Summary
Thymosin alpha 1 (Tα1) is a powerful modulator of immunity and inflammation. Despite years of studies, there are a few reports evaluating serum Tα1 in health and disease. We studied a cohort of healthy individuals in comparison with patients affected by chronic inflammatory autoimmune diseases. Sera from 120 blood donors (healthy controls, HC), 120 patients with psoriatic arthritis (PsA), 40 with rheumatoid arthritis (RA) and 40 with systemic lupus erythematosus (SLE), attending the Transfusion Medicine or the Rheumatology Clinic at the Policlinico Tor Vergata, Rome, Italy, were tested for Tα1 content by means of a commercial enzyme‐linked immunosorbent assay (ELISA) kit. Data were analysed in relation to demographic and clinical characteristics of patients and controls. A gender difference was found in the HC group, where females had lower serum Tα1 levels than males (P < 0·0001). Patients had lower serum Tα1 levels than HC (P < 0·0001), the lowest were observed in PsA group (P < 0·0001 versus all the other groups). Among all patients, those who at the time of blood collection were taking disease‐modifying anti‐rheumatic drugs (DMARD) plus steroids had significantly higher Tα1 levels than those taking DMARD alone (P = 0·044) or no treatment (P < 0·0001), but not of those taking steroids alone (P = 0·280). However, whichever type of treatment was taken by the patients, serum Tα1 was still significantly lower than in HC and there was no treatment‐related difference in PsA group. Further prospective studies are necessary to confirm and deepen these observations. They might improve our understanding on the regulatory role of Tα1 in health and disease and increase our knowledge of the pathogenesis of chronic inflammatory autoimmune diseases.
Keywords: autoimmune diseases, psoriatic arthritis, rheumatoid arthritis, systemic lupus erythematosus, thymosin α1
Introduction
Thymosin alpha 1 (Tα1) is a naturally occurring thymic peptide of 28 amino acids described by Goldstein and Coll 1. It derives from the N‐terminus tract of Prothymosin α (ProTα) that is cleaved by legumain, a lysosomal asparagine endopeptidase also present in mammals 2. Both Tα1 and legumain share a wide distribution in different tissues, suggesting that the ProTα processing to yield Tα1 represents a generalized process in mammals 3. Lymphoid tissues show high legumain and Tα1 levels, which argue for an important biological function of the peptide in this context.
Tα1 plays a key role in the control of immunity, tolerance and inflammation 4, 5. It regulates immune response via a primary action on the cells of the innate immune system and thus acts as an endogenous regulator of both inflammatory and adaptive immune responses 5. Tα1‐induced effects are context‐dependent 5. Consistently, we showed that Tα1 administration increased natural killer (NK) activity in mice immunosuppressed by cancer and/or cyclophosphamide but not in normal mice 6.
Tα1 use in the therapy of diseases associated with immune dysfunction, namely hepatitis B virus (HBV) and hepatitis C virus (HCV), some types of cancer, severe sepsis and as an adjuvant for vaccine enhancement 7, relies upon its ability to target different cells. Recent studies by nuclear magnetic resonance (NMR) spectroscopy reinforce this assumption and provide a model mechanism of action for which Tα1 undergoes a direct interaction with peculiar portions of cell membranes and then behaves as an activator of biological cascade(s) 8.
Arthritis and other rheumatic diseases share severe immune imbalances and abnormal release of mediators, resulting in damage to organs and systems. Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by synovitis, leading to destruction of cartilage and bone, functional limitation and disability 9. Psoriatic arthritis (PsA) is a chronic inflammatory arthritis associated closely with psoriasis, which differs from RA in both laboratory and clinical features 10. Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by inflammation and tissue damage due to circulating autoantibodies and immune complexes depositing in different tissues 11. The aetiology of such diseases is still uncertain, although the role of genetic and epigenetic factors has been emphasized 12. The pathophysiology of RA, PsA and SLE implies an intricate cytokine network participating in inflammation and in perpetuation of disease by positive feedback loops including abnormal T cell signalling and unbalanced T helper type 17 (Th17)/regulatory T cells (Tregs) ratio (but may involve the overall cytokine milieu), thus promoting systemic disease 13, 14, 15.
