Abstract
Vaginal microbicides are a promising means to prevent the transmission of HIV, empowering women by putting protection under their control. We have been using gel technology to develop microbicides in the intermediate texture space to overcome shortcomings of current solid and liquid forms. We recently formulated semisoft ovules from mixed polymer combinations of carrageenan and Carbopol 940P to overcome some of the flaws with our previous generation of formulations based solely on carrageenan. To determine the user acceptability of the reformulated gels, women first evaluated intact semisoft ovules before evaluating ovules that had been subjected to mechanical crushing to simulate samples that represent post-use discharge. Women then evaluated combinations of intact and discharge samples to understand how ovule textures correlated with texture of the resulting discharge samples. Carbopol concentration directly and inversely correlated with willingness to try for discharge samples and intact samples respectively. When evaluating intact samples, women focused on the ease of inserting the product and preferred firmer samples; conversely, when evaluating discharge samples, softer samples that resulted in a smooth paste were preferred. Significant differences between samples were lost when evaluating pairs as women made varying tradeoffs between their preference for ease of inserting intact ovules and acceptability of discharge appearance. Evaluating samples that represent different stages of the use cycle reveals a more holistic measure of product acceptability. Studying sensory acceptability in parallel with biophysical performance enables an iterative design process that considers what women prefer in terms of insertion as well as possibility of leakage.
Keywords: vaginal microbicides, acceptability, carrageenan, Carbopol 940, HIV, formulation development
1. Introduction
Microbicides have been an active area of research in the field of HIV prevention [1], and vaginal microbicides are a promising method for women to self-initiate prevention [2]. Several biologically effective microbicide candidates have been discontinued in Phase III clinical trials due to futility [3, 4]. Follow-up studies with clinical trial participants show that poor user adherence is a major factor influencing clinical efficacy of microbicide products [4, 5]. The microbicidal drug must be present at effective levels in the vaginal tissue to prevent viral transmission, which is only possible with good adherence to study protocols [6]. Two main classes of factors influence user adherence: acceptability of product features and the user's social and cultural context, including practical limitations. Product features that influence acceptability include product characteristics (smell, color, texture, appearance) [7] lubrication (which may affect the sexual experience [8, 9]), leakage [7, 9, 10] and perceived product efficacy. Social and cultural influences that hinder adherence include sigma or discrimination associated with use, partner complaints or discontent, beliefs regarding non-use by peers, potential drug side effects, or fear of harm from taking drugs when there is no illness, and even mistrust of stated research goals. [5]. Practical limitations included missed visits, lack of product replenishment, conflicts with participants’ schedules, and forgetfulness [5]. These two classes of factors may interact to further influence user adherence[11]. While developers have little control over various social, cultural and practical factors, understanding them and how they influence product features can aid in the design of the product to help maximize opportunities for success in clinical trials.
Several microbicide prototypes are currently in development or being tested in clinical trials [12]; these include vaginal gels [13], intravaginal rings [14, 15], tablets [16] and quick dissolving films [17, 18]. Studies have compared different physical forms of microbicides to determine appropriateness based on the country and culture [12, 19, 20]. We have developed carrageenan-based semisoft suppositories that fall in the intermediate design space between solids (intravaginal rings, tablets, films, fat-based suppositories) and viscous liquids (colloquially called “gels” in spite of not being gels in a strict rheological sense). The carrageenan-based semisoft suppositories are being developed to release the active pharmaceutical ingredient (API) and slowly disintegrate within the body so that any resultant discharge mimics vaginal mucous secretions. Our initial prototypes formulated with carrageenan alone successfully released drug [21], but did not disintegrate when exposed to a simulated vaginal environment (i.e., a small volume (5 mL) of vaginal simulant fluid at 37 °C). Accordingly, we are investigating addition of other polymers, such as carbopol, to carrageenan formulations to aid with breakdown in a simulated vaginal environment. Carbopol® has been previously investigated in vaginal gel and tablet formulations because of its mucoadhesive properties [22, 23] that facilitate longer vaginal retention time for delivery of the encapsulated active pharmaceutical ingredient [24]. Carbopol® 971P-NF has been previously investigated in combination with a mixture of kappa and lambda carrageenan for spermicidal applications [25] and Carbopol® 980 has been investigated with kappa carrageenan for mucoadhesive vaginal “gel” formulations [23]. However, in most of these formulations, the concentrations of carrageenan or cations used prevented gel formation, and the final products remained viscoelastic liquids. Leakage and messiness have been associated with use of these viscoelastic liquids (which are commonly referred to as gels in the microbicide literature) [26]. The formulations prepared in our efforts are viscoelastic solids and have a self-supporting shape/form that facilitates manual insertion, unlike viscoelastic liquids that require an applicator. Data from our focus group studies as well as other microbicide acceptability studies have shown that applicators may not be ideal, as hiding the applicator would be necessary for covert use of the product [10, 27-29] and disposal is a burden, especially for plastic applicators. On the other hand, previous research has shown that in some cultures around the world women are not comfortable touching parts of their reproductive system and would be more likely to use products that have an applicator [8, 30]. Therefore, we believe there is a need for products that have potential for both manual as well as applicator-assisted insertion, based on women's preferences and cost constraints, as well as a need for the potential for covert use.
During initial formulation efforts, we observed that addition of carbopol made suppositories more susceptible to fracturing with lesser force, resulting in a product resembling a mucous-like, smooth paste upon breakdown. However, previous data show softer suppositories were less preferred by women, as such items would be difficult to insert without an applicator [31]. When evaluating suppositories ex vivo (in the hand) with limited information regarding the life cycle of the product, women may focus disproportionately on imagined ease of insertion, especially as the orientation video shown as part of prior tests by our team may have cued participants to think about the performance of the product in terms of insertion (The video may be viewed here: http://journals.plos.org/plosone/article/asset?unique&id=info:doi/10.1371/journal.pone.0054975.s001). In focus groups conducted by our team, we found women drew upon their experiences with other vaginal products and assumed that the suppositories presented during the testing might melt at body temperature, similar to other vaginal suppositories available to deliver deodorant, prebiotics, vitamin-E, or to treat vaginal yeast infection [27].
During testing of personal care products (e.g., lipsticks, shampoo, and skin creams) the properties of the product during application and throughout the day ultimately determine product acceptability and continued use [32, 33]. For example in the ASTM method for Descriptive Skinfeel Analysis of Creams and Lotions, evaluation techniques at different phases of use including product delivery, pick-up, rub-out and after-feel have been systematically specified [34]. Similarly, it is important for researchers to design tests that not only focus on the application/insertion of the suppositories, but also on the subsequent characteristics, including feeling inside the body as well as feel and appearance of the resulting discharge. Notably, present work is part of a preclinical development effort, and it would not be ethical to test this product vaginally in the absence of toxicology and safety data; accordingly, we cannot collect data regarding the feeling of the product inside the body. However evaluating the product based on the feel and appearance of the possible discharge is still within the scope of the study.
In previous focus groups, women were given additional information on the suppositories: we explained that they do not melt inside the body (unlike other lipid-based suppositories that melt at body temperature turning to a smooth liquid-like consistency) but instead breakdown slowly while continuously releasing medication. On further explanation, the suppositories would break under force, resulting in chunks; the size and consistency of these would depend on the formulation and firmness. Based on the discussions in these focus groups, the moderator suggested participants rub and crush suppositories in between their palms, giving them insight into possible appearance and feel of the discharge from the product. This exercise helped in the designing the present study to evaluate the acceptability of the suppositories not only on appearance and firmness prior to and during (imagined) insertion, but also while considering the potential discharge, which might play a strong role in women's continued use of the product. Prior studies with microbicides, especially “gels,” have indicated leakage amount and appearance negatively affects women's acceptability of the product [10, 19, 35]. Accordingly, evaluating the characteristics of the simulated discharge was of strong interest in our development efforts.
