Abstract
We report nanoparticle-stabilized capsules (NPSCs) as a platform for co-delivery of survivin-targeted siRNA and tamoxifen. These capsules feature an inner oil core that provides a carrier for tamoxifen, and coated is coated on the surface with positvely charged nanoparticles self-assembled with siRNA. the multifaceted chemical nature of the NPSC system enables simultaneous delivery of both payloads directly into the cytosol in vitro. NPSC co-delivery of tamoxifen and survivin-targeted siRNA into breast cancer cells disables pathways that inhibit apoptosis, resulting in enhanced breast cell death.
Introduction
Breast cancer is the most common type of cancer affecting women worldwide; approximately 1 in 8 women will develop invasive breast cancer at some point during their lifetime.1 Traditional therapies for breast cancer include chemo- and radio-therapies, treatments that often lose effectiveness over time and have substantial off-target effects. There has been a significant drive in research to discover innovative methods for treating breast cancer in safer, more robust and successful ways.
Over the past decade there has been considerable effort to capitalize on the potential of small interfering RNA (siRNA) to act as an anti-cancer therapy.2–4 siRNA can be introduced into cells to induce the endogenous RNA interference (RNAi) mechanism, by which the mRNA of specific proteins are degraded, resulting in corresponding knockdown.5 By introducing siRNA targeted to the messenger RNA (mRNA) of proteins that promote cancer survival and expansion, the progression of cancer may be inhibited or even reversed outright.6
Due to the high specificity and low toxicity of siRNA, there have been numerous investigations toward its use as an alternative to traditional cancer treatments7–10. Because siRNA is both large and strongly negatively charged, a delivery platform is required to introduce exogenous siRNA into cells for induced RNAi.11 Many different vehicles have been synthesized for this purpose; however, the majority of these rely on endocytosis to enter cells, and therefore require an endosomal escape strategy for siRNA to reach the cytoplasm, where RNAi occurs.2,3,4 Without endosomal escape, the siRNA remains trapped, decreasing agent efficacy. There are several different strategies that have been developed for release, including the proton sponge effect, endosome pore formation, and photo-activated endosome disruption.12 While these methods differ in their specific mechanisms, they typically involve rupture of the endosomal membrane caused by a chemical or physical force. However, the escape efficiency of the delivered cargo is often poor, resulting in an inevitable decrease in potency for vehicles relying on endocytosis. Additionally, strategies for endosomal escape often show undesirable, non-specific cellular toxicity.
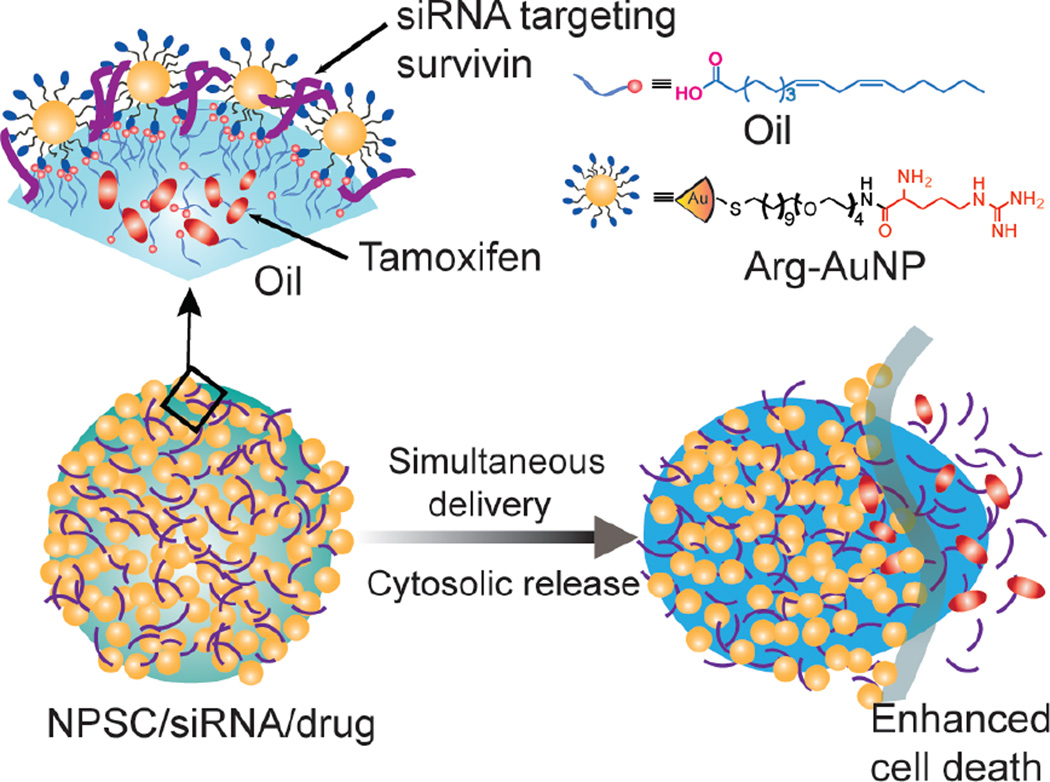
Nanoparticle stabilized capsules (NPSC) provide a potential solution to the issue of endosomal sequestration of siRNA.25 NPSCs consist of an linoleic acid oil core coated in arginine-functionalized gold nanoparticles (Arg-AuNP) that are electrostatically associated with negatively charged biomolecules such as siRNA (Figure 1). Instead of relying on endocytosis to enter cells, NPSCs use an entirely different mechanism, circumventing endosomal entrapment.25 Evidence suggests that the linoleic acid oil core of the NPSC causes the vehicle to utilize a cholesterol dependent “membrane fusion” mechanism: the oil core fuses with the lipid portion of the cell membrane upon contact, resulting in direct entry of associated cargoes into the cytosol of treated cells.25 By entering the cells via this alternative mechanism, NPSCs avoid the need to employ a means for endosomal escape, decreasing dosages and giving them enhanced promise as efficient, non-toxic co-delivery vehicles.
