Abstract
Purpose
There have been few studies on gender difference in the impact of a urine albumin-to-creatinine ratio (UACR) within the normal range on the risk of hypertension. We evaluated whether the association between the UACR below the microalbuminuria range and the incident risk of hypertension is different between men and women.
Materials and Methods
A total of 1173 individuals (442 men and 731 women) aged 40 to 70 years without hypertension was examined at baseline (2005–2008) and followed (2008–2011). We defined the UACR as the amount of albumin (mg/dL) divided by creatinine (g/dL) in randomly voided urine. The subjects were classified according to UACR tertile.
Results
During an average of 2.6 years of follow-up, 57 men (12.9%) and 66 women (9.0%) developed hypertension. In multivariable-adjusted models, the odds ratio for new-onset hypertension comparing the highest and lowest tertiles of UACR was 1.83 [95% confidence interval (CI) 0.85–3.94] in men and 2.69 (95% CI 1.27–5.73) in women. In stratified analyses by menopausal status, higher tertiles of UACR were associated with an increased risk of incident hypertension in postmenopausal women.
Conclusion
Higher normal UACR levels were associated with an increased risk of incident hypertension in women. The UACR could have a clinical role in predicting the development of hypertension.
Keywords: Albuminuria, hypertension, prospective study
INTRODUCTION
Hypertension is one of the most influential risk factors for mortality worldwide.1,2 Epidemiological studies have reported that hypertension is strongly associated with the risk of coronary heart disease, cerebrovascular disease, and chronic kidney disease.3,4,5 However, there remains a lack of understanding of the determinants of hypertension.
Microalbuminuria is a diagnostic criterion for chronic kidney disease6 and an independent predictor of hypertension, metabolic syndrome, type 2 diabetes and coronary heart disease.7,8,9 Early diagnosis and appropriate management of microalbuminuria could reduce the burden of hypertension-related morbidity and mortality in the general population.10 Urine albumin-to-creatinine ratio (UACR) calculation is a method by which an individual can be diagnosed with albuminuria. The method is easy and quite acceptable in large epidemiological studies.
Previous studies have reported that an increased UACR is associated with the development of hypertension.11,12 Recently, several longitudinal studies have demonstrated that higher UACR values within the normal range can be used to predict the development of hypertension.13,14,15 However, few studies have evaluated the gender difference in the association between a UACR below the microalbuminuria range and the risk of incident hypertension. We examined whether the association between a UACR in the normal range and the incident risk of hypertension differs by gender in a community-based prospective study.
MATERIALS AND METHODS
Study design and participants
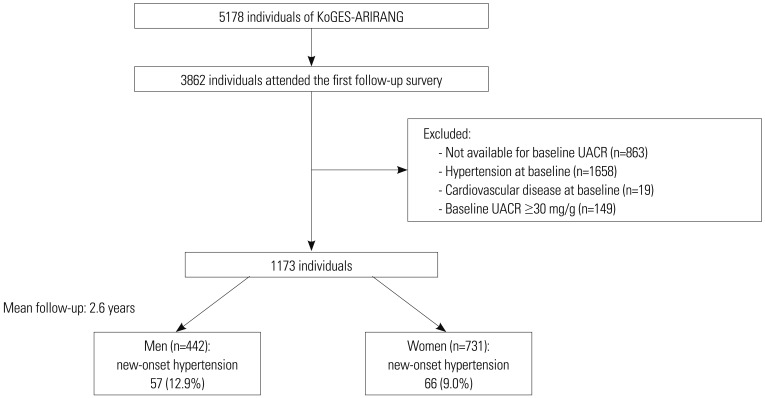
Our study data were obtained from the Korean Genome and Epidemiology Study on Atherosclerosis Risk of Rural Areas in the Korean General Population (KoGES-ARIRANG), which is an ongoing prospective study conducted in Wonju and Pyeongchang, Korea.16 A total of 5178 adults aged 40 to 70 years were included in the baseline survey (November 2005 to January 2008), of whom 3862 participants (74.6%) attended the first follow-up survey, which was carried out from April 2008 to January 2011. We excluded 863 subjects without baseline UACR measurements, 1658 subjects with hypertension at baseline, and 19 participants with a history or presence of cardiovascular disease. To include only subjects with a UACR within the normal range, we excluded those with a UACR of ≥30 mg/g (n=149).17 Ultimately, 1173 individuals (442 men and 731 women) were included in the analysis (Fig. 1). The protocol for this study was approved by the Institutional Review Board of Wonju Severance Christian Hospital, and all participants provided written informed consent for the study.