Here, we explored how adding varying levels carbopol to our carrageenan formulations affected women's perceived effectiveness, imagined ease of insertion, willingness to try and preferences for the new formulations. By presenting women with samples that simulate discharge, we aim to provide participants with more complete information regarding the product function and receive feedback on the post-use attributes. Finally, by presenting intact suppositories with corresponding simulated discharge samples, this study sheds new light on the relative importance of measuring product acceptability during different stages of use, and demonstrates how women make tradeoffs for an overall favorable product experience.
2. Methods
2.1 Materials
Commercial samples of kappa carrageenan (Gelcarin NF 911, batch number: 10707011) were kindly provided by FMC Biopolymers (Philadelphia, PA). Carbopol 940P was purchased from VWR International. Tenofovir (TFV) was provided by Gilead Sciences (Foster City, California) in kind. All other reagents such as sodium hydroxide, potassium chloride were purchased from VWR International (Bridgeport, NJ) and used as received.
2.2 Participant recruitment
Women (Test 1: n = 99 and Test 2: n = 94) were recruited as described elsewhere [36, 37] to evaluate prototypes ex vivo (in their hands) at the Sensory Evaluation Center at Penn State. All participants met the following criteria for inclusion: a) female; b) between 18 and 55 years of age; c) reported having had vaginal sex with a man in the last 12 months; d) were willing to manipulate prototypes with their hands and evaluate them using a computer-guided assessment in an isolated test booth. All procedures were approved by the Pennsylvania State University Institutional Review Board (protocol #36943). Participants provided informed consent and were reimbursed for their time.
2.3 Sample design and preparation
To study the effect of carbopol addition to carrageenan gels on user preferences, gels with varying ratios of kappa carrageenan to carbopol were prepared as shown in Table 1. To prepare mixed gels, carrageenan and carbopol gels (in 2× concentration) were prepared separately in 0.05 M potassium chloride (KCl) solution. Carbopol when dispersed in KCl solution has a low pH (2.5-3.5), which inhibits gelation of carrageenan. The pH of the carbopol dispersion was raised by addition of a 10N sodium hydroxide solution (0.7:1 v/w of carbopol) as per the manufacturer's instructions, and the mixture was allowed to hydrate at 40 °C in a shaking incubator (150 rpm) overnight. Carrageenan gels were prepared by addition of dry kappa carrageenan powder to KCl solution which was held at 85 °C for 2 hours. Carrageenan and carbopol gels were mixed by vigorous stirring and left to equilibrate at 85 °C for an additional 2 hours. Previous acceptability studies revealed that women preferred bullet shaped suppositories at size 3 mL prepared from gels with a small strain storage modulus (G’) of 12500 Pa (25 °C). As shown in Figure 1, bullet-shaped suppositories (31 mm long with a diameter of 11mm) were prepared by casting in molds, as described previously [31]. The different gel formulations, prepared with varying ratios of carbopol:carrageenan, were matched rheologically in their small strain storage modulus to have the desired G’ value of 12500 Pa at 25 °C. Small deformation rheological measurements were performed at a frequency of 1 Hz and strain of 1 % on a strain-controlled oscillatory rheometer (ARES, TA Instrument, New Castle, DE) using a cone and plate geometry (probe diameter = 25 mm, cone angle = 5.73°). The gel was loaded between the cone and plate while hot (80 °C) and the edges sealed with a light coating of mineral oil to prevent moisture evaporation. Data were recorded first on cooling to 15 °C, followed by heating to 60 °C at a rate of 5 °C per minute.
Table 1.
Composition of gels used to prepare suppositories matched in their small strain storage modulus (G’=12500 Pa at 25 °C)
| Formulations | Kappa Carrageenan (% w/v) | Carbopol (% w/v) | KCl (M) |
|---|---|---|---|
| 1.75% K | 1.75 | 0 | 0.05 |
| 2% K 0.7% C | 2 | 0.7 | 0.05 |
| 1.75% K 1% C | 1.75 | 1 | 0.05 |
| 1.5% K 1.1% C | 1.5 | 1.1 | 0.05 |
Figure 1.
Bullet shaped suppositories, 31 mm long with a diameter of 11 mm.
Samples for ex vivo handling by participants were presented in 0.75 oz transparent plastic cups (Solo Cup Company, Urbana, IL) labeled using 3 digit blinding codes kept at 25 °C with the lids sealed tightly until evaluated. To prepare samples for simulated post-coital discharge evaluation, suppositories were molded as described above. The suppositories were incubated with vaginal simulant fluid (VSF) for 4 hours at 37 °C, after which excess VSF was drained off, and the suppositories were placed in vacuum-sealed plastic bags to facilitate crushing. Vaginal simulant fluid (VSF) was prepared as described in [38], stored at 4 °C and equilibrated to 37 °C before adding to the suppositories. The suppositories were then crushed using a rolling pin. The crushed samples (3g, weight of a 3 mL suppository) were placed into 0.75 oz transparent plastic cups (Solo Cup Company, Urbana, IL) labeled using 3 digit blinding codes with 2 ml VSF and stored at 4 °C with the lids sealed tightly overnight. The samples of simulated discharge were allowed to equilibrate to room temperature prior to evaluation.
2.4 Sample evaluation
Prior to sample evaluation, participants were asked to watch a 90s video in which a female physician demonstrates how a participant should evaluate a prototype in her hand [36, 37]. Participants were instructed to: 1. Take the sample and put it into her non-dominant hand; 2. Gently stroke the sample with the index finger of her dominant hand; 3. Put the sample between her fingers and pinch gently (hand not specified verbally; shown as dominant hand in video); 4. Finally hold the sample between her fingers and imagine she was trying to insert the sample into her vagina (hand not specified). After watching the video, participants were provided with a consent form to read. Women provided their consent to participate by signing in with the research team, where they were assigned individual ID codes and sent to the testing area. All ratings were collected using Compusense® Cloud (Guelph, Ont).
2.4.1 Sample evaluation for Test 1
Participants were presented with four samples together on one tray: a carrageenan only suppository (control) and suppositories prepared with 3 different kappa carrageenan:carbopol formulations (Table 1) all with a G’ =12500 Pa (25 °C). Participants rated their perceived effectiveness, ease of insertion and willingness to try on separate 100-point visual analog scales for each of the suppositories. To avoid low willingness to try ratings due to low perceived STI risk, women were asked to assume they might need these products ‘to prevent potential infections, including Chlamydia, herpes and HIV.’ Verbal end anchors (e.g., not at all willing, extremely willing) were provided on the scales, and these were indented at 10% and 90% of the scale to minimize end use avoidance. Appropriateness of firmness was assessed using 5-point categorical just-about-right (JAR) scale. (Standard practice is to collect ‘overall liking’ ratings along with JAR data [39]; however in our previous microbicide studies, willingness-to-try has been consistently used in place of liking [36]). After various ratings were obtained for each sample, women were asked to rank all four suppositories in order from most to least preferred. Demographics, such as age, education, marital status, prior STI diagnosis, prior unplanned pregnancy, number of sexual partners in past year and number of vaginal deliveries, were collected at the end of the test (Table 2). Prior experience with other vaginal products was collected using a Check-All-That-Apply (CATA) question, summarized in Table 3.
Table 2.
