Abstract
Merging pharmaceutical and digital (mobile health, mHealth) ingredients to create new therapies for chronic diseases offers unique opportunities for natural products such as omega-3 polyunsaturated fatty acids (n-3 PUFA), curcumin, resveratrol, theanine, or α-lipoic acid. These compounds, when combined with pharmaceutical drugs, show improved efficacy and safety in preclinical and clinical studies of epilepsy, neuropathic pain, osteoarthritis, depression, schizophrenia, diabetes and cancer. Their additional clinical benefits include reducing levels of TNFα and other inflammatory cytokines. We describe how pleiotropic natural products can be developed as bioactive incentives within the network pharmacology together with pharmaceutical drugs and self-care interventions. Since approximately 50% of chronically-ill patients do not take pharmaceutical drugs as prescribed, psychobehavioral incentives may appeal to patients at risk for medication non-adherence. For epilepsy, the incentive-based network therapy comprises anticonvulsant drugs, antiseizure natural products (n-3 PUFA, curcumin or/and resveratrol) coupled with disease-specific behavioral interventions delivered by mobile medical apps. The add-on combination of antiseizure natural products and mHealth supports patient empowerment and intrinsic motivation by having a choice in self-care behaviors. The incentivized therapies offer opportunities: (1) to improve clinical efficacy and safety of existing drugs, (2) to catalyze patient-centered, disease self-management and behavior-changing habits, also improving health-related quality-of-life after reaching remission, and (3) merging copyrighted mHealth software with natural products, thus establishing an intellectual property protection of medical treatments comprising the natural products existing in public domain and currently promoted as dietary supplements. Taken together, clinical research on synergies between existing drugs and pleiotropic natural products, and their integration with self-care, music and mHealth, expands precision/personalized medicine strategies for chronic diseases via pharmacological-behavioral combination therapies.
Keywords: Drug-resistant epilepsy, refractory epilepsy, inflammation, addiction, cognitive behavioral therapy, brain-derived neurotropic factor, music, Mozart, video games
INTRODUCTION
In addition to search for more effective pharmacological treatments, therapies for chronic diseases have been challenged by medication non-adherence. Due to real-life factors, the complexity of health care delivery, and compromised patient behaviors, this global health care
problem is also being addressed by self-care interventions [1]. Another challenge to improve therapies for chronic diseases is to convert clinically-beneficial natural products into medical treatments due to their public domain status [2]. These two challenges can be simultaneously tackled by overlapping opportunities in digital/mobile medical technologies which can deliver disease self-management content, new intellectual property and patient engagement [3]. This review describes new opportunities to address the aforementioned challenges by combining natural products with pharmaceutical drugs and behavioral interventions. For natural products which improve clinical outcomes, one strategy to convert them into medical treatments is by combining them as psychobehavioral incentives together with pharmaceutical drugs and mobile medical software. In the first part, we review clinical and preclinical studies supporting combination therapies consisting of pleiotropic natural products and pharmaceutical drugs. In the second part, we describe medication non-adherence for chronic disorders and premise of using natural products as psychobehavioral incentives to improve therapy outcomes. In the third section, we review evidence for combining antiseizure drugs with natural products and behavioral interventions for the treatment of epilepsy, as an example of incentivized therapy for a chronic disease. Lastly, we provide translational and industry considerations of developing molecular-behavioral combination therapies for chronic medical conditions by merging pharmaceutical and digital ingredients via mHealth.
Pleiotropic Natural Products and their Combinations with Pharmaceutical Drugs
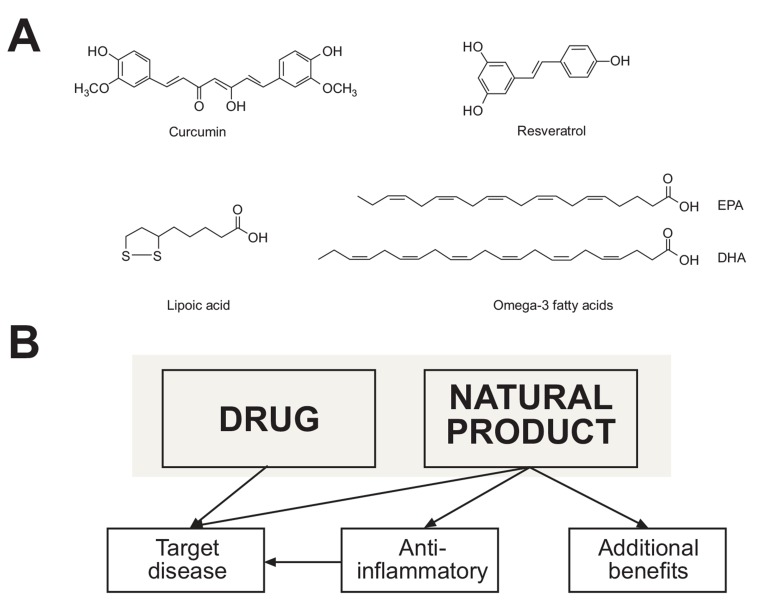
Pleiotropic natural products such as omega-3 polyunsaturated fatty acids (n-3 PUFA), α-lipoic acid, curcumin, L-theanine, or trans-resveratrol are extensively tested in clinical trials, while also commonly used as dietary supplements (Fig. 1A) [4-10]. These pleiotropic natural products produce multiple physiological responses through concurrent targeting of various receptors and signaling pathways, and/or scavenging free radicals. Reviews on mechanisms of action for these compounds have been published [11-15]. n-3 PUFA target peroxisome proliferator-activated receptors (PPARs) and NFκB transcription factors, selected G-protein coupled receptors, and can also affect cell membrane compositions and metabolic pathways for synthesis of pro-inflammatory eicosanoids [12, 13, 15-17]. α-Lipoic acid mediates antioxidant activity, chelation of metal ions, inhibition of NFκB and PPARs expression, and modulates lipid and glucose metabolism. Curcumin exerts antioxidant, anticancer and anti-inflammatory activities by targeting redox and inflammatory pathways via Nrf2, NFκB, COX-2, STAT and MAP kinases, as well as cyclin D1, MMP9, VEGF and CXCR4 [5, 11, 14, 18]. Resveratrol can activate α- and β- estrogen receptors, and also modulates diverse antioxidant, apoptotic and inflammatory pathways by activating or inhibiting PPARα, Nrf2, NFκB, SIRT1, AMPK, COX1/2 among others [8, 19-23]. These natural products modulate inflammatory cytokines, including TNFα, IL-1 and IL-6 [4, 24-27]. Neuropharmacological effects of theanine are mediated via several receptors (GABA(A), NMDA, AMPA, kainite), the nitric oxide pathway, and upregulated expression of brain-derived neurotropic factor (BDNF) [28-32]. Taken together, pleiotropic natural products can be considered as “a single compound with selective polypharmacology” (Fig. 1B) [33-35].