Given the known regulatory activity of Tα1 on immunity and inflammation, we have analysed a large cohort of healthy individuals in comparison with patients affected by PsA, RA or SLE, looking for a possible correlation, if any, between serum Tα1 levels and such diseases.
Methods
Patients and study design
We carried out a retrospective analysis on sera from 120 patients presenting with PsA, diagnosed according to the Classification Criteria for Psoriatic Arthritis (CASPAR) 16, 40 with RA and 40 with SLE, diagnosed according to the American College of Rheumatology (ACR) revised criteria 17, 18. All of them were out‐patients at the Rheumatology Clinic, Policlinico Tor Vergata, Rome, Italy. Sera from 120 consecutive blood donors of the Transfusion Medicine and Immunohaematology Section (SIMT) at the same institution served as healthy controls (HC).
The study was performed according to the Declaration of Helsinki and in accordance with the International Conference on Harmonization Good Clinical Practice Guidelines [ICH‐GCP E6 (R2)]. The Ethical Committee of the University of Rome Tor Vergata approved the study protocol. All patients and controls provided written informed consent before participating in any study‐related activities.
Individual medical histories, laboratory and/or clinical data at the time of blood sampling were recorded in database files and were gathered anonymously for research purpose. The clinical evaluation of patients was performed by using the Disease Activity Score (DAS) 44 and C‐reactive protein levels (CRP, 0–3 mg/l) 19, or the SLE Disease Activity Index 2000 (SLEDAI‐2K) 20, where appropriate.
Laboratory assays
Peripheral blood collection for routine laboratory tests was performed in patients and controls at the time of medical examination or at blood donation, respectively. Serum aliquots, obtained from peripheral blood by standard methods, were frozen at −80°C immediately after collection and not thawed until use for the purposes of this research.
The quantitative determination of serum Tα1 was performed by a competitive enzyme‐linked immunosorbent assay (ELISA) using a commercial kit (Tα1 ELISA kit; Immundiagnostik AG, Bensheim, Germany), according to the manufacturers' instructions. Plates were read at an optical density of 450 nm on an ELISA reader (Model 550; Bio‐Rad Laboratories, Hercules, CA, USA).
Statistical analysis
Statistical analysis was performed using the statistical environment r (version 3.2.5) 21. The Kruskal–Wallis rank sum test and the Mann–Whitney U‐test were used for statistical comparisons among patient groups. All tests were two‐sided and a P‐value < 0·05 was considered statistically significant. All P‐values for multiple pairways comparisons were adjusted by using Benjamini and Hochberg correction 22.
Results
Characteristics of the study population
Table 1 summarizes demographic and clinical characteristics of the study subjects. Overall, our study population consisted of 320 Caucasian individuals (133 males and 187 females) aged 18–72 years.
Table 1.
Demographic and clinical characteristics of patients and healthy controls
| HC | RA | PsA | SLE | |
|---|---|---|---|---|
| Number | 120 | 40 | 120 | 40 |
| Gender female/male | 56/64 | 33/7 | 62/58 | 36/4 |
| Age (years) | 41 (18–62) | 57 (38–70) | 52 (19–70) | 55 (25–72) |
| Disease Activity Score/Index | ||||
| DAS44* | n.a. | 4·7 (2·7–7·3) | 4·9 (2·1–8·1) | n.a. |
| SLEDAI‐2K † | n.a. | n.a. | n.a. | 4 (0–82) |
| Treatments ‡ | ||||
| DMARD, n (%) | n.a. | 14 (35) | 40 (34) | 16 (40) |
| CS, n (%) | n.a. | 4 (10) | 8 (7) | 7 (17·5) |
| DMARD+CS, n (%) | n.a. | 19 (47·5) | 27 (23) | 16 (40) |
| None, n (%) | n.a. | 3 (7·5) | 45 (37·5) | 1 (2·5) |
Data expressed as median (range) if not specified otherwise. HC = healthy controls; RA = rheumatoid arthritis; PsA = psoriatic arthritis; SLE = systemic lupus erythematosus. *DAS44 (range = 0–10) is a continuous measure consisting of four variables: the Ritchie articular index (RAI), a 44 swollen joint count, erythrocyte sedimentation rate (ESR) and a general health (GH) assessment measured on a visual analogue scale. Level of disease activity: low (DAS ≤ 2·4), moderate (2·4 < DAS ≤ 3·7), high (DAS > 3·7), remission (DAS <1·6) 19. †SLEDAI‐2K = SLE Disease Activity Index 2000. SLEDAI‐2K ≥ 4 indicate active disease 20. ‡Concomitant treatments at the time of blood collection: DMARD = disease modifying anti‐rheumatic drugs (methotrexate, sulphasalazine, hydroxicloroquine, azathioprine, cyclosporin; CS = corticosteroids (prednisone); none = no treatment; n.a. = not applicable.
