Summary
Malignant pleural effusion (MPE) is a poor prognostic sign for cancer patients, whereas the functional condition of MPE CD8+ T cells is unknown. Intracavitary immunotherapy with interleukin (IL)‐2 has been proven effective in controlling MPE. To elucidate the underlying mechanism, 35 lung cancer (LC) patients with MPE and 12 healthy donors were included in this study. For the IL‐2 therapy experiments, after draining partial MPE, we treated 14 patients by administrating IL‐2 (3 or 5 × 106 U in 50 ml saline) into the thoracic cavity. Before and after IL‐2 treatment (40‐48 h), the MPE and peripheral blood (PB) were obtained from the subjects. PB from healthy volunteers was collected as control. The expression of programmed cell death 1 (PD‐1), granzyme B (GzmB), interferon (IFN)‐γ and the proliferation were analysed in CD8+ T cells from MPE and PB. The CD8+ T cells in the MPE of LC patients showed lowest GzmB, IFN‐γ and proliferation but highest PD‐1 expression, compared with that in PB of LC patients and healthy donors. IL‐2 treatment reduced the expression of PD‐1, increased the expression of GzmB and IFN‐γ and enhanced the proliferation of CD8+ T cells in MPE. In addition, IL‐2 treatment reduced carcino‐embryonic antigen (CEA) level in MPE. These results indicate that MPE CD8+ T cells exhibit exhaustion phenotype which can be reversed by IL‐2 therapy.
Keywords: Granzyme B, interferon‐γ, IL‐2, malignant pleural effusion, programmed cell death‐1
Introduction
As one of the most malignant tumours with the fastest‐growing mortality, the incidence and fatality rates of lung carcinoma have increased in the past decades 1. One of the complications that occur as a result of advanced lung cancer (LC) is malignant pleural effusion (MPE). As well as causing shortness of breath or chest discomfort, MPE is also correlated with worse outcomes and a poor prognosis. The average life expectancy of MPE is as few as 6 months 2. Thus, controlling the development of MPE is essential for improving the prognosis of LC patients.
Recent studies have identified that cancer‐specific T cells are largely dysfunctional, exhibiting exhaustion phenotype, which contributes to tumour immune escape and metastasis 3, 4, 5. These exhausted CD8+ T cells manifest as a reduced secretion of cytokines, including interleukin (IL)‐2, granzyme B (GzmB) and interferon (IFN)‐γ, decreased cell viability and proliferation, as well as enhanced apoptosis 6, 7. In addition, elevated expression of programmed cell death 1 (PD‐1), an immune inhibitory receptor, is a signature for T cell exhaustion 8, 9. The activation of PD‐1 inhibits T lymphocyte activation and expansion and cytokine production 10, 11, 12, 13, while blocking PD‐1 breaks T cell immune tolerance to tumour cells and enhances anti‐tumour immunity 14, 15, 16, 17, 18. Akin to the tumour microenvironment, abundant immune cells, including CD8+ T cells, accumulate in the MPE 19. The exhausted phenotype of CD8+ T cells in the tumour microenvironment suggests the possibility that MPE CD8+ T cells might also be dysfunctional.
Traditional ways for controlling MPE include thoracentesis, intrapleural chemical, talc pleurodesis and indwelling pleural catheter 20, 21, 22, 23. Apart from these methods, immunotherapy has been broadly used to treat cancer by provoking the immune system 24, among which IL‐2 intrapleural administration in MPE is a representative method 25. IL‐2 therapy prolongs patients' survival 26, increases the numbers of CD3+ T cells and natural killer (NK) cells, enhances the immune response and reduces the incidence of MPE 27, 28, and the efficacy rate of controlling MPE was ∼59% with no significant side effects 29, 30, 31, 32, 33. In addition, a recent study suggested that IL‐2 administration enhanced CD8+ T cell responses and decreased inhibitory receptor levels, including PD‐1, on virus‐specific CD8+ T cells in chronic lymphocytic choriomeningitis virus (LCMV)‐infected mice 34. These findings suggest that intrapleural IL‐2 therapy may affect the functions of CD8+ T cells in MPE.
In this study, we obtained MPE and peripheral blood (PB) samples from LC patients and attempted to assess the CD8+ T cell phenotype in MPE prior to and after the well‐established IL‐2 intrapleural therapy compared with CD8+ T cells in PB of healthy controls. Our results indicate a novel notion that intrapleural IL‐2 therapy improves MPE by reversing CD8+ T cell exhaustion in MPE.