Fig. (1).
Structures of selected pleiotropic natural products (A), and their multiple functions within the network pharmacology (B). Pleiotropic natural products exert pharmacological properties via specific molecular targets and signaling pathways, while also delivering anti-inflammatory activities. Additional benefits of pleiotropic natural products may include antidepressant, cardiovascular, metabolic, neuroprotective, cognitive, antioxidant, or anticancer activities [11-15, 41].
Clinical studies of the pleiotropic natural products suggest their favorable tolerability, safety and efficacy in several medical indications. n-3 PUFA (omega-3-acid ethyl esters; Lovaza® by GlaxoSmithKline) was approved by the US Food and Drug Administration as an adjunct therapy for patients with severe hypertriglyceridemia [36]. Clinical data on metabolism and toxicity of resveratrol suggest that it is well tolerated, but clinical efficacy data remain limited [9, 37, 38]. Repeated administration of formulated curcumin (200 mg or 400 mg daily) in cancer patients was safe and well tolerated [39]. Six-week treatments with curcumin (1000 mg/day) appeared safe for patients with major depressive disorders [40]. Safety, tolerability and efficacy of α-lipoic acid was studied in diabetes, Alzheimer’s disease (AD), multiple sclerosis (MS), neuropathy and pain [27, 41-45]. Theanine has been clinically tested in patients with schizophrenia [46] and attention-deficit hyperactivity disorder (ADHD) [28]. The pleiotropic natural products are clinically tested as monotherapies or as adjunct therapies to existing medical treatments. Examples of clinical trials showing improved efficacy by combining selected natural products with analgesics, anti-seizure, anticancer, antidiabetic, or antidepressant drugs are shown in Table 1. New opportunities for n-3 PUFA also include cystic fibrosis [47] and asthma [48]. Despite positive effects of the natural products on specific medical conditions (n-3 PUFA in cardiovascular disease (CVD), α-LA for diabetic neuropathy, curcumin in depression), clinical trials of the natural products tested as monotherapies sometimes report inconclusive or negative results. There are also emerging reports which suggest prostate cancer or metabolic risks associated with n-3 PUFA supplementation [49, 50]. Taken together, current clinical results on the natural products suggest their efficacy in therapies in which additional anti-inflammatory and neuroprotective activities are beneficial for a patient.
Table 1.
Clinical studies showing clinical effects of combining pleiotropic natural products with pharmaceutical drugs.
| Indication | Drug | Natural Product | Refs. |
|---|---|---|---|
| ARTHRITIS | |||
| Osteoarthritis pain | NSAIDS | Curcumin | [51] |
| Knee osteoarthritis | NSAIDS, other analgesics | Curcumin and Glucosamine | [52] |
| Knee osteoarthritis | NSAIDS | Curcumin | [53] |
| Knee osteoarthritis | NSAIDS, paracetamol | n-3 PUFA | [54] |
| Rheumatoid arthritis | Diclofenac sodium | Curcumin | [55] |
| PAIN | |||
| Burning mouth syndrome | Gabapentin | α-Lipoic acid | [56] |
| Postoperative pain | Opioid analgesics | Capsaicin | [57] |
| Chronic neck pain | Superoxide dismutase | α-Lipoic acid | [58] |
| EPILEPSY | |||
| Drug resistant epilepsy | Antiepileptic drugs | n-3 PUFA | [59] |
| Drug resistant epilepsy | Antiepileptic drugs | n-3 PUFA | [60] |
| Refractory seizures | Antiepileptic drugs | n-3 PUFA | [61] |
| Intractable focal or generalized epilepsy | Antiepileptic drugs | n-3 PUFA | [62] |
| CANCER | |||
| Pancreatic cancer | Gemcitabine | Curcumin | [63] |
| Prostate cancer | Docetaxel | Curcumin | [64] |
| Leukemia | Imatinib | Curcumin | [65] |
| Breast cancer | Anthracycline-based drugs | n-3 PUFA | [66] |
| Lung cancer | First-line chemotherapy | n-3 PUFA | [67] |
| Breast cancer | Paclitaxel | n-3 PUFA | [68] |
| Prostate cancer | Lycopene | n-3 PUFA | [69] |
| Gastrointestinal | n-3 PUFA | [70] | |
| DIABETES | |||
| Type 2 | Insulin | Resveratrol | [71] |
| Type 2 | Insulin | Curcumin | [72] |
| Diabetes mellitus and hypertension | Quinapril | α-Lipoic Acid | NCT00795262 |
| Peripheral diabetic neuropathy | Prostaglandin E1 | α-Lipoic Acid | [73] |
| MENTAL DISORDERS | |||
| Depression | Fluoxetine | Curcumin | [40] |
| Reserpine (rats) | Curcumin | [74] | |
| Major depressive disorder | Escitalopram | Curcumin | [75] |
| Major depressive disorder | Selective serotonin reuptake inhibitors (SSRIs) | Creatine | [76] |
| Depression | Psychotropic drugs | n-3 PUFA | [77] |
| Schizophrenia | Antipsychotic drugs | Theanine | [78, 79] |
Preclinical studies on additive and synergistic effects between pharmaceutical drugs and the natural products encourage randomized clinical trials of their combinations as the adjunct therapies [80]. Table 2 summarizes results from studies in animal models of pain, epilepsy, cancer, diabetes and mental disorders. It is important to emphasize that such preclinical animal studies are scarce and, in most cases, their limitations also include a lack of isobolic analysis. Synergistic effects of curcumin with another pleiotropic natural product, epigallocatechin gallate [81, 82], offer new multi-targeted combinations with anticancer drugs [83-85], in addition to their anti-inflammatory activities [24, 86]. From the perspective of combination therapies with curcumin, n-3 PUFA, or resveratrol, pleiotropic natural products were also shown to modulate serotonergic and dopaminergic systems in the brain, thus offering potential for add-on antidepressant or anxiolytic benefits (Table 3). Clinical trials supporting preclinical finding on potential antidepressant benefits are emerging [77, 87, 88]. Since depression is a common comorbidity in epilepsy, pain and other chronic diseases, these additional mood-stabilizing activities may support mental health management during pharmacotherapies. When comparing therapeutic indications between clinical (Table 1) and preclinical (Table 2) studies in combining drugs with natural products, only epilepsy and cancer currently show immediate translational promise. However, in addition to “canonical” challenges when developing new drugs via preclinical and clinical studies, additional challenges of incorporating natural products into prescribed medications are having a public domain status and medication non-adherence (adding “one more pill” may rather discourage a patient, despite clinical benefits).
