Summary
In the context of psoriasis, T helper type 17 (Th17) cells infiltrate the inflammatory site and interact with local mesenchymal cells, including skin fibroblasts. The aim of this work was to study the interactions of skin‐derived fibroblasts with peripheral blood mononuclear cells (PBMC) with a focus on the Th17 pathway and to identify a mechanism which leads to a high interleukin (IL)−17 secretion. A co‐culture system between PBMC and skin fibroblasts was developed. Healthy and patient PBMC were added to non‐lesional or lesional skin fibroblasts at a 5:1 ratio for 48 h in the presence or not of activation with phytohaemagglutinin (PHA). Monocytes were removed or not by adherence before the co‐culture. An anti‐podoplanin antibody was also used during the co‐culture. Cytokine production (IL‐8, IL‐6, IL‐1β and IL‐17) was measured by enzyme‐linked immunosorbent assay (ELISA) and cell staining (CD3, CD4, IL‐17 and podoplanin) by flow cytometry. Without T cell receptor (TCR) activation, IL‐8, IL‐6 and IL‐1β production increased in PBMC‐fibroblast co‐culture compared to PBMC alone. No additional effect was observed with TCR activation, with no difference in the Th17 cell percentage in activated‐PBMC alone or co‐cultured. Conversely, IL‐17 production was increased highly only in co‐cultures between control and patient activated‐PBMC and skin fibroblasts. Removal of monocytes decreased cytokine production, notably that of IL‐17. Addition of an anti‐podoplanin antibody decreased IL‐17 secretion by 60%. Interactions between resting PBMC and fibroblasts induce the IL‐8, IL‐6 and IL‐1β production. PBMC activation and cell interactions are critical for a high IL‐17 secretion. Podoplanin contributes largely to this massive IL‐17 secretion.
Keywords: cell interactions, IL‐17, immune cells, podoplanin, psoriasis, skin fibroblasts
Introduction
Psoriasis is a chronic inflammatory disease characterized by a hyperproliferation of keratinocytes and an infiltrate of immune cells 1, 2. Although the pathogenesis is not yet totally understood, recent studies highlighted the involvement of the T helper type 17 (Th17) pathway in disease development and presentation 3, 4. Interleukin (IL)‐17, the major proinflammatory cytokine produced by Th17 cells, plays a key role in chronic inflammation 5, 6, 7. In psoriasis, Th17 frequency is increased in peripheral blood and in psoriatic lesions and IL‐17 secretion is up‐regulated at inflammatory sites 8, 9, 10, 11. The major role of IL‐17 in psoriasis pathogenesis has been confirmed by the impressive clinical improvement seen in patients undergoing treatment with IL‐17 inhibitors 12, 13.
The recruitment of activated immune cells, including Th17 cells, to the disease site leads to interactions with local cells. This promotes the proliferation of mesenchymal cells such as fibroblasts in the skin or synoviocytes in the synovium, leading to the chronicity of inflammation 2, 14. In the context of psoriasis, interaction molecules are crucial for the disease development 15. In another chronic inflammatory context, rheumatoid arthritis (RA), cell interactions between synovium‐derived mesenchymal cells and activated peripheral blood mononuclear cells (PBMC) promote IL‐17 secretion 16.
Among the immune cells, monocytes could play a key role as they secrete cytokines (IL‐6 and IL‐1β) involved in Th17 differentiation 17, 18, 19 and are activated in psoriatic patients 20. Furthermore, monocytes can express an interaction molecule, podoplanin (pdpn). Although its physiological functions are still poorly documented, pdpn is known to play a crucial role in the development of the lymphatic system, in cell motility and in epithelial–mesenchymal transition 21, 22. This transmembrane protein is also expressed in hyperproliferative psoriatic epidermis 23, 24 and modulates IL‐8 secretion during platelet–synoviocyte interactions in RA 25. This suggests that pdpn could be involved in the high level of proinflammatory cytokine secretion, specifically of IL‐17, during cell interactions.
Here, the aim was to study the role and the mechanism of the interactions between psoriatic skin fibroblasts and immune cells on the Th17 pathway, reproducing the early step of cell contact in situ. For this we used an in‐vitro model with activated immune cells in contact with skin fibroblasts from psoriasis patients to study the effect of blocking of cell interactions seen in psoriasis skin.
Materials and methods
Samples
Skin fibroblasts were obtained from skin biopsies of psoriatic patients who fulfilled the Classification Criteria for Psoriasis or Psoriatic Arthritis. Punch biopsies of 4 or 5 mm were performed after local anaesthesia with xylocaine 1%. Biopsies of lesional skin defined by a clinical inflammatory aspect and non‐lesional skin by a clinical normal aspect and 2 cm away from an active plaque were obtained from the same patient. The biopsies were minced and adhered in six‐well plates in Dulbecco's modified Eagle's medium (DMEM; Eurobio, Courtaboeuf, France) supplemented with 10% fetal bovine serum (heat‐inactivated FBS; Life Technologies, Carlsbad, CA, USA), 2 mM L‐glutamine and 100 U/ml penicillin/streptomycin. Cells were maintained at 37°C in a humidified 5% CO2 incubator and used between passages 4 and 9. PBMC from healthy donors or from patients were isolated by Ficoll‐Hypaque (Eurobio) density‐gradient centrifugation. Each individual signed an informed consent form. The protocol was approved by the committee for the protection of people participating in biomedical research under the number AC‐2010‐11‐64.
Co‐culture assays
Co‐culture was initiated by seeding fibroblasts overnight in 96‐well plates at a density of 2·104 cells/well in RPMI‐1640 medium (Eurobio) supplemented with 10% human AB serum (Etablissement Français du Sang, La Plaine Saint‐Denis, France), 2 mM L‐glutamine and 100 U/ml penicillin/streptomycin (complete RPMI). The next day, PBMC (1·105 cells/well) were seeded in complete RPMI at a ratio of 5 : 1, in the presence or absence of phytohaemagglutinin (PHA, 5 μg/ml) or antibodies against CD3 and CD28 (anti‐CD3/28, 5 µg/ml). After 48 h, supernatants and PBMC were collected for analysis.