Fig. 1. Flowchart of study population. UACR, urine albumin-to-creatinine ratio; KoGES-ARIRANG, Korean Genome and Epidemiology Study on Atherosclerosis Risk of Rural Areas in the Korean General Population.
Anthropometric and biochemical measurements
At baseline and at the first follow-up examination, study participants underwent a complete evaluation of medical history and answered a lifestyle questionnaire. Body weight and height were measured while participants wore light indoor clothing without shoes. Systolic and diastolic blood pressures were measured from the right arm using a standard mercury sphygmomanometer (Baumanometer, Copiague, NY, USA) after each participant had rested for 5 minutes. Two measurements were taken with at least 5-minute intervals, and the mean of the two blood pressure readings was used for the analyses. A suitable cuff size was chosen for each participant according to the mid-arm circumference. Muscle mass was measured via bioelectrical impedance analysis (Zeus 9.9, Jawon Medical Co., Ltd., Gyeongsan, Korea). Baseline data for past history of disease and current smoking and alcohol drinking status were collected using self-reported questionnaires. Subjects who answered "yes" to the question "Do you perform physical exercise regularly enough to make you sweat?" were placed in the regular exercise group.
Antecubital venous blood samples were collected from the participants after fasting for more than 12 hours or overnight. The fasting glucose and insulin levels were determined using a glucose oxidase-based assay and double-antibody radioimmunoassay (Biosource Europe SA, Nivelles, Belgium). Serum concentrations of high-density lipoprotein (HDL) and low-density lipoprotein cholesterol and triglyceride were determined using enzymatic methods (Advia 1650; Siemens, Tarrytown, NY, USA). High-sensitivity C-reactive protein (hs-CRP) was analyzed using the Denka Seiken assay (Tokyo, Japan). The estimated glomerular filtration rate (GFR) was calculated using the Modification of Diet in Renal Disease equation:18
| Estimated GFR=186×[Serum Creatinine (in mg/dL)]-1.154× [Age (in years)]-0.203×[0.742 (if female)] |
Urine albumin (mg/dL) and creatinine (g/dL) were measured from randomly voided urine. The UACR was defined as the amount of urine albumin divided by creatinine levels in urine. We defined the presence of microalbuminuria as a UACR between 30 and 300 mg/g and overt proteinuria as a UACR of greater than 300 mg/g.17 The intra-assay and inter-assay coefficients of variation of urinary albumin were 1.1% and 1.2% and were 0.5% and 1.4% for urinary creatinine, respectively.
Definition of hypertension
Based on the Eighth Joint National Committee guidelines,19 hypertension was defined as a systolic blood pressure of at least 140 mm Hg or a diastolic blood pressure of at least 90 mm Hg, or current usage of antihypertensive agents.
Statistical analysis
Data are expressed as means with standard deviations, medians with interquartile ranges, or frequencies with percentages. All analyses were performed separately for men and women and for menopausal status in women. The study population was divided into gender-specific tertiles of UACR values. The cutoff points of the UACR tertiles were 5.38 and 9.37 mg/g for men and 6.17 and 11.64 mg/g for women. Multivariable logistic regression was used to evaluate the independent association between baseline UACR values and incident hypertension. The study utilized three models with progressive degrees of adjustment. First, we conducted an age-adjusted analysis. Second, we additionally adjusted for baseline body mass index, muscle mass, systolic blood pressure, history of diabetes mellitus, smoking, alcohol consumption, and regular exercise. Finally, we further adjusted for baseline fasting serum glucose, triglyceride, HDL cholesterol, hs-CRP, and estimated GFR. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated with respect to increasing tertiles of UACR levels. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA). p values of <0.05 were considered to be statistically significant.