Table 2.
Preclinical studies showing effects of combining pleiotropic natural products with pharmaceutical drugs.
| Medical Condition | Drug | Natural Product | Major Outcome | Refs. |
|---|---|---|---|---|
| Epilepsy | Valproate | Curcumin | Curcumin enhanced antiseizure activity of subtherapeutic doses of valproate, provide anti-inflammatory and antioxidant effects. | [89] |
| Epilepsy | Carbamazepine | n-3 PUFA | n-3 PUFA increased protection from seizures in combination with carbamazepine | [90] |
| Depression | Corticorsterone | Curcumin | Curcumin and corticosterone reduces depressive-like behaviors, increased sucrose consumption and reduce mobility time | [91] |
| Depression | Fluoxetine, Mitrazapine | n-3 PUFA | n-3 PUFA potentiated subtherapeutic doses of antidepressant drugs | [92, 93] |
| Pain | Morphine | Ginsenoids | Ginsenoids and morphine yielded synergistic antinociception in nociceptive rats |
[94] |
| Pain | Morphine | n-3 PUFA | n-3PUFA potentiated analgesia and prevented tolerance to morphine | [95] |
| Cancer | Capecitabine | Curcumin | Curcumin potentiated anticancer activity of capecitabine through the NF-κB pathway |
[96] |
| Cancer | 5-Fluorouracil/ Oxaliplatin | Curcumin | Gastric tumor volume was significantly reduced in mice when curcumin was added to a combination of 5-FU/oxiplatin combination. | [97] |
| Cancer | 5-Fluorouracil | Resveratrol | Resveratrol potentiated 5-FU anticancer activity in skin cancer mouse model |
[98] |
| Diabetic Neuropathy | Enalapril | α-Lipoic acid | Enalapril with LA prevented vascular and neuronal damage in diabetic rats | [99] |
| Ischemic Stroke | Etanercept | α-Lipoic acid | Etanercept with LA enhanced recovery in cerebral infarct, motor deficit and stroke |
[100] |
| Schizophrenia | Clozapine | α-Lipoic acid | Clozapine with LA reversed schizophrenia-like symptoms in mice | [101] |
Medication Adherence as a New Challenge for Treating Chronic Diseases
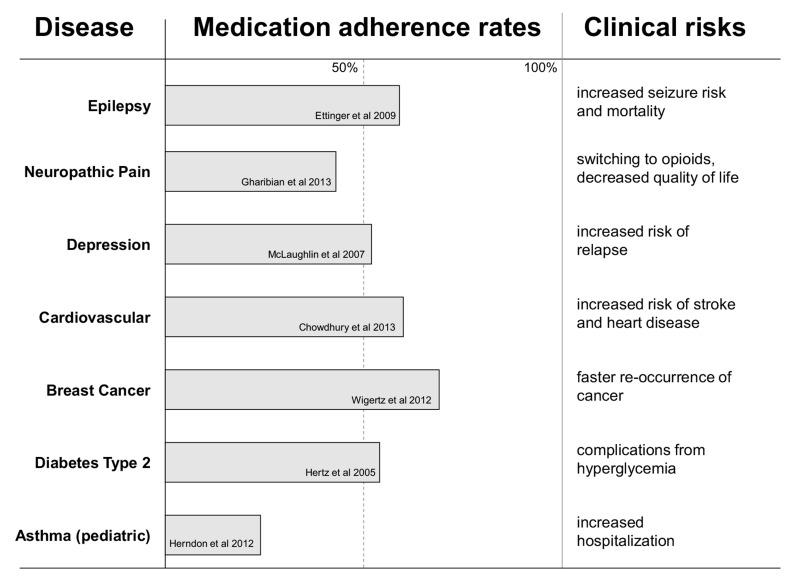
Approximately 50% of patients with chronic diseases struggle to take medications as prescribed [111], resulting in symptom exacerbation, and increased morbidity and mortality (Fig. 2). Adherence is defined by the World Health Organization as “the extent to which a person’s behavior - taking medication, following a diet, and/or executing lifestyle changes – corresponds with agreed recommendations from a health care provider”. Clinical consequences of non-adherence contribute to a decreased quality of life for patients and care givers. Financial consequences of decreased medication adherence include estimated $100-290 billion per year for the US health care insurance companies and patients [112], as well as revenue losses of $188 billion per year by the US pharmaceutical industry [113]. Global pharmaceutical industry have an estimated $564 billion loss per year due to patients’ non-adherence [113]. Therefore, improving medication adherence for chronic diseases is a win-win opportunity for patients and health care industry.
Fig. (2).
Medication adherence rates for selected chronic disorders and clinical consequences of non-adherence. Medication adherence values were obtained from medication-possession ratio (MPR) reported in retrospective studies [114-121]. For CVD, a meta-analysis value was used. Adherence data vary significantly in values depending on a population, type of study (prospective or retrospective) and methods used to measure adherence.
