Abstract
Background
Small fiber neuropathy is a well-recognized complication of type 2 diabetes and has been shown to be responsible for both neuropathic pain and impaired wound healing. In previous studies, we have demonstrated that ganglioside GM3 depletion by knockdown of GM3 synthase fully reverses impaired wound healing in diabetic mice. However, the role of GM3 in neuropathic pain and small fiber neuropathy in diabetes is unknown.
Purpose
Determine whether GM3 depletion is able to reverse neuropathic pain and small fibers neuropathy and the mechanism of the reversal.
Results
We demonstrate that GM3 synthase knockout and the resultant GM3 depletion rescues the denervation in mouse footpad skin and fully reverses the neuropathic pain in diet-induced obese diabetic mice. In cultured dorsal root ganglia from diet-induced diabetic mice, GM3 depletion protects against increased intracellular calcium influx in vitro.
Conclusions
These studies establish ganglioside GM3 as a new candidate responsible for neuropathic pain and small fiber neuropathy in diabetes. Moreover, these observations indicate that systemic or topically applied interventions aimed at depleting GM3 may improve both the painful neuropathy and the wound healing impairment in diabetes by protecting against nerve end terminal degeneration, providing a disease-modifying approach to this common, currently intractable medical issue.
Keywords: Neuropathic pain, small fiber neuropathy, GM3 ganglioside, obesity, glucose intolerance, diabetes
Background
Painful diabetic neuropathy (PDN) affects 25% of diabetic patients1–3 and has a substantial impact on quality of life,4 in addition to a high risk of pedal ulceration and amputation. Current therapies for diabetic neuropathic pain are only partially effective, despite the huge need for effective intervention. Moreover, the molecular and electrophysiological mechanisms underlying PDN are not well understood.
Pain is a physiological response to potentially dangerous noxious stimuli. However, pathological or “neuropathic pain” is associated with sustained excitability and pathological sustained activity of nociceptive primary afferent neurons, so that pain is produced in the absence of appropriate stimuli.5–7 The molecular mechanisms leading to neuropathic pain in diabetes are poorly understood.
Neuropathic pain is known to be associated with both pathological spontaneous ectopic activity and degeneration of unmyelinated C-fibers. For many painful neuropathies, such as PDN, loss of the distal intraepidermal free-end nerve terminals manifests in a reduction in the intraepidermal nerve fiber density.8,9 Indeed, degeneration of free-end nerve terminals, also called small fiber neuropathy (SFN), is a well-recognized complication of T2D.5,9–11 Intraepidermal nerve fiber densities are reduced in patients with impaired glucose tolerance and clinically overt diabetes,12 as well as in animal models of T2D, including the diet-induced obese (DIO) diabetic model.11 Although there is substantial interest in axonal degeneration in PDN, the mechanistic details of the molecular cascade linking diabetes and neuropathic pain to nerve fiber degeneration are incompletely understood.
Gangliosides are sialylated glycosphingolipids found in high concentrations in the nervous system and known to play an important role in lipid raft-based signaling. The precursor ganglioside, GM3, and its synthesizing enzyme, GM3 synthase (GM3S), are increased in expression in canonical insulin-responsive tissues13 and, as we have shown, in the skin of diabetic mouse models and human diabetic feet.14,15 Increasing evidence suggests that GM3 accumulation is a mediator of Tumor Necrosis Factor (TNF)-induced insulin resistance.13,16 We have recently shown that ganglioside GM3 plays a role in the impaired wound healing of diabetes, at least in part through its suppression of insulin/insulin-like growth factor-1 receptor signaling in epidermal cells.14,15 Depletion of ganglioside GM3 by GM3S knockout or topically applied spherical nucleic acid (SNA) nanoconjugate-mediated knockdown of GM3S fully reverses diabetic wound healing impairment.14,15
Nerve fiber degeneration has been shown to be associated with the poor wound healing in diabetes, but the impact of ganglioside alterations on diabetic nerve function has not been explored. We now show that GM3S depletion fully reverses neuropathic pain and small fibers degeneration in DIO diabetic mice by preventing increased calcium influx in diabetic dorsal root ganglia (DRG) sensory neurons. These studies establish ganglioside GM3 as a new therapeutic target for neuropathic pain and small fibers neuropathy in diabetes. Moreover, these observations suggest that systemic or regional interventions to deplete GM3 prevent nerve end terminal degeneration, thereby improving both the painful neuropathy and the wound healing impairment in diabetes.
Materials and methods
Animals
All animal experiments were approved by the Institutional Animal Care and Use Committee at Northwestern University. Animals were housed with food and water ad libitum and kept on 12-h light cycle. Wild-type (WT) C57BL/6 (Jackson Laboratories) and GM3 synthase knockout (GM3S KO) (backcrossed to C57BL/6 background) male mice were studied. Mice were weighed twice weekly.
High fat diet-induced (DIO) diabetes as a model of T2D
High fat diet (HFD) induces insulin resistance and is therefore used to create rodent models of T2D.11 WT and GM3S KO mice were fed with a 60% fat diet for 10 weeks beginning at 6 weeks of age. Control mice were fed a regular (11% fat) diet (RD). Glucose tolerance testing (GTT) was performed as previously described.11 Briefly, mice were fasted for 12 h, injected with a 45% D-glucose solution (2 mg glucose/kg body weight), and blood glucose was measured before and at 30, 60, and 120 min after glucose injection using an OneTouch UltraMini glucose meter and OneTouch Ultra test strips. The cutoff for diabetes was determined based on ≥2 SD above the mean for WT RD mice.