Materials and methods
Patients
We examined 35 patients with histologically diagnosed LC with MPE at the Xinqiao Hospital, the Third Military Medical University (Chongqing, China), between September 2013 and July 2016, and 12 healthy volunteers, as listed in the Supporting information, Tables S1 and S2. Patients who were confirmed with metastatic non‐small cell lung cancer (NSCLC) or small cell lung cancer (SCLC) with MPE and did not require recombinant human (rh)IL‐2 chemotherapy were included. The Eastern Cooperative Oncology Group Performance Status Scale (ECOG PS) was 0–2. The main exclusion criterion was the requirement for a high dose of immunosuppressive treatment (prednisone equivalent dose > 10 mg/day). Moreover, patients with fever showing evidence of ischaemic heart disease and significant arrhythmias were excluded. In the experiments examining PD‐1, GzmB and IFN‐γ in blood and MPE, patient characteristics are shown in the Supporting information, Table S1. This study meets the ethical principles and requirements of our committee (Ethics Committee, Xinqiao Hospital, Third Military Medical University) and complies with the Declaration of Helsinski. All participants in this study provided written informed consent.
Recombinant human (rh)IL‐2 treatment in the MPE of LC
The rhIL‐2 was dissolved in 50 ml saline solution and infused continuously into the pleural cavity after the MPE were drained. The rhIL‐2 dose depended upon patient status: if patients were in a poor state (poor vital signs or cachexia), 3 × 106 U were used, otherwise 5 × 106 U were used. The details are described in the Supporting information, Table S2. Before and after IL‐2 treatment (40–48 h), MPE and PB were obtained from the patients.
Flow cytometric analysis of PD‐1, GzmB and IFN‐γ on CD8+ T cell in the blood and MPE
Peripheral blood mononuclear cells (PBMC) from healthy volunteers and LC patients with MPE and mononuclear cells (MC) from MPE of LC patients were isolated by density gradient centrifugation using human PB lymphocyte separation medium (Ficoll; TBD, Tianjin, China). 1 × 106 cells were stained with antibodies including CD3‐fluorescein isothiocyanate (FITC), CD8‐allophycocyanin (APC), CD279‐phycoerythrin (PE) (PD‐1‐PE; BD Biosciences, San Jose, CA, USA) and analysis using the fluorescence activated cell sorter (FACS)Calibur (BD Biosciences).
For GzmB and IFN‐γ staining, cells (1 × 106) were activated with anti‐CD3 antibody (T&L Biotechnology, Beijing, China) and rhIL‐2 (Shuanglu Pharm, Beijing, China), treated with phorbol 12‐myristate 13‐acetate (PMA, 50 ng/ml; Sigma, St Louis, MO, USA), ionomycin (Ion, 1 μg/ml; Sigma) and GolgiPlug protein transport inhibitor (1 μl/ml; BD Biosciences) and were cultured subsequently for 4 h (37°C; 5% CO2). The cells were harvested and stained with CD3‐FITC and CD8‐peridinin chlorophyll (PerCP). After fixation and permeabilization using the Cytofix/CytopermPlus Fixation/Permeabilization Kit (BD Biosciences), intracellular staining was conducted using Alexa Fluor 647 mouse immunoglobulin (Ig)G1κ isotype control, Alexa Fluor 647 mouse anti‐human GzmB or IFN‐γ‐APC (human; BD Biosciences). GzmB and IFN‐γ expression was analysed with flow cytometry.
CD8+ T cell proliferation assay
The MC in the MPE and PBMC were stained with carboxy fluorescein succinimidyl ester (CFSE; Dojindo Laboratories, Shanghai, China); 1 × 107 PBMC/MC were incubated in phosphate‐buffered saline (PBS) with a final concentration of CFSE (2 μM) for 20 min at 37°C. After washing, the labelled cells (1 × 106 cells/well) were incubated in the presence of anti‐CD3 antibody and rhIL‐2 for 5 days. The cells were harvested and stained with CD3‐PE and CD8‐APC (BD Biosciences) and analysed by flow cytometry. The division percentage (D%), proliferation index (PI) and division index (DI) were used to quantify T cell division. D% = (the number of cell divisions)/(total cell numbers) × 100%. DI = the average number of divisions that a cell (present in the starting population) has undergone; PI = (sum of the cells in all generations)/(computed number of original parent cells theoretically present at the start of the experiment. PI is the proportional to the percentage of cells that have proliferated in culture. These indexes were calculated using FlowJo software (Tree Star, Ashland, OR, USA).