Figure 1.
Schematic presentation of NPSC-mediated co-delivery of both siSurv and tamoxifen for enhanced cancer therapy.
Building on the capabilities of siRNA, therapies have been designed that combine the targeted knockdown capabilities of siRNA with the more broadly cytotoxic power of small molecule drugs to create '1–2 punch'.2 A variety of platforms have been developed for the simultaneous delivery of siRNA and drugs, including polymers,13 liposomes,14 and mesoporous silica nanoparticles.15 In general, these nanoparticles encapsulate siRNA via electrostatic interactions, with anticancer drugs loaded by either non-covalent encapsulation or covalent conjugation.16 These co-delivery strategies have resulted in improved cancer therapies by countering biological compensation and by accessing multiple context-specific targets.
While co-delivery vehicles have shown promise, they face not only all of the challenges that are encountered in individual siRNA or small-molecule drug delivery, but also unique issues.17–22 The syntheses of vehicles that can tolerate both aqueous and hydrophobic cargoes can be cumbersome and unwieldy.21 Furthermore, endocytosis remains the primary method of uptake for these vehicles, which results in endosomal entrapment of both cargoes, reducing the total concentration of successfully delivered therapeutic.23 Hydrophobic small molecule drugs pose an additional challenge since they are not compatible with the aqueous environment of the body, and exhibit unfavorable pharmacokinetic and pharmacodynamic features.21 Because of their hydrophobicity, these molecules have retention and excretion characteristics that are significantly different from those of aqueous molecules. The challenging pharmacokinetics of these drugs also presents an issue for co-delivery as vehicle modifications meeting the requirements for hydrophobic drug incorporation may alter the release profile.21 Because of these challenges, there has been little effort towards developing potentially powerful drugs that do not have favorable solubility profiles; this trend can be reversed by developing effective delivery vehicles for hydrophobic drugs.24
We have previously reported the use of nanoparticle-stabilized capsules for independent deliveries of proteins, siRNA, and drugs.25–27 The outer positively charged shell of the “nanocapsule” structure allows interaction with negatively charged molecules such as siRNA and certain proteins, while the inner oil core facilitates encapsulation of hydrophobic chemotherapeutics. Here, we combine their capacities to associate with siRNA electrostatically and solubilize hydrophobic drugs in the oil layer to generate the first NPSC dual-delivery agents. By co-loading NPSCs concomitantly with siRNA and anti-cancer drugs, we not only reduced the number of doses required to treat the cells with multiple therapeutics, but also anticipate that the dual-loaded entities will have a greater anti-cancer therapeutic effect than individual treatments alone.
The therapeutic combination chosen for NPSC co-delivery is survivin-targeted siRNA (siSurv) and tamoxifen. One of the core elements of this strategy is delivery of siRNA targeted to the cancer-related protein survivin. Survivin is an inhibitor of apoptosis protein (IAP) that is over-expressed in nearly every type of cancer.18 The delivery of siSurv decreases survivin expression, thus preventing the caspase-inhibiting action of the functional survivin protein, enabling apoptosis.28 Tamoxifen (TM) is a highly hydrophobic cytostatic and cytotoxic chemotherapeutic used clinically for the treatment of estrogen receptor positive breast cancers.19,29 It has been shown that cancer cells that only receive a single treatment of tamoxifen alone can either evade apoptosis by over-expressing survivin, while those that are treated with siSurv alone do not have sufficient impetus to undergo apoptosis following survivin knockdown.30 For this reason, we concomitantly deliver the two via NPSCs to yield increased breast cancer cell death by both halting growth of cancer cells, thereby driving them towards apoptosis, and removing their ability to avoid it, in a single treatment.
