Summary
Adult‐onset Still's disease (AOSD) patients may show an evanescent salmon‐pink erythema appearing during febrile attacks and reducing without fever. Some patients may experience this eruption for many weeks. During AOSD, exceptionally high serum levels of ferritin may be observed; it is an iron storage protein composed of 24 subunits, heavy (H) subunits and light (L) subunits. The ferritin enriched in L subunits (L‐ferritin) and the ferritin enriched in H subunits (H‐ferritin) may be observed in different tissues. In this work, we aimed to investigate the skin expression of both H‐and L‐ferritin and the number of macrophages expressing these molecules from AOSD patients with persistent cutaneous lesions. We observed an increased expression of H‐ferritin in the skin, associated with an infiltrate in the biopsies obtained from persistent cutaneous lesions of AOSD patients. Furthermore, a positive correlation between H‐ferritin skin levels as well as the number of CD68+/H‐ferritin+ cells and the multi‐visceral involvement of the disease was observed. Our data showed an increased expression of H‐ferritin in the skin of AOSD patients, associated with a strong infiltrate of CD68+/H‐ferritin+ cells. Furthermore, a correlation between the levels of H‐ferritin as well as of the number of CD68+/H‐ferritin+ cells and the multi‐visceral involvement of the disease was observed.
Keywords: adult‐onset Still's disease, dermal immune system, ferritin, hyperferritinaemic syndrome, macrophage
Introduction
The skin is an organ that serves as an interface between the host and the environment, providing a mechanical and biological barrier against chemical, physical and pathogenic insults 1, 2. Anatomically, the skin comprises two distinct compartments: the epidermis, an avascular layer composed mainly of keratinocytes, melanocytes and the dermis, in which reside different immunocompetent cells, including dendritic cells (DCs) dermal macrophages and T cells 1, 2, 3, 4. During physiological conditions, macrophages are the most abundant haematopoietic population in the skin 5, 6, serving primarily as a first line of defence against potentially invading pathogens 7, 8, 9. Perivascular macrophages may also play a role in regulating local iron homeostasis 3, 4. These cells, in skin as well as in other organs, may display different functional phenotypes, and are divided into two subsets: M1 (classically activated) and M2 (alternatively activated) macrophages 10. M1 macrophages are activated by inflammatory stimuli and express higher levels of proinflammatory cytokines, such as tumour necrosis factor (TNF), interleukin (IL)‐1β and IL‐12. Conversely, M2 macrophages are induced by IL‐4 and IL‐13 and express higher levels of IL‐4 and IL‐10, thus promoting wound‐healing and immune modulatory activity 11.
Adult‐onset Still's disease (AOSD) is an inflammatory disease characterized by high spiking fevers, arthritis and severe multi‐visceral involvement, which needs to be treated by intensive immunosuppressive therapies 12, 13, 14, 15. The pathogenic mechanism for this condition is still largely unknown 12. A predisposing genetic background has also been suggested, although no consistent associations with human leucocyte antigen (HLA) haplotypes or other non‐HLA genes has been confirmed 12, 13, 14. An infectious trigger has been considered, due to the temporal relationship between the disease onset and different viral, bacterial and other pathogenic organism infections, suggesting the main role played by the innate immunity in AOSD. In fact, proinflammatory cytokines have been shown to be involved in the pathogenesis of the disease, such as IL‐1β, IL‐6, IL‐18, interferon (IFN)‐γ and TNF 12, 13, 14, 15. Classically, the disease is characterized by fever, the highest temperatures usually occurring in the evening. Fever may precede the onset of other manifestations, such as arthralgia and arthritis, which involve the wrists, knees and ankles 12, 13. A large percentage, up to 87%, of AOSD patients may show an evanescent salmon‐pink erythema that may be associated with an erythematous maculopapular eruption, found predominantly on the proximal limbs and trunk, appearing during febrile attacks and reducing without fever 16. Some patients may experience this eruption for many weeks, and it has been suggested that this type of persistent rash may be associated with a more severe outcome 16, 17. Although this eruption is well accepted as a major diagnostic criterion, the skin lesions are often misdiagnosed 16, 17.
Recently, it has been proposed that AOSD and other diseases, such as macrophage activation syndrome, catastrophic anti‐phospholipid syndrome and septic shock, might be included in a common entity called ‘hyperferritinaemic syndrome’ 18. Generally, serum ferritin levels are considered a non‐specific marker of the acute phase response, which is often ignored or not evaluated. Ferritin is an iron storage protein composed by 24 subunits, heavy (H) subunits and light (L) subunits 19, 20. Usually, the ferritin enriched in L subunits (L‐ferritin) has been found in the liver and in the spleen, whereas the ferritin enriched in H subunits (H‐ferritin) may be observed in the heart and kidneys 19, 20. Although the main modulator of this molecules is the iron availability, ferritin synthesis may also be regulated by different inflammatory cytokines such as IL‐1β and IL‐6 [20,21]. Increasing evidence suggests that circulating ferritin levels may not only reflect an acute phase response but play a critical role in inflammation, binding specific receptors on the surface of immune‐competent cells, thus contributing to the abnormal production of inflammatory cytokines, leading to the subsequent ‘cytokine storm’ 18, 19, 20, 21.