Statistical analysis
Data were analyzed by two-tailed unpaired Student’s t-test, with p < 0.05 considered to be significant.
Functional studies of sensation
For Von Frey behavioral studies, mice were habituated to the testing apparatus for 15 min/day beginning two days before behavioral testing.7,17–20 After this acclimation, each filament was applied to six spots spaced across the glabrous side of the hind paw. Mechanical stimuli were applied with seven filaments, each differing in the bending force delivered (10, 20, 40, 60, 80, 100, and 120 mN; 100 mN = 10.197 g force equivalence), but each fitted with a flat tip and a fixed diameter of 0.2 mm. The filaments were tested in order of ascending force, with each filament delivered for 1 s in sequence from the first to the sixth spot, alternating from one paw to the other. The interstimulus interval was 10–15 s.20 For the thermal studies (Ugo Basile), the presence of thermal hyperalgesia was determined by measuring foot withdrawal latency to heat stimulation (thermal paw withdrawal) using the Hargreaves’ plantar test apparatus.6,7 Briefly, each mouse was placed in a box with a temperature-maintained glass floor and, after habituation, an infrared heat generator was aimed at the mouse’s hind paw under the floor. The stimulus shuts off automatically when the hind paw moves or after 20 s. Reaction time was recorded, with a shortening of the withdrawal latency indicating thermal hyperalgesia. Measurements were repeated four times on each paw at 5 min intervals, with the latter three pairs of measurements used as data. Mice were tested on each of three successive days.
Statistical analysis for functional studies
The incidence of foot withdrawal was expressed as a percentage of six applications of each filament as a function of force. A Hill equation was fitted to the function (Origin version 6.0, Microcal Software) relating the percentage of indentations eliciting a withdrawal to the force of indentation. From this equation, the threshold force was obtained and defined as the force corresponding to a 50% withdrawal rate. The GB-Stat School Pack software (Dynamic Microsystem) was used to statistically evaluate all data. The one-way analysis of variance with a Dunnett’s Multiple Comparison test was used to analyze the difference between multiple experimental groups, with p < 0.05 considered to be significant.
Assessment of GM3S expression in DRG and sciatic nerve
DRG sensory neurons and sciatic nerves from WT RD and HFD mice were removed after 10–11 weeks on the selected diet. Total protein was then extracted from DRG and sciatic nerves from three mice in each group using cell lysis buffer (Cell Signaling Tech), and 30 g total protein from each group (WT RD and WT HFD) was loaded onto 10% SDS-PAGE gel. After separation, protein was transferred onto nitrocellulose membrane and the GM3S was determined by Western blotting using anti-GM3S antibody (Santa Cruz).
Detection of cutaneous innervation
Harvested feet were fixed in Bouin’s fixative for 12 h, and then the overlying footpad skin was dissected, submerged in 15% sucrose solution for 12 h, and embedded in Optimal Cutting Temperature Compound (Tissue-Tek). Cryostat sections (80 m) were blocked for 1 h at room temperature (RT) in 5% normal goat serum, 0.3% Triton X-100, and 1% Bovine Serum Albumin (BSA) diluted. Samples were incubated with anti-PGP 9.5 antibody (Millipore #AB1761-I, 1:850 dilution) at 4℃ for 12 h, followed by incubation with Alexa Fluor 488-conjugated goat anti-rabbit antibody (1:1000 dilution) for 1 h at RT. DAPI 1:10 (400 mM stock) was applied for 10 min at RT to counterstain cell nuclei.
Confocal analysis
Nerves were imaged by confocal microscopy (Nikon A1R) and composite Z-stack images were obtained. The epidermal–dermal junction was outlined by a blinded observer, and three other blinded reviewers counted the number of nerves crossing this junction for the junctional length. The dermis was traced using ImageJ and, after subtracting the background, the green fluorescence was quantified as a percentage of total area using the GFP channel.
Statistical analysis
Unpaired Student’s t-tests were used to analyze and compare the data and calculate p-values using Graph Pad web site, with p < 0.05 considered significant.
Culture and intracellular calcium imaging of DRG neurons
DRG sensory neurons from WT and GM3S KO mice (HFD and, as control RD) were removed after 10–11 weeks on the selected diet and cultured as previously described.17,21 Briefly, DRG neurons were acutely dissociated and digested with collagenase IV (1 µg/mL) and papain (30 U/mL) (Worthington Biochemical Corp, Lakewood, NJ). Cells were plated on laminin (20 µg/mL) or, as a control, poly-L-lysine (20 µg/mL)-coated glass coverslips and cultured at 37℃ with 5% CO2 for 48 h in adult neurogenic medium: F12 with L-glutamine, 0.5% FBS, 1 × N2 (Life Technologies), penicillin (100 µg/mL), and streptomycin (100 U/mL). The response of acutely cultured DRG neurons to different concentration of potassium (K+) (10, 25, and 50 mM) or capsaicin (cap) (1, 2.5, 5, and 10 µM) were recorded using intracellular calcium imaging as previously described.17 Acutely cultured DRG cells were loaded with fura-2 AM (3 µM, Invitrogen, Carlsbad, CA) for 25 min at RT in balanced salt solution (BSS) (NaCl (140 mM), Hepes (10 mM), CaCl2 (2 mM), MgCl2 (1 mM), glucose (10 mM), and KCl (5 mM)). Cells were rinsed with BSS, mounted onto a chamber that was placed onto the inverted microscope, and continuously perfused with BSS (2 mL/min). Intracellular calcium ([Ca2+]i was measured by digital video microfluorometry with an intensified CCD camera coupled to a microscope and MetaFluor software. Cells were illuminated with a 150 W xenon arc lamp, and the excitation wavelengths of the fura-2 (340/380 nm) were selected by a filter changer.