RESULTS
During an average 2.6 years of follow-up, 57 men (12.9%) and 66 women (9.0%) developed new-onset hypertension. Table 1 shows the baseline characteristics of the 1173 study subjects separated by gender, with and without incident hypertension. In both men and women, baseline body mass index and systolic blood pressure were significantly higher in subjects with incident hypertension than in those without incident hypertension. Diastolic blood pressure, urine albumin, and UACR were significantly higher in women who developed hypertension than in those who did not. Women with incident hypertension were older than those without incident hypertension at baseline. Additionally, baseline body mass index was significantly higher in subjects with incident hypertension than in those without incident hypertension in both premenopausal and postmenopausal women. Age, systolic and diastolic blood pressure, urine albumin, and UACR were significantly higher in postmenopausal women who developed hypertension than in those who did not (Table 2).
Table 1. Baseline Characteristics of Study Subjects with and without Incident Hypertension.
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Without incident hypertension | With incident hypertension | p value | Without incident hypertension | With incident hypertension | p value | |
| n (%) | 385 (87.1) | 57 (12.9) | 665 (91.0) | 66 (9.0) | ||
| Age, yrs | 55.1±8.2 | 56.1±8.8 | 0.425 | 51.9±8.1 | 57.0±8.1 | <0.001 |
| Body mass index, kg/m2 | 23.4±2.8 | 24.5±2.3 | 0.006 | 23.9±3.0 | 25.0±3.0 | 0.007 |
| Muscle mass, kg | 46.5±4.6 | 47.8±4.7 | 0.058 | 35.6±3.9 | 35.8±3.7 | 0.661 |
| Systolic BP, mm Hg | 116.0±9.8 | 120.9±9.1 | <0.001 | 114.3±10.9 | 120.3±10.3 | <0.001 |
| Diastolic BP, mm Hg | 74.6±6.8 | 75.9±6.2 | 0.184 | 72.9±7.6 | 75.3±6.5 | 0.013 |
| Fasting glucose, mg/dL | 95.4±21.6 | 95.3±12.4 | 0.953 | 89.5±10.9 | 90.9±10.3 | 0.308 |
| HDL-cholesterol, mg/dL | 45.1±10.9 | 45.2±11.7 | 0.939 | 48.6±10.3 | 46.6±9.7 | 0.145 |
| LDL-cholesterol, mg/dL | 113.0±31.7 | 117.5±28.2 | 0.306 | 117.0±30.6 | 124.1±29.0 | 0.072 |
| Triglycerides, mg/dL | 122.0 (82.0, 182.0) | 123.0 (101.0, 179.0) | 0.710* | 98.0 (74.0, 138.0) | 112.5 (82.0, 153.0) | 0.054* |
| hs-CRP, mg/L | 0.79 (0.41, 1.91) | 0.59 (0.47, 1.50) | 0.645* | 0.53 (0.29, 1.19) | 0.66 (0.41, 1.27) | 0.055* |
| Urine albumin, mg/dL | 0.79 (0.43, 1.38) | 0.92 (0.50, 1.68) | 0.235* | 0.76 (0.43, 1.11) | 0.82 (0.74, 1.17) | 0.010* |
| Urine creatinine, g/dL | 0.133 (0.094, 0.169) | 0.127 (0.081, 0.165) | 0.393* | 0.094 (0.064, 0.128) | 0.081 (0.062, 0.111) | 0.139* |
| Urine albumin to creatinine ratio, mg/g | 6.85 (3.90, 10.76) | 7.81 (5.77, 11.98) | 0.121* | 8.50 (4.95, 13.15) | 10.88 (7.14, 16.55) | 0.002* |
| Estimated GFR | 78.0 (72.1, 85.7) | 79.7 (69.7, 86.1) | 0.953* | 75.1 (69.7, 80.7) | 73.0 (65.9, 79.1) | 0.190* |
| Current smokers (%) | 168 (43.6) | 22 (38.6) | 0.566 | 7 (1.1) | 0 (0.0) | 0.861 |
| Current drinkers (%) | 235 (61.0) | 40 (70.2) | 0.237 | 174 (26.2) | 15 (22.7) | 0.645 |
| Regular exercise (%) | 101 (26.2) | 15 (26.3) | 1.000 | 185 (27.8) | 11 (16.7) | 0.071 |
BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; GFR, glomerular filtration rate; SD, standard deviation.
Values are expressed as mean (SD), number (%), or median (25th, 75th percentiles).
*p value from Mann-Whitney U test.