The group of patients consisted of 200 individuals (69 males and 131 females) aged 19–72 years, diagnosed clinically as RA, PsA or SLE according to the criteria specified in Methods. The HC group consisted of 120 consecutive blood donors (64 males and 56 females) aged 18–62 years. As shown in Table 1, the two major groups, namely HC and PsA, exhibited a similar gender prevalence, whereas the two smaller groups, i.e. RA and SLE, were composed mainly of female individuals, as expected based on the epidemiology of these diseases 23.
At the time of blood collection, the patients had active disease, as assessed by calculating DAS44 or SLEDAI‐2K scores (Table 1), and most of them were receiving treatment with disease‐modifying anti‐rheumatic drugs (DMARD) and/or corticosteroids (CS) (Table 1).
Laboratory findings
Serum Tα1 levels varied considerably among different individuals in the HC group as well as in the patient group, and even within the same diagnostic group.
In the HC group, females had significantly lower serum Tα1 levels than males [Tα1 ng/ml median (interquartile range, IQR) = 28 ·74 (17·98–70·25) versus 78·96 (40·80–130·13), respectively; P < 0·0001, Fig. 1a].
Figure 1.
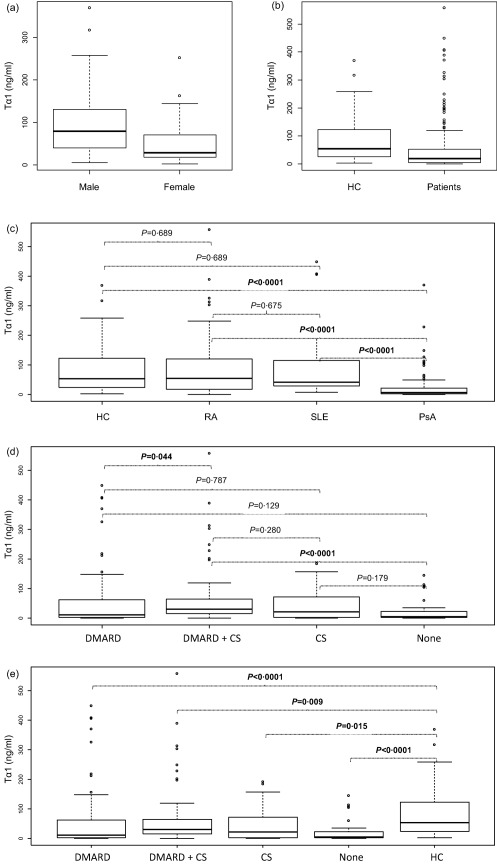
Data are shown as box‐plots, where each box represents the 25th–75th percentiles. Lines inside the box represent the median value. P‐values were calculated by Mann–Whitney U‐test (comparisons between two groups) or Kruskal–Wallis rank sum test (comparisons between more than two groups). All P‐values for multiple pairways comparisons were adjusted using Benjamini and Hochberg correction 22. Box‐plots represented serum thymosin alpha 1 (Tα1) levels according to gender in HC (a), to HC and patients (b), to the clinical diagnosis (c), to the treatment (d) and by comparing HC towards all treatment groups (e). HC = healthy controls; PsA = psoriatic arthritis; RA = rheumatoid arthritis; SLE = systemic lupus erythemathosus; DMARD = disease modifying anti‐rheumatic drugs; CS = corticosteroids; none = no treatment.