Medication adherence is a patient behavioral component of pharmacotherapy. Why does a patient struggle with long-term compliance with taking prescribed medication? For each patient, factors affecting adherence vary, and can be caused by comorbidities (anxiety, stress, depression, fatigue), financial burden, strict medication schedules, miscommunications with doctors, nurses or pharmacists, poor expectations and suboptimal education, or/and limited knowledge and involvement in managing symptoms [122, 123]. Persistence in taking medication can be challenged by drug-related adverse side effects, or by managing a large number of prescription medications [124-126]. Additional reasons are as trivial as forgetfulness and travel, and/or as serious as life style choices and ignorance. Patients with type 1 or 2 diabetes describe fear and embarrassment of injecting insulin as the leading cause of non-compliance [127]. Studies suggest that depression and adverse childhood experiences (ACEs) can be predictors of medication adherence [128-130]. Patient-related factors can be addressed by educating and empowering patients to develop persistence in self-management of pharmacotherapies and disease-related symptoms [3, 131].
INCENTIVIZED THERAPIES
Psychobehavioral Incentives to Improve Medication Adherence
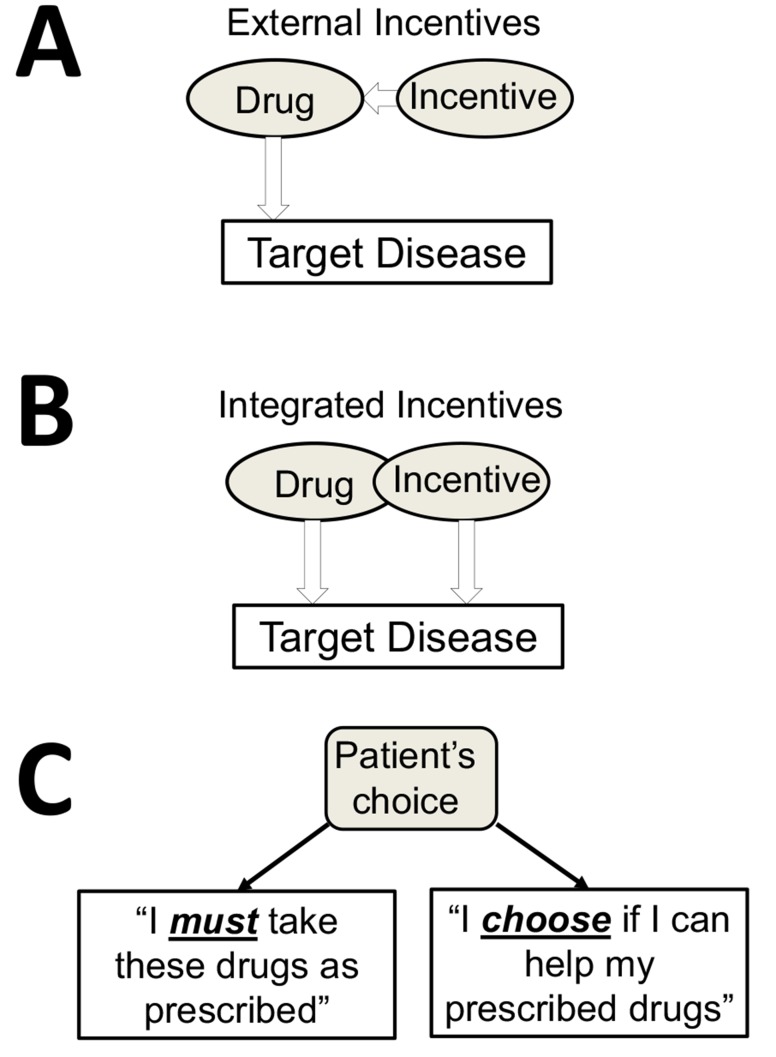
Incentives have been explored as a strategy to improve patient’s motivation to comply with his/her pharma- cotherapy. This strategy is illustrated in Fig. 3A. Rewarding a patient with incentives is a challenging task, since tangible external rewards can become counterproductive [132]. Financial incentives to reinforce medication adherence face such considerations as ethical, cost-effectiveness and sustainability [133-135]. Incentivizing diabetic patients was recently reviewed in the context of positive perception while
Fig. (3).
A psychobehavioral basis of integrating pleiotropic natural product into the network pharmacology in which the compound becomes both an incentive and an active ingredient of the combination therapy. (A) - External incentives are tested to support medication adherence [134]. (B) – A new concept of combining a pharmaceutical drug with a specific natural product which becomes pharmacologically-active incentive. (C) Having a choice as a psychobehavioral incentive targeting an intrinsic motivation of a patient. Shared-decision making and daily self-administration of natural product support self-care.
decreasing intrinsic motivation by the patients [136, 137]. As alternatives to external incentives, motivational components of mobile and digital technologies for diabetes patients can address the sustainability challenges [138].
Integrating incentives into medical treatment is a new concept in which pharmacological and psychobehavioral elements are combined together into a network therapy (Figs. 3B and 3C). Such psychobehavioral element is a patient’s choice of using (or not using) a pleiotropic natural product and mHealth, thus becoming an empowerment tool for her/his engagement in the therapy. A choice is used as a tool to improve intrinsic motivation [139, 140]. Empowering patients through choices and shared decision-making has been studied [141-144], including epilepsy patients [145]. Clinical applications of patient empowerment using mHealth technologies were described for cancer, diabetes and other chronic medical indications [146-149]. Disease-specific serious games which incorporate choices and engagement show improved self-management behaviors in patients [150-152].
Integrating psychobehavioral incentives with phar- macotherapies can be presented in the context of network pharmacology (Figs. 1B, 3 and 4), a term usually reserved for describing multi-drug combination therapies and derived from system biology [33, 153]. Development and applications of multi-target, network pharmacology for the treatment of complex chronic diseases has been established for cancer [154, 155], neurotropic pain [156, 157], epilepsy [158], depression [159] and diabetes [160, 161]. Psychological contribution of the placebo effect to pharmacological outcomes has been extensively studied for several chronic medical conditions [162, 163]. Recently, adding psycho- behavioral elements to the network pharmacology has been facilitated by disease-specific mobile medical apps [3]. Below, we describe specific examples of how mHealth technologies can further integrate pleiotropic natural products into molecular-behavioral combination therapies.