Statistical analysis
Differences between the control and various treatment groups were assessed by analysis of variance with p < 0.05 considered significant.
Results
GM3S depletion fully reverses neuropathic pain in DIO mice
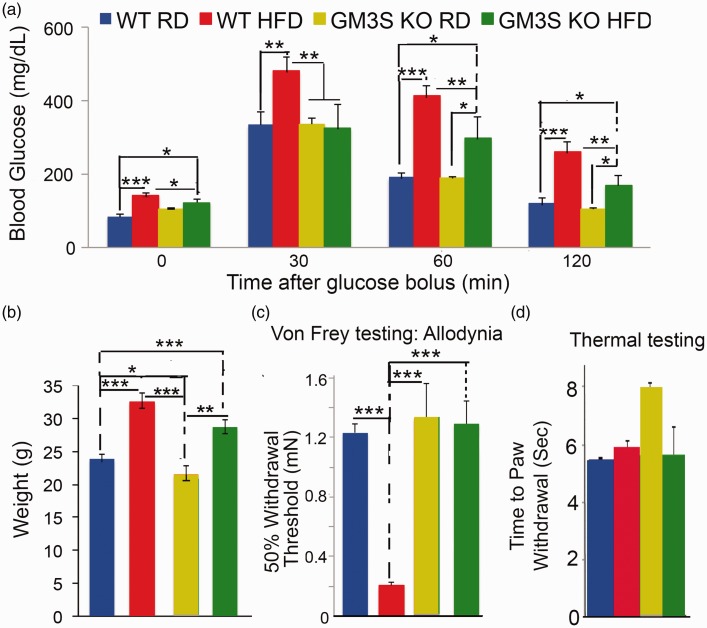
GM3S KO mice (courtesy of Dr R. Proia, NIH)22 have previously been described and show no detectable GM3 or downstream gangliosides (such as GM2, GM1, and GD3).15,22,23 GM3S KOs and their WT littermate controls were fed either a RD containing 11% fat or a HFD consisting of 60% fat for 10 weeks before testing. Two hour GTTs showed higher glucose levels in the WT and GM3S KO mice on a HFD than in the WT and GM3S KO mice on a RD (WT HFD vs. RD, p < 0.001; GM3S KO HFD vs. RD, p < 0.05). GM3S KO mice exhibited variably improved glucose tolerance, with a significantly lower 2 h level of glucose overall in GM3S KO HFD than in WT HFD mice (p < 0.01) (Figure 1(a)).
Figure 1.
GM3S depletion fully reverses neuropathic pain in DIO mice. GM3 synthase knockout (GM3S KO) mice and their WT littermate controls were fed either a Regular Diet (RD) or a high fat diet (HFD) for 10 weeks. (a) GM3S knockout variably improves glucose tolerance. When compared with RD-fed mice, HFD-fed mice had significantly higher baseline fasting and 30, 60, and 120 min glucose levels (mg/dL). Overall, glucose levels in the GM3S KO HFD mice were slightly higher than those in the WT RD mice but were much lower than in WT HFD mice. However, the improvement was variable in GM3S KO HFD mice. (b) Obesity is not impacted by GM3 depletion. Mean weights were higher in WT HFD (n = 10) and GM3S KO HFD (n = 24) mice compared with WT RD (n = 8) and GM3S KO RD (n = 11) mice but not in WT HFD versus GM3S KO HFD mice (p = 0.08). (c) GM3S depletion fully reverses neuropathic pain in DIO mice. Pain behavior was assessed using Von Frey filaments. In WT HFD diabetic mice, the withdrawal threshold required to elicit a response was reduced compared with WT RD non-diabetic mice, demonstrating the development of neuropathic pain. No difference was noted in GM3S KO HFD mice compared with WT RD or GM3S KO RD, showing that GM3S depletion fully reverses neuropathic pain in DIO mice. (d) All mouse groups show normal mean thermal sensitivity. Values are expressed as mean ± SE.*p < 0.05, **p < 0.01, ***p < 0.001.
As previously shown, the mean weights after 10 weeks on the HFD were higher in WT (mean ± standard error (SE), 32.5 ± 1.4 g) and GM3S KO (28.7 ± 1.0 g) mice when compared with WT (23.9 ± 0.7 g) or GM3S KO (21.5 ± 1.3 g) on the RD (p < 0.001) but were similar between WT and GM3S HFD mice (p = 0.08) (Figure 1(b)). These data suggest that GM3S depletion has no ameliorative effect on diet-induced obesity, but partially improves the glucose intolerance induced by HFD in some mice.