Demographic information. Numbers in right two columns indicate the % of participants (n=99 for test 1 and n=94 for test 2)
| Age | Test 1 % Participants | Test 2 % Participants |
|---|---|---|
| 18-24 years old | 17.2 | 22.3 |
| 25-29 years old | 28.3 | 29.8 |
| 30-34 years old | 17.2 | 14.9 |
| 35-39 years old | 14.1 | 10.6 |
| 40-44 years old | 16.2 | 13.8 |
| 45-50 years old | 6.1 | 8.5 |
| 50-55 years old | 1.0 | 0 |
| Marital Status | ||
| Now married | 53.5 | 53.2 |
| Divorced/Separated | 10.1 | 8.5 |
| Never married | 36.4 | 38.3 |
| Vaginal Births | ||
| None | 63.6 | 67 |
| One | 19.2 | 13.8 |
| Two | 14.1 | 13.8 |
| Three or more | 3.0 | 5.3 |
| Sexual Partners in the past 12 months | ||
| Zero | 5.1 | Choice not offered |
| One | 72.7 | 78.7 |
| 2 to 3 | 18.2 | 15.9 |
| 4 to 5 | 2.0 | 4.3 |
| 6 to 10 | 2.0 | 1.1 |
| More than 10 | 0.0 | 0 |
| STI Diagnosis | ||
| Yes | 9.1 | 12.8 |
| No | 90.9 | 87.2 |
| Unplanned Pregnancy | ||
| Yes | 15.2 | 14.9 |
| No | 84.9 | 85.1 |
Table 3.
Prior vaginal product usage from a Check All That Apply (CATA) question, so column totals may exceed 100%. Numbers in right two columns indicate the % of participants (n=99 for test 1 and n=94 for test 2).
| Vaginal products used | Test 1 | Test 2 |
|---|---|---|
| Vaginal contraceptive products such as NuvaRing® | 10.1 | 8.5 |
| Spermicidal gels and films | 3.0 | 2.1 |
| Yeast infection medicines such as Vagisil® and Monistat | 23.3 | 23.4 |
| Douche | 6.1 | 3.2 |
| Menstruation products such as tampons | 75.8 | 77.7 |
| Lubrication products such as KY® gels, liquibeads and Vitamin-E suppositories | 27.3 | 36.2 |
2.4.2 Sample evaluation for Test 2
Based on the results of Test 1 (described below), formulations with both the highest and lowest carbopol concentration used in the previous test (Table 1) were retained with the carrageenan only control to explore the effect of evaluating simulated discharge on willingness to try scores. Participants first received tray 1 with 3 intact suppositories, which they evaluated in the same manner as Test 1. After evaluating the suppositories, participants returned their tray to receive a 2nd tray with the simulated discharge samples. Participants were instructed to remove the cup lid, visually inspect the samples and rate the acceptability of the discharge appearance on a 100-point visual analog scale. Instructions for the evaluation of the discharge samples were provided using a demonstration video embedded in the Compusense® questionnaire. Participants were instructed to 1. Using the provided spoon, scoop a small amount of sample into the palm of your non-dominant hand 2. Using the thumb of your dominant hand, rub the sample between your thumb and palm, as shown in the video 3. Now imagine this is how the product feels after several hours inside your body, and that the sample could come out in this state during sex or with your natural discharge.
Women rated their perceived effectiveness and willingness to try on separate 100-point visual analog scales and were given open-ended comment boxes to indicate what they liked or disliked about the sample presented. After evaluating all 3 samples, participants were asked to rank the samples in order of preference. Participants then returned the tray to receive a 3rd tray on which pairs of samples were presented, each pair consisting of the intact suppositories along with it's corresponding simulated discharge (both of which had been previously evaluated by the participant separately on tray 1 and 2). Women were informed that the simulated discharge samples were prepared from suppositories presented on tray 1, but were manipulated to show how they might break down inside your body. Participants were then asked to consider the samples as pairs, thinking about both how the product would feel during insertion into the vagina (without an applicator) and also how it would feel and look over the next several hours considering that it may come out during sex or with their natural discharge. Given these dual considerations, they were asked to again rank the samples in order of preference. An open comment box was provided for participants to provide feedback regarding the test products after evaluating all the samples on the 3 trays. Demographic information collected after evaluating all samples is summarized in Table 2 and Table 3.
2.5. Mechanical Characterization of Elongation Properties of the Gels
To characterize the large-deformation rheological properties of carrageenan gels upon carbopol addition, the gels were molded into oval rings (outer length of oval = 5.90 cm, center-to-center length = 2.48 cm, radius of outer arc = 1.71 cm, width of gel leg = 1.03 cm, depth of gel = 1.0 cm) and stretched in tension at 5 mm/s on two 1.4 cm aluminum dowel pins attached to a TAXT2i Texture Analyzer (Stable Micro Systems, Halsemere, Surrey, UK) [31, 40]. Eight to ten measurements of tensile force and deformation at fracture were made for each gel type and firmness. Dowel pins and the contacting surfaces of the ring were lightly coated with mineral oil to reduce friction. The force and deformation at fracture were measured to calculate the stress at fracture (σ) and strain at fracture (ε) using the equations:
where: σ is the true stress at fracture; F is the force at fracture; A0 the initial cross sectional area (m2); ΔL the change in leg length (m); Ca the average circumference; and ϵ the Hencky or true strain at fracture.
To quantify the change in force required to fracture suppositories upon carbopol addition to carrageenan, compression force required to fracture the symmetrical spherical suppositories (size 3 mL) was measured. As fracture behavior might be altered by exposure to vaginal fluids, we quantified the compression force required to fracture spherical suppositories that had been soaked in 5 mL VSF at 37 °C with constant shaking. Compression force was measured at 0 (No exposure to VSF), 2 and 24 h using the compression platen probe in the TAXT2i Texture Analyzer (Stable Micro Systems, UK). Compression force required to fracture ovules was measured while compressing it at 1 mm/s for 10mm or until the force exceeded 100g and the inflection point in the force curve was recorded as the compression force.
2.6. Characterization of Drug Release
To quantify potential changes in the drug release profile due to carbopol addition, release was compared between carrageenan only controls and formulations with carbopol. The rate of release of the antiretroviral drug tenofovir (TFV) was determined for spherical suppositories loaded with 40 mg TFV, based on the dose of TFV employed in clinical trials with 4 mL of 1% TFV gel [13]. The acidic pH of TFV in water (pH = 3–4) disrupted carrageenan gel formation, as the pKa of carrageenan is 4.9. Therefore, the required concentration of TFV was dissolved in deionized water and the pH of the TFV solution was adjusted to 5.2 by addition of sodium hydroxide. Mixed gels were then prepared as described above and characterized in a rheometer (see above) to confirm the storage modulus matched the desired value of G’ = 12,500 at 25 °C within 10%. Drug release studies were performed in 5 mL VSF in 50 mL screw-cap test tubes placed in a shaking incubator at 150 rpm and 37 °C for 24 hours. Dissolution studies with 5 mL VSF were designed to better mimic the vaginal environment, as the average volume of vaginal fluid obtained from healthy donors has been shown to vary in the range of 0.5–8 mL/day [41, 42].