In this paper, we demonstrate that NPSCs are highly effective in the simultaneous delivery of anti-Survivin siRNA and tamoxifen to MCF7 human breast cancer cells. We confirm NPSC-mediated delivery of both to the cytoplasm, and ensure decreased survivin mRNA levels following treatment with siSurv. To demonstrate the enhanced anti-cancer efficiency of the dual-loaded siRNA, we used cell viability assays and apoptotic staining. As a result, we find that not only does NPSC encapsulation increase the effectiveness of tamoxifen treatment, but that the siRNA/drug combination is more effective in inducing cancer cell death than individual treatments alone. These results illustrate that the NPSC vehicle holds promise as an effective treatment vector for cancer by facilitating the effective co-delivery of various therapeutics.
Methods
General methods
All standard reagents and chemicals used were purchased from Fisher Scientific or Sigma-Aldrich, except where noted. Chloroauric acid used for gold nanoparticle synthesis was bought from Strem Chemicals Inc. (Newburyport, MA). Validated siSurv was purchased from Sigma-Aldrich. Scrambled siRNA and FAM-labeled scrambled siRNA were purchased from Invitrogen (Carlsbad, CA). Transmission electron microscopy (TEM) was performed on a JEOL-2010 microscope with an accelerating voltage of 200 kV. Particle size was measured using a Malvern Zetasizer (Nano series, Malvern Instruments Inc, USA) with a He-Ne laser (633 nm) and a backscattering angle of 173°. Confocal microscopy images were obtained on an Eclipse Ti-E microscope (Nikon, Tokyo, Japan) using a 63× or 10× objective.
NPSC, drug and siRNA complexation
The arginine-functionalized AuNP (Arg-AuNP) and NPSC were synthesized according to our previous report.25–27 Briefly, tamoxifen was dissolved into linoleic acid at 120 mg/mL. Then, 1 µL of linoleic acid was mixed with 500 µL of phosphate buffer (5 mM, pH = 7.4) containing 1 µM Arg-AuNP and agitated by an amalgamator at 5000 rpm for 100 s to form emulsions. Then, 10 µL of the emulsion was added into 90 µL of 5 mM phosphate buffer containing 2.5 µM Arg-AuNP and incubated for 10 min. at room temperature to afford NPSC/drug/siRNA complex.
Cell culture
MCF7 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). Cells were cultured at 37 °C under humidified atmosphere with 5% CO2. High-glucose DMEM (4.5 g/L) with 10% fetal bovine serum (FBS), 1% antibiotic (100 U/mL penicillin and 100 µg/mL streptomycin) was used for culturing cells.
Fluorescently labeled siRNA and oil delivery
For confocal laser scanning microscopy (CLSM) imaging of cellular uptake of the NPSC/siRNA complex, MCF7 cells (8.0 × 104 cells) were seeded in each well of a 4 chamber Lab-Tek II chambered coverglass system (Nunc, NY) one day prior to the experiment. On the day of delivery, the culture medium was replaced with Opti-MEM containing 40 nM NPSC/FAM-siRNA/Nile Red; the cells were subsequently incubated for 2 h at 37 °C. After 2 h, the media was removed, and the cells were washed once with cold phosphate buffer saline (PBS); fluorescence imaging was performed in PBS using an Eclipse Ti-E microscope.
NPSC/siSurv delivery for evaluation of survivin expression
MCF7 cells (2 × 104 cells/well) were seeded in a 24-well plate for 24 h prior to delivery. On the day of transfection, the cells were washed with PBS three times, followed by the addition of various siSurv or scrambled siRNA formulations in Opti-MEM, which were incubated with the cells for 3 h. The Opti-MEM was replaced with fresh culture media, and the cells were incubated for an additional 48 h prior to further analysis.
RNA extraction
Approximately 1.5 µg of RNA was harvested using the Pure Link RNA Mini kit (Ambion) following the manufacturer’s instructions. Superscript IV reverse transcriptase was used for conversion of approximately 150 ng of RNA to cDNA, along with RNaseOut, 10 mM dNTPs, and 50 µm random hexamers (Thermo Fisher Scientific), also following manufacturer’s instructions.
Real Time Polymerase Chain Reaction (RT-PCR)
RTPCR was performed on cDNA as prepared above using a CFX Connect Real Time System with iTaq Universal SYBR Green Supermix (Biorad). All DNA primers were purchased from Integrated DNA Technologies (Caralville, Iowa). The following sequences were used: GAPDH Forward (5’- CTT CTT TTG CGT CGC CAG CC-3’), Reverse (5’-ATT CCG TTG ACT CCG ACC TTC-3’); Survivin Forward (5’- GAC GAC CCC ATA GAG GAA CA- 3’), Reverse (5’-CTT GAC AGA AAG GAA AGC GCA-3’). The samples were incubated as follows: the samples were first activated at 50 °C for 2 min then 95 °C for 2 min. Then denaturing occurred at 95 °C for 5 min followed by annealing at 60 °C. The denature/anneal process was repeated over 40 cycles. At the conclusion a melting analysis was performed holding at 95 °C for 10 min, then 60 °C for 5 min and then back to 95 °C for 5 min. Relative survivin expression was determined by comparing the Ct value of survivin to that of GAPDH, used as a housekeeping gene, by the 2^ΔΔCt method.31 Three biological replicates were performed for each control group and three technical replicates were used for each biological replicate.