PBMC stimulation with rh‐IL‐2 in vitro
PBMC (3 × 106 cells/well) from LC patients were seeded in six‐well plates with or without 2000 U/ml of IL‐2 treatment for 48 h. The cells were then harvested for PD‐1, GzmB and IFN‐γ staining. For the groups staining with CFSE, the cells were cultured with anti‐CD3 antibody and rhIL‐2 for 72 h.
Measurement of carcinoembryonic antigen (CEA) in MPE of LC
The MPE supernatant of LC patients was collected for CEA examination by the Etiologies Department of Xiaoqiao Hospital, Third Military Medical University (Chongqing, China).
Statistical analysis
Reduction percentage of CEA and PD‐1 was calculated using the following equation: reduction (%) = 100 × (1–y/x)%, where y and x represent the levels of CEA or PD‐1 in MPE after and before IL‐2 treatment, respectively.
Statistical significance was calculated by paired and unpaired t‐tests, as indicated in the figure legends, with P < 0·05 considered statistically significant.
Results
Immune dysfunction of CD8+ T cells in MPE of LC
We first assessed the expression of GzmB, IFN‐γ and the proliferation of CD8+ T cells from three different groups that comprised PB from healthy volunteers and LC patients with MPE, as well as from MPE (Figs 1 and 2). In accordance with our recent study 17, the percentage of GzmB‐expressing CD8+ T cells in PB of LC patients was significantly higher than that in MPE (P < 0·01). Of note, the percentage of GzmB+CD8+ T cells in PB of LC patients was higher than that in PB of healthy controls, but there were no significant differences in GzmB between PB from LC patients and healthy controls (Fig. 1a). Consistently, after stimulation with phorbol 12‐myristate 13‐acetate (PMA) and ionomycin, MPE CD8+ T cells produced less IFN‐γ than PB CD8+ T cells from either healthy controls or LC patients (P < 0·05; Fig. 1b).
Figure 1.
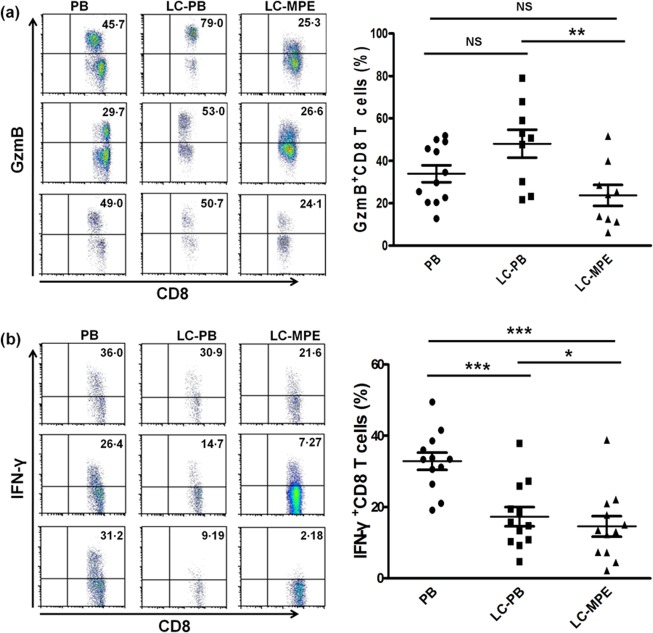
Immune function of CD8+ T cells in the MPE of LC is immunosuppressed. (a,b) Cytokine expression in CD3+CD8+ T cells in PB and MPE: GzmB (a) and IFN‐γ (b). Representative dot‐plots were gated on CD3+CD8+ T cells in flow cytometry analysis. *P < 0·05; **P < 0·01; ***P < 0·001.
Figure 2.