The patients' serum Tα1 levels were significantly lower than in HC [18·38 (3·74–52·82) versus 53·08 (24·39–122·74), respectively; P < 0·0001, Fig. 1b]. Analysing data according to the clinical diagnosis (Fig. 1c), serum Tα1 was globally significantly different among the four groups (P < 0·0001). In particular, serum Tα1 levels of PsA patients were dramatically lower than in HC [6·93 (2·05–21·27) versus 53·08 (24·39–122·74), respectively; P < 0·0001], but also significantly lower than in RA [6·93 (2·05–21·27) versus 54·73 (18·76–115·95), respectively; P < 0·0001] or SLE patients [6·93 (2·05–21·27) versus 41·37 (29·34–113·12), respectively; P < 0·0001]. Serum Tα1 levels of RA or SLE patients were not significantly different from those of HC, although showing a trend towards lower values, especially in SLE.
Among all patients (Fig. 1d), serum Tα1 was once again globally significantly different among the treatment groups (P < 0·0001). In particular, the patients who at the time of blood collection were taking DMARD plus CS, had higher Tα1 levels than those who were taking DMARD alone [29·87 (15·36–63·84) versus 10·87 (2·62–60·08), respectively; P = 0·044] or no treatment [29·87 (15·36–63·84) versus 4·56 (2·50–22·13), respectively; P < 0·0001], but not of those taking steroids alone (P = 0·280; Fig. 1d). However, serum Tα1 of patients taking any of the treatment types was still significantly lower than in HC (Fig. 1e), and there was no treatment‐related difference in the PsA group (data not shown).
Finally, no relevant abnormalities of blood cells count, haemoglobin, cholesterol and albumin were observed (Table 2). The median ESR and CRP were high in RA patients and within the normal range for SLE or PsA patients. No significant correlation was found between Tα1 levels and blood cell counts or blood chemistry profile, except than a trend in ESR (P = 0·073).
Table 2.
Laboratory characteristics of patients and healthy controls
| Tests | HC | RA | PsA | SLE |
|---|---|---|---|---|
| Hb (g/dl) | 14·8 (10·9–15·60) | 13·0 (7·6–15·7) | 13·8 (8·6–16·9) | 13·3 (9·4–15·3) |
| WBC × 1000/ml | 6·11 (3·46–9·98) | 7·45 (2·99–14·3) | 7·11 (0·75–16·70) | 5·30 (3·40–15·2) |
| Lymph × 1000/ml | 1·98 (1·09–4·40) | 1·97 (0·93–5·43) | 2·10 (1·07–3·89) | 1·39 (0·61–4·10) |
| Neu × 1000/ml | 3·31 (1·52–7·61) | 4·88 (1·27–9·56) | 3·99 (1·09–12·56) | 2·91 (1·03–11·0) |
| Plt × 1000/ml | 228 (139–235) | 266 (206 – 569) | 253 (138–471) | 223 (93–384) |
| Albumin (g/dl) | 4·60 (3·80–5·37) | 4·07 (3·27–4·69) | 4·29 (3·42–4·83) | 4·01 (3·83–4·72) |
| Cholesterol (mg/dl) | 184 (117–323) | 205 (179–266) | 206 (146–291) | 208 (175–287) |
| CRP (mg/l) | n.a. | 5·5 (0·0–58·3) | 2·62 (0·0–46·0) | 0·30 (0·0–19) |
| ESR (mm/h) | n.a. | 32 (2–120) | 16 (2–75) | 21·5 (2–84) |
Data expressed as median (range). HC = healthy controls; RA = rheumatoid arthritis; PsA = psoriatic arthritis; SLE = systemic lupus erythematosus; Hb = haemoglobin; WBC = white blood cells; Lymph = lymphocytes; Neu = neutrophils; Plt = platelets; CRP = C‐reactive protein, normal values (0–3 mg/l); ESR = erythrocyte sedimentation rate, normal values (0–30 mm/h); n.a. = not applicable.