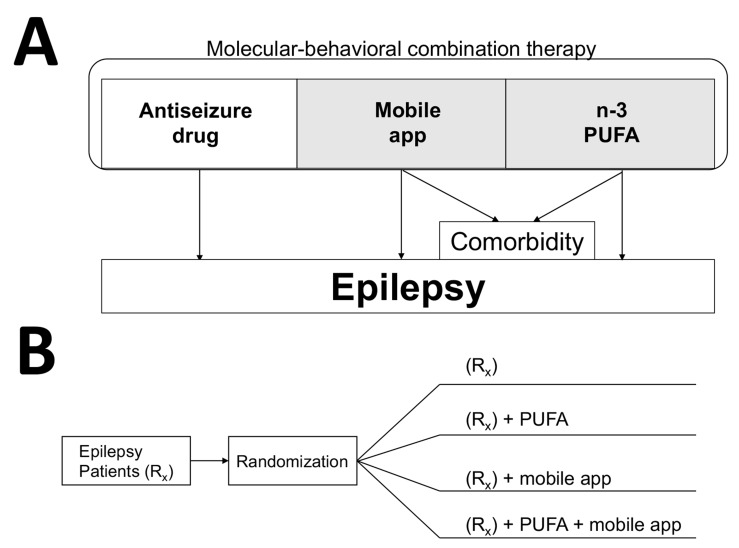
Fig. (4).
Molecular-behavioral combination therapy for epilepsy patients. (A) Three components of the molecular-behavioral combination therapy are antiseizure pharmaceutical drug, n-3 PUFA as pharmacologically-active incentives, and mobile app delivering antiseizure music and epilepsy self-management content [3]. Given high prevalence of depression as comorbidity for chronic disorders, the add-on therapy (in shaded area) can be specifically prescribed for patients with refractory epilepsy and experiencing symptoms of depression. Mobile apps and software for treating depression and anxiety show promise in clinical trials [211-213]. In addition to n-3 PUFA, curcumin also can be developed as psychobehavioral incentives due to its antiseizure and antidepressant activities. (B) An example of a parallel study design to test the molecular-behavioral combination therapy in form of a drug-device combination product. Patients with refractory epilepsy are usually given two or more antiseizure drugs (Rx group). To establish efficacy of all possible combinations, such four-arm study can last 6-12 months, and can further include crossover, as was previously used to establish efficacy of n-3 PUFA in patients with refractory epilepsy [60], or/and a delayed start/prerandomization to reduce placebo responses [214]. Determining pharmacokinetic interactions between natural products and antiseizure drugs are important to ensure the safety of the drug-drug combination study (2nd and 4th arm), even though natural products may have prescription medicine status (e.g. n-3 PUFA as Lovaza® or Vascepa®).
Incentivized Therapy for Epilepsy
Innovating medical treatments for epilepsy patients is driven by needs to address both the refractory epilepsy and significant medication non-adherence. Approximately 30% of epilepsy patients are refractory to antiseizure drugs despite taking more than one medication. Consequently this medical condition is subject to non-pharmacological treatments such as the ketogenic diet, vagus nerve stimulation, deep brain stimulation, or brain surgery [164]. Since only 48% of epilepsy patients become seizure-free after taking their first antiseizure drug [165-168], the incentivized therapies may offer better seizure control for newly diagnosed patients. (150,000-200,000 people in the US and approximately 2.4 million people world-wide are diagnosed with epilepsy each year, according to statistics presented by the World Health Organization and the Epilepsy Foundation). Additional challenge for effective treatments for epilepsy patients in prevalence of depression and anxiety as comorbidities [169]. To improve the therapies of pharmacoresistant epilepsy, medication non-adherence and comorbidities, the drug-device combination therapy consisting of antiseizure medications and a mobile medical app for epilepsy patients was proposed [3]. Herein we describe the incentivized therapy for epilepsy which comprises antiseizure pharmaceutical drugs, antiseizure natural products, and behavioral and self-management components delivered by the mHealth technology. We also review preclinical and clinical evidence for incorporating these elements into the network therapy.
Pleiotropic natural products such as n-3 PUFA, curcumin or resveratrol were shown to exhibit anticonvulsant activities in epilepsy patients as well as in animal models of epilepsy and status epilepticus. Data from clinical trials suggest that n-3 PUFA may be effective in reducing frequency of seizures in patients with refractory epilepsy [170]. DeGiorgio et al. showed in randomized controlled trial (RCT) that a 10-week treatment with fish oil daily dose of 1080 mg DHA/EPA was effective in reducing seizure frequency by 33% in refractory epilepsy adult patients taking 1-4 anticonvulsant drugs [60]. Previous studies with adult epilepsy patients taking fish oil for seizure control were inconclusive [62, 171, 172], however these studies were with higher doses, consistent with the recent finding from the DeGiorgio et al. study [60]. The importance of dosing n-3 PUFA in humans and animal studies was previously discussed [173]. In the recent study, pediatric patients with refractory epilepsy were subject to a 3-month treatment with daily fish oil containing 240 mg DHA and 360 mg EPA, resulting in over 50% of patients becoming seizure-free [59]. While these clinical trials were carried out with a small number of patients, the positive results are also supported by preclinical data in animal models of epilepsy [90, 174]. The mechanism by which n-3 PUFA exert their antiepileptic effects is not known although PUFA was shown to affect neuronal excitability in the hippocampus [175, 176] and also exhibit anti-neuroinflammatory properties [177]. The main PUFA component in the brain is DHA, and some models suggest that n-3 PUFA may modulate neuronal excitability via changing levels of sodium-potassium- chloride cotransporters NKCC1 and NKCC2 by affecting lipid rafts through brain cholesterol [178], and shifting conductance of potassium channels [179, 180]. Noteworthy, fatty acids were shown to suppress seizures [181, 182], at least in part due to binding to ionotropic glutamate AMPA receptors [183].
Curcumin and resveratrol exhibit antiseizure activities in animal models of epilepsy. Combinations of curcumin and subtherapeutic doses of antiepileptic drugs (valproate, phenytoin, phenobarbitone and carbamazepine) showed increased protection against seizures in the MES and PTZ models of epilepsy [89]. In the PTZ-kindled mice model of epilepsy, curcumin was effective in a dose-dependent manner attenuating seizure severity and reducing depression-like behavior and memory impairment [184]. In the PTZ-treated rat model of chronic epilepsy, curcumin attenuated cognitive impairment, expression of proinflammatory cytokins in the hippocampus and glial activation [185]. Curcumin also exhibited antiepileptogenic activities in the kainate-induced temporal lobe epilepsy and post-status epilepticus in rats [186-188]. Similar effects were observed for resveratrol in the PTZ-kindled rats [189] and its anti-inflammatory activity via the AMPK/mTOR signaling pathway was found in the model of status epilepticus [190]. The use of resveratrol in epilepsy and status epilepticus has been discussed [191, 192]. Potential pharmacokinetic interactions between resveratrol and carbamazepine may prevent this particular combination to treat epileptic seizures [193], similarly to potential interactions between n-3 PUFA and carbamazepine [194].