GM3S depletion fully reverses neuropathic pain in DIO mice
The effect of GM3S depletion on diabetic neuropathic pain was assessed in DIO mice using Von Frey filaments. In these functional studies, results were tallied by observers who were blinded to subgroup (although obesity in HFD mice was easily noted). The withdrawal threshold required to elicit a response was significantly reduced in WT HFD diabetic mice when compared with WT RD non-diabetic mice, showing the development of neuropathic pain (Figure 1(c)). In contrast, the withdrawal threshold required to elicit a response in GM3S KO HFD mice resembled that in WT RD and GM3S KO RD non-diabetic controls, suggesting that GM3S depletion fully reverses neuropathic pain in DIO mice (Figure 1(c)). Thermal testing is normal in DIO mice, as previously noted,11 and similarly was normal in our WT HFD, GM3 KO HFD, and GM3 KO RD mice (Figure 1(d)).
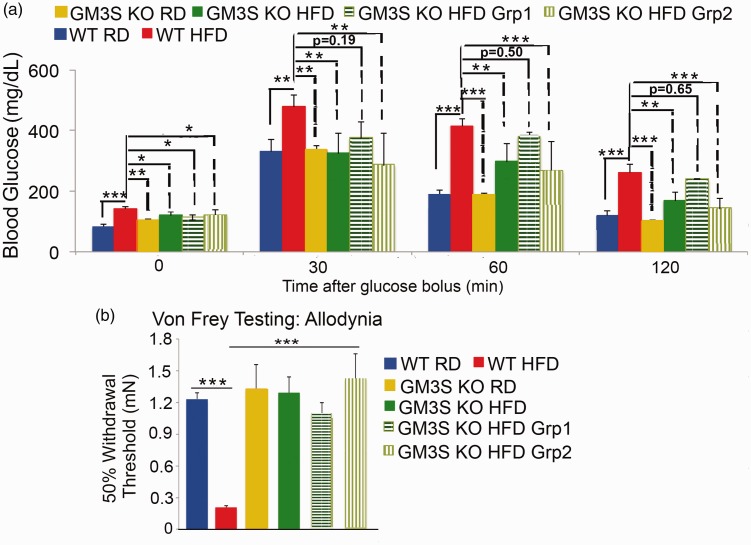
Given that GM3S knockout variably improves mouse glucose tolerance, we were able to divide GM3S KO mice into diabetic and non-diabetic to assess the role of improved glucose tolerance versus reduction of GM3S itself on the observed reversal of the neuropathy. Cutoff levels for diabetes in mice depend on a wide variety of factors, including strain, age, sex, and environment of the mice subjected to GTTs.24 To compare “diabetic” versus “non-diabetic” GM3S KO HFD mice, we first established cutoff levels by assessing glucose tolerance in 80 WT littermate RD mice, setting the cutoff at 2 SDs above the mean for glucose at 2 h after glucose challenge (≥182 mg/dL). GM3S KO HFD mice were then divided into two groups: (i) GM3S KO HFD Group 1 with GTT 2 h glucose levels ≥182 mg/dL (diabetic, n = 6); and (ii) GM3S KO HFD Group 2 with glucose levels < 182 mg/dL (non-diabetic, n = 17) (Figure 2(a)). Regardless of diabetic status, the withdrawal threshold required to elicit a response in GM3S KO HFD mice was significantly higher than WT HFD and was not different from non-diabetic control WT RD mice (Figure 2(b)), suggesting a primary neuroprotective effect of GM3 depletion.
Figure 2.
GM3S depletion fully reverses neuropathic pain, even in diabetic mice. GM3S KO HFD mice were divided into diabetic (Group 1; 2 h GTT glucose levels ≥182 mg/dL) or non-diabetic (Group 2, <182 mg/dL) based on a threshold of ≥ 2 SD above the mean for 2 h glucose levels in our WT RD mice (n = 80). As shown in (a), the fasting baseline levels of glucose in the two GM3S KO HFD groups remained higher than WT RD baseline levels, but the 2 h glucose levels in Group 2 were not higher than those of WT RD mice (WT RD, n = 9; WT HFD, n = 9; GM3S KO RD, n = 9; GM3S KO HFD overall, n = 23; GM3S KO HFD Group 1, n = 6; GM3S KO HFD Group 2, n = 17). (b) The Von Frey Pain Behavior Test demonstrated that the withdrawal threshold required to elicit a response in GM3S KO HFD Groups 1 and 2 was significantly higher than WT HFD, but similar to WT RD and GM3S KO RD mice. Values are expressed as mean ± SE. *p < 0.05, **p < 0.01, ***p < 0.001.