Serum stability gel electrophoresis assay
NPSC complexes containing 300 pmol siRNA were incubated in 5 mM PB containing 10% FBS at 37°C for 2, 4 and 7 hours. At those time points, 1 µL 50 mM SDS was added and the solution was heated to 60°C for 5 minutes to denature the nucleases in the FBS. The siRNA was then released from the complexes by addition of 1 µL of 25 mg/mL heparin solution. The solutions were then added to a 1% w/v agarose gel (40 mM Tris-HCl, 1% v/v acetic acid, 1 mM EDTA) which was then subjected to electrophoresis at 120 v for 15 minutes.
Cytotoxicity of NPSC/siRNA complex
MCF7 cells (2 × 104 cells/well) were seeded in a 24-well plate 24 h prior to the experiment. On the day of experiment, cells were washed with PBS and treated with varied concentrations of NPSC and siRNA/tamoxifen complexes in OptiMem (prepared similarly to NPSC/siSurv complex solution described above) for 3 h. The treatment media was replaced with fresh culture media, and cells were incubated for an additional 48 h. For cells treated with free tamoxifen, tamoxifen was dissolved in DMSO and added directly to cell solutions. The cell viability was determined using Alamar Blue assay reagent (Invitrogen, CA) using a SpectraMax M2 plate reader exciting at 560 nm and detecting emission at 590 nm. Three biological replicates were performed for viability determination.
Apoptosis Assay
MCF7 cells (2.0 × 104 cells) were seeded in each well of a 4 chamber Lab-Tek II chambered coverglass system (Nunc, NY) a day before the experiment. On the day of, cells were washed with cold PBS and treated with various concentrations of NPSC and siRNA/tamoxifen complexes in OptiMEM for 3 h, followed by replacement of media. For cells treated with free tamoxifen, tamoxifen was dissolved in DMSO and added directly to cell solutions. The cells were incubated for an additional 48 h, after which the media was replaced with fresh growth media containing 1 mM YOPRO-1 (ThermoFisher Scientific) apoptotic staining reagent, and incubated for 30 min at 37 °C. After removing medium, the cells were washed and replaced with cold phosphate buffer saline (PBS) for fluorescence imaging under an Eclipse Ti-E microscope.
Results and Discussion
Prior to co-delivery experiments, NPSCs were fabricated and characterized. NPSCs were prepared as described previously.25–27 Previous work has optimized the formulation of the NPSCs so essentially all gold nanoparticles in solution are incorporated into the NPSC complex.26 In order to determine the quality of the NPSCs synthesized, TEM images and DLS measurements were obtained to determine the relative size and morphology of the fabricated capsules (Figure S1). These measurements demonstrated a consistent spherical morphology and a stable size of approximately 150 nm in diameter, with no population of unbound nanoparticle (Figure S2). Additionally, DLS and serum stability assays were utilized to demonstrate the relative stability and siRNA protection capabilities of NPSCs in serum conditions. These assays demonstrated that the NPSC were able to stay intact in serum for a significant period of time (Figure S3 and S4).