The mHealth technologies specific for epilepsy patients comprise of mobile medical apps, online resources and wearables intended to help epilepsy self-management, including detecting and monitoring seizures [195-197]. Rapid progress in wireless and mobile EEG interfaces [198, 199] or the EDA-sensors offers unique opportunities for biofeedback-based streaming of digital content developed specifically for epilepsy patients [3]. Examples of online and mobile technologies and apps for epilepsy patients are studied and reviewed [195, 196, 200]. Design features of mobile medical apps intended to reduce frequency of epileptic seizures were previously described [3]. Pharmaceutical industry has been incorporating these new developments, including applications for epilepsy patients. For example, the UCB Pharma partnered with MC10 company in developing the Biostamp, a sensor for detecting seizure movements in epilepsy patients. Among many advances, wearable and mobile app technologies also include the SmartWatch (by Smart Monitor), Embrace (by Empatica), Seizalarm app (Seizalarm) or My Epilepsy (by the Epilepsy Foundation).
Behavioral therapy and self-management for epilepsy patients have been extensively studied and offer self-care strategies to reduce seizure frequency [201-206]. For example, significant reduction of seizures in epilepsy patients was observed during and after six months of psychological and self-management intervention [201]. A randomized trial of behavioral therapy showed a significant reduction of seizure frequency in older adults with epilepsy [206]. Similar positive results were observed in randomized trial of behavioral interventions in patients with refractory epilepsy [205]. Behavioral-educational interventions were also effective in reducing seizures in children with epilepsy [202]. Clinical efficacy of Mozart’s music, including sonata K.448, in reducing seizures in patients with refractory epilepsy [207-209], supports listening to this music for 10 minutes daily as an additional self-care intervention [3]. Mobile medical apps are positioned to reduce and prevent seizures given feasibility and convenience for delivering and monitoring self-care and behavioral interventions. The gamification of mobile medical apps ensure engaging and rewarding content, making patients more eager to engage with their therapies, including medication adherence and disease-related, healthy habits. A recent review emphasizes further needs to study effectiveness of epilepsy self-management strategies [210].
Incentivized therapy for epilepsy incorporating antiseizure pharmaceutical drugs and natural products and self-care delivered by mHealth is illustrated in Fig. 4. The add-on administration of n-3 PUFA (1g or 0.6 g per day for adult or pediatric patients, respectively), when coupled with mobile medical app, may further improve control of seizures by engaging a patient with the therapy including additional antiseizure natural product and self-care behaviors. Epilepsy-specific behavioral therapy (including awareness of avoiding and managing stress and other seizure-precipitating triggers), educational elements, medication reminders and antiseizure music are provided to the patient via mHealth, hence delivering “behavioral” component of the resulting molecular-behavioral combination therapy. The psychobehavioral incentives of having a choice, feeling empowered, and shared-decision making regarding the add-on therapy targets patient intrinsic motivation (Fig. 3B and 3C), further supporting long-term compliance and persistence with the therapy. Epilepsy patients express their desires to be actively involved in choices and shared-decision making [145].
Incorporation of Behavioral Interventions and Self-Care into the Incentivized Therapies for Chronic Diseases
Chronic diseases such as neuropathic pain, epilepsy, cancer, diabetes, CVD, arthritis, asthma, or mental disorders are medical conditions that last at least a year, or are permanent. Because of the long duration, chronic medical conditions challenge patients, caregivers and healthcare systems, and also pose a major economic burden [215, 216]. An additional challenge for managing a chronic disease happens after achieving remission and discontinuation of pharmacotherapies. The Center for Disease Control and Prevention (CDC) emphasized importance of behavioral components (such as physical activity, nutrition) which can prevent progression of chronic diseases [217]. Supporting healthy habits during the therapy and also after reaching remission (during the inactive-disease state) is critical for long-term improvements in therapy outcomes and health-related quality of life.
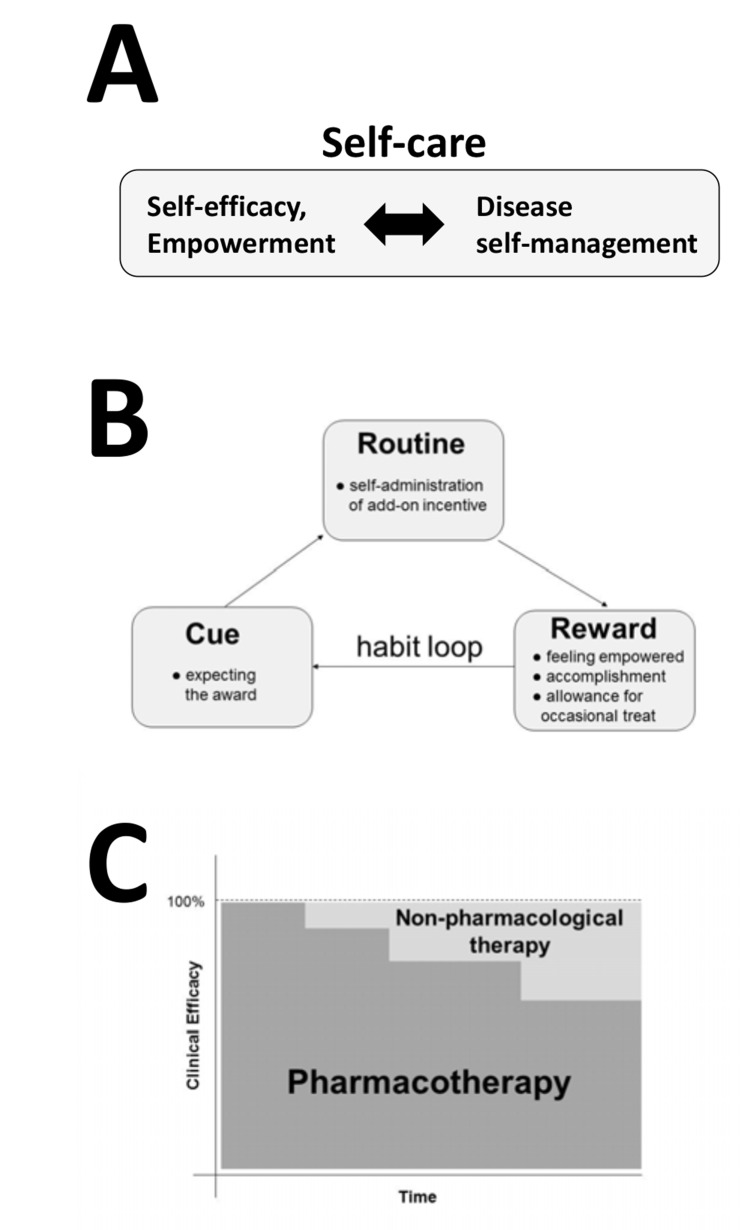