Transwell assay
Fibroblasts were seeded in 24‐well plates at a density of 1·105 cells/well in complete RPMI. After overnight incubation, PBMC were added directly on top of fibroblasts or in a cell culture insert (0·4 μm pore size) at a concentration of 5·105 cells/well in the presence or absence of PHA (5 μg/ml). After 48 h, supernatants and PBMC were recovered for analysis.
Enzyme‐linked immunosorbent assays (ELISA)
IL‐17A, IL‐8, IL‐6 and IL‐1β productions were evaluated from culture supernatants with commercially available Duoset ELISA kits (DY317, DY208, DY206 and DY201 catalogue numbers, respectively), according to the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA).
Flow cytometry
Allophycocyanin (APC), fluorescein isothiocyanate (FITC), phycoerythrin (PE), PE‐cyanin‐7 or eFluor 450‐conjugated antibodies (eBiosciences, San Diego, CA, USA) were used to stain cells. EFluor 450‐CD3 (48‐0038), PE‐Cy7‐CD4 (25‐0049) and PE‐pdpn (12‐9381) were used for surface staining, according to the manufacturer's instructions. PBMC were fixed and permeabilized for APC‐IL‐17A (17‐7179) intracellular staining. Cells were incubated in cold phosphate‐buffered saline (PBS) and 2% paraformaldehyde for 1 h in the dark for fixation step. Cells were permeabilized in PBS with 0.2% Tween at 37°C for 15 min before intracellular staining. Analysis was performed with kaluza software.
Monocyte contribution
One of the simplest and most common ways to isolate monocytes is by adherence 26, 27. To remove part of the monocytes, PBMC were preincubated 2 h at 37°C. This method allowed the removal at least 50% of the monocytes, as defined by CD14 staining and IL‐1β production.
Inhibition of pdpn
A dose–response curve was performed to determine the optimum concentration of anti‐human pdpn (14‐9381; eBiosciences, San Diego, CA, USA). PBMC were preincubated for 4 h with different concentrations (0, 1, 5, 10 and 20 μg/ml) before co‐culture assay. According to the highest inhibition of cytokine production (data not shown), all experiments studying pdpn were performed with 5 μg/ml. A monoclonal antibody directed against the BetV1 allergen and not involved in the cell interactions was used as control antibody at the same concentration (anti‐BetV1 Ab; Dendritics, Lyon, France).
Statistical analysis
Statistical analyses for co‐culture assays were performed using two‐way analysis of variance (anova). For Transwell, monocytes and pdpn experiments a paired non‐parametric Wilcoxon test was used. For pdpn staining, an unpaired Mann–Whitney test was used. All analyses were performed with Graph Pad Prism version 6 software. P‐values less than or equal to 0·05 were considered significant.
Results
Interactions between skin fibroblasts and PBMC induce IL‐8, IL‐6 and IL‐1β production
PBMC and skin fibroblasts produce proinflammatory chemokines and cytokines such as IL‐8, a mediator involved in the migration of neutrophils in psoriasis, and IL‐6 and IL‐1β, both involved in Th17 differentiation. In culture of psoriatic skin fibroblasts alone, either non‐lesional or lesional, the production of IL‐8, IL‐6 and IL‐β was low and PHA activation had no effect (Fig. 1a–c). In contrast, skin fibroblasts were fully responsive to IL‐17, as the production of IL‐8 and IL‐6 was increased with IL‐17 versus control (non‐lesional skin fibroblasts: 4·5 ± 1·4 versus 6·3 ± 2·2 ng/ml for IL‐6 and 27·0 ± 6·1 versus 48·3 ± 9·4 ng/ml for IL‐8; lesional skin fibroblasts: 5·2 ± 1·4 versus 12·9 ± 5·1 ng/ml for IL‐6 and 40·4 ± 9·4 versus 81·9 ± 24·8 ng/ml for IL‐8). Similar results for IL‐8 and IL‐6 were found in culture of PBMC alone, with or without PHA (Fig. 1a,b). In PBMC alone, IL‐1β production was detectable in the control condition (i.e. without PHA activation) (98·6 ± 96·3 pg/ml), but PHA activation highly increased its secretion (1422·8 ± 789·8 pg/ml, P = 0·03, Fig. 1c). IL‐8, IL‐6 and IL‐1β production was increased significantly in the control condition in PBMC‐non‐lesional skin fibroblast co‐culture versus PBMC alone (688·1 ± 390·9 ng/ml versus 64·3 ± 39·6 ng/ml, P ≤ 0·001; 216·1 ± 94·9 ng/ml versus 9·3 ± 7·8 ng/ml, P ≤ 0·002; and 1241·2 ± 1166·5 pg/ml versus 98·6 ± 96·3 pg/ml, P ≤ 0·05, respectively, Fig. 1a–c); and also in PBMC‐lesional skin fibroblast co‐culture (835·1 ± 395·4 ng/ml versus 64·3 ± 39·6 ng/ml, P ≤ 0·001; 311·2 ± 147·0 ng/ml versus 9·3 ± 7·8 ng/ml, P ≤ 0·002; and 1325·9 ± 1258·8 pg/ml versus 98·6 ± 96·3 pg/ml, P ≤ 0·05, respectively, Fig. 1a–c). Activation of PBMC by PHA had no additional effect in co‐culture (Fig. 1). These data indicated that cell–cell contact was sufficient to activate the proinflammatory cytokine production. Moreover, a tendency to higher production was observed in co‐cultures with lesional compared to co‐cultures with non‐lesional skin fibroblasts, mainly for IL‐6 (Fig. 1).
Figure 1.