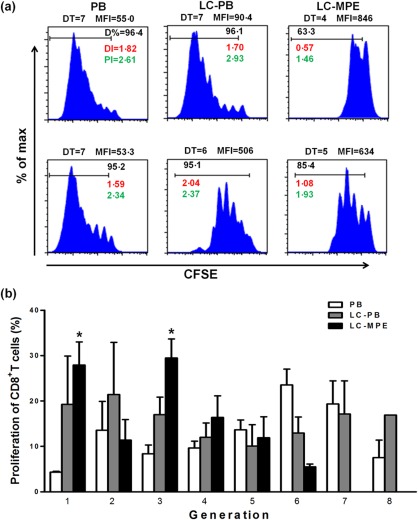
CD8+ T cells in the MPE of LC show the lowest proliferation capacity. (a) Proliferation of CD3+CD8+ T cells [stained with carboxy fluorescein succinimidyl ester (CFSE)] in PB of healthy control and LC patients, as well as in the MPE of LC patients. (b) The percentage in each generation with CFSE dilution. DT = division time; MFI = mean fluorescent intensity; DI% = division percentage; PI = proliferation index; DI = division index. The non‐paired t‐test, *P < 0·05, PB versus MPE.
We then investigated the proliferation of CD8+ T cells from MPE and PB. We found that MPE CD8+ T cells showed the lowest proliferation capacity, while CD8+ T cell proliferation in the PB of LC patients and healthy controls was significantly higher (Fig. 2a,b). These results illustrate that the function of CD8+ T cells in MPE is impaired.
CD8+ T cells express a high level of PD‐1 in MPE of LC
To validate further the functional condition of MPE CD8+ T cells, we evaluated the percentage of PD‐1‐expressing CD8+ T cells in PB of healthy subjects and LC patients, as well as in MPE (Fig. 3a,b). The fraction of PD‐1‐expressing CD8+ T cells was highest in MPE (P < 0·01) and lowest in PB of healthy donors (P < 0·05). PD‐1‐expressing CD8+ T cells in MPE were 1·58‐fold greater than those in the PB of patients (P < 0·01). The aforementioned results demonstrate that CD8+ T cells in the MPE of LC patients display exhaustion phenotype.
Figure 3.
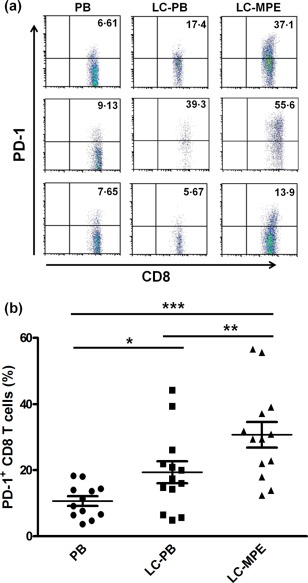
High PD‐1 expression of CD8+ T cells in the MPE of LC patients. (a) Frequencies of PD‐1‐expressing CD8+ T cells in 13 blood samples from LC patients with MPE, 12 blood samples for healthy controls and 13 MPE samples for LC, non‐paired t‐test. (b) Representative dot‐plots of PD‐1 staining gated on CD3+CD8+ T cells. *P < 0·05; **P < 0·01; ***P < 0·001.
IL‐2 reversed the exhaustion phenotype of CD8+ T cells
Intrapleural administration of IL‐2 was reported to be effective in alleviating tumour‐associated malignant pleurisy 35. We next wondered whether or not IL‐2 played a role in regulating T cell function. We collected 14 specimens and perfused IL‐2 into the thorax to treat the MPE. We found that the fraction of PD‐1‐expressing CD8+ T cells was reduced significantly after IL‐2 treatment (Fig. 4a). In addition, the production of GzmB and IFN‐γ by CD8+ T cells was increased markedly in the MPE (Fig. 4b,c). Furthermore, IL‐2 treatment enhanced the proliferation of MPE CD8+ T cells, as the mean fluorescence intensity (MFI) of CFSE was reduced significantly (Fig. 5a). To investigate further whether the same phenomena also occurred in LC‐PB, the function and proliferation of CD8+ T cells were determined after cells stimulated with or without rhIL‐2 for 48 h in vitro. Compared with vehicle, the percentage of PD‐1‐expressing in CD8+ T cells and the production of GzmB and IFN‐γ did not change significantly after IL‐2 treatment (Fig. 4). The IL‐2 treatment also enhanced the proliferation of LC‐PB CD8+ T cells (Fig. 5b). These results demonstrate that the magnitude change of CD8+ T cells in LC‐PB CD8+ T cells by IL‐2 treatment is not as significant as that in MPE.
Figure 4.