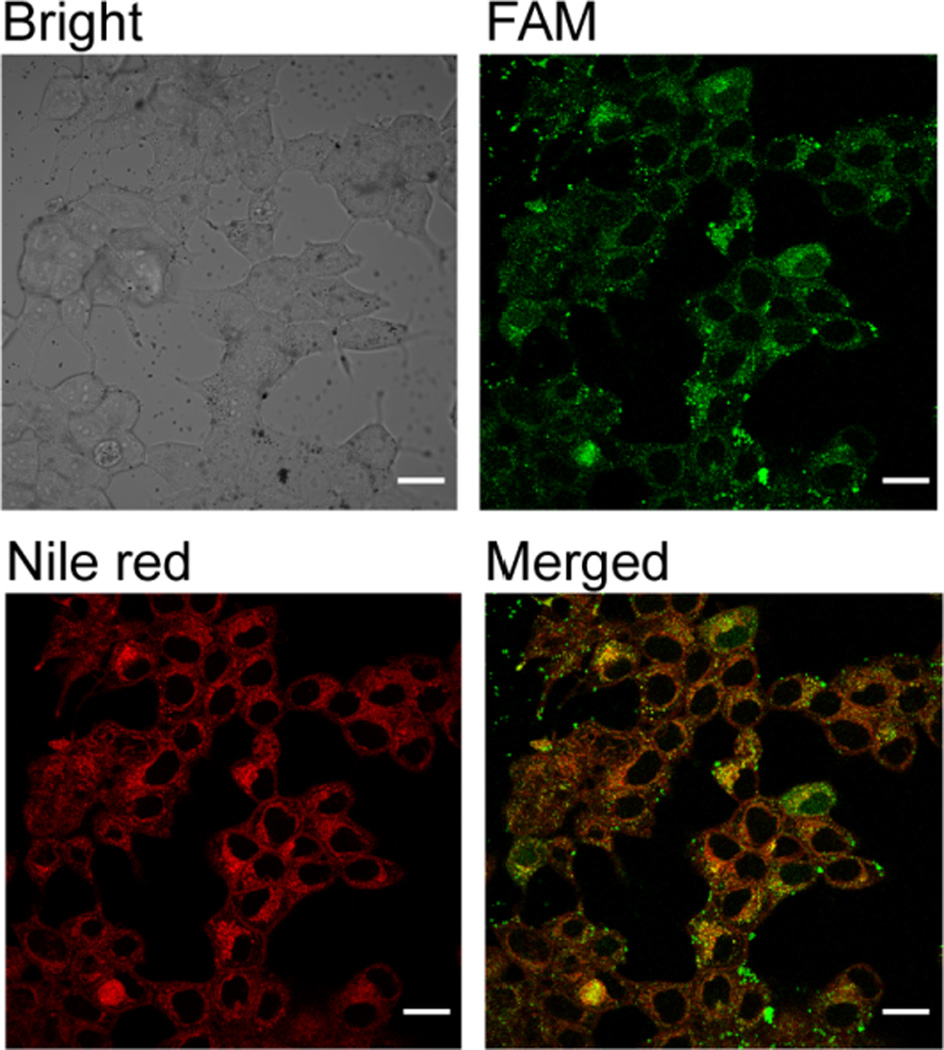
Having confirmed the successful fabrication of NPSCs, we determined whether NPSCs could deliver siRNA and a hydrophobic small molecule simultaneously into the cytosol. We have previously reported that the NPSCs can efficiently deliver drugs or siRNA individually into the cytosol of treated cells.25–27 To confirm co-delivery, FAM-labeled scramble sequence siRNA (FAM-siRNA) was incorporated onto the surface of NPSCs that had Nile Red dissolved in the interior oil core. Using confocal laser scanning microscopy (CLSM) (Figure 2) to monitor uptake, we observed significant and diffuse green and red fluorescence throughout the cytosol following treatment. This pattern suggests that the fluorophore has free access to the entire cytosol since an endocytotic route would lead to endosomal entrapment,25 which should be apparent via “punctuate” fluorescence signal and is not observed.25 Additionally, there is significant overlap of the red and green fluorescence signals, providing evidence for co-localization of both cargoes.
Figure 2.
Confocal microscopy images of MCF-7 cells following 2 hour treatment with NPSC/FAM-siRNA/Nile red complex. Scale bar: 20 µm.
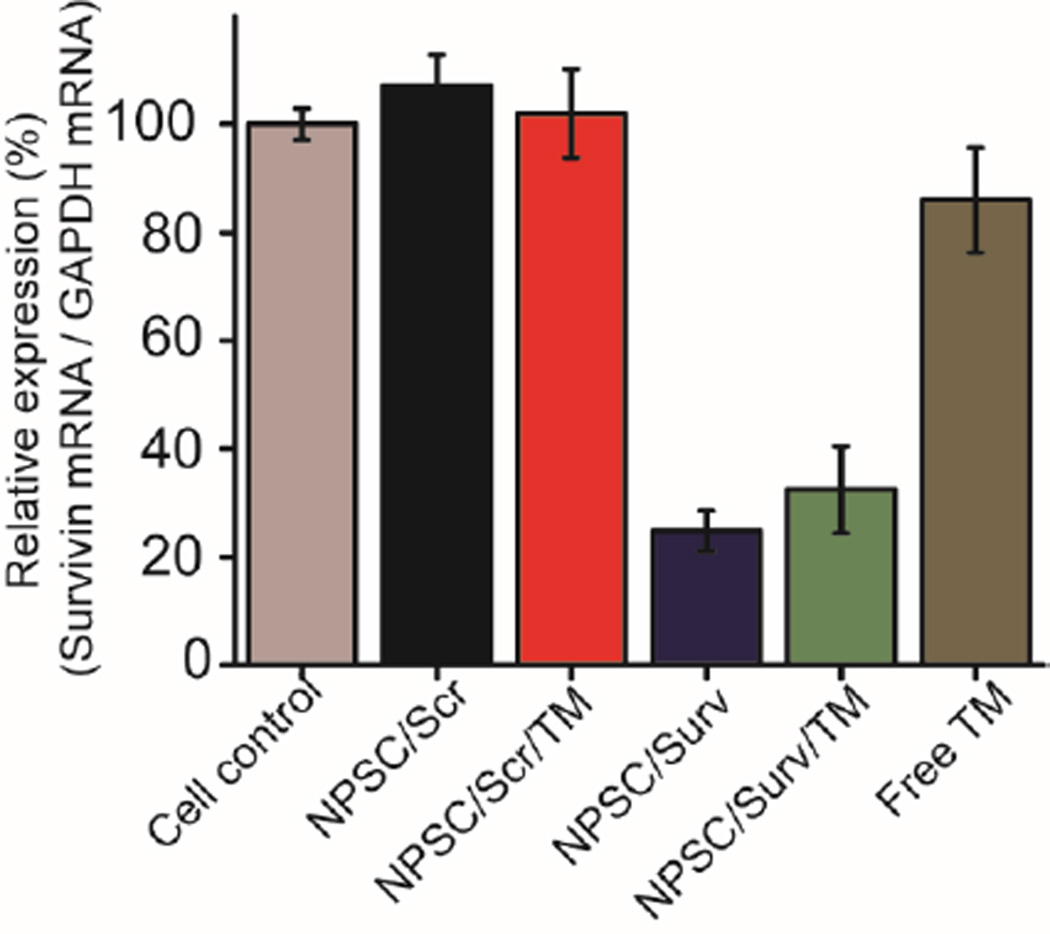
To confirm the ability of NPSCs to effectively deliver siRNA for knock down of survivin, Real Time-Polymerase Chain Reaction (RT-PCR) was used to determine the change in mRNA expression following treatment (Figure 3). Both the NPSCs loaded with siSurv alone and NPSCs loaded with tamoxifen and siSurv resulted in an approximately 70% decrease in survivin mRNA levels. Concurrently, the control groups that did not receive siSurv treatment showed minimal survivin mRNA change compared to untreated control. This data is evidence of the ability of NPSCs to efficiently deliver siRNA into the cytosol to elicit specific RNAi to occur. The ability of NPSCs to deliver siRNA for targeted knockdown in serum conditions was likewise demonstrated (Figure S5).