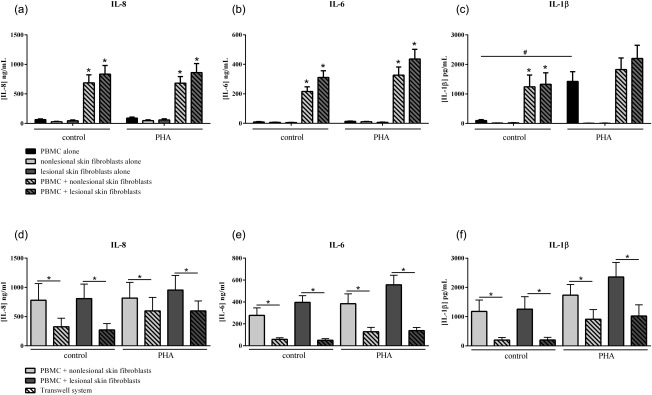
Effect of interactions between skin fibroblasts and peripheral blood mononuclear cells (PBMC) on interleukin (IL)−8, IL‐6 and IL‐1β production. Healthy PBMC and psoriatic skin fibroblasts were cultured alone or in co‐culture at a ratio of 5 : 1 for 48 h in the presence or absence of phytohaemagglutinin (PHA) (5 μg/ml). The Transwell system was used to inhibit cell–cell contact. The production of IL‐8 (a,d), IL‐6 (b,e) and IL‐1β (c,f) in cell supernatants was measured by enzyme‐linked immunosorbent assay (ELISA). *Compares PBMC alone and co‐cultures (a,b,c); #compares control and PHA (a,b,c); *compares co‐cultures and Transwell system (d,e,f); *#, P ≤ 0·05. Results are represented as mean ± standard error of the mean (s.e.m.), n = 15 experiments from five psoriatic patients (skin fibroblasts) and three healthy donors (PBMC) (a,b,c) and n = 9 experiments for Transwell experiments (d,e,f).
To investigate the importance of cell–cell contact, a Transwell system was used. The insert prevents direct cell interactions but allows the exchange of soluble factors. In this system, the production of IL‐8, IL‐6 and IL‐1β was decreased significantly compared to control (326·3 ± 334·4 ng/ml versus 780·0 ± 706·7 ng/ml for IL‐8, 58·8 ± 32·2 ng/ml versus 277·4 ± 136·9 ng/ml for IL‐6 and 199·4 ± 150·3 pg/ml versus 1177·8 ± 1011·3 pg/ml for IL‐1β, P ≤ 0·008 with non‐lesional skin fibroblasts; 272·4 ± 246·2 ng/ml versus 807·8 ± 485·9 ng/ml for IL‐8, 50·5 ± 30·0 ng/ml versus 396·1 ± 129·3 ng/ml for IL‐6 and 200·2 ± 171·4 pg/ml versus 1255·7 ± 1087·3 pg/ml for IL‐1β, P ≤ 0·008 with lesional skin fibroblasts, Fig. 1d–f). A significant decrease of IL‐8, IL‐6 and IL‐1β production with the Transwell was also observed with PHA (Fig. 1d–f). This confirmed that cell interactions were sufficient and required to activate the proinflammatory cytokine production.
PBMC activation and cell interactions are needed to have a high IL‐17 secretion
The production of IL‐17 has been associated with the Th17 cells 28. The role of cell interactions on the IL‐17 pathway was studied to distinguish intracellular staining from actual secreted IL‐17. The Th17 cells were gated in the CD3+CD4+ population. IL‐17+ cells were observed in culture of PBMC alone or in co‐cultures with non‐lesional or lesional skin fibroblasts. However, no difference in the percentage of IL‐17+ T cells was observed (Fig. 2a). The effect of PHA activation showed a tendency towards a higher Th17 cell percentage, but was not significant (1·7 ± 1·5% versus 2·4 ± 1·2% for PBMC alone; 2·4 ± 2·0% versus 3·7 ± 2·0% with non‐lesional skin fibroblasts and 2·1 ± 1·8% versus 3·7 ± 1·8% with lesional skin fibroblast, Fig. 2a).
Figure 2.
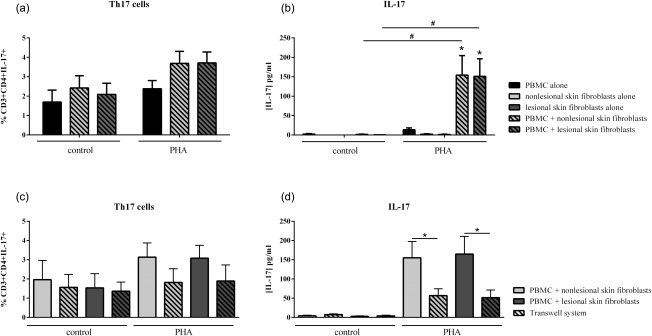
Effect of interactions between skin fibroblasts and peripheral blood mononuclear cells (PBMC) on T helper type 17 (Th17) differentiation and interleukin (IL)‐17 secretion. Healthy PBMC and psoriatic skin fibroblasts were cultured alone or in co‐culture at a ratio of 5 : 1 for 48 h in the presence or absence of phytohaemagglutinin (PHA) (5 μg/ml). The Transwell system was used in co‐cultures to inhibit cell–cell contact. PBMC were recovered after 48 h and stained with surface markers CD3 and CD4 and with intracellular IL‐17A. The percentage of CD3+CD4+IL‐17A+ is represented (a,c). IL‐17A production was measured in supernatants after 48 h by enzyme‐linked immunosorbent assay (ELISA) (b,d). *Compares PBMC alone and co‐cultures and # compares control and PHA (a,b). *Compares co‐cultures and Transwell system (c,d). *P ≤ 0·05. Error bars represent standard error of the mean (s.e.m.), n = 15 for (a,b) and n = 9 for (c,d).
Actual IL‐17 secretion in supernatants was measured by ELISA. In the control condition without PHA, IL‐17 production was almost undetectable in PBMC and co‐cultures (Fig. 2b). T cell receptor (TCR) activation by PHA did not increase IL‐17 secretion significantly in PBMC alone compared to the control condition (13·3 ± 14·1 pg/ml versus 2·2 ± 3·5 pg/ml, Fig. 2b). In contrast, there was a massive production of IL‐17 in co‐culture with activated PBMC (154·2 ± 151·9 pg/ml with non‐lesional skin fibroblasts, P = 0·0006 and 151·1 ± 134·0 pg/ml with lesional skin fibroblasts, P = 0·0007, Fig. 2b). PBMC activation by anti‐CD3/CD28 gave similar results to PHA (Fig. 3a). These results demonstrated that the combination of TCR activation and cell–cell contact was required to induce a high IL‐17 secretion. An important conclusion is that the results with fluorescence activated cell sorter (FACS) staining of intracellular IL‐17 do not reflect the amount of IL‐17 which is secreted, and thus functional.