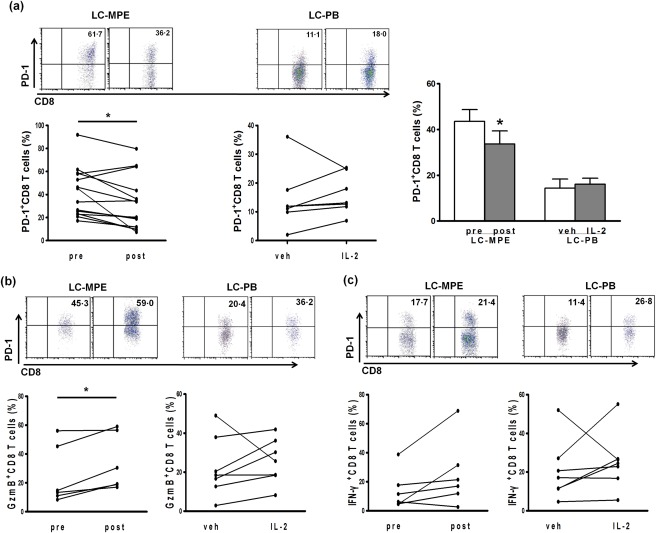
IL‐2 regulates PD‐1 expression and immune function in MPE and LC‐PB CD8+ T cells. (a) PD‐1 expression on the CD8+ T cell surface in 14 MPE samples of LC patients by IL‐2 treatment, and seven PB of LC patients in vitro. (b,c) The proportion of CD3+CD8+ T cells producing GzmB and IFN‐γ. *P < 0·05; **P < 0·01; ***P < 0·001.
Figure 5.
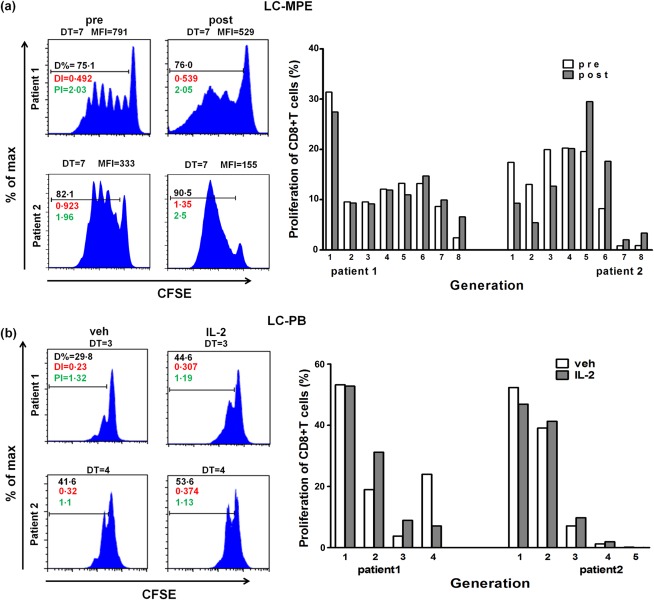
IL‐2 restores MPE CD8+ T cell proliferation. (a) CD8+ T cells in the MPE were stained with CFSE and then incubated for 5 days (37°C). (b) Proliferation of CD3+CD8+ T cells (stained with CFSE) in PB of LC patients, with IL‐2 treatment in vitro. The proliferation was examined with flow cytometry.
IL‐2 reduced CEA levels in MPE
To validate the correlation between IL‐2 therapy and clinical outcome we determined the CEA level, a key indicator for tumour malignancy, in MPE. Consistent with the results from PD‐1 expression, IL‐2 treatment reduced CEA level in the supernatant of the MPE (P < 0·01; Fig. 6). These results indicate that IL‐2 treatment reverses significantly the exhaustion of MPE CD8+ T cells.
Figure 6.
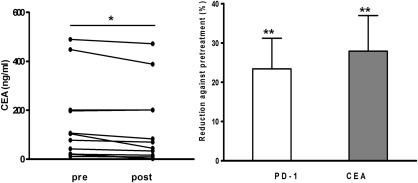
IL‐2 treatment reduces CEA in the MPE of LC. (a) CEA concentration in the MPE of LC, t‐test. (b) The percentage reduction of PD‐1 and CEA after IL‐2 treatment in the MPE of LC, t‐test. **P < 0·01; post‐treatment versus pretreatment.