Discussion
To the best of our knowledge, this is the first study where Tα1 serum levels of patients with chronic inflammatory autoimmune diseases have been analysed in relation to their demographic, clinical and laboratory characteristics and in comparison with a large cohort of healthy individuals.
The rationale of our study stems from the consideration of the potential role of malfunctioning Treg cells in chronic inflammatory immune and autoimmune diseases and from the notion that Tα1 is a natural circulating hormone peptide capable of influencing many components of the inflammatory/autoimmune cascade at a time 5. Indeed, although being capable of activating adaptive and innate immunity, including dendritic cells (DC), Tα1 can also attenuate the immunogenic/inflammatory activity of myeloid DCs through an indoleamine 2,3‐dioxygenase (IDO)‐dependent pathway 24. This finding qualifies Tα1 as a unique immune regulatory and pleiotropic peptide capable of the fine‐tuning and control of the quality of immune response. The hypothetical benefits of using thymic preparations in autoimmune diseases had not has already been proposed 25, but information on the circulating levels of thymic hormones in such patients was lacking.
Here we show, for the first time to our knowledge, that healthy female individuals have significantly lower Tα1 serum levels than the males. This evidence adds to other gender differences reported in the literature, i.e. a more intense anti‐viral response observed in females which induces rapid virus clearance, but if excessively high or prolonged can result in chronic and/or inflammatory diseases 26 or a lower production of the immunosuppressive cytokine IL‐10 after stimulation with selected Toll‐like receptor ligands or viruses 26, 27. These findings may contribute to explaining the high prevalence of autoimmune diseases in females 23.
We then show that patients affected by chronic inflammatory autoimmune diseases show a trend towards lower Tα1 serum levels than in HC. In particular, PsA patients present with the lowest Tα1 levels, which are dramatically lower than in HC but also significantly lower than in RA and SLE patients. This finding is not surprising, as there are several differences among chronic inflammatory autoimmune diseases, not only in terms of clinical symptoms but also regarding laboratory and immunological data. As an example, despite the superficial similarity of clinical manifestations, RA and PsA show many differences at clinical, anatomical and molecular levels, conditioning different clinical response and outcome 28.
The fact that the majority of our study subjects were aged between 20 and 60 years did not allow us to find the expected correlation of Tα1 levels with age 29. Similarly, the fact that our patients had active disease, and most of them were receiving treatment at the time of the blood collection, prevented us from finding significant relationships between Tα1 levels and disease activity scores or inflammation markers. Regarding the ESR and CRP, it is known that normal values of both can be found occasionally in active SLE or active PsA 16, 18.
In the total group of patients, the concomitant use of DMARDs and steroids had a positive impact on serum Tα1 levels. However, the values were always significantly lower than in HC and there were no treatment‐related differences in the PsA group. In agreement with the literature, our findings thus reinforce the assumption that chronic inflammatory autoimmune diseases constitute a heterogeneous group of diseases with regard to clinical manifestations, laboratory and immunological data and therapeutic response 28. Nevertheless, in the clinical practice they are subject to the same treatment options. Hopefully, these findings will be the object of future and larger studies, in line with recent interest in exploring the effects of various anti‐rheumatic drugs, including biologicals, on immune functions in various rheumatological conditions 14.
Finally, there was no relation between Tα1 levels and blood cell count, haemoglobin, cholesterol and albumin, suggesting that those values should not have influenced Tα1 measurements. We paid attention to albumin levels, as we have reported recently that Tα1 uses serum albumin as a carrier 30.
The retrospective character of this research, the lack of drug‐naive patients and the quantification of Tα1 in a single serum sample for each individual represent important limitations of our study. Given these limitations, the present research is a preliminary exploration of the relation between serum Tα1 and chronic inflammatory autoimmune diseases. Our findings indicate a need of more information on the complex interplay between different cell subsets in chronic inflammatory autoimmune diseases, and also in relation to the Tα1 circulating levels. Indeed, the biological effects of anti‐rheumatic drugs, their toxicity and the different therapeutic response, remain to be understood fully. Conversely, Tα1 has shown an excellent safety profile compared to other immunomodulatory agents 4, 7.