Patient self-care and disease self-management become important behavioral components of patient-centered health care [218, 219]. Self-care can be considered as a combination of disease self-management and self-efficacy, where self-management can be described as patient’s skills and behaviors affecting disease symptoms, and self-efficacy is patient’s motivation and perceived ability to engage in self-management behaviors. The relationship between self-management and self-efficacy is shown in Fig. 5A. Incorporation of disease-specific behavioral and self-care components into patient daily life has been facilitated using mHealth technologies. For example, listening to the antiseizure music and avoiding seizure-triggers are daily behavioral components to help epilepsy patients to reduce and prevent seizures. There is accumulating clinical evidence for other non-pharmacological, behavioral interventions for specific chronic medical conditions. Therapeutic physical exercise was validated for rheumatoid arthritis (RA) [220, 221], major depressive disorder [222], neurodegenerative disorders [223], neuropathic pain [224], pediatric cancer [225], or perioperative management, postoperative recovery and outcomes [226]. Physical exercise programs for patients with RA may additionally improve their health-related quality of life by improving sleep and fatigue [227]. Yoga improved disease-symptoms for patients with low-back pain [228-230], cancer-related fatigue [231, 232], osteoarthritis [233-235], epilepsy [236], asthma [237], diabetes [238] and PTSD [239]. Music-supported therapies include medical indications such as pain, epilepsy, stroke, myocardial infart, dementia, depression, anxiety, and other psychiatric disorders (references in [3] and [240-242]). The pleiotropic nature of music is mediated by neurochemical changes in the brain, endocrinological and immune systems [243-245], activating the mesolimbic system and dopaminergic neurotransmission [246-251]. Recent studies suggest that exposure to Mozart and Bach music can upregulate brain-derived neurotropic factor (BDNF) [252, 253]. Nutrition-based interventions have shown clinical efficacy in reducing seizures in epilepsy patients [254-256], but more studies on pharmacokinetic interactions with antiepileptic drugs are needed [257, 258]. Nutrition has been effective non-pharmacological treatment for CVD [259], diabetes [260], or arthritis including combinations with physical exercise [261]. Taken together, cognitive behavioral therapy, music, nutrition, physical exercise and yoga are already being combined into behavioral interventions and self-care using mHealth [262-264].
Self-administration of an optional (add-on) treatment targets patient intrinsic motivation, and can promote long-lasting behavioral changes by engaging habit-forming mechanisms [265, 266]. As illustrated in Fig. 5B, an opportunity to self-administer the clinically-efficacious dose of the pleiotropic natural product and other self-care behaviors, leads to activating the reward system and creating a habit-forming loop. Engaging habit-forming neuronal networks in the brain may facilitate health-promoting behavioral benefits [267, 268]. mHealth and gamification principles offer additional tools to engage, monitor and virtually reward a patient [269-271]. While self-administration of pleiotropic natural products can improve pharmacotherapy outcomes, it is nonpharmacological treatments that hold long-term potential for introducing new healthy routines in chronically-ill patients. This aspect becomes critical once a chronically-ill patient reaches a stage of clinical remission and inactive disease state, since she/he is in a vulnerable state due to behavioral and life-style contribution to the disease and the therapy outcomes. Additional benefit of incorporating clinically-efficacious behavioral interventions is the opportunity to reduce dosing of pharmaceutical-based therapies (Fig. 5C). While this application is apparent for patients with neuropathic pain being treated with opioids, or arthritis patients taking NSAIDs and other anti-inflammatory medications, future clinical studies will evaluate its potential use in treating epilepsy, depression, cardiovascular and other chronic medical conditions [4, 5, 21, 25, 60, 191, 192].
Fig. (5).
Roles of self-care and the reward system in the incentivized therapies. Due to the long duration of chronic medical conditions, healthy habits and self-care can slow down progression of the chronic disease, improve the therapy outcomes and the health-related quality of life after reaching remission. (A) Self-care consists of disease self-management (patient behaviors related to management and prevention of disease symptoms), self-efficacy and empowerment (perceived abilities to cope with, control and manage disease symptoms). Digital technologies become effective means to deliver empowerment and disease self-management content [148, 150]. (B) Patients activate the habit-forming mechanism by a self-administration of an incentive. The habit-forming loop scheme is adapted from “The Power of Habit” by Charles Duhigg. (C) Integration of incentive-based nonpharmacological interventions with pharmacotherapies can lead to decreasing the dose of pharmaceutical drugs, an opportunity instantly applicable in the treatment of chronic, neuropathic pain with opioids, or arthritis with NSAIDs and other anti-inflammatory drugs. Randomized clinical trials will determine if this strategy also applies to epilepsy patients or those with depression to decrease dosing or number of medications.
Industry and Translational Considerations
This article describes the use of pleiotropic natural products in combinations with existing pharmaceutical drugs and self-care delivered by the mHealth technology. From the regulatory point of view, this is a multifaceted challenge, requiring judicious and cooperative endeavor to develop such integrated medical treatments. Incorporating natural product or self-care delivered by mHealth already creates numerous challenges, and integrating two or more modalities is even more difficult. Clinical development of drug-device combinations can be facilitated by a natural product having the status of prescribed medication, or/and mobile app reaching the status of regulated medical device.