Discussion
The general approach for the palliative management of symptomatic pleural effusions is chest tube drainage. Many agents, including IL‐2, have been administered into the pleural space and have been shown to be effective in controlling effusions 23. However, the underlying mechanism was unclear. In this study, we showed that the function of MPE CD8+ T cells was impaired with the exhaustion phenotype, which was reversed by IL‐2 treatment. These findings suggest that MPE T cells are in an exhausted condition, which could be reversed by intrapleural IL‐2 therapy.
T cell exhaustion leads to the dysfunction of CD8+ T cells in tumour progression. Exhausted T cells with high PD‐1 expression are defective in producing cytokines including IFN‐γ, tumour necrosis factor (TNF)‐α and IL‐2 and proliferate poorly 8. In this study, we found that the percentages of CD8+ T cells expressing GzmB and IFN‐γ were reduced, and the proliferation declined in the MPE compared with that in the PB of LC patients. Our results were in accord with the literature, which reported that PD‐1 expression on tumour‐infiltrating CD8+ T cells surface was higher than that in blood, and that the production of IL‐2, IFN‐γ and proliferation declined significantly 6. These results suggest that T cells in MPE are functionally exhausted, as in tumour tissues.
The down‐regulation of PD‐1 expression in CD8+ T cells and the restoration of biological functions of IL‐2 have been proved in the LCMV chronic viral infection mouse model 34. As we observed T cell exhaustion in MPE, we hypothesized that IL‐2 treatment may be an effective way to alleviate MPE by reverse MPE T cell exhaustion 25, 36. Indeed, we found that IL‐2 therapy reduced PD‐1 expression, reversed exhaustion, increased the production of GzmB, IFN‐γ and regained the immune function of CD8+ T cells in the MPE of LC patients. These results suggest that reverse T cell exhaustion, including anti‐PD‐1 and IL‐2 treatment, can be effective ways to manage MPE in LC patients 24, 37.
Accumulating evidence has indicated that down‐regulation of CEA is associated with a good prognosis in cancer patients after anti‐tumour therapy 38, 39. In this study, we found the CEA levels were reduced after IL‐2 therapy in MPE, implying that the malignancy of MPE was attenuated after IL‐2 therapy.
The side effects of IL‐2 treatment are correlated highly with dosage. A low dose of IL‐2 can cause vomiting, emesis and fever (∼ 38°C) for several hours. In contrast, high dose of IL‐2 treatment often lead to more severe side effects, such as fever, chills, sickness, emesis, fatigue, rash, hypotension, arrhythmia, myocardial ischaemia and abnormal hepatic and renal function. Fortunately, these adverse effects could disappear after IL‐2 withdrawal 40, 41. In our study, we observed that some patients had fever, vomiting, emesis and fatigue after IL‐2 treatment which recovered within several hours; no serious side effects were observed.
In summary, our study demonstrates for the first time that MPE T cells are phenotypically exhausted, which can be reversed by IL‐2 treatment. IL‐2 treatment also reduced CEA in the MPE. These findings suggest that targeting mechanisms controlling T cell exhaustion (including PD‐1) would be an effective treatment strategy for controlling MPE of LC patients.
Disclosure
The authors confirm that there are no disclosures.
Author contributions
Z. B. and L. Y. S. conceived and designed the experiments. H. C. Y., Z. Y. H., W. T. and C. L. performed the experiments. H. C. Y., W. Y. S., L. Q. J., L. Y. S. and Z. B. analysed the data. H. C. Y., Z. Y. H., W. T., C. L., G. Z. H., L. Y. S. and Z. B. contributed reagents, materials and analysis tools. H. C. Y., W. T., Z. Y. H. and L. Y. S. wrote the paper.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Patient characteristics in experiments of programmed cell death 1 (PD‐1), granzyme B (GzmB) and interferon (IFN)‐γ expression without interleukin (IL)‐2 therapy.
Table S2. Patient characteristics in interleukin (IL)‐2 therapy experiments.
Acknowledgements
We are grateful to L. J. Y., Z. Y. W. and S. J. H. for help with collecting and managing the patient samples. This work was supported by National Natural Science Foundation of China Grants (numbers 81222031 and 81472435), Outstanding Youth Scientist Foundation of Chongqing (numbers CSTC, 2008BA5035) and Youth 1000 Talents Program.