In this work, we investigated the tissue expression of both H‐ and L‐ferritin and the number of macrophages expressing these molecules in the inflammatory cutaneous infiltrate from AOSD patients. In addition, we performed an analysis to evaluate whether these results might correlate with the multi‐visceral involvement of the disease.
Patients and methods
During the last 10 years we obtained skin samples from 10 consecutive patients with active AOSD with persistent (more than 24–36 h) cutaneous lesions, referred to the Rheumatology Clinic of L'Aquila University and to the Rheumatology Clinic of Palermo University. In addition, we obtained peripheral blood mononuclear cells (PBMCs) in six of the enrolled patients. These blood samples were collected at the same time of the skin biopsies during AOSD flare. All these patients fulfilled the criteria proposed by Yamaguchi for AOSD 22. Serum levels of ferritin, erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP) and Pouchot's score evaluating the multi‐visceral involvement of the disease 23 were recorded. The Pouchot's score ranges from 0 to 12 and is calculated by assigning and adding 1 point for each of the following manifestations occurring during a disease flare: fever, evanescent rash, pleuritis, pneumonia, pericarditis, hepatomegaly or abnormal liver function tests, splenomegaly, lymphadenopathy, white blood cells > 15 000/mm3, sore throat, myalgias and abdominal pain. Ten skin biopsies, obtained during aesthetical surgical interventions for melanocytic lesions, were considered as healthy controls (HCs). The demographic characteristics of enrolled patients are reported in Table 1.
Table 1.
Clinical data of enrolled adult‐onset Still's disease (AOSD) patients.
| Women (men) | 8 (2) |
| Disease duration, median (range) months | 12 (6; 18) |
| Erythrocyte sedimentation rate median (range) mm/h | 85 (43; 103) |
| C‐reactive protein median (range) mg/dL | 108.5 (20; 300) |
| Serum ferritin median (range) ng/ml | 1218 (564; 3343) |
| Pouchot's score median (range) | 6 (4; 8) |
The local ethics committee approved this study. It was performed according to the Good Clinical Practice guidelines, and written informed consent was obtained from all patients according to the Declaration of Helsinki.
Histological analysis of biopsies
Sequential sections (thickness 3 µm) were obtained from skin biopsies and fixed by formaldehyde and embedded by paraffin. For conventional smear preparations, conventional glass smear slides were fixed with 95% ethanol for at least 15 min and then treated with water for 1 min, haematoxylin for 1 min, running water for 15 min, eosin for 30 s, 95% ethanol for 10 min and 100% ethanol for 10 min. Stained slides were coverslipped with Permount. Haematoxylin and eosin images were acquired using an Olympus BX53 fluorescence microscope with CellSens software (Olympus America Inc., Center Valley, PA, USA).
For immunofluorescence, antigen retrieval was carried out using target retrieval solution (Dako, Glostrup, Denmark). Sections were treated with Dako protein block (Dako) to block non‐specific binding and successively with anti‐H‐ferritin, anti‐L‐ferritin, anti‐CD11, anti‐CD68, anti‐CD163, anti‐IL‐1β, anti‐IL‐12, anti‐IFN‐γ and anti‐TNF antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The immunoreaction was revealed by using a secondary fluorescence antibody (Alexa Fluor 488‐conjugated and Alexa Fluor 555‐conjugated; Invitrogen, Carlsbad, CA, USA), and negative controls were obtained by omitting the primary antibody. Cell nuclei were visualized using 4′,6‐diamidino‐2‐phenylindole (DAPI). Sections were examined and photographed under a light microscope (Olympus BX53). Immunofluorescence images were acquired using an Olympus BX53 fluorescence microscope with CellSens software (Olympus America Inc.). The number of CD68/H‐ferritin‐ and CD68/L‐ferritin positive cells was determined as follows: 10 random high‐power microscopic fields for each area (10 000 μm2), showing an immune‐inflammatory infiltrate, were selected and the numbers of CD68/H‐ferritin and CD68/L‐ferritin immunoreactive cells were counted by using NIHimageJ 1.43 (http://rsbweb.nih.gov/ij/) freeware. The mean values of positive cells in each area were added and divided for the number of areas in the section.
PBMC isolation
PBMC preparations were used in these studies. The heparinized blood was layered carefully onto Ficoll (density 1·077 g/ml; Fresenius Kabi Norge AS for Axis‐Shield PoC AS, Oslo, Norway) and centrifuged at 800 g for 30 min without brake to obtain a density gradient separation. After centrifugation, the mononuclear cell layer was recovered and washed twice with phosphate‐buffered saline (PBS; Sigma, St Louis, MO, USA).