Figure 3.
RTPCR of normalized survivin mRNA levels in MCF-7 cells 48 hours after treatment. Cell control (non-treated), NPSC/Scr (Scr: 40 nM), NPSC/Scr/TM (Scr: 40 nM, and TM: 2.6 µM), NPSC/Surv (Surv: 40 nM), NPSC/Surv/TM (Surv: 40 nM, and TM: 2.6 µM), and free TM (10 µM).
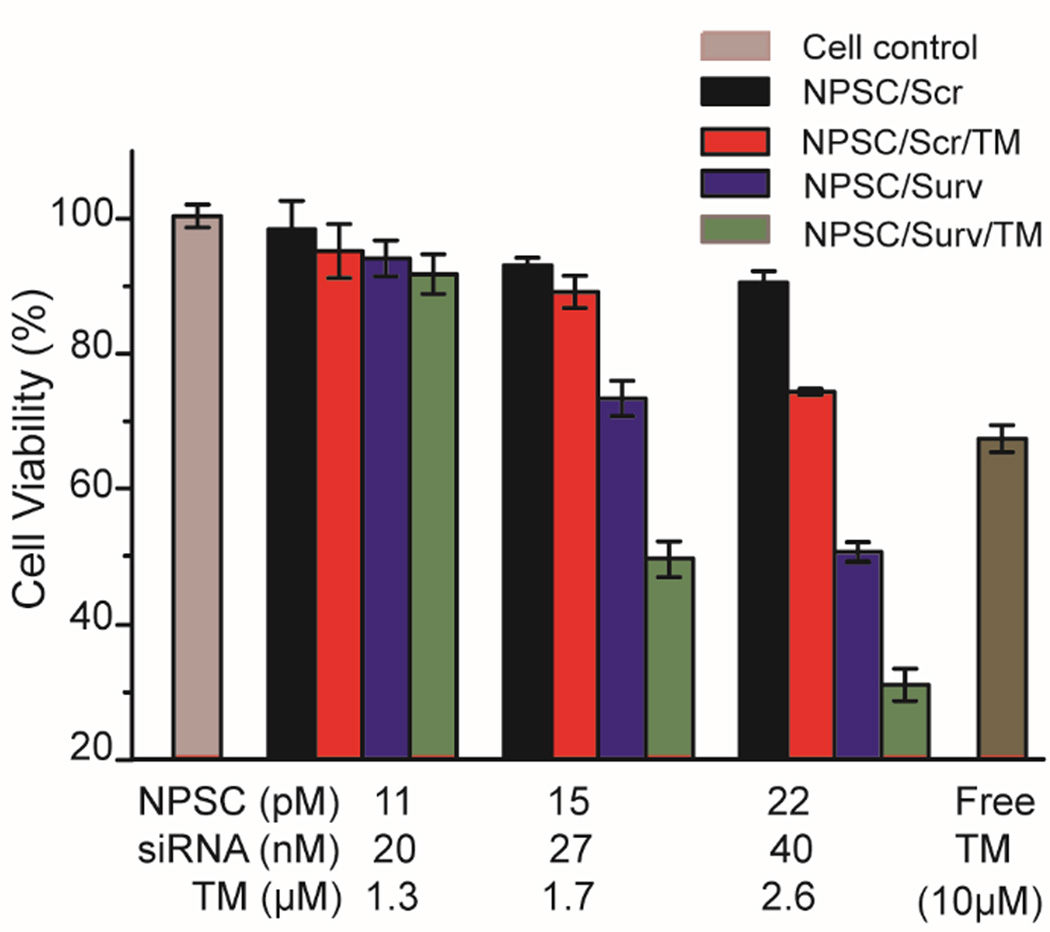
We next investigated the therapeutic potential of siRNA and tamoxifen co-delivery using NPSCs. To evaluate the efficacy of this combination, an Alamar Blue cell viability assay was performed (Figure 4). As shown in Figure 4, the NPSCs with both siSurv and tamoxifen reduce cell viability more than either of the individual treatments at any concentration level. With 40 nM siRNA and 2.6 µM tamoxifen, the combination outperforms either individual treatment by a minimum of 30% more decrease in cell viability, resulting in 80% decrease overall. Loading either siSurv or tamoxifen individually into the NPSCs shows dose-dependent decrease in cell viability. 40 nM siSurv/NPSC treatment resulted in a 50% decrease in cell viability, while 2.6 µm tamoxifen/NPSC treatment caused a 27% decrease in cell viability. This result suggests that not only is the combination of tamoxifen and siSurv efficient at killing breast cancer cells, but that NPSCs are an effective platform for co-delivery. Interestingly, NPSC delivery of tamoxifen results in a greater decrease in cell viability than free tamoxifen even at much higher doses. Because NPSCs show nearly undetectable toxicity at high doses (Figure S6), this may be the result of active tamoxifen delivery into the cell membrane via the unique membrane fusion entry mechanism of the capsules versus the passive diffusion that occurs with free tamoxifen.19 This is another major benefit of the NPSC system as the increased potency reduces the required concentration for a therapeutic effect, decreasing the risk of side effects that are common with chemotherapeutics. The apparent increase in cell death following co-delivery was further