To confirm the crucial role of cell interactions in IL‐17 production, the Transwell system was used as above. In the control condition, this contact inhibition had no effect on Th17 differentiation, as the percentage of IL‐17+ cells was similar between co‐culture and the Transwell system (Fig. 2c). Conversely, without cell interactions in the activation condition, IL‐17 secretion was reduced massively (155·0 ± 98·7 pg/ml versus 56·8 ± 44·6 pg/ml with non‐lesional skin fibroblasts, P = 0·008 and 164·7 ± 108·0 pg/ml versus 51·4 ± 42·0 pg/ml with lesional skin fibroblasts, P = 0·008, Fig. 2d). This Transwell experiment demonstrated clearly that physical interactions between activated PBMC and fibroblasts were crucial for high levels of IL‐17 secretion, as would be the case in psoriasis skin.
In addition, we confirmed that IL‐17 secretion could be induced with a specific TCR activation. Co‐cultures were realized in the presence of antibodies against CD3 and CD28 (anti‐CD3/28) in comparison with PHA. As observed in Fig. 3a, IL‐17 production increased only in co‐cultures with activation, and the level was similar to PHA or anti‐CD3/28 (non‐lesional skin fibroblasts: 169·2 ± 67·4 pg/ml with PHA and 215·5 ± 124·1 pg/ml with anti‐CD3/28; lesional skin fibroblasts: 203·0 ± 99·4 pg/ml with PHA and 240·7 ± 99·5 pg/ml with anti‐CD3/28, Fig. 3a).
Figure 3.
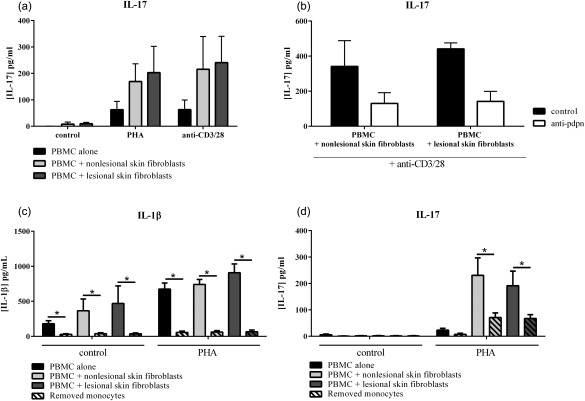
Role of monocytes in proinflammatory cytokine production. Healthy peripheral blood mononuclear cells (PBMC) were cultured alone or in co‐culture with psoriatic skin fibroblasts at a ratio of 5 : 1 for 48 h in the presence or absence of phytohaemagglutinin (PHA) (5 μg/ml) or anti‐CD3/28 (5 µg/ml). An antibody against podoplanin was added to the culture (b), and monocytes were removed or not by adherence before culture (c,d). The production of IL‐17 (a,b,d) and IL‐1β (c) was measured in supernatants after 48 h by enzyme‐linked immunosorbent assay (ELISA). *Compares conditions with and without monocytes. *P ≤ 0·05. Results are represented as mean ± standard error of the mean (s.e.m.).
Monocytes contribute to induce proinflammatory cytokine production, including IL‐17
With regard to the role of monocytes in the production of IL‐8, IL‐6 and IL‐1β, their potential contribution was investigated using our co‐culture system. As monocytes are a large source of IL‐1β in PBMC, the reduction of IL‐1β production reflected the effect of monocyte removal. However, a good response to PHA implies the presence of some monocytes, as obtained by adherence. As observed in Fig. 3c, removal of monocytes by adherence inhibited the production of IL‐1β significantly in all conditions (P = 0·004). Interestingly, IL‐17 production was also decreased significantly in co‐cultures between activated‐PBMC and skin fibroblasts without monocytes (P = 0·008, Fig. 3d). IL‐8 and IL‐6 are proinflammatory cytokines produced by many cell types, including monocytes and skin fibroblasts. In all conditions, IL‐8 secretion was decreased significantly (P ≤ 0·02, data not shown). Except in the control condition of PBMC alone, IL‐6 production was also decreased in conditions without monocytes (P = 0·004, data not shown). These results showed that monocytes have a major role in proinflammatory cytokine production, and notably in the massive IL‐17 secretion resulting from cell interactions between immune cells and mesenchymal cells in the persistence of inflammation.
Pdpn plays a major role in high IL‐17 secretion during co‐culture between activated‐PBMC and psoriatic skin fibroblasts
To identify a mechanism for the interactions, we focused our attention on membrane molecules and considered the possible role of pdpn. Pdpn can be expressed by different cell types: skin fibroblasts, synoviocytes and by immune cells such as monocytes and Th17 cells. Focusing upon pdpn expressed by monocytes and T cells, PBMC were preincubated with anti‐pdpn antibody. In co‐cultures with activated‐PBMC, the presence of anti‐pdpn antibody inhibited IL‐17 secretion significantly by 64·1 ± 18·0% with non‐lesional skin fibroblasts (245·6 ± 177·8 versus 103·3 ± 69·2 pg/ml, P = 0·002, Fig. 4a) and by 56·2 ± 20·8% with lesional skin fibroblasts (190·7 ± 136·3 versus 97·7 ± 73·4 pg/ml, P = 0·002, Fig. 4a). IL‐1β secretion was also inhibited (64·0 ± 22·3% of inhibition, 926·7 ± 215·7 versus 364·2 ± 263·6 pg/ml with non‐lesional skin fibroblasts, P = 0·002 and 53·8 ± 27·0% of inhibition, 991·7 ± 283·1 versus 462·1 ± 264·9 pg/ml with lesional skin fibroblasts, P = 0·002, Fig. 4b), but neither IL‐8 secretion (Fig. 4c) nor IL‐6 secretion (Fig. 4d). In the presence of TCR activation with anti‐CD3/28, the addition of anti‐pdpn antibody also inhibited IL‐17 secretion by 60% with non‐lesional skin fibroblasts (341·0 ± 104·2 versus 130·3 ± 43·2 pg/ml, Fig. 3b) and by 70% with lesional skin fibroblasts (441·1 ± 24·2 versus 141·6 ± 40·8 pg/ml, Fig. 3b). IL‐1β secretion was inhibited by around 60% in both co‐cultures while IL‐6 and IL‐8 secretion was not affected (data not shown). These results indicated that anti‐pdpn antibody had an inhibitory effect on IL‐17 and IL‐1β, independently of PBMC activation.
