Miller RR, 3rd, Dong L, Nelson NC, Brown SM, Kuttler KG, Probst DR, et al. Multicenter implementation of a severe sepsis and septic shock treatment bundle. Am J Respir Crit Care Med. 2013;188:77–82. doi: 10.1164/rccm.201212-2199OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 5.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 6.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Crit Care Med. 2007;35:1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 7.Marshall JC, Reinhart K International Sepsis Forum. Biomarkers of sepsis. Crit Care Med. 2009;37:2290–2298. doi: 10.1097/CCM.0b013e3181a02afc. [DOI] [PubMed] [Google Scholar]
- 8.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 9.Dupuy AM, Philippart F, Péan Y, Lasocki S, Charles PE, Chalumeau M, et al. Role of biomarkers in the management of antibiotic therapy: an expert panel review: I - currently available biomarkers for clinical use in acute infections. Ann Intensive Care. 2013;3:22. doi: 10.1186/2110-5820-3-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones AE, Saak K, Kline JA. Performance of the Mortality in Emergency Department Sepsis score for predicting hospital mortality among patients with severe sepsis and septic shock. Am J Emerg Med. 2008;26:689–692. doi: 10.1016/j.ajem.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Li C, Jia Y. Evaluation of the Mortality in Emergency Department Sepsis score combined with procalcitonin in septic patients. Am J Emerg Med. 2013;31:1086–1091. doi: 10.1016/j.ajem.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. doi: 10.1186/cc8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Keefe SC, Marshall FF, Issa MM, Harmon MP, Petros JA. Thrombocytosis is associated with a significant increase in the cancer specific death rate after radical nephrectomy. J Urol. 2002;168(4 Pt 1):1378–1380. doi: 10.1016/S0022-5347(05)64453-9. [DOI] [PubMed] [Google Scholar]
- 14.Silvestre J, Póvoa P, Coelho L, Almeida E, Moreira P, Fernandes A, et al. Is C-reactive protein a good prognostic marker in septic patients? Intensive Care Med. 2009;35:909–913. doi: 10.1007/s00134-009-1402-y. [DOI] [PubMed] [Google Scholar]
- 15.Samraj RS, Zingarelli B, Wong HR. Role of biomarkers in sepsis care. Shock. 2013;40:358–365. doi: 10.1097/SHK.0b013e3182a66bd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CC, Chen SY, Tsai CL, Wu SC, Chiang WC, Wang JL, et al. Prognostic value of mortality in emergency department sepsis score, procalcitonin, and C-reactive protein in patients with sepsis at the emergency department. Shock. 2008;29:322–327. doi: 10.1097/shk.0b013e31815077ca. [DOI] [PubMed] [Google Scholar]
- 17.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26:1364–1370. doi: 10.1200/JCO.2007.12.9791. [DOI] [PubMed] [Google Scholar]
- 18.Moshage HJ, Janssen JA, Franssen JH, Hafkenscheid JC, Yap SH. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation. J Clin Invest. 1987;79:1635–1641. doi: 10.1172/JCI113000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franch-Arcas G. The meaning of hypoalbuminaemia in clinical practice. Clin Nutr. 2001;20:265–269. doi: 10.1054/clnu.2001.0438. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85:599–610. doi: 10.1093/bja/85.4.599. [DOI] [PubMed] [Google Scholar]
- 21.Emerson TE., Jr Unique features of albumin: a brief review. Crit Care Med. 1989;17:690–694. doi: 10.1097/00003246-198907000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, Kim J, Kim K, Jo YH, Rhee J, Kim TY, et al. Albumin and C-reactive protein have prognostic significance in patients with community-acquired pneumonia. J Crit Care. 2011;26:287–294. doi: 10.1016/j.jcrc.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Artero A, Zaragoza R, Camarena JJ, Sancho S, González R, Nogueira JM. Prognostic factors of mortality in patients with community-acquired bloodstream infection with severe sepsis and septic shock. J Crit Care. 2010;25:276–281. doi: 10.1016/j.jcrc.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Vincent JL, Dubois MJ, Navickis RJ, Wilkes MM. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003;237:319–334. doi: 10.1097/01.SLA.0000055547.93484.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siggaard-Andersen O, Fogh-Andersen N. Base excess or buffer base (strong ion difference) as measure of a non-respiratory acid-base disturbance. Acta Anaesthesiol Scand Suppl. 1995;107:123–128. doi: 10.1111/j.1399-6576.1995.tb04346.x. [DOI] [PubMed] [Google Scholar]