Several attempts to measure serum Tα1 have been performed in past years 29, 31, 32. Recent reports attest a renewed interest in this topic, especially in cancer, but they rely upon small numbers of subjects and the results are not univocal 33. Interestingly in our study, performed on a large cohort of individuals, the median serum Tα1 level found in HC is very close to the biologically active concentration of Tα1, as shown in past in‐vitro experiments 4, 5, 34.
Further prospective and longitudinal studies are necessary to confirm and extend our observations. They might improve our understanding of the regulatory role of Tα1 in health and disease and increase our knowledge on the pathogenesis of chronic inflammatory autoimmune diseases.
Disclosure
E. G. is a Thymosin patent holder. The other authors declare no disclosures.
Author contributions
E. G., F. P. and R. P. conceived the study and wrote the paper; R. G., I. C. and C. B. performed the experiments and discussed the results; A. V. and D. D. C. performed statistical analyses and reviewed the paper; M. S. C., P. T. and P. C. collected and analysed the clinical data and wrote the paper; G. A. and V. C. collected and analysed blood donor data.
Acknowledgements
This study was supported by a grant from MIUR to E. G. (Project grant: MIUR 10484/R). We are indebted to Dr M. T. Miele for his invaluable advice in linguistic revision of the manuscript.
References
- 1. Goldstein AL, Guha A, Zatz MM, Hardy MA, White A. Purification and biological activity of thymosin, a hormone of the thymus gland. Proc Natl Acad Sci USA 1972; 69:1800–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sarandeses CS, Covelo G, Díaz‐Jullien C, Freire M. Prothymosin alpha is processed to thymosin alpha 1 and thymosin alpha 11 by a lysosomal asparaginyl endopeptidase. J Biol Chem 2003; 278:13286–93. [DOI] [PubMed] [Google Scholar]
- 3. Franco FJ, Diaz C, Barcia M, Freire M. Thymosin alpha 1 is a native peptide in several tissues. Biochim Biophys Acta 1992; 1120:43–8. [DOI] [PubMed] [Google Scholar]
- 4. Garaci E, Favalli C, Pica F et al Thymosin alpha 1: from bench to bedside. Ann NY Acad Sci 2007; 1112:225–34. [DOI] [PubMed] [Google Scholar]
- 5. Romani L, Bistoni F, Montagnoli C et al Thymosin alpha1: an endogenous regulator of inflammation, immunity, and tolerance. Ann NY Acad Sci 2007; 1112:326–38. [DOI] [PubMed] [Google Scholar]
- 6. Garaci E, Mastino A, Pica F, Favalli C. Combination treatment using thymosin alpha 1 and interferon after cyclophosphamide is able to cure Lewis lung carcinoma in mice. Cancer Immunol Immunother 1990; 32:154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldstein AL, Goldstein AL. From lab to bedside: emerging clinical applications of thymosin alpha 1. Expert Opin Biol Ther 2009; 9:593–608. [DOI] [PubMed] [Google Scholar]
- 8. Nepravishta R, Mandaliti W, Eliseo T et al Thymosin α1 inserts N terminus into model membranes assuming a helical conformation. Expert Opin Biol Ther 2015; 15:S71–81. [DOI] [PubMed] [Google Scholar]
- 9. Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet 2001; 358:903–11. [DOI] [PubMed] [Google Scholar]
- 10. Chimenti MS, Ballanti E, Perricone C, Cipriani P, Giacomelli R, Perricone R. Immunomodulation in psoriatic arthritis: focus on cellular and molecular pathways. Autoimmun Rev 2013; 12:599–606. [DOI] [PubMed] [Google Scholar]
- 11. Rother N, van der Vlag J. Disturbed T cell signaling and altered Th17 and regulatory T cell subsets in the pathogenesis of systemic lupus erythematosus. Front Immunol 2015; 6:610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Renaudineau Y, Beauvillard D, Padelli M, Brooks WH, Youinou P. Epigenetic alterations and autoimmune disease. J Dev Orig Health Dis 2011; 2:258–64. [DOI] [PubMed] [Google Scholar]