2.7. Statistical Analyses
Data were collected using Compusense® Cloud (Guelph, ONT, Canada) and analyzed using JMP v9.0.2 (Cary, NC, USA). Effects of carbopol addition on the perceived effectiveness, imagined ease of insertion and willingness to try were analyzed using ANOVA, with participant as a random effect and formulation as a fixed effect. Tukey's Honest Significant Difference (HSD) was used for post hoc comparisons, with p < 0.05 considered significant. Response distributions for the JAR categories were compared using the Cochran-Mantel Haenszel (CMH) test [39]. Ranking data were analyzed via Friedman's test; rank-sums were calculated and compared using least significant differences [43]. Similarly, the effect of suppository type on gel compression and elongation was analyzed via ANOVA. The least square means (L.S.M.) were then compared across different suppository types using Tukey's HSD test with p < 0.05 considered significant. For the TFV release data in VSF mean and standard error were calculated (n = 7) for each time point. For comparing the initial rates of diffusion (0–2 h) across the suppository types, the slopes for individual samples were computed from the release data. The least square means (L.S.M.) of slopes were calculated and compared using Tukey's HSD test with p < 0.05 considered significant.
3. Results
3.1 Effect of Carbopol addition on Women's Preferences
To determine the effect of carbopol addition, 99 women evaluated 4 suppositories comprising of a control (carrageenan only) and three different ratios of kappa carrageenan:carbopol for test 1 (Table 1). The carrageenan control scored significantly higher that the carbopol containing samples with regard to perceived effectiveness, imagined ease of insertion and willingness to try (Figure 2a-c). For perceived effectiveness, there was no significant difference between the three carbopol-containing samples, indicating carbopol addition affected perceived effectiveness but not in a concentration dependent manner (at least in the range tested). However carbopol concentration did influence the imagined ease of insertion and willingness to try ratings, with the highest carbopol concentration scoring significantly lower than the two lower carbopol concentrations for both ratings. The JAR data sheds light on possible reasons for this drop, as the percentage of women endorsing ‘Too Soft’ increased with increasing carbopol concentration (Figure 3). As women were instructed to imagine insertion without an applicator, softer samples containing carbopol may be perceived difficult to manipulate for insertion, which in turn presumably affects their willingness to try ratings. The ranking distribution is consistent with the willingness to try ratings (Table 4), with the carrageenan control being the most favored, followed by the two lower carbopol concentration samples, and the highest carbopol sample being the least favored.
Figure 2.
Effect of kappa carrageenan and carbopol concentration on (A) perceived effectiveness (B) imagined ease of insertion and (C) willingness to try, which were measured on a 100 point visual analog scale. Indented semantic anchors at 10 (e.g., “not at all willing”) and 90 (e.g., “extremely willing”) were provided to minimize end avoidance bias. Upper case letters denote statistically significant differences between samples with varying carrageenan and carbopol concentrations using Tukey's honest significant difference (HSD) test.
Figure 3.
Carrageenan to carbopol ratio influences distributions of responses on a just-about-right scale for firmness from A) Test 1 and B) Test 2. X-axis is the proportion of responses for each category on the just-about-right scale for firmness. Numbers inside the bars are the raw number of participants endorsing that category.
Table 4.
Rank order of different formulations in Test 1 with varying carrageenan and carbopol concentrations, in order of preference from most preferred (1) to least preferred (4) along with calculated Rank Sum. Upper case letters denote statistically significant differences between the rank sum for the different formulations.
| Rank Order | 1 | 2 | 3 | 4 | Rank Sum | Significant difference |
|---|---|---|---|---|---|---|
| 1.75% K | 57 | 20 | 11 | 11 | 174 | A |
| 2% K 0.7% C | 15 | 41 | 28 | 15 | 241 | B |
| 1.75% K 1% C | 14 | 24 | 42 | 19 | 264 | B |
| 1.5% K 1.1% C | 13 | 14 | 18 | 54 | 311 | C |
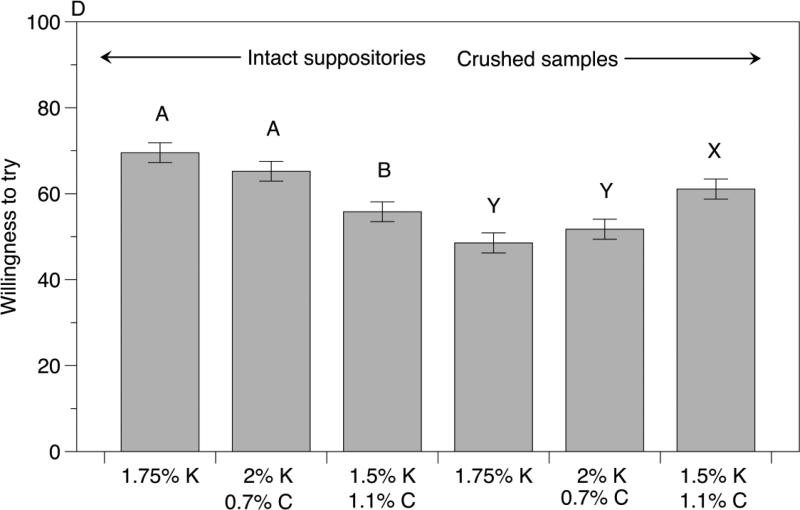
In test 2, 94 women evaluated 3 samples (carrageenan control, and the highest and lowest carbopol concentrations from test 1). When they evaluated intact suppositories on tray 1, highest carbopol concentration sample differed significantly from the other two samples for the perceived effectiveness and willingness to try ratings as well as based on the rank sum differences (Figure 4 and Table 5). Notably, we saw a concentration dependent effect of carbopol concentration on the imagined ease of insertion ratings with the significant differences across all three samples (Figure 4B). Critically, when women evaluated crushed samples as simulated discharge, we saw a reversal in preferences with higher carbopol samples scoring significantly higher than the lower carbopol and carrageenan control in terms of the perceived effectiveness, discharge appearance acceptability and willingness to try ratings (Figure 4). Again we see a carbopol concentration dependent effect on the rank sum values, but instead with the higher carbopol sample being the most preferred, followed by lower carbopol sample and the carrageenan only control being the least preferred (Table 5). Finally, when women evaluated sample pairs comprising the intact samples and their corresponding crushed discharge simulant, there were no significant differences in ranking among the 3 sample pairs (Table 5). Even though the differences among the three formulations tested were no longer significant when women looked at pairs of intact and crushed samples, 44% women selected the pair containing the highest carbopol concentration sample, suggesting that discharge appearance may be a bigger driving factor in the decision.
Figure 4.
Effect of kappa carrageenan and carbopol concentration on (A) Perceived effectiveness of intact suppositories and crushed samples (B) imagined ease of insertion for intact suppositories (C) Acceptability of discharge appearance (D) willingness to try for intact suppositories and crushed samples measured on a 100 point visual analog scale during Test 2. Indented semantic anchors at 10 (e.g., “not at all willing”) and 90 (e.g., “extremely willing”) were provided to minimize scale end avoidance bias. Upper case letters denote statistically significant differences between samples with varying carrageenan and carbopol concentrations across the sample type (intact suppository or crushed samples) using Tukey's honest significant difference (HSD) test.
Table 5.