Table 2. Baseline Characteristics of Premenopausal and Postmenopausal Women.
| Premenopausal women | Postmenopausal women | |||||
|---|---|---|---|---|---|---|
| Without incident hypertension | With incident hypertension | p value | Without incident hypertension | With incident hypertension | p value | |
| n (%) | 286 (95.6) | 13 (4.4) | 369 (88.1) | 50 (11.9) | ||
| Age, yrs | 45.6±3.7 | 46.5±3.2 | 0.391 | 56.8±7.1 | 60.3±6.1 | 0.001 |
| Body mass index, kg/m2 | 24.0±3.1 | 25.9±2.3 | 0.022 | 23.8±3.0 | 24.7±3.2 | 0.038 |
| Muscle mass, kg | 36.9±3.6 | 38.1±2.2 | 0.230 | 34.6±3.7 | 35.1±3.7 | 0.338 |
| Systolic BP, mm Hg | 113.2±10.7 | 118.1±8.5 | 0.107 | 115.3±11.0 | 121.3±10.3 | <0.001 |
| Diastolic BP, mm Hg | 72.4±7.9 | 73.8±5.1 | 0.508 | 73.3±7.3 | 75.8±6.9 | 0.022 |
| Fasting glucose, mg/dL | 89.0±9.9 | 90.8±11.4 | 0.502 | 89.9±11.6 | 90.5±10.0 | 0.728 |
| HDL-cholesterol, mg/dL | 48.8±9.9 | 47.1±14.2 | 0.679 | 48.4±10.6 | 46.0±8.3 | 0.065 |
| LDL-cholesterol, mg/dL | 108.9±28.5 | 119.0±26.5 | 0.209 | 123.0±30.5 | 124.2±28.9 | 0.786 |
| Triglycerides, mg/dL | 86.0 (67.0, 14.0) | 111.0 (83.0, 153.0) | 0.052* | 110.0 (81.0, 152.0) | 111.5 (82.0, 150.0) | 0.825* |
| hs-CRP, mg/L | 0.39 (0.24, 0.98) | 0.45 (0.39, 0.72) | 0.315* | 0.66 (0.36, 1.35) | 0.90 (0.49, 1.68) | 0.178* |
| Urine albumin, mg/dL | 0.75 (0.44, 1.11) | 0.79 (0.49, 0.85) | 0.961* | 0.76 (0.41, 1.10) | 0.88 (0.76, 1.28) | 0.007* |
| Urine creatinine, g/dL | 0.105 (0.069, 0.135) | 0.086 (0.068, 0.156) | 0.991* | 0.089 (0.063, 0.120) | 0.078 (0.062, 0.107) | 0.226* |
| Urine albumin to creatinine ratio, mg/g | 7.83 (4.68, 12.24) | 6.81 (4.87, 11.18) | 0.753* | 9.02 (5.26, 13.57) | 12.25 (8.52, 18.60) | 0.001* |
| Estimated GFR | 77.8 (72.1, 83.2) | 77.9 (67.6, 80.6) | 0.366* | 73.3 (67.8, 78.1) | 72.6 (65.2, 78.1) | 0.549* |
| Current smokers (%) | 3 (1.0) | 0 (0.0) | 1.000 | 4 (1.1) | 0 (0.0) | 1.000 |
| Current drinkers (%) | 96 (33.6) | 6 (46.2) | 0.524 | 74 (20.1) | 9 (18.0) | 0.878 |
| Regular exercise (%) | 87 (30.4) | 4 (30.8) | 1.000 | 94 (25.5) | 5 (10.0) | 0.025 |
BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; GFR, glomerular filtration rate; SD, standard deviation.
Values are expressed as mean (SD), number (%), or median (25th, 75th percentiles).
*p value from Mann-Whitney U test.
Multivariable logistic regression models were used to evaluate the independent association between baseline UACR tertiles and new-onset hypertension (Table 3). In men, the OR for incident hypertension in the highest UACR tertile was 1.83 (95% CI 0.85–3.94) compared to the lowest UACR tertile (P for trend=0.124) after adjustment for age, baseline body mass index, muscle mass, systolic blood pressure, diabetes mellitus, smoking, alcohol consumption, regular exercise, fasting glucose, triglyceride, HDL cholesterol, hs-CRP, and GFR. In women, the OR for incident hypertension in the highest UACR tertile was 2.69 (95% CI 1.27–5.73) compared to the lowest UACR tertile (P for trend=0.014) after adjustment for confounding factors described above.