Quantitative reverse transcription–polymerase chain reaction (qRT–PCR) analysis
Total RNA was extracted from PBMCs and reverse‐transcribed into complementary DNA (cDNA) with the ThermoScript RT–PCR system (Invitrogen). The RT–PCR was run in triplicate. FTH and FTL gene expression was performed by using SYBR green kits (Applied Biosystems, Bleiswijk, the Netherlands). Primers were designed on the basis of the reported sequences [Primer bank NCBI; β‐actin: 5′‐CCTGGCACCCAGCACAAT‐3′ (forward) and 5′‐AGTACTCCGTGTGGATCGGC‐3′ (reverse); H‐ferritin: 5′‐TCCTACGTTTACCTGTCCATGT‐3′ (forward) and 5′‐GTTTGTGCAGTTCCAGTAGTGA‐3′ (reverse); L‐ferritin: 5′‐CAGCCTGGTCAATTTGTACCT‐3′ (forward) and 5′‐GCCAATTCGCGGAAGAAGTG‐3′ (reverse). Results were analysed after 45 cycles of amplification using the ABI 7500 Fast Real Time PCR System.
Statistical analysis
GraphPad Prism version 5.0 software was used for statistical analyses. Due to the non‐parametric distribution of our data, the Mann–Whitney U‐test was used as appropriate for analyses. Spearman's correlation analysis and linear regression were performed to evaluate the possible correlation among the tissue expression of both H‐ and L‐ferritin and the number of double‐positive cells for CD68 and H‐ferritin (CD68+/H‐ferritin+ or double‐positive cells for CD68 and L‐ferritin (CD68+/L‐ferritin+) and clinical and laboratory data. Spearman's correlation analysis and linear regression were also performed to evaluate the possible correlation among the tissue expression of H‐ferritin and the proinflammatory cytokines, IL‐1β, TNF and IFN‐γ. Statistical significance was expressed by a P‐value < 0·05.
Results
Increased expression of H‐ferritin in AOSD skin
Immunofluorescence analysis showed increased H‐ferritin expression in the skin samples of AOSD patients when compared with HCs (Fig. 1). Conversely, no differences were observed for L‐ferritin when compared to HCs. Furthermore, a significant increase of H‐ferritin was detected when compared with L‐ferritin in AOSD patients (P < 0·001) and when compared with both H‐ferritin and L‐ferritin in HCs (P < 0·001; P < 0·001). The statistical analysis between the tissue expression of H‐ferritin and Pouchot's score of the same patients showed a positive correlation between H‐ferritin and the multi‐visceral involvement of the disease (P < 0018301). In addition, we reported a positive correlation among the tissue expression of H‐ferritin and both serum ferritin (P < 0·05) and CRP (P < 0·05), respectively. Figure 2 shows the results.
Figure 1.
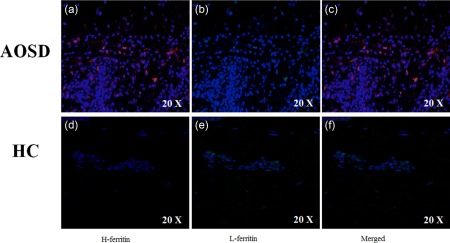
Expression of H‐ferritin in skin samples of adult‐onset Still's disease (AOSD) patients. Immunofluorescence analyses in skin samples of AOSD patients show: (a) increased expression of H‐ferritin in the tissue; (b) on the contrary, no expression of L‐ferritin may be observed; (c) among the infiltrating macrophages, only one cell displays both the molecules. Immunofluorescence analyses in skin samples of healthy controls (HCs) show: no expression of H‐ferritin (d) and of L‐ferritin (e). (f) No co‐localization between H‐ferritin and L‐ferritin.
Figure 2.
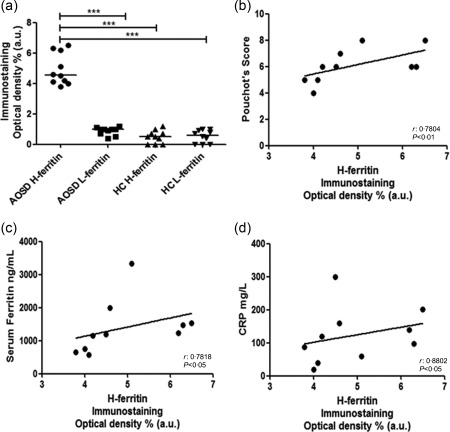
Expression of H‐ferritin in skin and correlation with of multi‐visceral involvement of adult‐onset Still's disease (AOSD) patients. (a) H‐ferritin immunostaining optical density shows a significant increase of H‐ferritin tissue expression levels when compared with the levels of L‐ferritin and healthy controls (HCs) (***P < 0·001). (b) A positive correlation between H‐ferritin and the Pouchot's score was observed. (c) A positive correlation between the H‐ferritin tissue level and serum ferritin was observed. (d) A positive correlation between H‐ferritin and systemic inflammatory status (C‐reactive protein) was observed.