GM3S is increased in diabetic nerves
In diabetes, GM3 is now known to be increased in canonical insulin target tissues, as well as epidermis. However, the expression of GM3S has never been assessed in peripheral nerves. Isolated WT HFD diabetic mouse DRGs and sciatic nerve had levels of expression of GM3S that were 1.7-fold and 24-fold, respectively, those in WT RD non-diabetic mice (Suppl. Figure 1). GM3S KO mice are unable to make GM3S or GM3.22
GM3S depletion rescues SFN in DIO diabetes
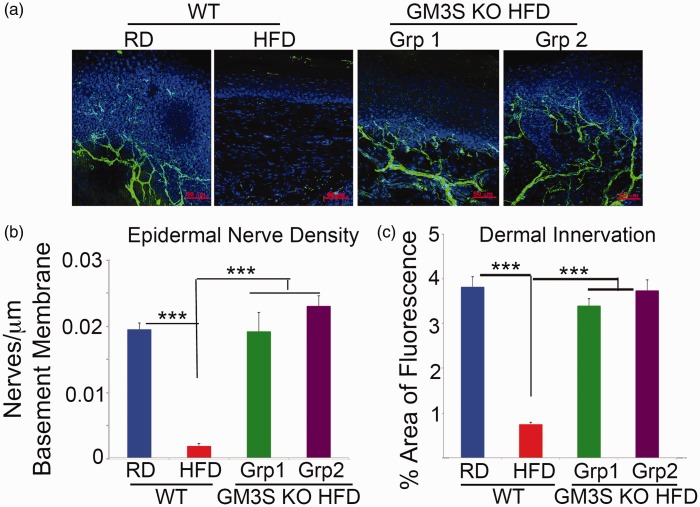
Denervation of epidermal footpad skin was observed in WT HFD diabetic mice, consistent with the presence of diabetic neuropathy. In contrast, even diabetic GM3S KO HFD mice had preservation of footpad skin innervation, as measured by the number of nerves crossing into the epidermis (0.02 nerves/µm basement membrane; Figures 3(a),(b)) and dermal nerve density (Figures 3(a),(c)).
Figure 3.
GM3 depletion rescues denervation in mouse footpad skin in diet-induced diabetes. (a) Representative confocal images of PGP 9.5 expression in 80 µm mouse footpad sections. Epidermal nerve density (b) is expressed as the number of nerves crossing the epidermis as a function of epidermal–dermal junction length. Dermal innervation (c) is expressed as the percentage of dermis with fluorescence. Data represent 16 feet (n = 8) from each group with three non-contiguous sections analyzed per foot. Grp 1 = diabetic GM3S KO HFD mice; Grp 2 = non-diabetic GM3 KO HFD mice. Values are expressed as mean ± SE. ***p < 0.001.
GM3S depletion prevents hyperexcitability-associated calcium influx into diabetic DRG neurons
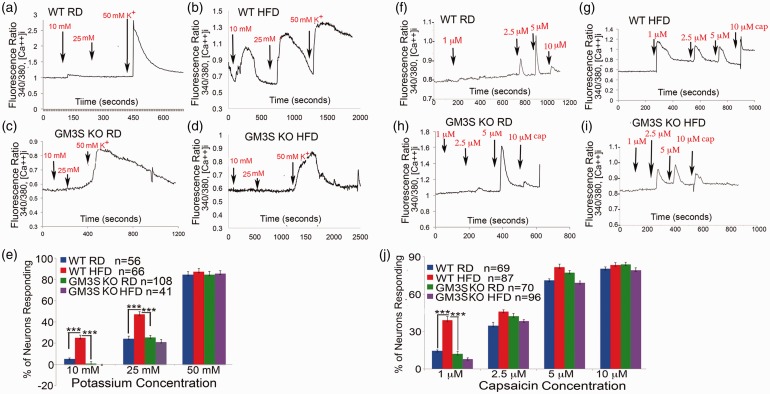
SFN is a well-recognized complication of T2D,5,9–11 and we observed denervation of the epidermal footpad skin in our animal model of HFD-induced diabetes (Figure 3(a), WT HFD). Calcium toxicity has recently been shown to contribute to DRG neuritis and small fiber degeneration in a genetic form of SFN associated with a mutation in the Nav 1.7 sodium channel.25 To examine a potential role for neuronal hyperexcitability in diabetic mice using (Ca)I into DRG neurons as a surrogate for overall excitability, we performed Ca imaging studies in response to different concentrations of known DRG neuron excitatory stimuli: high potassium (K+) and capsaicin in acutely isolated HFD-induced diabetic and RD-fed non-diabetic control mouse DRGs.21
DRG neurons from WT HFD mice were much more sensitive to both high K+ and capsaicin stimulation than WT RD DRG neurons. Even low concentrations of K+ (10 mM) and capsaicin (1 µM) increased (Ca)I in WT HFD-induced diabetic DRGs (25.0 ± 2.7% are responsive, mean ± SE, Figures 4(b) and 4(e) and 39.0 ± 3.0% responsive, Figures 4(g) and 4(j), respectively) but not in non-diabetic WT RD mouse DRGs (5.0 ± 0.9% responsive, Figures 4(a) and 4(e)) and 14 ± 0.9% responsive, Figures 4(f) and 4(j), respectively) (both p < 0.001). GM3S KO HFD DRGs, even from mice with 2 h GTT glucose levels ≥ 182 mg/dL, had significantly lower increases in (Ca)I than WT HFD DRGs (p < 0.001) in response to 10 mM K + (0 ± 0.3%) and 1 µM capsaicin (8 ± 0.9%) (Figures 4(d), 4(e), 4(i), and 4(j)). GM3S KO HFD DRG (Ca)I responses were no different from those in WT RD DRGs, confirming the ability of GM3 depletion to reduce hyperexcitability and associated (Ca)I into DRG neurons. The basal F340/F380 ratios (measuring free cytosolic calcium concentration) for neurons in all four experimental groups were the same (Suppl. Table 1). Similarly, the number of neurons sensitive to the highest concentrations of K+ (50 mM) and capsaicin (10 µM) was the same in all the groups (Figures 4(e) and 4(j)). Considering that TRPV1 expression, reflected by capsaicin sensitivity, is restricted to a major portion of nociceptors, this population of neurons is likely to be similar between DRG culture from WT RD and GM3S KO mice (Figure 4(j)).