C. Y. H. and Y. H. Z. contributed equally to this work and share the first authorship.
Y. S. L. and B. Z. share senior authorship.
References
- 1. Torre LA, Bray F, Siegel RL et al Global cancer statistics, 2012. CA Cancer J Clin 2015; 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Ruckdeschel JC. Management of malignant pleural effusions. Semin Oncol 1995; 22:58–63. [PubMed] [Google Scholar]
- 3. Ahmadzadeh M, Johnson LA, Heemskerk B et al Tumor antigen‐specific CD8+ T cells infiltrating the tumor express high levels of PD‐1 and are functionally impaired. Blood 2009; 114:1537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prado‐Garcia H, Romero‐Garcia S, Aguilar‐Cazares D, Meneses‐Flores M, Lopez‐Gonzalez JS. Tumor‐induced CD8+ T‐cell dysfunction in lung cancer patients. Clin Dev Immunol 2012; 2012:741741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thommen DS, Schreiner J, Muller P et al Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res 2015; 3:1344–55. [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y, Huang S, Gong D, Qin Y, Shen Q. Programmed death‐1 upregulation is correlated with dysfunction of tumor‐infiltrating CD8+ T lymphocytes in human non‐small cell lung cancer. Cell Mol Immunol 2010; 7:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waki K, Yamada T, Yoshiyama K et al PD‐1 expression on peripheral blood T‐cell subsets correlates with prognosis in non‐small cell lung cancer. Cancer Sci 2014; 105:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12:492–9. [DOI] [PubMed] [Google Scholar]
- 9. Konishi J, Yamazaki K, Azuma M et al B7‐H1 expression on non‐small cell lung cancer cells and its relationship with tumor‐infiltrating lymphocytes and their PD‐1 expression. Clin Cancer Res 2004; 10:5094–100. [DOI] [PubMed] [Google Scholar]
- 10. Chen DS, Irving BA, Hodi FS. Molecular pathways: next‐generation immunotherapy – inhibiting programmed death‐ligand 1 and programmed death‐1. Clin Cancer Res 2012; 18:6580–7. [DOI] [PubMed] [Google Scholar]
- 11. Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the PD‐1/PD‐L1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev 2013; 12:1091–100. [DOI] [PubMed] [Google Scholar]
- 12. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death‐1 ligand 1 interacts specifically with the B7‐1 costimulatory molecule to inhibit T cell responses. Immunity 2007; 27:111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Akbay EA, Koyama S, Carretero J et al Activation of the PD‐1 pathway contributes to immune escape in EGFR‐driven lung tumors. Cancer Discov 2013; 3:1355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Topalian SL, Hodi FS, Brahmer JR et al Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012; 366:2443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brahmer JR, Drake CG, Wollner I et al Phase I study of single‐agent anti‐programmed death‐1 (MDX‐1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010; 28:3167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. John LB, Kershaw MH, Darcy PK. Blockade of PD‐1 immunosuppression boosts CAR T‐cell therapy. Oncoimmunology 2013; 2:e26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zarour HM. Reversing T‐cell dysfunction and exhaustion in cancer. Clin Cancer Res 2016; 22:1856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ohaegbulam KC, Assal A, Lazar‐Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD‐1 and PD‐L1 pathway. Trends Mol Med 2015; 21:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scherpereel A, Grigoriu BD, Noppen M et al Defect in recruiting effector memory CD8+ T‐cells in malignant pleural effusions compared to normal pleural fluid. BMC Cancer 2013; 13:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martin DE, Arnau OA, Martorell CM, Canto Armengod A. Thoracocentesis for the assessment of lung cancer with pleural effusion. Arch Bronconeumol 2002; 38:479–84. [DOI] [PubMed] [Google Scholar]
- 21. Shoji T, Tanaka F, Yanagihara K, Inui K, Wada H. Phase II study of repeated intrapleural chemotherapy using implantable access system for management of malignant pleural effusion. Chest 2002; 121:821–4. [DOI] [PubMed] [Google Scholar]