No difference of H‐ferritin mRNA expression in PBMCs in AOSD
We then analysed both the relative H‐ferritin and L‐ferritin mRNA expression in PBMCs of AOSD patients. Our results showed no significant differences in both H‐ferritin and L‐ferritin relative mRNA expression when AOSD patients were compared with HCs (Fig. 3).
Figure 3.
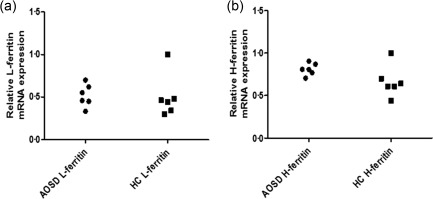
H‐ferritin and L‐ferritin relative mRNA expression in peripheral blood mononuclear cells (PBMCs) in adult‐onset Still's disease (AOSD) patients. No significant difference of both H‐ferritin (a) and L‐ferritin (b) relative mRNA expression in PBMCs was shown between AOSD patients and healthy controls (HCs).
Proinflammatory cytokines and H‐ferritin in AOSD skin
Immunofluorescence analysis showed an increased expression of proinflammatory cytokines in the skin samples of AOSD patients when compared with HCs (Figs 4 and 5). Specifically, a significant increase of IL‐1β was detected in AOSD samples when compared with HCs (P < 0·001). We observed a statistically significant correlation (P < 0·001) between the tissue expression of H‐ferritin and IL‐1β of the same patients. In addition, we showed co‐localization between H‐ferritin and IL‐1β in the skin samples of our patients (Fig. 4). We noted a significant increase of TNF and IFN‐γ in the skin of our AOSD patients when compared with HCs (P < 0·001 for each comparison) (Fig. 5). However, no statistically significant correlations were observed among the tissue levels of these cytokines and the levels of H‐ferritin tissue expression.
Figure 4.
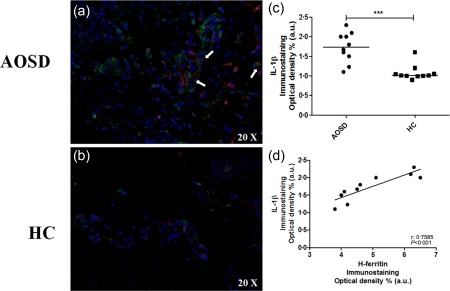
Expression interleukin (IL)‐1β in skin of adult‐onset Still's disease (AOSD) patients and its correlation with H‐ferritin. Immunofluorescence analyses in skin samples of AOSD patients show: (a) increased expression of interleukin (IL)‐1β in the skin. Furthermore, arrows indicate infiltrating cells which co‐express both IL‐1β and H‐ferritin. (b) Lack of IL‐1β expression in healthy controls (HCs). (c) IL‐1β immunostaining optical density shows a significant increase of IL‐1β tissue levels when compared with HCs (***P < 0·001). (d) IL‐1β immunostaining optical density shows a significant correlation between IL‐1β and H‐ferritin.
Figure 5.
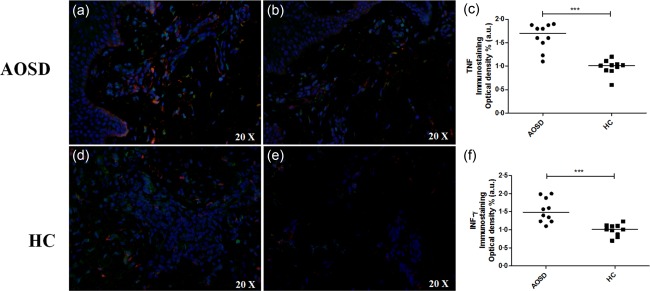
Increased expression of tumour necrosis factor (TNF) and interferon (IFN)‐γ in skin of adult‐onset Still's disease (AOSD) patients. Immunofluorescence analyses in skin samples of AOSD patients show: (a) expression of IFN‐γ in AOSD patients; (b) expression IFN‐γ in healthy controls (HCs); (c) IFN‐γ immunostaining optical density shows a significant increase expression IFN‐γ when comparing AOSD patients with HCs (***P < 0·001); (d) expression of TNF in AOSD; (e) expression of TNF in HCs; (f) TNF immunostaining optical density shows a significant increase expression TNF when comparing AOSD patients with HCs (***P < 0·001).