confirmed by staining of apoptotic nuclei with YOPRO-1 (Figure S7), where cells treated with NPSCs carrying both chemotherapeutic cargoes displayed a major increase in fluorescent nuclei, versus controls. This experiment provides evidence that this treatment strategy will have a wide therapeutic window that is beneficial for long term cancer treatments.
Figure 4.
Alamar blue evaluation of MCF-7 cell viability. Cells were treated with NPSC/Scr, NPSC/Scr/TM, NPSC/Surv, NPSCs/Surv/TM complexes, and free TM at indicated siRNA and drug concentrations. The error bars represent the standard deviations of three parallel measurements.
Conclusion
We have reported the fabrication and development of a combination siRNA and hydrophobic anti-cancer drug delivery platform using NPSCs. NPSCs composed of Arg-AuNP and linoleic acid provide an ideal combination of chemical functionalities and interacting forces to effectively bind and encapsulate anti-survivin siRNA and tamoxifen, and are able to deliver them efficiently to the cytosol in a non-endocytotic manner. Not only did the co-delivery of both therapeutics result in the increased efficacy of cancer cell death, incorporation of tamoxifen in the NPSCs resulted in higher cell death than tamoxifen alone, a fact that could be attributed to active delivery of tamoxifen in aqueous media by the capsules. NPSC-mediated co-delivery is highly promising in terms of being able to maximize the usefulness of different therapies and reduce the probability of adverse side effects that are currently common in most cancer therapies.21 The NPSC platform can be extended to a variety of combination therapies involving different small molecule drugs and biologics in order to produce advanced cancer therapies. Future work will develop the NPSC platform as a therapeutic vehicle further utilizing the co-delivery capabilities of the vehicle to deliver different cargo combinations, providing new therapeutic approaches for different disease models.