- 26.Chaudry IH. Cellular mechanisms in shock and ischemia and their correction. Am J Physiol. 1983;245:R117–R134. doi: 10.1152/ajpregu.1983.245.2.R117. [DOI] [PubMed] [Google Scholar]
- 27.Mecher C, Rackow EC, Astiz ME, Weil MH. Unaccounted for anion in metabolic acidosis during severe sepsis in humans. Crit Care Med. 1991;19:705–711. doi: 10.1097/00003246-199105000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Noritomi DT, Soriano FG, Kellum JA, Cappi SB, Biselli PJ, Libório AB, et al. Metabolic acidosis in patients with severe sepsis and septic shock: a longitudinal quantitative study. Crit Care Med. 2009;37:2733–2739. doi: 10.1097/ccm.0b013e3181a59165. [DOI] [PubMed] [Google Scholar]
- 29.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 30.Howell MD, Talmor D, Schuetz P, Hunziker S, Jones AE, Shapiro NI. Proof of principle: the predisposition, infection, response, organ failure sepsis staging system. Crit Care Med. 2011;39:322–327. doi: 10.1097/CCM.0b013e3182037a8e. [DOI] [PubMed] [Google Scholar]
- 31.Davis JW, Kaups KL, Parks SN. Base deficit is superior to pH in evaluating clearance of acidosis after traumatic shock. J Trauma. 1998;44:114–118. doi: 10.1097/00005373-199801000-00014. [DOI] [PubMed] [Google Scholar]
- 32.Sankoff JD, Goyal M, Gaieski DF, Deitch K, Davis CB, Sabel AL, et al. Validation of the Mortality in Emergency Department Sepsis (MEDS) score in patients with the systemic inflammatory response syndrome (SIRS) Crit Care Med. 2008;36:421–426. doi: 10.1097/01.CCM.0B013E3181611F6A0. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen HB, Van Ginkel C, Batech M, Banta J, Corbett SW. Comparison of predisposition, insult/infection, response, and organ dysfunction, acute physiology and chronic health evaluation II, and mortality in emergency department sepsis in patients meeting criteria for early goal-directed therapy and the severe sepsis resuscitation bundle. J Crit Care. 2012;27:362–369. doi: 10.1016/j.jcrc.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Wilhelm J, Hettwer S, Hammer D, Schürmann M, Christoph A, Amoury M, et al. Outcome prediction using clinical scores and biomarkers in patients with presumed severe infection in the emergency department. Med Klin Intensivmed Notfmed. 2012;107:558–563. doi: 10.1007/s00063-012-0147-5. [DOI] [PubMed] [Google Scholar]
- 35.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 36.Thomas-Rueddel DO, Poidinger B, Weiss M, Bach F, Dey K, Häberle H, et al. Hyperlactatemia is an independent predictor of mortality and denotes distinct subtypes of severe sepsis and septic shock. J Crit Care. 2015;30:439.e1–439.e6. doi: 10.1016/j.jcrc.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 37.Sivayoham N, Rhodes A, Cecconi M. The MISSED score, a new scoring system to predict mortality in severe sepsis in the emergency department: a derivation and validation study. Eur J Emerg Med. 2014;21:30–36. doi: 10.1097/MEJ.0b013e328364a8d4. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen HB, Lynch EL, Mou JA, Lyon K, Wittlake WA, Corbett SW. The utility of a quality improvement bundle in bridging the gap between research and standard care in the management of severe sepsis and septic shock in the emergency department. Acad Emerg Med. 2007;14:1079–1086. doi: 10.1197/j.aem.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 39.Jiang LB, Zhang M, Jiang SY, Ma YF. Early goal-directed resuscitation for patients with severe sepsis and septic shock: a meta-analysis and trial sequential analysis. Scand J Trauma Resusc Emerg Med. 2016;24:23. doi: 10.1186/s13049-016-0214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mouncey PR, Osborn TM, Power GS, Harrison DA, Sadique MZ, Grieve RD, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]