Table 3. OR for New-Onset Hypertension According to Baseline UACR Tertile.
| UACR at baseline | ||||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | P for trend | |
| Men | ||||
| UACR, mg/g | <5.38 | 5.38–9.36 | ≥9.37 | |
| No. of subjects | 147 | 147 | 148 | |
| No. of new-onset hypertension | 14 (9.5%) | 20 (13.6%) | 23 (15.5%) | |
| OR (95% CI) for new-onset hypertension | ||||
| Model 1 | 1.00 | 1.47 (0.71–3.04) | 1.71 (0.84–3.48) | 0.146 |
| Model 2 | 1.00 | 1.58 (0.75–3.33) | 1.66 (0.79–3.48) | 0.187 |
| Model 3 | 1.00 | 1.65 (0.78–3.50) | 1.83 (0.85–3.94) | 0.124 |
| Women | ||||
| UACR, mg/g | <6.17 | 6.17–11.63 | ≥11.64 | |
| No. of subjects | 243 | 244 | 244 | |
| No. of new-onset hypertension | 11 (4.5%) | 24 (9.8%) | 31 (12.7%) | |
| OR (95% CI) for new-onset hypertension | ||||
| Model 1 | 1.00 | 2.20 (1.05–4.64) | 2.62 (1.27–5.40) | 0.008 |
| Model 2 | 1.00 | 2.59 (1.20–5.57) | 2.73 (1.29–5.78) | 0.012 |
| Model 3 | 1.00 | 2.57 (1.19–5.56) | 2.69 (1.27–5.73) | 0.014 |
UACR, urine albumin-to-creatinine ratio; OR, odds ratio; CI, confidence interval; BMI, body mass index; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; GFR, glomerular filtration rate.
Model 1: adjusted for age, Model 2: Model 1+additionally adjusted for baseline BMI, muscle mass, systolic blood pressure, diabetes mellitus, smoking, alcohol consumption, regular exercise, Model 3: Model 2+additionally adjusted for baseline fasting serum glucose, triglyceride, HDL cholesterol, hs-CRP, estimated GFR.
Table 4 presents the ORs for new-onset of hypertension according to baseline UACR tertiles and menopausal status in women. Women were divided into premenopausal and postmenopausal groups. Compared to the lowest UACR tertile, the ORs for incident hypertension in the highest UACR tertile were 0.70 (95% CI 0.15–3.28) in premenopausal women (P for trend=0.718) and 3.93 (95% CI 1.53–10.11) in postmenopausal women (P for trend=0.006).
Table 4. OR for New-Onset Hypertension According to Baseline UACR Tertile and Menopausal Status.
| UACR at baseline | ||||
|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | P for trend | |
| Premenopausal women | ||||
| UACR, mg/g | <5.65 | 5.65–10.74 | ≥10.75 | |
| No. of subjects | 99 | 100 | 100 | |
| No. of new-onset hypertension | 4 (4.0%) | 5 (5.0%) | 4 (4.0%) | |
| OR (95% CI) for new-onset hypertension* | 1.00 | 1.18 (0.27–5.20) | 0.70 (0.15–3.28) | 0.718 |
| Postmenopausal women | ||||
| UACR, mg/g | <6.54 | 6.54–12.33 | ≥12.34 | |
| No. of subjects | 139 | 140 | 140 | |
| No. of new-onset hypertension | 7 (5.0%) | 19 (13.6%) | 24 (17.1%) | |
| OR (95% CI) for new-onset hypertension* | 1.00 | 3.49 (1.33–9.17) | 3.93 (1.53–10.11) | 0.006 |
UACR, urine albumin-to-creatinine ratio; OR, odds ratio; CI, confidence interval; BMI, body mass index; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; GFR, glomerular filtration rate.
*Adjusted for baseline age, baseline BMI, muscle mass, systolic blood pressure, diabetes mellitus, smoking, alcohol consumption, regular exercise, fasting serum glucose, triglyceride, HDL cholesterol, hs-CRP, estimated GFR.
DISCUSSION
The present study demonstrated gender-specific associations between a UACR within the normal range and incident hypertension in a Korean elderly population. We observed that higher UACR levels within the normal range are associated with an increased risk of incident hypertension, and these relationships were more pronounced and significant in women, particularly postmenopausal women. The associations were independent of baseline age, body mass index, systolic blood pressure, smoking, regular exercise, fasting serum glucose, triglyceride, HDL cholesterol, and hs-CRP.