- 13. Bowes J, Budu‐Aggrey A, Huffmeier U et al Dense genotyping of immune‐related susceptibility loci reveals new insights into the genetics of psoriatic arthritis. Nat Commun 2015; 6:6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Conigliaro P, Triggianese P, Perricone C et al Restoration of peripheral blood natural killer and B cell levels in patients affected by rheumatoid and psoriatic arthritis during Etanercept treatment. Clin Exp Immunol 2014; 177:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chimenti MS, Triggianese P, Conigliaro P, Candi E, Melino G, Perricone R. The interplay between inflammation and metabolism in rheumatoid arthritis. Cell Death Dis 2015; 6:e1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor W, Gladman D, Helliwell P et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum 2006; 54:2665–73. [DOI] [PubMed] [Google Scholar]
- 17. Arnett FC, Edworthy SM, Bloch DA et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31:315–24. [DOI] [PubMed] [Google Scholar]
- 18. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725. [DOI] [PubMed] [Google Scholar]
- 19. Van der Heijde DM, van't Hof M, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 1993; 20:579–81. [PubMed] [Google Scholar]
- 20. Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002; 29:288–91. [PubMed] [Google Scholar]
- 21. R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: 2016. ISBN 3‐900051‐07‐0, Available at: https://www.R-project.org/ [Google Scholar]
- 22. Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med 1990; 9:811–8. [DOI] [PubMed] [Google Scholar]
- 23. Quintero OL, Amador‐Patarroyo MJ, Montoya‐Ortiz G, Rojas‐Villarraga A, Anaya JM. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J Autoimmun 2012; 38:J109–19. [DOI] [PubMed] [Google Scholar]
- 24. Romani L, Bistoni F, Perruccio K et al Thymosin a1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood 2006; 108:2265–74. [DOI] [PubMed] [Google Scholar]
- 25. Lavastida MT, Goldstein AL, Daniels JC. Thymosin administration in autoimmune disorders. Thymus 1981; 2:287–95. [PubMed] [Google Scholar]
- 26. Torcia MG, Nencioni L, Clemente AM et al Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL 10 production in males. PLOS ONE 2012; 7:e39853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat Rev Immunol 2010; 10:594–604. [DOI] [PubMed] [Google Scholar]
- 28. Veale DJ, Fearon U. What makes psoriatic and rheumatoid arthritis so different? RMD Open 2015; 1:e000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McClure JE, Lameris N, Wara DW, Goldstein AL. Immunochemical studies on thymosin: radioimmunoassay of thymosin alpha 1. J Immunol 1982; 128:368–75. [PubMed] [Google Scholar]
- 30. Mandaliti W, Nepravishta R, Sinibaldi Vallebona P, Pica F, Garaci E, Paci M. Thymosin α1 interacts with exposed phosphatidylserine in membrane models and in cells and uses serum albumin as carrier. Biochemistry 2016; 55:1462–72. [DOI] [PubMed] [Google Scholar]
- 31. Sherman KE, Jones CC, Goldstein AL, Naylor PH. Low thymosin alpha‐1 concentrations in patients chronically infected with the hepatitis B virus. Viral Immunol 1991; 4:195–9. [DOI] [PubMed] [Google Scholar]
- 32. Molinero P, Soutto M, Benot S, Hmadcha A, Guerrero JM. Melatonin is responsible for the nocturnal increase observed in serum and thymus of thymosin alpha1 and thymulin concentrations: observations in rats and humans. J Neuroimmunol 2000; 103:180–8. [DOI] [PubMed] [Google Scholar]
- 33. Jou YC, Tsai YS, Hsieh HY et al Plasma thymosin‐α1 level as a potential biomarker in urothelial and renal cell carcinoma. Urol Oncol 2013; 31:1806–11. [DOI] [PubMed] [Google Scholar]
- 34. Serafino A, Pierimarchi P, Pica F et al Thymosin α1 as a stimulatory agent of innate cell‐mediated immune response. Ann NY Acad Sci 2012; 1270:13–20. [DOI] [PubMed] [Google Scholar]