CD68+/H‐ferritin+ macrophages in AOSD skin
We analysed the number of CD68+ macrophages in the cutaneous inflammatory infiltrate in our samples. The double staining between the H‐ferritin and CD68 confirmed an increased presence of macrophages expressing H‐ferritin in the inflammatory infiltrate when compared with HCs (P < 0·001) (Fig. 6). We did not observe any co‐localization between CD68 and L‐ferritin in the same samples. Our analyses show a positive correlation between the number of CD68+/H‐ferritin+ in the skin of AOSD patients and the multi‐visceral involvement of the disease (P < 0·05). Subsequently, we performed a double staining between the H‐ferritin and IL‐12, a well‐known M1 macrophage marker, and CD163, which is considered a M2 macrophage marker 24, 25. In our samples, we observed a strong infiltrate of cells co‐expressing H‐ferritin and IL‐12, suggesting that M1 macrophages colonize the affected skin. Conversely, our data failed to show any co‐localization between H‐ferritin and CD163 (Fig. 7).
Figure 6.
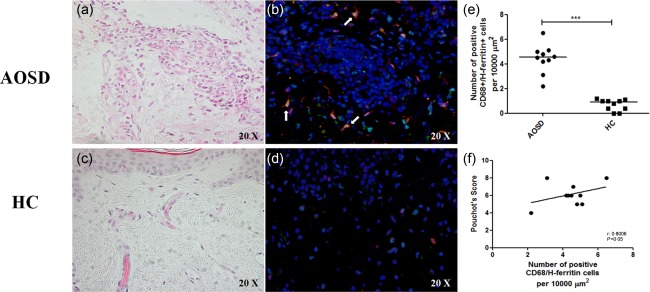
CD68 macrophages expressing H‐ferritin in skin infiltrate of adult‐onset Still's disease (AOSD) patients. Haematoxylin and eosin staining of AOSD patients (a) and healthy controls (HCs) (c). In AOSD patients, an infiltrate enriched in monocytes/macrophages cells may be observed. Only few neutrophils and eosinophils may be detected. The immunofluorescence analysis shows: (b) a large number of CD68+/H‐ferritin+ cells may be identified in the skin of AOSD patients; conversely, (d) only one CD68+/H‐ferritin+ cell may be observed in the skin of HCs. (e) A significantly increased number of CD68+/H‐ferritin+ cells is present in AOSD skin when compared to HCs (***P < 0·001). (f) A positive correlation between CD68+/H‐ferritin+ cells and the Pouchot's score was observed.
Figure 7.
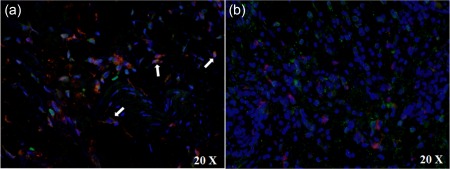
Co‐localization of H‐ferritin and M1 macrophage marker in skin infiltrate of adult‐onset Still's disease (AOSD) patients.
DCs marker and H‐ferritin in AOSD skin
A double staining between the H‐ferritin and CD11, a marker of DCs 26, was performed in our samples. Our data failed to show any co‐localization between H‐ferritin and CD11 (Fig. 8).
Figure 8.
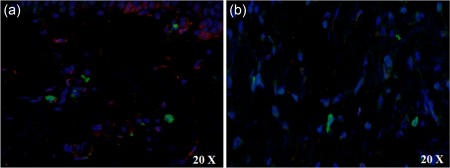
Dendritic cells marker and H‐ferritin in skin of adult‐onset Still's disease (AOSD) patients.
Discussion
In this work, we observed an increased expression of H‐ferritin in the skin associated with an infiltrate characterized mainly by CD68+/H‐ferritin+ macrophages in the biopsies obtained from persistent cutaneous lesions of AOSD patients. Although the persistence of cutaneous lesions in AOSD is uncommon, this clinical feature is associated with the onset of systemic complications and a more severe prognosis 16, 17.
As already mentioned, it has been proposed recently that AOSD should be included in hyperferritinaemic syndrome, in which the higher levels of ferritin may not only be considered a consequence of the inflammation, but actively playing a role in the pathogenic mechanism which will lead to the ‘cytokine storm’, a syndrome in which disruption of the balance of a helpful inflammatory response, switching it from being beneficial to destructive, may cause an uncontrolled up‐regulation of proinflammatory molecules such as IL‐1β, TNF, IL‐8 and IL‐6 18, 19, 20. This exaggerated cytokine production may lead to a disease characterized by hypotension, fever and diffuse oedema that may evolve to multi‐organ dysfunction and death 27.
In this work, we observed an increased expression of H‐ferritin in the skin of our patients. It must be pointed out that H‐ferritin, specifically binding surface receptors on the immune cells, may play a role during their activation and production of inflammatory molecules such as IL‐1β and IL‐6 18, 19, 20, 21. Although the low number of enrolled patients limited us to achieve definitive conclusions, the skin expression of H‐ferritin cells correlated strongly with multi‐visceral involvement. This result may confirm previous reports showing that the persistence of skin lesions correlates with the degree of systemic activity, and although the majority of papers discussing these persistent AOSD‐related skin lesions are case reports or small series, some authors found a significant correlation of AOSD activity with a poor outcome 28, 29, 30, 31, 32, 33. With regard to the increased H‐ferritin expression in skin lesions, we found a strong correlation between this expression and a more severe disease, paralleling previous studies regarding different target organs of AOSD 34, 35.