Figure 4.
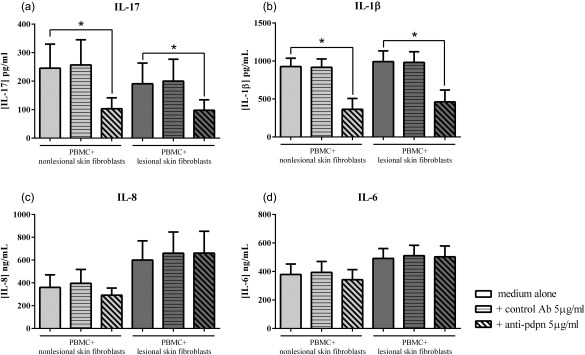
Major role of podoplanin (pdpn) in high interleukin (IL)‐17 secretion. Healthy peripheral blood mononuclear cells (PBMC) were preincubated for 4 h with or without human anti‐pdpn or irrelevant control antibody before co‐culture with psoriatic skin fibroblasts for 48 h in the presence of phytohaemagglutinin (PHA) (5 μg/ml). Levels of IL‐17 (a), IL‐1β (b), IL‐8 (c) and IL‐6 (d) were measured in the supernatants after 48 h by enzyme‐linked immunosorbent assay (ELISA). *P ≤ 0·05. Results are represented as mean ± standard error of the mean (s.e.m.), n = 7. The values for control antibody were similar to those with medium alone (245·6 ± 177·8 versus 256·8 ± 187·4 pg/ml for IL‐17, 926·7 ± 215·7 versus 917·4 ± 213·5 pg/ml for IL‐1β, 360·5 ± 170·4 versus 396·6 ± 187·4 ng/ml for IL‐8 and 378·7 ± 142·8 versus 393·9 ± 148·5 ng/ml for IL‐6, with non‐lesional skin fibroblasts; 190·7 ± 136·3 versus 200·2 ± 143·1 pg/ml for IL‐17, 991·7 ± 283·1 versus 981·8 ± 280·3 pg/ml for IL‐1β, 600·1 ± 283·8 versus 660·1 ± 312·2 ng/ml for IL‐8 and 491·5 ± 121·6 versus 511·2 ± 126·5 ng/ml for IL‐6, with lesional skin fibroblasts).
To study the regulation of its expression during cell interactions after 48 h in co‐culture, cells were recovered and stained for pdpn. PBMC alone showed a very low percentage of pdpn+ cells (4·3 ± 2·1% in control; 3·2 ± 4·1% with PHA, Fig. 5a,d). However, the percentage of pdpn+ cells increased in control co‐cultures, as psoriatic skin fibroblasts express this protein (24·0 ± 3·1% with non‐lesional skin fibroblasts; 18·3 ± 4·2% with lesional skin fibroblasts, Fig. 5b–d). Interestingly, PHA activation increased the number of pdpn+ cells in co‐cultures significantly compared to control (P = 0·03, Fig. 5b–d). Furthermore, the addition of anti‐pdpn did not affect the differentiation of Th17 cells (2.0 ± 0·4% versus 1·8 ± 0·3% with non‐lesional skin fibroblasts; 1·4 ± 0·4% versus 1·4 ± 0·3% with lesional skin fibroblasts, Fig. 5e). These results were consistent with the increase of IL‐17 secretion associated with over‐expression of pdpn on both skin fibroblasts and activated PBMC.
Figure 5.
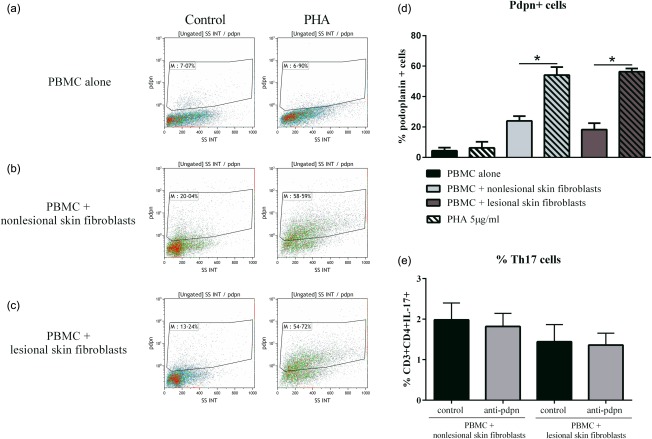
Expression of podoplanin (pdpn) in peripheral blood mononuclear cells (PBMC) alone or in co‐culture. Healthy PBMC were cultured alone or in co‐culture with psoriatic skin fibroblasts at a ratio of 5 : 1 for 48 h in the presence or absence of phytohaemagglutinin (PHA) (5 μg/ml). Cells were recovered after 48 h and stained with anti‐pdpn antibody. Dot‐plot of one experiment is represented (a,b,c). The percentage of pdpn+ cells in the different conditions is represented (d). Co‐cultures with anti‐pdpn antibody were performed and PBMC were recovered after 48 h and stained for surface markers CD3 and CD4, and with intracellular IL‐17A. The percentage of CD3+CD4+IL‐17A+ is represented (e). *P ≤ 0·05. Error bars represent standard error of the mean (s.e.m.) (d).