- 22. Dresler CM, Olak J, Herndon JE II et al Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest 2005; 127:909–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Massarelli E, Onn A, Marom EM et al Vandetanib and indwelling pleural catheter for non‐small‐cell lung cancer with recurrent malignant pleural effusion. Clin Lung Cancer 2014; 15:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zitvogel L, Kroemer G. Targeting PD‐1/PD‐L1 interactions for cancer immunotherapy. Oncoimmunology 2012; 1:1223–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Masotti A, Fumagalli L, Morandini GC. Intrapleural administration of recombinant interleukin‐2 in non‐small cell lung cancer with neoplastic pleural effusion. Monaldi Arch Chest Dis 1997; 52:225–8. [PubMed] [Google Scholar]
- 26. Ishida T, Kohdono S, Hamatake M et al Malignant pleurisy and intrathoracic dissemination in carcinoma of the lung: diagnostic, therapeutic and prognostic implications. Int Surg 1995; 80:70–4. [PubMed] [Google Scholar]
- 27. Bjermer L, Gronberg H, Roos G, Henriksson R. Interleukin‐2‐administration intravenously and intrapleurally in a patient with primary pulmonary adenocarcinoma. Cellular responses in peripheral blood, intrapleural fluid and bronchoalveolar lavage. Biotherapy 1993; 6:1–7. [DOI] [PubMed] [Google Scholar]
- 28. Viallat JR, Boutin C, Rey F et al Intrapleural immunotherapy with escalating doses of interleukin‐2 in metastatic pleural effusions. Cancer 1993; 71:4067–71. [DOI] [PubMed] [Google Scholar]
- 29. Lissoni P, Barni S, Ardizzoia A et al Intracavitary administration of interleukin‐2 as palliative therapy for neoplastic effusions. Tumori 1992; 78:118–20. [DOI] [PubMed] [Google Scholar]
- 30. Yasumoto K, Ogura T. Intrapleural application of recombinant interleukin‐2 in patients with malignant pleurisy due to lung cancer. A multi‐institutional cooperative study. Biotherapy 1991; 3:345–9. [DOI] [PubMed] [Google Scholar]
- 31. Yasumoto K, Nagashima A, Nakahashi H et al Effect of postoperative intrapleural instillations of interleukin‐2 in patients with malignant pleurisy due to lung cancer. Biotherapy 1993; 6:133–8. [DOI] [PubMed] [Google Scholar]
- 32. Astoul P, Viallat JR, Laurent JC, Brandely M, Boutin C. Intrapleural recombinant IL‐2 in passive immunotherapy for malignant pleural effusion. Chest 1993; 103:209–13. [DOI] [PubMed] [Google Scholar]
- 33. Astoul P, Bertault‐Peres P, Durand A et al Pharmacokinetics of intrapleural recombinant interleukin‐2 in immunotherapy for malignant pleural effusion. Cancer 1994; 73:308–13. [DOI] [PubMed] [Google Scholar]
- 34. West EE, Jin HT, Rasheed AU et al PD‐L1 blockade synergizes with IL‐2 therapy in reinvigorating exhausted T cells. J Clin Invest 2013; 123:2604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Walker‐Renard PB, Vaughan LM, Sahn SA. Chemical pleurodesis for malignant pleural effusions. Ann Intern Med 1994; 120:56–64. [DOI] [PubMed] [Google Scholar]
- 36. Chen YM, Ting CC, Peng JW et al Restoration of cytotoxic T lymphocyte function in malignant pleural effusion: interleukin‐15 vs. interleukin‐2. J Interferon Cytokine Res 2000; 20:31–9. [DOI] [PubMed] [Google Scholar]
- 37. Nagato T, Celis E. A novel combinatorial cancer immunotherapy: poly‐IC and blockade of the PD‐1/PD‐L1 pathway. Oncoimmunology 2014; 3:e28440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stremitzer S, Stift J, Graf A et al CEA change after neoadjuvant chemotherapy including bevacizumab and clinical outcome in patients undergoing liver resection for colorectal liver metastases. Ann Surg Oncol 2015; 22:1315–23. [DOI] [PubMed] [Google Scholar]
- 39. Kim CW, Yu CS, Yang SS et al Clinical significance of pre‐ to post‐chemoradiotherapy s‐CEA reduction ratio in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Ann Surg Oncol 2011; 18:3271–7. [DOI] [PubMed] [Google Scholar]
- 40. Klapper JA, Downey SG, Smith FO et al High‐dose interleukin‐2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer 2008; 113:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwartzentruber DJ, Lawson DH, Richards JM et al gp100 peptide vaccine and interleukin‐2 in patients with advanced melanoma. N Engl J Med 2011; 364:2119–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Patient characteristics in experiments of programmed cell death 1 (PD‐1), granzyme B (GzmB) and interferon (IFN)‐γ expression without interleukin (IL)‐2 therapy.
Table S2. Patient characteristics in interleukin (IL)‐2 therapy experiments.