These results might suggest that ferritin up‐regulation, under the influence of proinflammatory cytokines such as IL‐1β, IFN‐γ and TNF, which we found expressed largely in the skin of our patients, might further induce the H‐ferritin expression which, amplifying the production of proinflammatory cytokines, thus generating a vicious loop which perpetuates the inflammatory state 12, 13, 14, 15, 34, 35, 36, 37. Of interest, we observed a significant increase of tissue levels of IL‐1β which co‐localize with H‐ferritin, confirming the pivotal role of IL‐1β during this disease 12, 13, 14, 15.
In addition, our results, showing a statistical correlation among the levels of H‐ferritin in the skin of AOSD patients, serum ferritin and levels of CRP, suggest a possible role of the innate immune system of the skin in maintaining the degree of systemic inflammation.
At present, it is well known that the histopathology of the AOSD‐associated evanescent rash, characterized typically by a mixed inflammatory infiltrate surrounding the perivascular areas and without epidermal changes, differs strongly from the persistent rash, in which the eruption shows two main findings: (i) a pattern of dyskeratosis, present mainly in the superficial layers of the epidermis without accompanying basilar dyskeratosis; and (ii) a sparse superficial dermal infiltrate often containing neutrophils but without vasculitis. Although dyskeratosis in the lower epidermis is typically absent, few cases have shown focal vacuolar interface alterations and rare necrotic keratinocytes in the lower epidermis 28, 29, 30, 31, 32, 33. Of interest, the description of the mixed infiltrates, associated with AOSD, does not point out the presence and the possible role that skin macrophages may play in the induction and maintenance of the skin rash. In the skin samples obtained from AOSD patients we focused upon the inflammatory infiltrates, and especially the monocyte/macrophage population. Interestingly, we identified a specific subset of macrophages, displaying a specific CD68+/H‐ferritin+ phenotype that cannot be observed in normal skin, suggesting a close relationship of these cells with the observed hyperferritaemia. At present, in our study, we still cannot conclude if the CD68+ macrophages are producing ferritin or phagocytosing cells actively. However, it is well known that different macrophage cell lines may release H‐ferritin after inflammatory stimuli 36, 37, 38. In fact, it has been shown in one animal model that cultured macrophages may secrete both H‐ and L‐ferritin, confirming their role in serum ferritin production through a non‐classical secretory pathway 37. To our knowledge, our paper is the first demonstration of a statistically significant correlation between the number of CD68+/H‐ferritin+ macrophages infiltrating the skin of AOSD patients and the multi‐visceral involvement of the disease. This datum confirms the possible role played by dermal macrophages and the innate immune system of the skin to induce and sustain the degree of systemic inflammation. Furthermore, our result parallels what has already been observed with other biomarkers of macrophage activation, such as macrophage–colony stimulating factor, IFN‐γ, sCD163 and macrophage inhibitory factor, which correlate significantly with the AOSD multi‐visceral involvement 39, 40, 41, 42. In addition, we show a co‐localization between H‐ferritin and IL‐12, confirming that M1‐macrophages largely infiltrate the skin of AOSD patients. M1‐macrophages exhibit anti‐microbial properties physiologically by release of inflammatory mediators, and although these cells play a pivotal role in host defence, a possible dysregulation of these cells may lead to different pathological inflammatory conditions 4, 10, 11. In fact, during AOSD, the exaggerated production of IL‐1β, IFN‐γ and TNF may promote the recruitment and/or the proliferation of these M1 macrophages expressing H‐ferritin 34, 35, 36, 37.
The increased number of CD68+/H‐ferritin+ cells in the inflammatory cutaneous infiltrate as well as the increased expression H‐ferritin, which correlate significantly with inflammatory markers and multi‐visceral involvement, strongly suggest the possible role of these cells in the AOSD pathogenesis.
In conclusion, our data show an imbalance between the production of L‐ and H‐ferritin in the skin of AOSD patients, associated with a strong infiltrate of CD68+/H‐ferritin+ macrophages in the same samples. Furthermore, a correlation between the levels of H‐ferritin as well as the number of infiltrating CD68+/H‐ferritin+ macrophages and the multi‐visceral involvement of the disease was observed. Taken together, our data suggest that CD68+/H‐ferritin+ macrophages may be involved in the induction and maintenance of the skin lesions as well as in the systemic inflammation, which is a specific hallmark of the disease. Further studies are ongoing in our laboratory in order to elucidate the role of ferritin and CD68+/H‐ferritin+ macrophages in AOSD.