Use of an autologous system of co‐culture gives similar results and excludes the allogeneic reaction
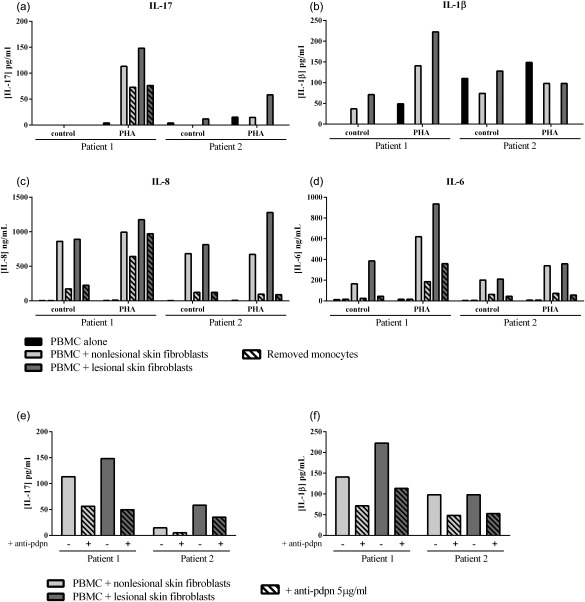
To exclude an allogeneic reaction, the experiments were realized by using PBMC and skin fibroblasts from the same patients. As observed in Fig. 6a–d, the results on cytokine production (IL‐17, IL‐1β, IL‐8 and IL‐6, Fig. 6a–d, respectively) in co‐cultures and with the removal of monocytes were similar to those with healthy PBMC. Furthermore, when the anti‐pdpn antibody was added in autologous co‐culture, the secretion of IL‐17 (Fig. 6e) and IL‐1β (Fig. 6f) was decreased while the production of IL‐8 and IL‐6 was unchanged (non‐lesional skin fibroblasts: 994·4 ± 103·7 versus 1039·0 ± 29·2 ng/ml for IL‐8 and 518·4 ± 30·3 versus 492·7 ± 41·6 ng/ml for IL‐6; lesional skin fibroblasts: 1131·2 ± 53·3 versus 1082·1 ± 27·4 ng/ml for IL‐8 and 835·3 ± 16·2 versus 798·7 ± 44·3 ng/ml for IL‐6, data not shown). The results using an autologous system were coherent with the results obtained with healthy PBMC and thus excluded the contribution of an allogeneic reaction.
Figure 6.
Exclusion of allogeneic reaction by using an autologous system. Peripheral blood mononuclear cells (PBMC) and psoriatic skin fibroblasts from the same patient (autologous system) were cultured alone or in co‐culture at a ratio of 5 : 1 for 48 h in the presence or absence of phytohaemagglutinin (PHA) (5 μg/ml) and monocytes were removed or not by adherence before culture (a,b,c,d). Autologous cells were co‐cultured in the presence of PHA at a 5 : 1 ratio for 48 h with or without anti‐pdpn antibody (e,f). Levels of IL‐17 (a,e), IL‐1β (b,f), IL‐8 (c) and IL‐6 (d) were measured in the supernatants after 48 h by enzyme‐linked immunosorbent assay (ELISA). Results are represented as mean ± standard error of the mean (s.e.m.); n = 2.
Discussion
Cell interactions between mesenchymal and immune cells are critical for the local production of proinflammatory cytokines, with an effect on cell survival 16, 17, 29, 30. In psoriasis, the role of interactions on proinflammatory cytokine secretion, with a main focus on IL‐17, was investigated to differentiate intracellular staining from secreted IL‐17 and to evaluate the mechanisms involved in these cell interactions.
A co‐culture system between skin fibroblasts and PBMC was used to reproduce the in‐vivo early step of cell–cell interaction between migrating immune cells and local mesenchymal cells and the use of an autologous cell system validated this model. Our findings demonstrated that cell interactions were sufficient to provide a necessary activation state for the secretion of IL‐8, IL‐6 and IL‐1β. These results were in agreement with a previous study showing that bone marrow mesenchymal cells in interaction with PBMC increased IL‐6 and IL‐1β mRNA 16. These observations reflect how high levels of proinflammatory cytokines, including IL‐8, IL‐6 and IL‐1β, can be found in psoriatic lesions 31. The migrating preactivated immune cells interact with resident cells as skin fibroblasts, and this leads to an increased production of proinflammatory cytokines. The variability of cytokine production between non‐lesional and lesional skin fibroblasts could depend upon the site of biopsy and the degree of inflammation. Fibroblasts in the psoriatic lesion and nearby may have a more severe inflammatory phenotype, and thus induce a higher proinflammatory cytokine secretion.
Among these cytokines, IL‐6 and IL‐1β are involved in Th17 differentiation. Th17 cells, in turn, secrete IL‐17, which acts on different targets, including skin fibroblasts 32, and this promotes skin inflammation. Considering the results on IL‐6 and IL‐1β, the effect of cell interactions on Th17 pathway was studied to differentiate intracellular from secreted IL‐17. The percentage of CD3+CD4+ IL‐17+ cells was evaluated in PBMC alone or in co‐culture. Cell contact alone had no major effect on Th17 differentiation. Only TCR activation had a modest effect. This indicated that early Th17 differentiation requires cell activation more than cell–cell contact.
When looking at IL‐17 production, and in contrast to IL‐8, IL‐6 and IL‐1β, cell interactions were not sufficient to induce high IL‐17 secretion in supernatants. This production required two signals, TCR activation and cell–cell contact. Moreover, activation of skin fibroblasts by TNF alone in co‐culture without TCR stimulation had no effect on IL‐17 production (data not shown). Thus, IL‐17 secretion during cell interactions was dependent upon both T cell activation and fibroblast contact. Transwell experiments confirmed that cell interactions, and thus the fibroblast contribution, were crucial to induce a massive IL‐17 secretion even in presence of TCR activation.
These results outline a key point which has not been taken into account previously: there is a major discrepancy between intracellular staining and secreted IL‐17. The intracellular presence of IL‐17 inside Th17 cells does not imply its high secretion. Activated cells have to interact physically with mesenchymal cells, derived from either skin, synovium, bone marrow. TCR activation and cell–cell contact are two necessary mechanisms leading to high IL‐17 production, and this differs from its intracellular expression. Both mechanisms are present during psoriasis pathogenesis 2, and this could explain not only the presence, but even more the secretion, of IL‐17 in psoriatic skin lesions 8, 9, 10, 11.