Figure 4.
GM3S depletion prevents increased intracellular calcium influx in DRG neurons in diet-induced diabetes. (a–d) Representative tracings of intracellular calcium responses ([Ca++]i) to different concentration of potassium (K+) (10–50 mM) of acutely cultured DRG sensory neurons from control WT RD (a, n = 56), WT HFD (b, n = 66), GM3S KO RD (c, n = 108), and GM3S KO HFD (d, n = 41) mice. (e) Percentage of DRG neurons from each group responding to different concentration of potassium (K+) (10, 25, and 50 mM). Values are expressed as mean ± SE. ***p < 0.001. (f–i) Representative tracings of intracellular calcium responses to different concentrations of capsaicin (cap) (1–10 µM) of DRG sensory neurons from the subgroups. (i) Percentage of DRG neurons from WT control mice fed with regular diet (WT RD), WT diabetic mice fed with high fat diet (WT HFD), GM3KO mice fed with RD (GM3KO RD), and GM3KO mice fed with high fat diet (GM3KO HFD) responding to different concentration of capsaicin (1, 2.5, 5, and 10 µM). Values are expressed as mean ± SE. ***p < 0.001.
Discussion
SFN is a well-recognized complication of T2D and has been shown to be responsible for both neuropathic pain and impaired wound healing.26–28 In previous studies, we demonstrated that ganglioside GM3 depletion produced by GM3S knockout15 or regional knockdown of GM3S by topically applied siRNA nanoconjugates14 fully reverses impaired wound healing in DIO diabetic mice,14,15 at least in part through increasing insulin/IGF-1 receptor signaling and accelerating epidermal cell migration. In the present studies, we have demonstrated that GM3S knockout, which results in GM3 depletion, also fully reverses neuropathic pain and small fibers degeneration in diabetes, possibly by preventing increased (Ca)I and neuronal hyperexcitability in DRG sensory neurons. While GM3S depletion ameliorates glucose tolerance in the mice, which could indirectly improve neuronal health, even diabetic GM3S KO mice showed preservation of cutaneous nerves, suggesting a primary effect of GM3 depletion on protecting nerves.
Neuropathic pain is associated with sustained pathological excitability of nociceptive primary afferent neurons, so that pain is produced in the absence of appropriate stimuli.5–7 We have previously shown that DRG hyperexcitability is increased in diabetic DRGs21 and animal models of neuropathic pain.17,18 That GM3 depletion both reduces (Ca)I and prevents pathological nociceptor hyperexcitability further supports this association. In our experiments on cultured DRG neurons, increased (Ca)I in response to both high K+ and capsaicin was evident in WT HFD mice, however increased thermal hypersensitivity was not observed.11 Although culture conditions may alter DRG responses differently from DRG responses in vivo, our data suggest that thermal hypersensitivity is regulated by influences beyond TRPV1.
Degeneration of myelinated and unmyelinated fibers in the DRG and dorsal horn has been noted previously from GM3 accumulation in double knockout mice missing the two enzymes that metabolize GM3 to more complex gangliosides, GM2/GD2 and GD3 synthases.29,30 Double knockout mice have perineural cutaneous inflammation in association with neurological changes and develop acral ulcerations that heal poorly,29 although the molecular mechanism of the neuropathy is not well understood.
How GM3 accumulation induces nerve degeneration is unknown. The present studies provide evidence that preventing GM3 accumulation in diabetic mice protects against increased (Ca)I, which leads to DRG neurite degeneration and contributes to nerve degeneration in a genetic model of SFN.25 Indeed, increased (Ca)I is widely thought to be a major contributor to pathways leading to axonal degeneration.31–33 Our demonstration that GM3 depletion protects from both increased (Ca)I and small fiber degeneration in diabetic mice is consistent with the possibility. It is unclear, however, whether GM3 itself has a deleterious effect or largely affects neurons through its metabolism to more complex gangliosides, particularly ganglioside GM1. GM1 has been shown to have a direct excitatory effect on DRG neurons.34 In addition, the association of tetrodotoxin-resistant channels (Nav 1.8) in DRG sensory neurons with lipid rafts, which are highly enriched with gangliosides, and in particular GM1, has been found to regulate sensory neuron excitability.34 GM1 is also known to accumulate in association with endoplasmic reticulum stress and calcium-dependent mitochondrial apoptosis,35 which have been described in diabetic neuropathy.36 Nevertheless, the development of neuropathy in the double knockout mice, with increases in GM3 but depletion of GM1 through metabolic blockade, suggests that GM3 itself, rather than more complex gangliosides, is the effector.14,15 Selective concurrent knockdown of GM2/GD2 synthase and GD3 synthase in cultured diabetic neurons, as we have previously described in keratinocytes,14,15 will allow future investigation of the specific effect of GM3.