The exact mechanism for the association between the UACR and incidence of hypertension is unclear, although several possible explanations were discussed in previous studies. Glomerular endothelial dysfunction may be an underlying precursor of hypertension.13 Microalbuminuria could be an indicator of microvascular endothelial injury and is also involved in the progression of chronic kidney disease.20,21 Several studies have explored the relationship between endothelial dysfunction, arterial stiffness, and blood pressure.22,23 Elevation of UACR could also be associated with loss of vasorelaxation and vascular calcification in patients with microalbuminuria.24,25 Additionally, the development of hypertension may be due to the upregulation of angiotensin-converting enzymes in proteinuric-conditions and the albumin-triggered activation of the intrarenal renin-angiotensin system.26,27
Recent studies have reported that high normal ranges of UACR are associated with the development of hypertension.13,14,15,28 The Framingham Offspring Study and an Indo-Asian population study reported an association between a high normal level of albuminuria and the risk of hypertension for 2 and 2.9 years of follow-up, respectively.13,15 However, separate analyses by gender were not performed in these previous studies. Our results showed that the ORs for men were lower than those for women and not significant in the association between UACR and incident hypertension. This gender difference could be attributed to the different levels and variations in UACR between men and women. Women have higher baseline UACR values than men due to lower muscle mass and less urinary excretion of creatinine.29 The non-significant results in men may have also been due to the small sample size. Further studies are required to understand the profound pathophysiology of the gender difference in the effects of UACR on hypertension.
Menopausal status could also affect the relationship between UACR and incident hypertension. Our results showed that postmenopausal women had higher baseline UACR levels than premenopausal women. A previous study reported that the supplementation of estrogen in postmenopausal women lowers the UACR level and thereby lowers the risk of albuminuria.30 In stratified analyses by menopausal status, postmenopausal women showed a significant association between UACR and incident hypertension. Our findings are consistent with those of the Nurses' Health Study.31 One possible explanation might be the lower levels of estrogen in postmenopausal women. Previous studies reported that sex hormones, particularly estrogen, modulate mesangial expansion, matrix deposition, collagen synthesis, and activation of the renin-angiotensin system in the kidneys.32,33,34 The lack of estrogen in postmenopausal women may lead to the progression of hypertension in relation to the UACR.
The strengths of our study include its prospective design, gender-separated analyses, and adjustment for possible confounding factors. However, the present study also had several limitations. First, the sample in the present study was taken from a single spot urine collection for analyzing UACR instead of 24-hour urinary albumin excretion, which may have led to improper diagnosis of albuminuria in certain participants. However, spot urine for analyzing UACR correlates well with total amounts of urinary albumin excretion in 24-hour urine and is generally used to detect decreased kidney function.35,36 Second, bioelectrical impedance analysis is not generally considered as a reference method for evaluating muscle mass. However, a bioelectrical impedance analysis can provide a simple and rapid measurement of muscle mass for epidemiological studies. Third, the study was restricted to middle-aged and elderly Koreans living in rural areas. Generalization of the results may be limited. Fourth, we analyzed the follow-up data for only 2.6 years. Further studies with a longer follow-up period are needed.
In conclusion, the present study provides epidemiological evidence that a higher UACR within the normal range can independently predict the development of hypertension in women. UACR measurement may be an easy and non-invasive marker for predicting incident hypertension. Further studies are required to understand the gender-specific impact of UACR on the risk of hypertension.