Supplementary Material
Notes and references
- 1.American Cancer Society. Cancer Facts & Figures. [accessed January 28, 2016];2015 http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2015/ [Google Scholar]
- 2.Tobin LA, Xie Y, Tsokos M, Chung SI, Merz AA, Arnold MA, Li G, Malech HL, Kwong KF. Biomaterials. 2013;34:2980–2990. doi: 10.1016/j.biomaterials.2013.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma X, Zhao Y, Ng KG, Zhao Y. Chem. Eur. J. 2013;19:15593–15603. doi: 10.1002/chem.201302736. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Wang Y, Zhao M, Tan C, Li Y, Le X, Ji L, Mao Z. Biomaterials. 2012;33:2780–2790. doi: 10.1016/j.biomaterials.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Lapierre J, Salomon W, Cardia J, Bulock K, Lam JT, Stanney WJ, Ford G, Smith-Anzures B, Woolf T, Kamens J, Khvorova A, Samarsky D. RNA. 2011;17:1032. doi: 10.1261/rna.2399411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Liu S, Zhang G, Zhou C, Zhu H, Zhou X, Quan L, Bai J, Xu N. Breast Cancer Res. 2005;7:R220. doi: 10.1186/bcr975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gary DJ, Puri N, Won YY. J. Control. Release. 2007;121:64–73. doi: 10.1016/j.jconrel.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 8.Santel A, Aleku M, Keil O, Endruschat J, Esche V, Fisch G, Dames S, Löffler K, Fechtner M, Arnold W, Giese K, Klippel A, Kaufmann J. Gene Ther. 2006;13:1222–1234. doi: 10.1038/sj.gt.3302777. [DOI] [PubMed] [Google Scholar]
- 9.Medarova Z, Pham W, Farrar C, Petkova V, Moore A. Nat. Med. 2007;13:372–377. doi: 10.1038/nm1486. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Wang Z, Liu D, Yan X, Wang F, Niu G, Yang M, Chen X. Angew. Chem. Int. Ed. Engl. 2014;53:1997–2001. doi: 10.1002/anie.201309985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 12.Varkouhi AK, Scholte M, Storm G, Haisma HJ. J. Control. Release. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Zhu C, Jung S, Luo S, Meng F, Zhu X, Park TG, Zhong Z. Biomat. 2010;31:2408–2416. doi: 10.1016/j.biomaterials.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 14.Shim G, Han SE, Yu YH, Lee S, Lee HY, Kim K, Kwon IC, Park TG, Kim YB, Choi YS, Kim CW, Oh YK. J Control. Release. 2011;155:60–66. doi: 10.1016/j.jconrel.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 15.Chen AM, Zhang M, Wei D, Stueber D, Taratula O, Minko T, He H. Small. 2009;5:2673–2677. doi: 10.1002/smll.200900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar V, Mondal G, Slavik P, Rachagani S, Batra SK, Mahato RI. Mol. Pharm. 2015;12:1289–1298. doi: 10.1021/mp500847s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y, Huo S, Hardie J, Liang X, Rotello VM. Expert Opin. Drug Deliv. 2016;5247:1–13. doi: 10.1517/17425247.2016.1134486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groner B, Weiss A. BioDrugs. 2014;28:27–39. doi: 10.1007/s40259-013-0058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung RK, Whittaker PA. Pharmacol. Ther. 2005;107:222–239. doi: 10.1016/j.pharmthera.2005.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng A, Kallio A, Härkönen P. Endocrinology. 2007;148:2764–2777. doi: 10.1210/en.2006-1269. [DOI] [PubMed] [Google Scholar]
- 21.Lehenkari P, Parikka V, Rautiala T, Weckström M, Dahllund J, Härkönen PL, Väänänen HK. J Bone Miner. Res. 2003;18:473–481. doi: 10.1359/jbmr.2003.18.3.473. [DOI] [PubMed] [Google Scholar]
- 22.Aagaard L, Rossi JJ. Adv. Drug Deliv. Rev. 2007;59:75–86. doi: 10.1016/j.addr.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bae KH, Lee K, Kim C, Park TG. Biomaterials. 2011;32:176–184. doi: 10.1016/j.biomaterials.2010.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y, Tang R, Duncan B, Jiang Z, Yan B, Mout R, et al. Angew. Chem. Int. Ed. 2015;54:506–510. doi: 10.1002/anie.201409161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang XC, Samanta B, Agasti SS, Jeong Y, Zhu ZJ, Rana S, Miranda OR, Rotello VM. Angew. Chemie. 2011;123:497–501. doi: 10.1002/anie.201005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang R, Kim CS, Solfiell DJ, Rana S, Mout R, Velazquez-Delgado EM, Chompoosor A, Jeong Y, Yan B, Zhu Z, Kim C, Hardy JA, Rotello VM. ACS Nano. 2013;7:6667–6673. doi: 10.1021/nn402753y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kappler M, Bache M, Bartel F, Kotzsch M, Panian M, Würl P, Blümke K, Schmidt H, Meye A, Taubert H. Cancer Gene Ther. 2004;11:186–193. doi: 10.1038/sj.cgt.7700677. [DOI] [PubMed] [Google Scholar]
- 29.Brünner N, Bronzert D, Vindeløv LL, Rygaard K, Spang-Thomsen M, Lippman ME. Cancer Res. 1989;49:1515–1520. [PubMed] [Google Scholar]
- 30.George J, Banik NL, Ray SK. Neuro. Oncol. 2010;12:1088–1101. doi: 10.1093/neuonc/noq079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmittgen TD, Livak KJ. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.