Rank order of different formulations presented in Test 2 with varying carrageenan and carbopol concentrations, in order of preference from most preferred (1) to least preferred (3) along with calculated Rank Sum. Upper case letters denote statistically significant differences between the rank sum for the different formulations. Lower number indicates greater preference.
| Intact suppositories | Rank 1 | Rank 2 | Rank 3 | Rank sum | Significance |
|---|---|---|---|---|---|
| 1,75% K | 60 | 20 | 14 | 142 | C |
| 2% K 0.7% C | 25 | 60 | 9 | 172 | B |
| 1.5% K 1.1% C | 9 | 14 | 71 | 250 | A |
| Crushed samples | Rank 1 | Rank 2 | Rank 3 | Rank sum | Significance |
|---|---|---|---|---|---|
| 1,75% K | 8 | 29 | 57 | 237 | A |
| 2% K 0.7% C | 19 | 47 | 28 | 197 | B |
| 1.5% K 1.1% C | 67 | 18 | 9 | 130 | C |
| Paired samples | Rank 1 | Rank 2 | Rank 3 | Rank sum | Significance |
|---|---|---|---|---|---|
| 1.75% K | 28 | 32 | 34 | 194 | A |
| 2% K 0.7% C | 25 | 38 | 31 | 194 | A |
| 1.5% K 1.1% C | 41 | 24 | 29 | 176 | A |
Data from our previous quantitative and qualitative work each indicate women's vaginal product use as well as other factors (e.g., perceived risk, age, and number of vaginal deliveries) may potentially influence women's perceived effectiveness, imagined ease of insertion and willingness to try microbicide suppositories [27, 31]. Effect of individual demographic variables on perceived effectiveness, imagined ease of insertion and willingness to try were tested using a nested ANOVA model. Women who had previously used yeast infection medications gave higher ratings for willing to try compared to non-users (77 vs 64, p=0.0009 in test 1; and 71 vs 61, p=0.0323 in test 2), and these differences varied based on the formulation during test 1 (Formulation × Use Interaction p=0.0017). When evaluating the crushed samples during test 2, yeast infection medication users gave higher ratings for discharge appearance acceptability (64 vs 52, p=0.0024), perceived effectiveness (61 vs 51, p=0.0096) and willingness to try (66 vs 50, p=0.0007), as compared to non-users. Women who reported having been previously diagnosed with an STI gave higher willingness to try ratings compared to other women (78 vs 66, p=0.0417 and 75 vs 62, p=0.0185, for tests 1 and 2 respectively). Women reporting an unplanned pregnancy in test 1 gave higher imagined ease of insertion ratings (69 vs 55, p=0.0069) compared to other women, and we saw a similar trend in test 2 that it did not reach statistical significance (61 vs 53, p=0.1585). Finally, women with multiple (2 or more) partners in the past 12 months in test 2 showed a trend for higher willingness to try ratings (71 vs 62, p=0.0602).
Based on overall comments and stated reasons for liking/disliking the crushed samples, women would ideally like a sample firm enough to insert without an applicator while still resulting in smooth discharge without visible chunks. Some women expected that the discharge would eventually dissolve into the vaginal tissue similar to lotion, as opposed to coming out in visible chunks. There was good consensus among women regarding liking of the clear nature of the discharge (irrespective of formulation), which some thought would be good for hygiene and cleanup as well as avoid suspicion from partners. As women were not provided with information regarding the way the drug would be released from the suppositories, there were several comments regarding preference governed by their perception of where the medication would be concentrated in the milieu of the discharge. Representative comments that highlight these points are given below.
At first, I did not like how the product was in bigger chunks, but after feeling it, I realized that I liked this feature because it seems it might stay in the body for longer this way. When touching it, it easily broke down into smoother/softer pieces. (2% K, 0.7% C, Caucasian age 30-34 years)
The pieces were too big and hard - it would be better if it dissolved more inside the body - which would make me think it to be more effective and it would come out more like discharge and not be as disturbing for the other partner (1.75% K, Caucasian age 25-29 years)
I liked that it looked like something that already comes out of our bodies during and after sex. It seems like something I could clean up easily and comfortably. (1.5% K, 1.1% C, Caucasian age 18-24 years)
3.2. Characterization of Elongation Properties of Combination Gels
The stress (σ) and strain (ε) at fracture for rings prepared from the combination (kappa carrageenan / carbopol) gels were determined to study the effect of carbopol addition on the large-scale deformation properties of the gels (Figure 5). With carbopol addition, the stress that gel rings could withstand before fracturing under tensile force decreased with increasing carbopol concentration. The lowest carbopol concentration gel (0.7% C) withstood stress comparable to the kappa carrageenan control, while the two higher carbopol concentrations withstood significantly lower force than the control. Conversely the highest carbopol concentration gel withstood higher strain under tension before fracturing compared to control, with the lower two carbopol concentrations in between.
Figure 5.
Stress and strain at fracture for kappa carrageenan and carbopol combination gels, calculated using force and deformation required to fracture gel rings. Upper case letters denote statistically significant differences (p < 0.05) in the stress and strain between the different ratios of carrageenan:carbopol gels.
Preferably, the suppositories being developed will break down within the body releasing the medication, while also mimicking vaginal mucous secretions when they erode, so that they may be covertly eliminated. By measuring the compression force required to fracture the ovules upon exposure to a simulated vaginal environment for 24 hours, we are able to quantify the softening of the ovule expected inside the body. We measured the compression force required to fracture ovules prior to VSF exposure (depicted by 0h in Figure 6) for comparison to the forces exerted during handling and insertion as those might result in the suppository breaking. We have previously seen from results of our focus groups that if the product fractured/cracked during insertion, then women did not believe it would be effective and would chose to use another intact ovule. Here, the fracture force and distance before breaking for gels containing carbopol was significantly lower than the kappa carrageenan control for the different time points tested. The compression force and distance required to fracture ovules also decreased upon exposure to VSF, however we did not see significant differences in the fracture force between 2 and 24 hours. This may be attributed to lack of sink conditions and presence of KCl in the VSF.
Figure 6.
Force to fracture in compression (A) required to break spherical suppositories and compression distance at break (B) after suppositories have been in contact with vaginal simulant fluid for 0 - 24 h. Upper case letters denote statistically significant differences (p < 0.05) between different kappa carrageenan and carbopol combinations measured at the same time point.
3.3 Characterization of drug release
The release of tenofovir (TFV) from spherical suppositories was characterized over 24 h in 5 mL VSF. For diffusion into 5 mL VSF, 45-50 % of TFV was released in the first 2 h, 60 % within 6 h and 70 % in 24 h (Figure 7). Previous studies with 5 mL of dissolution medium indicated that release plateaus at 24 h [21]. Recognizing the limitation of insufficient sink conditions, for the 5 mL drug diffusion studies, the dissolution medium was replenished with fresh VSF at 24 h to stimulate additional release. About 85-90% of the TFV within all the different gel combination suppositories was released in the next 24 h (Figure 7). There was no significant difference in the initial release rate (0-2 h) of TFV from the different combinations of carrageenan and carbopol gels as well as between the amount of TFV released at each time point over 48 hours.
Figure 7.
Effect of addition of carbopol to carrageenan gels on release of tenofovir from spherical suppositories in 5 mL vaginal simulant fluid. Release of TFV by diffusion from suppositories of size 3 mL loaded with 40 mg TFV each was studied for 48 h. Plotted are mean and standard error (n = 7) of the cumulative amount of TFV released.
4. Discussion
Vaginal microbicides hold great potential to empower women with a method they can self-initiate to prevent sexual transmission of HIV and other STIs. Prior clinical trials with vaginal microbicide candidates have shown mixed results, with differences in efficacy depending on participants’ product usage and compliance to study protocol [4, 5, 13]. Women's lack of adherence to study protocols has been cited as one of the factors contributing to the decreased effectiveness of the microbicide candidates [5]. There are several factors that affect women's adherence to such protocols: some are product dependent [8, 10, 44] while others are dependent on the social and cultural background of the place where the trials are conducted [5, 45]. While it is important to investigate social and cultural barriers to microbicide use and adherence and to devise strategies to circumnavigate these barriers, it is also important to assess and optimize acceptability of products prior to advancing to the clinical trial stage, as clinical trials are expensive, time consuming and personally invasive. Thus, it is important to only move forward with products that have been shown to be both biologically efficacious and well accepted by women. In vivo assessment of the vehicle – be it a gel, ring or suppository – without the drug would be the gold standard for acceptability studies; however, it is also true that women are less likely to even try certain products based on their visual appearance or concept of operation [46, 27]. Thus, the very first step in acceptability research needs to understand women's beliefs related to product appearance or features while designing products that instill confidence and trust. Creative use of sensory testing methods is a good strategy to identify these beliefs using focus groups [27], creating different product iterations and then testing them in large scale quantitative tests [31, 36, 37].