The critical involvement of cell interactions indicated the need to identify a molecular mechanism. The contribution of monocytes was first investigated, as they secrete the key cytokines involved in Th17 differentiation and are activated in patients with psoriasis 20. To study this involvement, monocytes were removed by adherence before co‐culture. The reduction of IL‐1β production confirmed that the removal was efficient. Interestingly, the production of IL‐8 and IL‐6 was also decreased significantly. Furthermore, this removal inhibited largely the IL‐17 production induced by the cell interactions between activated PBMC and fibroblasts. This suggested that monocytes are a major player in the process inducing proinflammatory cytokine production and specifically the massive IL‐17 secretion. In return, IL‐17 will increase the production of IL‐1β and other cytokines by monocytes.
The mechanism by which monocytes are involved in the interaction‐induced IL‐17 production remained to be identified. We selected pdpn as a candidate. Indeed, this interaction molecule is expressed by human monocytes 33 and RA synoviocytes 25. Furthermore, pdpn is expressed in hyperproliferative psoriatic epidermis 23, 24, but its contribution to disease remains to be clarified further. Based on these observations, PBMC were preincubated before co‐culture with the anti‐pdpn antibody to block the pdpn expressed by monocytes and T cells. Our results demonstrated that the inhibition of the pdpn‐mediated interaction decreased the secretion of IL‐17 and IL‐1β by at least 50%, but not of IL‐8 and IL‐6. Furthermore, pdpn expression was increased in co‐culture with TCR activation which correlates with the massive IL‐17 production. These results suggested that cell interactions of fibroblasts with activated‐immune cells increased pdpn expression that contributed to high IL‐17 secretion. Pdpn could be one mechanism by which monocytes and their activation contribute to IL‐17 secretion during cell interactions.
The mechanistic knowledge on cellular pdpn functions comes in part from cancer studies 21. Pdpn is involved in cell motility, and notably in dendritic cell (DC) migration. DCs express the pdpn receptor C‐type lectin‐like receptor 2 (CLEC‐2), and the interaction with pdpn expressed by stromal cells induces DC migration to lymph nodes 34. Furthermore, pdpn can bind CCL21, a chemotactic factor for mature DC and T cell migration 21. CCR7 ligands, including CCL21, can then stimulate the production of IL‐23 by DCs leading to the IL‐23‐dependent generation of pathogenic Th17 cells 35. Moreover, different animal models have indicated the contribution of the pdpn pathway to various inflammatory diseases 36, 37. In the human situation, pdpn expression is up‐regulated in RA synovium 38, and pdpn‐mediated interaction between RA synoviocytes and platelets modulates IL‐8 production 25. Furthermore, pdpn expressed by fibroblasts may contribute to tissue organization in germinal centres as well as during tumour formation 39. Clearly, more studies are needed, but the present results indicate the link between pdpn, IL‐17 and chronic inflammation.
In conclusion, this study provides an important concept by showing that cell interactions between psoriatic skin fibroblasts and blood‐derived immune cells play a major role in proinflammatory cytokine production, leading to a massive IL‐17 secretion distinct from, and not reflected by, intracellular expression. Monocytes and the interaction molecule pdpn contribute widely to this high IL‐17 secretion. In this context, pdpn could be a potential therapeutic target to block Th17 cell activity during chronic inflammation.
Author contributions
M. N. carried out the experiments and drafted the manuscript; she is supported by the Institut Universitaire de France. N'D. N.‐T. participated in the experiments and helped to revise the manuscript; she is supported by the IHU prometteur OPERA. P. M. conceived the study and helped to draft the manuscript. He is a senior member of and supported by the Institut Universitaire de France. All authors read and approved the final manuscript.
Disclosure
The authors have no conflicts of interest to declare.
Acknowledgements
The authors acknowledge Dr Ana‐Maria Moldovan, Hospital Edouard Herriot, for providing skin biopsies. These studies were supported in part by the IHU prometteur OPERA.
References
- 1. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007; 370:263–71. [DOI] [PubMed] [Google Scholar]
- 2. Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med 2009; 361:496–509. [DOI] [PubMed] [Google Scholar]
- 3. Di Cesare A, Di Meglio P, Nestle FO. The IL‐23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 2009; 129:1339–50. [DOI] [PubMed] [Google Scholar]
- 4. Martin DA, Towne JE, Kricorian G et al The emerging role of IL‐17 in the pathogenesis of psoriasis: preclinical and clinical findings. J Invest Dermatol 2013; 133:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maddur MS, Miossec P, Kaveri SV et al Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol 2012; 181:8–18. [DOI] [PubMed] [Google Scholar]
- 6. Miossec P, Kolls JK. Targeting IL‐17 and TH17 cells in chronic inflammation. Nat Rev Drug Discov 2012; 11:763–76. [DOI] [PubMed] [Google Scholar]
- 7. Miossec P, Korn T, Kuchroo VK. Interleukin‐17 and type 17 helper T cells. N Engl J Med 2009; 361:888–98. [DOI] [PubMed] [Google Scholar]
- 8. Benham H, Norris P, Goodall J et al Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res Ther 2013; 15:R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kagami S, Rizzo HL, Lee JJ et al Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 2010; 130:1373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kryczek I, Bruce AT, Gudjonsson JE et al Induction of IL‐17+ T cell trafficking and development by IFN‐gamma: mechanism and pathological relevance in psoriasis. J Immunol 2008; 181:4733–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lowes MA, Kikuchi T, Fuentes‐Duculan J et al Psoriasis vulgaris lesions contain discrete populations of Th1 and Th17 T cells. J Invest Dermatol 2008; 128:1207–11. [DOI] [PubMed] [Google Scholar]