ACKNOWLEDGEMENTS
This study was supported in part by a grant of the Korea Centers for Disease Control and Prevention (2005-E71013-00, 2006-E71002-00, 2007-E71013-00, 2008-E71004-00, 2009-E71006-00, 2010-E71003-00).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R Prospective Studies Collaboration. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 3.Blood Pressure Lowering Treatment Trialists' Collaboration. Turnbull F, Neal B, Ninomiya T, Algert C, Arima H, et al. Effects of different regimens to lower blood pressure on major cardiovascular events in older and younger adults: meta-analysis of randomised trials. BMJ. 2008;336:1121–1123. doi: 10.1136/bmj.39548.738368.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 5.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 6.Lambers Heerspink HJ, Brinkman JW, Bakker SJ, Gansevoort RT, de Zeeuw D. Update on microalbuminuria as a biomarker in renal and cardiovascular disease. Curr Opin Nephrol Hypertens. 2006;15:631–636. doi: 10.1097/01.mnh.0000247496.54882.3f. [DOI] [PubMed] [Google Scholar]
- 7.Inoue T, Iseki K, Higashiuesato Y, Nagahama K, Matsuoka M, Iseki C, et al. Proteinuria as a significant determinant of hypertension in a normotensive screened cohort in Okinawa, Japan. Hypertens Res. 2006;29:687–693. doi: 10.1291/hypres.29.687. [DOI] [PubMed] [Google Scholar]
- 8.Palaniappan L, Carnethon M, Fortmann SP. Association between microalbuminuria and the metabolic syndrome: NHANES III. Am J Hypertens. 2003;16(11 Pt 1):952–958. doi: 10.1016/s0895-7061(03)01009-4. [DOI] [PubMed] [Google Scholar]
- 9.Pan CY, Ho LT, Soegondo S, Prodjosudjadi W, Suwanwalaikorn S, Lim SC, et al. Prevalence of albuminuria and cardiovascular risk profile in a referred cohort of patients with type 2 diabetes: an Asian perspective. Diabetes Technol Ther. 2008;10:397–403. doi: 10.1089/dia.2007.0296. [DOI] [PubMed] [Google Scholar]
- 10.Garrison RJ, Kannel WB, Stokes J, 3rd, Castelli WP. Incidence and precursors of hypertension in young adults: the Framingham Offspring Study. Prev Med. 1987;16:235–251. doi: 10.1016/0091-7435(87)90087-9. [DOI] [PubMed] [Google Scholar]
- 11.Gerber LM, Schwartz JE, Pickering TG. Albumin-to-creatinine ratio predicts change in ambulatory blood pressure in normotensive persons: a 7.5-year prospective study. Am J Hypertens. 2006;19:220–226. doi: 10.1016/j.amjhyper.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 12.Brantsma AH, Bakker SJ, de Zeeuw D, de Jong PE, Gansevoort RT. Urinary albumin excretion as a predictor of the development of hypertension in the general population. J Am Soc Nephrol. 2006;17:331–335. doi: 10.1681/ASN.2005111153. [DOI] [PubMed] [Google Scholar]
- 13.Wang TJ, Evans JC, Meigs JB, Rifai N, Fox CS, D'Agostino RB, et al. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation. 2005;111:1370–1376. doi: 10.1161/01.CIR.0000158434.69180.2D. [DOI] [PubMed] [Google Scholar]
- 14.Hirayama A, Konta T, Hozawa A, Kawasaki R, Watanabe T, Shibata Y, et al. Slight increase in urinary albumin excretion within the normal range predicts incident hypertension in a community-based Japanese population: the Takahata study. Hypertens Res. 2015;38:56–60. doi: 10.1038/hr.2014.117. [DOI] [PubMed] [Google Scholar]
- 15.Jessani S, Levey AS, Chaturvedi N, Jafar TH. High normal levels of albuminuria and risk of hypertension in Indo-Asian population. Nephrol Dial Transplant. 2012;27(Suppl 3):iii58–iii64. doi: 10.1093/ndt/gfr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JY, Ahn SV, Yoon JH, Koh SB, Yoon J, Yoo BS, et al. Prospective study of serum adiponectin and incident metabolic syndrome: the ARIRANG study. Diabetes Care. 2013;36:1547–1553. doi: 10.2337/dc12-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis. 1999;33:1004–1010. doi: 10.1016/s0272-6386(99)70442-7. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 19.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 20.Ito S, Nagasawa T, Abe M, Mori T. Strain vessel hypothesis: a viewpoint for linkage of albuminuria and cerebro-cardiovascular risk. Hypertens Res. 2009;32:115–121. doi: 10.1038/hr.2008.27. [DOI] [PubMed] [Google Scholar]