In assessment of personal care products such as soap, shampoo, and creams; cosmetics such as lipsticks and foundations; or household products such as laundry detergents and cleaners, there are different sets of attributes that are important to the consumer across the product life cycle [47, 48]. For example, while conducting sensory testing for a shampoo, its smell, appearance, and how it feels on the hand before application, form the pre-use attributes that influence acceptability. Factors including how the product lathers in the hand and in the hair, as well as the aroma released during application form in-use product attributes. Finally, the shampoo's effect on hair texture, appearance, and even smell hours after the product has been applied form post-use product attributes [33]. For a consumer, the sum of all these attributes provide the holistic product experience, which either favors or hinders continued product use. Similarly, for microbicide products, multiple pre- and in-use product attributes need to be considered, especially since they are to be used before, during, or after sexual experiences. Cultural and personal practices surrounding sexual activity may also shape needs or preferences for intravaginal product attributes, further supporting the need for acceptability throughout the microbicide life cycle [26].
Until now we have focused on the pre-use product attributes by assessing the perceived effectiveness, imagined ease of insertion and willingness to try based on the product attributes such as size, shape and firmness of suppositories [31, 36, 37]. Since this work is part of a preclinical development effort, we cannot ethically assess in-use intravaginal product attributes. However, we can indirectly assess some of the external in-use product attributes that may affect women's willingness to use the product by having women assess the appearance and feel of the simulated discharge. Based on the gel properties tested in the current study, there is a clear tradeoff of suppository firmness with size and feel of the resulting discharge. While evaluating intact suppositories and simulated discharge, participants are specifically thinking of imagined ease of insertion and discharge appearance respectively. However when presented with the sample pairs with opposite trends for ratings, the ranking task compels tradeoffs, which may differ amongst participants. Based on the importance assigned by women to the pre- or post-use features, the ranking distribution varies across participants but we do not see significant differences between samples. It is important for formulators to understand the tradeoffs that are in play while making decisions regarding preferred firmness/composition, to design products that would be considered easy to insert while producing discharge that is well tolerated during and after use, including sex.
Among the women who explicitly mentioned how they made the tradeoff through the open comment section, women were more likely to consider the discharge appearance compared to the ease of insertion, which is reflected in the ranking distribution of the paired samples. Some women mentioned that they like the clear nature of the simulated discharge, as it would appear natural and resemble mucous secretions, which may raise fewer concerns from sexual partners and be easy to clean. Although the emphasis on covert use in the microbicide field has become less critical as some recent clinical trials have shown that women would prefer to disclose microbicide product use to primary partners [10, 20], a product that still offers the potential for covert use would be desirable as the need for disclosure is known to be different for casual/transactional partners [49, 50]. Some of the other reasons women mentioned for liking the simulated discharge were being smooth, soft, uniform in appearance and easy to dissolve. These reasons were mentioned for all formulations but more frequently for the higher carbopol (1.5% K, 1.1% C) containing samples. Impact on sexual pleasure and lubrication has been previously studied in acceptability studies for vaginal microbicides [28, 11]. The lubricating nature of vaginal gels has been shown to make sex less painful and more pleasurable. Thus the carbopol containing gels may have the potential to contribute to the lubricity of the microbicide formulation.
Some women believed the medication may be contained in the chunks and preferred the chunkier discharge samples whereas others thought the medication would be evenly distributed and preferred the smoother paste like samples. Some women indicated that they would not believe the drug was working if large, harder chunks were present in the discharge. This idea of perceived product efficacy has emerged recently, and vaginal microbicide researchers are paying increasing attention to consumer perception and the sensory aspects of products. Morrow et al. recently explored meaning-making in relation to sensory properties of vaginal products [9, 51], and demonstrated consumers assign beliefs about products and efficacy based on sensory properties. They in turn hypothesize that these meanings affect women's willingness-to-try and actual use of vaginal products. We have previously explored perceived product efficacy in terms of physical attributes of softgels using quantitative consumer tests and qualitative focus groups [37, 52]. The present test with simulated discharge furthers our understanding of use reactions based on different phases of product use. Since no differences in drug release were observed between the formulations, the consistency of the suppository or its discharge has no fundamental relation to the effectiveness of the drug; nonetheless, understanding such perceptions remains critical. Educating women about the product and how it would breakdown may also be vital to maintaining continued use of such delivery systems. While the discharge resulting from the breakdown of softgel ovules of different formulations inside the body is still under investigation (using modeling of different forces in the vagina), preliminary bench top experiments were used to devise methods to prepare samples to simulate post coital discharge. As the intention of this test was to observe how women's perception of the suppositories would vary with the consistency of the simulated discharge, pressure and duration of rolling was varied to ensure larger chunks were retained in the 1.75% K formulation, middle sized chunks in the 2% K 0.7% C formulation, and smallest chunks (almost no chunks) in the 1.5% K 1.1% C formulation. This ensured a gradient in the size of the remaining pieces of the suppository after simulated breakdown, which we observed in bench top experiments (light crushing within a flexible plastic tube under a weight on a tilt-table for several hours) would be similar the actual experience if these suppositories broke down in vivo. The texture of the chunks varied as a function of the formulation. The size and firmness of chunks varied inversely to the carbopol concentration, which enabled us to simultaneously determine the effect of chunk size and texture on discharge acceptability. While we understand that these samples may not be truly representative of the discharge in the vaginal environment, they still provide insight into the relationship between the size and texture of chunks and willingness to try the softgel ovules.
Ultimately, in vivo assessment by women is required to assess final product acceptability and adherence. However there is a limit on the number of product alternatives that can be studied in clinical trials due to expense. One approach to overcome this barrier is to first assess a wider range of product concepts to narrow the field of possible products that are selected to move forward in the development process. Prior research from our team suggests shape, size, and firmness all affected women's reported willingness to try a hypothetical microbicide, and these effects were dependent on whether or not an applicator would be provided with the microbicide [37]. The current work demonstrates that not only these initial product properties, but also the post-insertion properties of the product, may be vital for continued use. This is especially important for products designed not just as a treatment, where a woman may feel more compelled to use a product despite unpleasant attributes, but as a preventative measure, where a woman would need to use the product consistently without the added pressure of having a condition or infection.
Acknowledgments
This work was supported by National Institutes of Health grants from the National Institute of Allergy and Infectious Diseases (AI094514) to J.E.H. and G.R.Z. The authors would like to thank Mia Andrisani and Yuqing Wang for their assistance with mixed gel preparation and physical testing using rheology and texture analysis. The authors also thank Dr. Alyssa Bakke for her comments on the manuscript.
Footnotes
Conflict of Interest Disclosure
All the authors declare that they have no conflict of interest.
Informed Consent
‘All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all participants for being included in the study.’ The procedures were approved by the Pennsylvania State University Institutional Review Board (protocol #36943). Participants provided informed consent and were reimbursed for their time.