Author contributions
P. R.: study conception and design, data interpretation, literature search, figure creation, writing, paper revision and acceptance; P. C.: data collection, data interpretation, literature search, paper revision and acceptance; F. C. I.: data collection, literature search, paper revision and acceptance; P. D. B.: data collection, data interpretation, literature search, paper revision and acceptance; V. L.: data collection, data interpretation, literature search, paper revision and acceptance; F. C. A.: data collection, data interpretation, literature search, paper revision and acceptance; O. B.: data collection, literature search, paper revision and acceptance; G. G.: data collection, literature search, paper revision and acceptance; S. D. B.: data collection, literature search, paper revision and acceptance; G. T.: data collection, data interpretation, literature search, paper revision and acceptance; R. G.: study design, data interpretation, writing, paper revision and acceptance. All authors gave final approval for submitting the manuscript for review and agree to be accountable for all aspects of the work.
Disclosure
None of the authors have any disclosures to declare.
Acknowledgements
The authors thank Mrs Federica Sensini for her technical assistance.
References
- 1. Tay SS, Roediger B, Tong PL et al The skin‐resident immune network. Curr Dermatol Rep 2013; 3:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marzano AV, Fanoni D, Antiga E et al Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and Sweet's syndrome. Clin Exp Immunol 2014; 178:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Di Meglio P, Perera GK, Nestle FO. The multitasking organ: recent insights into skin immune function. Immunity 2011; 35:857–69. [DOI] [PubMed] [Google Scholar]
- 4. Schultze JL, Schmieder A, Goerdt S. Macrophage activation in human diseases. Semin Immunol 2015; 27:249–56. [DOI] [PubMed] [Google Scholar]
- 5. Dupasquier M, Stoitzner P, van Oudenaren A et al Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J Invest Dermatol 2004; 123:876–9. [DOI] [PubMed] [Google Scholar]
- 6. Dupasquier M, Stoitzner P, Wan H et al The dermal microenvironment induces the expression of the alternative activation marker CD301/mMGL in mononuclear phagocytes, independent of IL‐4/IL‐13 signaling. J Leukoc Biol 2006; 80:838–49. [DOI] [PubMed] [Google Scholar]
- 7. Goren I, Allmann N, Yogev N et al A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin‐driven lysozyme M‐specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol 2009; 175:132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mirza R, Di Pietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 2009; 175:2454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lucas T, Waisman A, Ranjan R et al Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010; 184:3964–77. [DOI] [PubMed] [Google Scholar]
- 10. Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol 2015; 33:643–75. [DOI] [PubMed] [Google Scholar]
- 11. Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 2009; 27:451–83. [DOI] [PubMed] [Google Scholar]
- 12. Gerfaud‐Valentin M, Jamilloux Y, Iwaz J, Sève P. Adult‐onset Still's disease. Autoimmun Rev 2014; 13:708–22. [DOI] [PubMed] [Google Scholar]
- 13. Efthimiou P, Paik PK, Bielory L. Diagnosis and management of adult onset Still's disease. Ann Rheum Dis 2006; 65:564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maria AT, Le Quellec A, Jorgensen C et al Adult onset Still's disease (AOSD) in the era of biologic therapies: dichotomous view for cytokine and clinical expressions. Autoimmun Rev 2014; 13:1149–59. [DOI] [PubMed] [Google Scholar]
- 15. Cipriani P, Ruscitti P, Carubbi F et al Tocilizumab for the treatment of adult‐onset Still's disease: results from a case series. Clin Rheumatol 2014; 33:49–55. [DOI] [PubMed] [Google Scholar]
- 16. Cozzi A, Papagrigoraki A, Biasi D et al Cutaneous manifestations of adult‐onset Still's disease: a case report and review of literature. Clin Rheumatol 2016; 35:1377–82. [DOI] [PubMed] [Google Scholar]
- 17. Yamamoto T. Cutaneous manifestations associated with adult‐onset Still's disease: important diagnostic values. Rheumatol Int 2012; 32:2233–7. [DOI] [PubMed] [Google Scholar]
- 18. Rosário C, Zandman‐Goddard G, Meyron‐Holtz EG et al The hyperferritinemic syndrome: macrophage activation syndrome, Still's disease, septic shock and catastrophic antiphospholipid syndrome. BMC Med 2013; 11:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zandman‐Goddard G, Shoenfeld Y. Hemophagocytic syndrome with hyperferritinemia: a stormy immunological response. Isr Med Assoc J 2013; 15:187–8. [PubMed] [Google Scholar]
- 20. Recalcati S, Invernizzi P, Arosio P et al New functions for an iron storage protein: the role of ferritin in immunity and autoimmunity. J Autoimmun 2008; 30:84–9. [DOI] [PubMed] [Google Scholar]