- 21.Satoh M. Endothelial dysfunction as an underlying pathophysiological condition of chronic kidney disease. Clin Exp Nephrol. 2012;16:518–521. doi: 10.1007/s10157-012-0646-y. [DOI] [PubMed] [Google Scholar]
- 22.Figueiredo VN, Yugar-Toledo JC, Martins LC, Martins LB, de Faria AP, de Haro Moraes C, et al. Vascular stiffness and endothelial dysfunction: correlations at different levels of blood pressure. Blood Press. 2012;21:31–38. doi: 10.3109/08037051.2011.617045. [DOI] [PubMed] [Google Scholar]
- 23.Higashi Y, Kihara Y, Noma K. Endothelial dysfunction and hypertension in aging. Hypertens Res. 2012;35:1039–1047. doi: 10.1038/hr.2012.138. [DOI] [PubMed] [Google Scholar]
- 24.Clausen P, Jensen JS, Jensen G, Borch-Johnsen K, Feldt-Rasmussen B. Elevated urinary albumin excretion is associated with impaired arterial dilatory capacity in clinically healthy subjects. Circulation. 2001;103:1869–1874. doi: 10.1161/01.cir.103.14.1869. [DOI] [PubMed] [Google Scholar]
- 25.Psyrogiannis A, Kyriazopoulou V, Vagenakis AG. Medial arterial calcification is frequently found in patients with microalbuminuria. Angiology. 1999;50:971–975. doi: 10.1177/000331979905001202. [DOI] [PubMed] [Google Scholar]
- 26.Largo R, Gómez-Garre D, Soto K, Marrón B, Blanco J, Gazapo RM, et al. Angiotensin-converting enzyme is upregulated in the proximal tubules of rats with intense proteinuria. Hypertension. 1999;33:732–739. doi: 10.1161/01.hyp.33.2.732. [DOI] [PubMed] [Google Scholar]
- 27.Cao W, Zhou QG, Nie J, Wang GB, Liu Y, Zhou ZM, et al. Albumin overload activates intrarenal renin-angiotensin system through protein kinase C and NADPH oxidase-dependent pathway. J Hypertens. 2011;29:1411–1421. doi: 10.1097/HJH.0b013e32834786f0. [DOI] [PubMed] [Google Scholar]
- 28.Park SK, Moon SY, Oh CM, Ryoo JH, Park MS. High normal urine albumin-to-creatinine ratio predicts development of hypertension in Korean men. Circ J. 2014;78:656–661. doi: 10.1253/circj.cj-13-0745. [DOI] [PubMed] [Google Scholar]
- 29.Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of the albumin/creatinine ratio to detect microalbuminuria: implications of sex and race. J Am Soc Nephrol. 2002;13:1034–1039. doi: 10.1681/ASN.V1341034. [DOI] [PubMed] [Google Scholar]
- 30.Schopick EL, Fisher ND, Lin J, Forman JP, Curhan GC. Postmenopausal hormone use and albuminuria. Nephrol Dial Transplant. 2009;24:3739–3744. doi: 10.1093/ndt/gfp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forman JP, Fisher ND, Schopick EL, Curhan GC. Higher levels of albuminuria within the normal range predict incident hypertension. J Am Soc Nephrol. 2008;19:1983–1988. doi: 10.1681/ASN.2008010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis. 1995;25:515–533. doi: 10.1016/0272-6386(95)90119-1. [DOI] [PubMed] [Google Scholar]
- 33.Sandberg K, Ji H. Sex and the renin angiotensin system: implications for gender differences in the progression of kidney disease. Adv Ren Replace Ther. 2003;10:15–23. doi: 10.1053/jarr.2003.50006. [DOI] [PubMed] [Google Scholar]
- 34.Kwan G, Neugarten J, Sherman M, Ding Q, Fotadar U, Lei J, et al. Effects of sex hormones on mesangial cell proliferation and collagen synthesis. Kidney Int. 1996;50:1173–1179. doi: 10.1038/ki.1996.425. [DOI] [PubMed] [Google Scholar]
- 35.Guy M, Borzomato JK, Newall RG, Kalra PA, Price CP. Protein and albumin-to-creatinine ratios in random urines accurately predict 24 h protein and albumin loss in patients with kidney disease. Ann Clin Biochem. 2009;46(Pt 6):468–476. doi: 10.1258/acb.2009.009001. [DOI] [PubMed] [Google Scholar]
- 36.Borch-Johnsen K, Feldt-Rasmussen B, Strandgaard S, Schroll M, Jensen JS. Urinary albumin excretion. An independent predictor of ischemic heart disease. Arterioscler Thromb Vasc Biol. 1999;19:1992–1997. doi: 10.1161/01.atv.19.8.1992. [DOI] [PubMed] [Google Scholar]