References
- 1.Drug Delivery and Development of Anti-HIV Microbicides. CRC Press; 2014. [Google Scholar]
- 2.Stockman JK, Syvertsen JL, Robertson AM, Ludwig-Barron NT, Bergmann JN, Palinkas LA. Women's Perspectives on Female-Initiated Barrier Methods for the Prevention of HIV in the Context of Methamphetamine Use and Partner Violence. Women's Health Issues. 2014;24(4):e397–e405. doi: 10.1016/j.whi.2014.04.001. doi: http://dx.doi.org/10.1016/j.whi.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. Tenofovir-Based Preexposure Prophylaxis for HIV Infection among African Women. New England Journal of Medicine. 2015;372(6):509–18. doi: 10.1056/NEJMoa1402269. doi:doi:10.1056/NEJMoa1402269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rees H, Delaney-More S, Baron D, Lombard C, Gray G, Myer L, et al., editors. FACTS 001 Phase III Trial of Pericoital Tenofovir 1% Gel for HIV Prevention in Women.. Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, WA.. 2015. [Google Scholar]
- 5.van der Straten A, Stadler J, Montgomery E, Hartmann M, Magazi B, Mathebula F, et al. Women's Experiences with Oral and Vaginal Pre-Exposure Prophylaxis: The VOICE-C Qualitative Study in Johannesburg, South Africa. PLoS ONE. 2014;9(2):e89118. doi: 10.1371/journal.pone.0089118. doi:10.1371/journal.pone.0089118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdool Karim SS, Kashuba ADM, Werner L, Karim QA. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378(9787):279–81. doi: 10.1016/S0140-6736(11)60878-7. doi:10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Berg JJ, Rosen RK, Bregman DE, Thompson LA, Jensen KM, Kiser PF, et al. “Set it and forget it”: women's perceptions and opinions of long-acting topical vaginal gels. Aids Behav. 2014;18(5):862–70. doi: 10.1007/s10461-013-0652-4. doi:10.1007/s10461-013-0652-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrow K, Rosen R, Richter L, Emans A, Forbes A, Day J, et al. The acceptability of an investigational vaginal microbicide, PRO 2000 gel, among women in a phase I clinical trial. Journal of Women's Health. 2003;12(7):655–66. doi: 10.1089/154099903322404302. doi:Doi 10.1089/154099903322404302. [DOI] [PubMed] [Google Scholar]
- 9.Morrow K, Underhill K, Berg JJ, Vargas S, Rosen RK, Katz DF. User-Identified Gel Characteristics: A Qualitative Exploration of Perceived Product Efficacy of Topical Vaginal Microbicides. Arch Sex Behav. 2014:1–9. doi: 10.1007/s10508-013-0235-5. doi:10.1007/s10508-013-0235-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bentley ME, Fullem AM, Tolley EE, Kelly CW, Jogelkar N, Srirak N, et al. Acceptability of a microbicide among women and their partners in a 4-country phase I trial. Am J Public Health. 2004;94(7):1159–64. doi: 10.2105/ajph.94.7.1159. doi:Doi 10.2105/Ajph.94.7.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greene E, Batona G, Hallad J, Johnson S, Neema S, Tolley EE. Acceptability and adherence of a candidate microbicide gel among high-risk women in Africa and India. Cult Health Sex. 2010;12(7):739–54. doi: 10.1080/13691051003728599. doi:10.1080/13691051003728599. [DOI] [PubMed] [Google Scholar]
- 12.Woodsong C, Holt JD. Acceptability and preferences for vaginal dosage forms intended for prevention of HIV or HIV and pregnancy. Adv Drug Deliv Rev. 2015;92:146–54. doi: 10.1016/j.addr.2015.02.004. doi:10.1016/j.addr.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, et al. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. doi:10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fetherston SM, Boyd P, McCoy CF, McBride MC, Edwards K-L, Ampofo S, et al. A silicone elastomer vaginal ring for HIV prevention containing two microbicides with different mechanisms of action. Eur J Pharm Sci. 2013;48(3):406–15. doi: 10.1016/j.ejps.2012.12.002. doi: http://dx.doi.org/10.1016/j.ejps.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Devlin B, Nuttall J, Wilder S, Woodsong C, Rosenberg Z. Development of dapivirine vaginal ring for HIV prevention. Antiviral Research. 2013;100(Supplement(0)):S3–S8. doi: 10.1016/j.antiviral.2013.09.025. doi: http://dx.doi.org/10.1016/j.antiviral.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 16.Pereira LE, Clark MR, Friend DR, Garber DA, McNicholl JM, Hendry RM, et al. Pharmacokinetic and safety analyses of tenofovir and tenofovir-emtricitabine vaginal tablets in pigtailed macaques. Antimicrobial Agents and Chemotherapy. 2014;58(5):2665–74. doi: 10.1128/AAC.02336-13. doi:10.1128/AAC.02336-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole AM, Patton DL, Rohan LC, Cole AL, Cosgrove-Sweeney Y, Rogers NA, et al. The Formulated Microbicide RC-101 Was Safe and Antivirally Active Following Intravaginal Application in Pigtailed Macaques. PLoS ONE. 2010;5(11):e15111. doi: 10.1371/journal.pone.0015111. doi:10.1371/journal.pone.0015111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akil A, Parniak MA, Dezzuitti CS, Moncla BJ, Cost MR, Li M, et al. Development and Characterization of a Vaginal Film Containing Dapivirine, a Non-nucleoside Reverse Transcriptase Inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug delivery and translational research. 2011;1(3):209–22. doi: 10.1007/s13346-011-0022-6. doi:10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nel AM, Mitchnick LB, Risha P, Muungo LT, Norick PM. Acceptability of vaginal film, soft-gel capsule, and tablet as potential microbicide delivery methods among African women. Journal of Women's Health (Larchmt) 2011;20(8):1207–14. doi: 10.1089/jwh.2010.2476. doi:10.1089/jwh.2010.2476. [DOI] [PubMed] [Google Scholar]
- 20.Hammett TM, Mason TH, Joanis CL, Foster SE, Harmon P, Robles RR, et al. Acceptability of formulations and application methods for vaginal microbicides among drug-involved women: results of product trials in three cities. Sexually Transmitted Diseases. 2000;27(2):119–26. doi: 10.1097/00007435-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Zaveri T, Hayes JE, Ziegler GR. Release of Tenofovir from Carrageenan-based Vaginal Suppositories. Pharmaceutics. 2014;6:366–77. doi: 10.3390/pharmaceutics6030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chatterjee A, Bhowmik BB, Thakur YS. Formulation, In Vitro and In Vivo Pharmacokinetics of Anti-HIV Vaginal Bioadhesive Gel. Journal of Young Pharmacists : JYP. 2011;3(2):83–9. doi: 10.4103/0975-1483.80290. doi:10.4103/0975-1483.80290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andrade AO, Parente ME, Ares G. Screening of mucoadhesive vaginal gel formulations. Brazilian Journal of Pharmaceutical Sciences. 2014;50:931–41. [Google Scholar]
- 24.Bhabani Shankar N, Prasant Kumar R, Udaya Kumar N, Benoy Brata B. Development and Characterization of Bioadhesive Gel of Microencapsulated Metronidazole for Vaginal Use. Iranian Journal of Pharmaceutical Research : IJPR. 2010;9(3):209–19. [PMC free article] [PubMed] [Google Scholar]