While our studies have discovered increased GM3S expression in peripheral nerves and a direct impact of ganglioside alteration on DRG neurons, the possibility that ganglioside modulation also affects neurons secondarily deserves future exploration. Disturbances in epidermal–neuronal signaling in inflamed or damaged skin lead to abnormal sensory transmission that underlies associated pain.37,38 Keratinocytes are known to produce a variety of factors that can potentially modulate and directly initiate nociceptive responses. It has recently been shown that optogenetic activation of keratinocytes can evoke pain behaviors, suggesting that robust communication occurs between keratinocytes and sensory afferents in the skin that transmit nociceptive stimuli.39 These findings support the idea that keratinocytes, as activators of cutaneous neurons, have a central role in the onset and maintenance of such abnormal transmission. We have already demonstrated that keratinocytes from GM3S KO mice have activated basal and ligand-stimulated IR and IGF1 R signaling. The result is accelerated migration and proliferation, particularly in the presence of excess glucose, which usually suppresses IR/IGF1R signaling and inhibits keratinocyte proliferation and migration.15 In contrast, GM3 and exposure to inducers of GM3 synthesis, such as excessive glucose and chronic low-dose TNF, suppress mouse keratinocyte migration and proliferation.15 It is possible that GM3 deletion modulates sensory afferents and pain behavior indirectly through activation of keratinocyte signaling pathways that affect nerves or the immune system, providing an accessible target for therapeutic intervention.
From a translational perspective, the results of the present experiments suggest a novel disease-modifying treatment for diabetic neuropathy and the recalcitrant wound healing impairment through systemic or possibly topically applied interventions to deplete GM3. Indeed, topically applied SNAs are able to penetrate skin and deliver GM3S-depleting siRNA,14 raising the possibility that topical delivery of GM3S siRNA SNA to the footpad can prevent or reverse neuropathic pain.
Author contributions
Mouse breeding, administration of HFD, testing for diabetes, and evaluation by immunofluorescence of epidermal and innervation were performed by the Paller laboratory (HW, XW, KF, and ASP). The Menichella laboratory (NDJ and DMM) performed the Von Frey, thermal behavioral, and calcium imaging studies. From the Miller Laboratory (AS, DR, and RJM), DR cultured the DRG neurons and AS supervised the calcium imaging studies. DMM and XQ performed the statistical analyses. DMM, ASP, and RJM supervised the project. DMM drafted the manuscript, which was then edited by ASP and RJM. All authors read and approved the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIAMS R21 NIH AR062898 and R01 NIH AR068375 (ASP), the Foglia Family Endowment, NIH 5R25NS070694-04 Research Education Program for Trainees in Neurology (DMM), the Dixon Young Investigator Grant: Northwestern Memorial Foundation (DMM), Cancer Center Support Grant (NCI CA060553), NIH K08 NS079482-01 (DMM), NIH 5R01DA013141-14 (RJM), and NIH/Rush University Medical Center 1R01AR064251-01 (RJM). Core support was provided by Northwestern’s Skin Disease Research Center (NIAMS P30AR057216), Mouse Histology and Phenotyping Laboratory (NCI CCSG P30CA060553), and Advanced Imaging Facility (NCI P30CA060553).
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2011; 34: S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyck PJ, Kratz KM, Lehman KA, et al. The Rochester Diabetic Neuropathy Study: design, criteria for types of neuropathy, selection bias, and reproducibility of neuropathic tests. Neurology 1991; 41: 799–807. [DOI] [PubMed] [Google Scholar]
- 3.Spallone V, Lacerenza M, Rossi A, et al. Painful diabetic polyneuropathy: approach to diagnosis and management. Clin J Pain 2012; 28: 726–743. [DOI] [PubMed] [Google Scholar]
- 4.daCosta DiBonaventura M, Cappelleri JC, Joshi AV. A longitudinal assessment of painful diabetic peripheral neuropathy on health status, productivity, and health care utilization and cost. Pain Med 2011; 12: 118–126. [DOI] [PubMed] [Google Scholar]
- 5.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10: 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Hehn CA, Baron R, Woolf CJ. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012; 73: 638–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JM, Song XJ, LaMotte RH. Enhanced excitability of sensory neurons in rats with cutaneous hyperalgesia produced by chronic compression of the dorsal root ganglion. J Neurophysiol 1999; 82: 3359–3366. [DOI] [PubMed] [Google Scholar]
- 8.Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008; 131: 1912–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Divisova S, Vickova E, Srotova I, et al. Intraepidermal nerve-fibre density as a biomarker of the course of neuropathy in patients with Type 2 diabetes mellitus. Diabetes Med 2016; 33: 650–654. [DOI] [PubMed] [Google Scholar]
- 10.Shun CT, Chang YC, Wu HP, et al. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain 2004; 127: 1593–1605. [DOI] [PubMed] [Google Scholar]
- 11.Obrosova IG, Ilnytska O, Lyzogubov VV, et al. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes 2007; 56: 2598–2608. [DOI] [PubMed] [Google Scholar]