- 21. Wang W, Knovich MA, Coffman LG et al Serum ferritin: past, present and future. Biochim Biophys Acta 2010; 1800:760–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yamaguchi M, Ohta A, Tsunematsu T et al Preliminary criteria for classification of adult Still's disease. J Rheumatol 1992; 19:424–30. [PubMed] [Google Scholar]
- 23. Pouchot J, Sampalis JS, Beaudet F et al Adult Still's disease: manifestations, disease course, and outcome in 62 patients. Medicine (Baltimore) 1991; 70:118–36. [PubMed] [Google Scholar]
- 24. Chong BF, Tseng LC, Hosler GA et al A subset of CD163+ macrophages displays mixed polarizations in discoid lupus skin. Arthritis Res Ther 2015; 17:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang W, He KF, Yang JG et al Infiltration of M2‐polarized 514 macrophages in infected lymphatic malformations: possible role 515 in disease progression. Br J Dermatol 2016. doi: 10.1111/bjd.14471 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26. Heier I, Søyland E, Krogstad AL et al Sun exposure rapidly reduces plasmacytoid dendritic cells and inflammatory dermal dendritic cells in psoriatic skin. Br J Dermatol 2011; 165:792–801. [DOI] [PubMed] [Google Scholar]
- 27. D'Elia RV, Harrison K, Oyston PC et al Targeting the ‘cytokine storm’ for therapeutic benefit. Clin Vaccine Immunol 2013; 20:319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Suzuki K, Kimura Y, Aoki M et al Persistent plaques and linear pigmentation in adult‐onset Still's disease. Dermatology 2001; 202:333–5. [DOI] [PubMed] [Google Scholar]
- 29. Sarkar RN, Bhattacharya R, Bhattacharyya K et al Adult onset Still's disease with persistent skin lesions complicated by secondary hemophagocytic lymphohistiocytosis. Int J Rheum Dis 2014; 17:118–21. [DOI] [PubMed] [Google Scholar]
- 30. Kikuchi N, Satoh M, Ohtsuka M et al Persistent pruritic papules and plaques associated with adult‐onset Still's disease: report of six cases. J Dermatol 2014; 41:407–10. [DOI] [PubMed] [Google Scholar]
- 31. Fortna RR, Gudjonsson JE, Seidel G et al Persistent pruritic papules and plaques: a characteristic histopathologic presentation seen in a subset of patients with adult‐onset and juvenile Still's disease. J Cutan Pathol 2010; 37:932–7. [DOI] [PubMed] [Google Scholar]
- 32. Lee JY, Hsu CK, Liu MF et al Evanescent and persistent pruritic eruptions of adult‐onset still disease: a clinical and pathologic study of 36 patients. Semin Arthritis Rheum 2012; 42:317–26. [DOI] [PubMed] [Google Scholar]
- 33. Wolgamot G, Yoo J, Hurst S et al Unique histopathologic findings in a patient with adult‐onset Still disease. Am J Dermatopathol 2007; 29:194. [DOI] [PubMed] [Google Scholar]
- 34. Ruscitti P, Ciccia F, Cipriani P et al The CD68+/H‐ferritin+ cells colonize the lymph nodes of the patients with Adult onset Still's disease and are associated with increased extracellular level of H‐ferritin in the same tissue. Correlation with disease severity and implication for pathogenesis. Clin Exp Immunol 2016; 183:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ruscitti P, Cipriani P, Di Benedetto P et al Increased level of H‐ferritin and its imbalance with L‐ferritin, in bone marrow and liver of patients with adult onset Still's disease, developing macrophage activation syndrome, correlate with the severity of the disease. Autoimmun Rev 2015; 14:429–37. [DOI] [PubMed] [Google Scholar]
- 36. Wei Y, Miller SC, Tsuji Y et al Interleukin 1 induces ferritin heavy chain in human muscle cells. Biochem Biophys Res Commun 1990; 169:289–96. [DOI] [PubMed] [Google Scholar]
- 37. Cohen LA, Gutierrez L, Weiss A et al Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood 2010; 116:1574–84. [DOI] [PubMed] [Google Scholar]
- 38. Chen TT, Li L, Chung DH et al TIM‐2 is expressed on B cells and in liver and kidney and is a receptor for Hferritin endocytosis. J Exp Med 2005; 202:955e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Colafrancesco S, Priori R, Alessandri C et al sCD163 in AOSD: a biomarker for macrophage activation related to hyperferritinemia. Immunol Res 2014; 60:177–83. [DOI] [PubMed] [Google Scholar]
- 40. Matsui K, Tsuchida T, Hiroishi K et al High serum level of macrophage‐colony stimulating factor (M‐CSF) in adult‐onset Still's disease. Rheumatology (Oxf) 1999; 38:477–8. [DOI] [PubMed] [Google Scholar]
- 41. Jung S‐Y, Park Y‐B, Ha Y‐J et al Serum calprotectin as a marker for disease activity and severity in adult‐onset Still's disease. J Rheumatol 2010; 37:1029–34.] [DOI] [PubMed] [Google Scholar]
- 42. Zou Y‐Q, Lu L‐J, Li S‐J et al The levels of macrophage migration inhibitory factor as an indicator of disease activity and severity in adult onset Still's disease. Clin Biochem 2008; 41:519–24. [DOI] [PubMed] [Google Scholar]