- 25.Maguire RA, Zacharopoulos VR, Phillips DM. Carrageenan - Based Nonoxynol - 9 Spermicides for Prevention of Sexually Transmitted Infections. Sexually Transmitted Diseases. 1998;25(9):494–500. doi: 10.1097/00007435-199810000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Giguere R, Carballo-Dieguez A, Ventuneac A, Mabragana M, Dolezal C, Chen BA, et al. Variations in microbicide gel acceptability among young women in the USA and Puerto Rico. Cult Health Sex. 2012;14(2):151–66. doi: 10.1080/13691058.2011.630099. doi:Doi 10.1080/13691058.2011.630099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaveri T, Powell KA, Morrow Guthrie K, Ziegler GR, Hayes JE. Preferences and Acceptability among Women for Vaginal Semisoft Ovules: A Mixed Methods Study. BMC Women's Health. 2015 Under Review. [Google Scholar]
- 28.Hoffman S, Morrow KM, Mantell JE, Rosen RK, Carballo-Dieguez A, Gai F. Covert Use, Vaginal Lubrication, and Sexual Pleasure: A Qualitative Study of Urban US Women in a Vaginal Microbicide Clinical Trial. Arch Sex Behav. 2010;39(3):748–60. doi: 10.1007/s10508-009-9509-3. doi:DOI 10.1007/s10508-009-9509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joglekar N, Joshi S, Kakde M, Fang G, Cianciola M, Reynolds S, et al. Acceptability of PRO2000 Vaginal Gel among HIV un-infected Women in Pune, India. Aids Care. 2007;19(6):817–21. doi: 10.1080/09540120601133576. doi:10.1080/09540120601133576. [DOI] [PubMed] [Google Scholar]
- 30.Hardy E, de Padua KS, Hebling EM, Osis MJ, Zaneveld LJ. Women's preferences for vaginal antimicrobial contraceptives. V: attitudes of Brazilian women to the insertion of vaginal products. Contraception. 2003;67(5):391–5. doi: 10.1016/s0010-7824(03)00026-x. doi:S001078240300026X [pii] [DOI] [PubMed] [Google Scholar]
- 31.Zaveri T, Primrose RJ, Surapaneni L, Ziegler GR, Hayes JE. Firmness Perception Influences Women's Preferences for Vaginal Suppositories. Pharmaceutics. 2014;6(3):512–29. doi: 10.3390/pharmaceutics6030512. doi:10.3390/pharmaceutics6030512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brummer R, Godersky S. Rheological studies to objectify sensations occurring when cosmetic emulsions are applied to the skin. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 1999;152(1–2):89–94. doi: http://dx.doi.org/10.1016/S0927-7757(98)00626-8. [Google Scholar]
- 33.Churchill A, Meyners M, Griffiths L, Bailey P. The cross-modal effect of fragrance in shampoo: Modifying the perceived feel of both product and hair during and after washing. Food quality and preference. 2009;20(4):320–8. doi: http://dx.doi.org/10.1016/j.foodqual.2009.02.002. [Google Scholar]
- 34.ASTM. E 1490-03 Standard Practice for Descriptive Skinfeel Analysis of Creams and Lotions. 2003 [Google Scholar]
- 35.Rosen RK, Morrow KM, Carballo-Dieguez A, Mantell JE, Hoffman S, Gai F, et al. Acceptability of tenofovir gel as a vaginal microbicide among women in a phase I trial: A mixed-methods study. J Womens Health. 2008;17(3):383–92. doi: 10.1089/jwh.2006.0325. doi:DOI 10.1089/jwh.2006.0325. [DOI] [PubMed] [Google Scholar]
- 36.Li B, Zaveri T, Ziegler GR, Hayes JE. Shape of vaginal suppositories affects willingness-to-try and preference. Antiviral Res. 2012;97(3):280–84. doi: 10.1016/j.antiviral.2012.12.024. doi:10.1016/j.antiviral.2012.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li B, Zaveri T, Ziegler GR, Hayes JE. User Preferences in a Carrageenan-Based Vaginal Drug Delivery System. PLoS ONE. 2013;8(1):e54975. doi: 10.1371/journal.pone.0054975. doi:10.1371/journal.pone.0054975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owen DH, Katz DF. A vaginal fluid simulant. Contraception. 1999;59(2):91–5. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 39.Rothman L, Parrker M. Just about Right (Jar) Scales: Design, Usage, Benefits, and Risks. American Society for Testing & Materials; 2009. [Google Scholar]
- 40.DeMars LL, Ziegler GR. Texture and structure of gelatin/pectin-based gummy confections. Food Hydrocolloid. 2001;15(4-6):643–53. doi:Doi 10.1016/S0268-005x(01)00044-3. [Google Scholar]
- 41.Godley MJ. Quantitation of vaginal discharge in healthy volunteers. British journal of obstetrics and gynaecology. 1985;92(7):739–42. doi: 10.1111/j.1471-0528.1985.tb01457.x. [DOI] [PubMed] [Google Scholar]
- 42.Stone A, Gamble CJ. The quantity of vaginal fluid. American journal of obstetrics and gynecology. 1959;78(2):279–81. doi: 10.1016/0002-9378(59)90173-5. [DOI] [PubMed] [Google Scholar]
- 43.Lawless HT, Heymann H. Food science texts series. 2nd ed. Springer; New York: 2010. Sensory evaluation of food: principles and practices. [Google Scholar]
- 44.Morrow K, Fava JL, Rosen RK, Vargas S, Shaw JG, Kojic EM, et al. Designing preclinical perceptibility measures to evaluate topical vaginal gel formulations: relating user sensory perceptions and experiences to formulation properties. Aids Res Hum Retrov. 2014;30(1):78–91. doi: 10.1089/aid.2013.0099. doi:10.1089/AID.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Braunstein S, van de Wijgert J. Preferences and practices related to vaginal lubrication: implications for microbicide acceptability and clinical testing. Journal of Women's Health (Larchmt) 2005;14(5):424–33. doi: 10.1089/jwh.2005.14.424. doi:10.1089/jwh.2005.14.424. [DOI] [PubMed] [Google Scholar]
- 46.Rosen M, van den Berg JJ, Vargas S, Senocak N, Shaw JG, Buckheit RW, et al. Meaning-making matters in product design: Users' sensory perceptions and experience evaluations of long-acting vaginal gels and intravaginal rings. Contraception. 2015 doi: 10.1016/j.contraception.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim I-A, Van Hout D, Lee H-S. Development of A Consumer-Relevant Lexicon for Testing Kitchen Cleansers Considering Different Product Usage Stages. Journal of Sensory Studies. 2015 n/a-n/a. doi:10.1111/joss.12179. [Google Scholar]
- 48.Laitala K, Kjeldsberg M, Klepp IG. Troubles with the Solution: Fabric Softeners and Odour Properties. Tenside Surfactants Detergents. 2012;49(5):362–8. doi:10.3139/113.110203. [Google Scholar]
- 49.Koo HP, Woodsong C, Dalberth BT, Viswanathan M, Simons-Rudolph A. Context of Acceptability of Topical Microbicides: Sexual Relationships. The Journal of social issues. 2005;61(1):67–93. doi: 10.1111/j.0022-4537.2005.00394.x. doi:10.1111/j.0022-4537.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han L, Lv F, Xu P, Zhang G, Juniper NS, Wu Z. Microbicide Acceptability among Female Sex Workers in Beijing, China: Results from a Pilot Study. Journal of Women's Health. 2009;18(9):1377–84. doi: 10.1089/jwh.2008.1239. doi:10.1089/jwh.2008.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morrow Guthrie K, Vargas S, Shaw JG, Rosen RK, van den Berg JJ, Kiser PF, et al. The Promise of Intravaginal Rings for Prevention: User Perceptions of Biomechanical Properties and Implications for Prevention Product Development. PLoS ONE. 2015;10(12):e0145642. doi: 10.1371/journal.pone.0145642. doi:10.1371/journal.pone.0145642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaveri T, Powell KA, Li B, Ziegler GR, Hayes JE, editors. Improving acceptability of vaginal drug delivery systems by using sensory methods.. The Society of Sensory Professionals 3rd Technical and Professional Conference; Jersey City, New Jersey. 2012 Oct 10-12. [Google Scholar]