- 12.Sumner CJ, Sheth S, Griffin JW, et al. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003; 60: 108–111. [DOI] [PubMed] [Google Scholar]
- 13.Nagafuku M, Sato T, Sato S, et al. Control of homeostatic and pathogenic balance in adipose tissue by ganglioside GM3. Glycobiology 2015; 25: 303–318. [DOI] [PubMed] [Google Scholar]
- 14.Randeria PS, Seeger MA, Wang XQ, et al. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc Natl Acad Sci USA 2015; 112: 5573–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang XQ, Lee S, Wilson H, et al. Ganglioside GM3 depletion reverses impaired wound healing in diabetic mice by activating IGF-1 and insulin receptors. J Invest Dermatol 2014; 134: 1446–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tagami S, Inokuchi Ji J, Kabayama K, et al. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem 2002; 277: 3085–3092. [DOI] [PubMed] [Google Scholar]
- 17.Bhangoo SK, Ren D, Miller RJ, et al. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun 2007; 21: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhangoo SK, Ripsch MS, Buchanan DJ, et al. Increased chemokine signaling in a model of HIV1-associated peripheral neuropathy. Molec Pain 2009; 5: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LaMotte CC. Lamina X of primate spinal cord: distribution of five neuropeptides and serotonin. Neuroscience 1988; 25: 639–658. [DOI] [PubMed] [Google Scholar]
- 20.Ma C, Shu Y, Zheng Z, et al. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol 2003; 89: 1588–1602. [DOI] [PubMed] [Google Scholar]
- 21.Menichella DM, Abdelhak B, Ren D, et al. CXCR4 chemokine receptor signaling mediates pain in diabetic neuropathy. Molec Pain 2014; 10: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashita T, Hashiramoto A, Haluzik M, et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci USA 2003; 100: 3445–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamashita T, Wu YP, Sandhoff R, et al. Interruption of ganglioside synthesis produces central nervous system degeneration and altered axon-glial interactions. Proc Natl Acad Sci USA 2005; 102: 2725–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayala JE, Samuel VT, Morton GJ, et al. Consortium NIHMMPC: standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 2010; 3: 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estacion M, Vohra BP, Liu S, et al. Ca2+ toxicity due to reverse Na+/Ca2+ exchange contributes to degeneration of neurites of DRG neurons induced by a neuropathy-associated Nav1.7 mutation. J Neuro 2015; 114: 1554–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev 2013; 93: 137–188. [DOI] [PubMed] [Google Scholar]
- 27.Gibran NS, Jang YC, Isik FF, et al. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res 2002; 108: 122–128. [DOI] [PubMed] [Google Scholar]
- 28.Cheng C, Singh V, Krishnan A, et al. Loss of innervation and axon plasticity accompanies impaired diabetic wound healing. PLoS One 2013; 8: e75877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue M, Fujii Y, Furukawa K, et al. Refractory skin injury in complex knock-out mice expressing only the GM3 ganglioside. J Biol Chem 2002; 277: 29881–29888. [DOI] [PubMed] [Google Scholar]
- 30.Sugiura Y, Furukawa K, Tajima O, et al. Sensory nerve-dominant nerve degeneration and remodeling in the mutant mice lacking complex gangliosides. Neuroscience 2005; 135: 1167–1178. [DOI] [PubMed] [Google Scholar]
- 31.Wang JT, Medress ZA, Barres BA. Axon degeneration: molecular mechanisms of a self-destruction pathway. J Cell Biol 2012; 196: 7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehning EJ, Doshi R, Isaksson N, et al. Mechanisms of injury-induced calcium entry into peripheral nerve myelinated axons: role of reverse sodium-calcium exchange. J Neurochem 1996; 66: 493–500. [DOI] [PubMed] [Google Scholar]
- 33.Persson AK, Hoeijmakers JG, Estacion M, et al. Sodium channels, mitochondria, and axonal degeneration in peripheral neuropathy. Trends Molec Med 2016; 22: 377–90. [DOI] [PubMed] [Google Scholar]
- 34.Qiao GF, Cheng ZF, Huo R, et al. GM1 ganglioside contributes to retain the neuronal conduction and neuronal excitability in visceral and baroreceptor afferents. J Neurochem 2008; 106: 1637–1645. [DOI] [PubMed] [Google Scholar]
- 35.Sano R, Annunziata I, Patterson A, et al. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell 2009; 36: 500–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamid HS, Mervak CM, Munch AE, et al. Hyperglycemia- and neuropathy-induced changes in mitochondria within sensory nerves. Ann Clin Transl Neurol 2014; 1: 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urashima R, Mihara M. Cutaneous nerves in atopic dermatitis. A histological, immunohistochemical and electron microscopic study. Virchows Arch 1998; 432: 363–370. [DOI] [PubMed] [Google Scholar]
- 38.Kinkelin I, Motzing S, Koltenzenburg M, et al. Increase in NGF content and nerve fiber sprouting in human allergic contact eczema. Cell Tissue Res 2000; 302: 31–37. [DOI] [PubMed] [Google Scholar]
- 39.Baumbauer KM, DeBerry JJ, Adelman PC, et al. Keratinocytes can modulate and directly initiate nociceptive responses. eLife 2015; 4: 09674. [DOI] [PMC free article] [PubMed] [Google Scholar]