- 12. Langley RG, Elewski BE, Lebwohl M et al Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014; 371:326–38. [DOI] [PubMed] [Google Scholar]
- 13. Mease PJ, Genovese MC, Greenwald MW et al Brodalumab, an anti‐IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med 2014; 370:2295–306. [DOI] [PubMed] [Google Scholar]
- 14. Firestein GS. Evolving concepts of rheumatoid arthritis. Nature 2003; 423:356–61. [DOI] [PubMed] [Google Scholar]
- 15. Conrad C, Boyman O, Tonel G et al Alpha1beta1 integrin is crucial for accumulation of epidermal T cells and the development of psoriasis. Nat Med 2007; 13:836–42. [DOI] [PubMed] [Google Scholar]
- 16. Eljaafari A, Tartelin ML, Aissaoui H et al Bone marrow‐derived and synovium‐derived mesenchymal cells promote Th17 cell expansion and activation through caspase 1 activation: contribution to the chronicity of rheumatoid arthritis. Arthritis Rheum 2012; 64:2147–57. [DOI] [PubMed] [Google Scholar]
- 17. Acosta‐Rodriguez EV, Napolitani G, Lanzavecchia A et al Interleukins 1beta and 6 but not transforming growth factor‐beta are essential for the differentiation of interleukin 17‐producing human T helper cells. Nat Immunol 2007; 8:942–9. [DOI] [PubMed] [Google Scholar]
- 18. Wilson NJ, Boniface K, Chan JR et al Development, cytokine profile and function of human interleukin 17‐producing helper T cells. Nat Immunol 2007; 8:950–7. [DOI] [PubMed] [Google Scholar]
- 19. Yang L, Anderson DE, Baecher‐Allan C et al IL‐21 and TGF‐beta are required for differentiation of human T(H)17 cells. Nature 2008; 454:350–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kouris A, Pistiki A, Katoulis A et al Proinflammatory cytokine responses in patients with psoriasis. Eur Cytokine Netw 2014; 25:63–8. [DOI] [PubMed] [Google Scholar]
- 21. Astarita JL, Acton SE, Turley SJ. Podoplanin: emerging functions in development, the immune system, and cancer. Front Immunol 2012; 3:283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin‐Villar E, Megias D, Castel S et al Podoplanin binds ERM proteins to activate RhoA and promote epithelial–mesenchymal transition. J Cell Sci 2006; 119:4541–53. [DOI] [PubMed] [Google Scholar]
- 23. Honma M, Fujii M, Iinuma S et al Podoplanin expression is inversely correlated with granular layer/filaggrin formation in psoriatic epidermis. J Dermatol 2013; 40:296–7. [DOI] [PubMed] [Google Scholar]
- 24. Honma M, Minami‐Hori M, Takahashi H et al Podoplanin expression in wound and hyperproliferative psoriatic epidermis: regulation by TGF‐beta and STAT‐3 activating cytokines, IFN‐gamma, IL‐6, and IL‐22. J Dermatol Sci 2012; 65:134–40. [DOI] [PubMed] [Google Scholar]
- 25. Rey D, Manuel J. Izquierdo et al Podoplanin‐mediated interaction of rheumatoid arthritis synovial fibroblasts with platelets modulates IL‐8 expression. Arthritis Rheum 2012; 64:1183. [Google Scholar]
- 26. Bennett WE, Cohn ZA. The isolation and selected properties of blood monocytes. J Exp Med 1966; 123:145–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson WD Jr, Mei B, Cohn ZA. The separation, long‐term cultivation, and maturation of the human monocyte. J Exp Med 1977; 146:1613–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T‐cell repertoire: the Th17 lineage. Curr Opin Immunol 2006; 18:349–56. [DOI] [PubMed] [Google Scholar]
- 29. Cho ML, Yoon CH, Hwang SY et al Effector function of type II collagen‐stimulated T cells from rheumatoid arthritis patients: cross‐talk between T cells and synovial fibroblasts. Arthritis Rheum 2004; 50:776–84. [DOI] [PubMed] [Google Scholar]
- 30. Miranda‐Carus ME, Balsa A, Benito‐Miguel M et al IL‐15 and the initiation of cell contact‐dependent synovial fibroblast‐T lymphocyte cross‐talk in rheumatoid arthritis: effect of methotrexate. J Immunol 2004; 173:1463–76. [DOI] [PubMed] [Google Scholar]
- 31. Baliwag J, Barnes DH, Johnston A. Cytokines in psoriasis. Cytokine 2015; 73:342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ha HL, Wang H, Pisitkun P et al IL‐17 drives psoriatic inflammation via distinct, target cell‐specific mechanisms. Proc Natl Acad Sci USA 2014; 111:E3422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hur J, Jang JH, Oh IY et al Human podoplanin‐positive monocytes and platelets enhance lymphangiogenesis through the activation of the podoplanin/CLEC‐2 axis. Mol Ther 2014; 22:1518–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Acton SE, Astarita JL, Malhotra D et al Podoplanin‐rich stromal networks induce dendritic cell motility via activation of the C‐type lectin receptor CLEC‐2. Immunity 2012; 37:276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kuwabara T, Ishikawa F, Yasuda T et al CCR7 ligands are required for development of experimental autoimmune encephalomyelitis through generating IL‐23‐dependent Th17 cells. J Immunol 2009; 183:2513–21. [DOI] [PubMed] [Google Scholar]
- 36. Miyamoto Y, Uga H, Tanaka S et al Podoplanin is an inflammatory protein upregulated in Th17 cells in SKG arthritic joints. Mol Immunol 2013; 54:199–207. [DOI] [PubMed] [Google Scholar]
- 37. Peters A, Pitcher LA, Sullivan JM et al Th17 cells induce ectopic lymphoid follicles in central nervous system tissue inflammation. Immunity 2011; 35:986–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ekwall AK, Eisler T, Anderberg C et al The tumour‐associated glycoprotein podoplanin is expressed in fibroblast‐like synoviocytes of the hyperplastic synovial lining layer in rheumatoid arthritis. Arthritis Res Ther 2011; 13:R40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoshino A, Ishii G, Ito T et al Podoplanin‐positive fibroblasts enhance lung adenocarcinoma tumor formation: podoplanin in fibroblast functions for tumor progression. Cancer Res 2011; 71:4769–79. [DOI] [PubMed] [Google Scholar]