For patients, many natural products are currently available as supplements, for which health claims and benefits are regulated by federal agencies [272]. Because dietary supplements market is becoming increasingly competitive due to fast growth, there are new tensions and opportunities to innovate these products. Converting natural products from dietary supplements into regulated medical treatments requires clinical studies on optimizing dosing, duration and drug-drug interactions to achieve maximum efficacy and safety for each specific medical indication. However, the lack of intellectual property protection of natural products is a challenge for developing them as medical treatments. A problem of patenting natural products was previously exemplified with curcumin [273, 274], whereas patenting guidelines also change for newly-discovered natural products [2]. Improving bioavailability of curcumin [275, 276], or generating derivatives of resveratrol [277, 278] are viable patenting strategies to improve their efficacy. To this end, combining natural products with disease-specific behavioral interventions via mHealth offers an additional level of intellectual property protection by merging copyrights (lasting over 50 years) with a pharmacologically-active compound which exists in public domain [3]. This strategy creates new regulatory challenges and requires closely working with the regulatory agencies to ensure the safety of patients and following proper regulatory mechanisms. Additional benefits of the regulatory pathway for developing natural products in combinations with mHealth include new strategies to innovate and validate new medical interventions, instead of following costly litigations (e.g. the 2015 Vascepa® case related to off-label claims).
Converting mobile apps into a regulated medical device requires pivotal clinical trials in support of regulatory clearance [279, 280]. Current regulatory guidelines indicate that apps delivering disease self-management content are likely exempt from premarket approval, subject to the discretion of the regulatory agencies. For combinations of mobile apps with natural products, their intended use for specific medical condition, marketing, and labelling must be supported by appropriate clinical studies, and subject to premarket notification or approval. A mobile app for tracking the use of dietary supplements, MyDS, was developed by the National Institute of Health Office for Dietary Supplements (the app was discontinued in June 2015) [281]. Regulatory challenges and growth of mHealth have resulted in excessive number of health-related and wellness apps, creating a problem of credibility and confusion [282], thus also creating opportunities for digital technologies to be part of clinically-validated integrative medicine [283]. The mHealth technology is becoming a part of personalized medicine [284], and several companies have been pioneering development of mobile medical apps and games as medical treatments. Health care providers support incorporation of mHealth into primary care practice and reimbursement policies [285].
Given multiple components of the incentivized therapy, the discovery and characterization of synergistic effects among natural products, pharmaceutical drugs and mobile medical apps is challenging at both preclinical and clinical levels. At a preclinical level, it is important to test combinations of natural products and pharmaceutical drugs using isobolic analysis, or sub-effective therapeutic doses. At a clinical level, large-scale, controlled testing is challenging due to additional placebo-related responses, driven by spontaneous remissions, methodological bias and psychobiological mechanisms of positive expectations and learning [162, 163, 214, 286]. The neurobiological mechanisms of the placebo effect are mediated by specific neurotransmitters, including dopamine, neuropeptides and their receptors located in different brain structures [162, 163]. To this end, the expectation-driven placebo effect may overlap with music and therapeutic games due to activating dopaminergic signaling in the patient’s reward system [3]. One possible mechanism of such additional placebo-like effects is that music and judiciously-timed digital images can produce temporal expectations [287-290]. To best of our knowledge, the synergistic effects between pharmacological and non-pharmacological treatments have not been demonstrated.
Optimal design of randomized and placebo-controlled clinical trials for any new therapy is critical for demonstrating its efficacy [291]. Even for testing monotherapies, there are challenges to account for placebo effects and responses that are often disease-specific and interwoven with natural fluctuations of chronic disease symptoms. In the case of epilepsy, the placebo effects and responses were observed for both antiseizure drugs and medical devices, and several study designs have been proposed to mitigate placebos [214], including adjusting “time to prerandomization” [292], or “delay start” [293]. Clinical testing of combinations of antiseizure drugs with natural products (n-3 PUFA) was accomplished using randomized and placebo-controlled crossover design with three periods in which patients with refractory epilepsy were receiving low- or high-dose of n-3 PUFA or placebo while taking their antiseizure medications [60]. Testing more complex combination therapies, such as those containing pharmaceutical drugs, natural products and mobile medical apps (e.g. shown in Fig. 4A) will require judicious design of parallel and randomized trials to account for placebo and nocebo effects, likely adding additional challenges associated with multiple testing sites. In addition to universal ethical aspects of reducing or enhancing the placebo effects in patients during the treatment of a chronic disease, additional ethical aspect of including the placebo arm in epilepsy patients is the increase of SUDEP (sudden unexplained death in epilepsy) [294].
From a translational perspective, the long-term premise of developing the combinations of pharmaceutical drugs, natural products and self-care is the creation of the molecular-behavioral combination therapies in which seamless integration of behavioral and pharmaceutical interventions is delivered. As illustrated in Fig. 4, the molecular-behavioral combination therapy for patients with epilepsy can consist of the antiseizure drug, n-3 PUFA and behavioral, self-management content (like listening to antiseizure music, avoiding seizure-triggers). Such strategy is also favorable for patients with neurological disorders in which inflammatory and behavioral components significantly contribute to disease symptoms. Large-scale, randomized and controlled clinical studies on interactions between behavioral and pharmacological interventions will shed more light on mutual relationships, yielding new rational strategies for personalized, precision and preventive medicine [295-297]. Translational perspectives for developing broad-spectrum approach and integrative therapies are discussed for mental disorders [298, 299] and cancer [80]. Better understanding of interactions between natural products and drug targets, and mechanisms by which enriched environment (nutrition, physical exercise), cell metabolism and epigenetics may affect pharmacological properties of drug candidates [300, 301], will facilitate design of incentivized therapies combining the most effective components.
CONCLUSION
Pleiotropic natural products or nonpharmacological treatments can be incorporated into pharmacotherapies as psychobehavioral incentives to improve therapy outcomes for chronically-ill patients. Such integrative approach results in molecular-behavioral combination therapies, possible to deliver by digital medical technologies. There is an apparent need for controlled clinical trials to account for the placebo responses and to validate the effectiveness of these pharmacological and non-pharmacological combinations. Incentivized medical treatments offer an opportunity to improve medication adherence and clinical efficacy of existing pharmaceutical drugs. In addition, the ability to catalyze habit-changing healthy behaviors in patients favors improvements in the health-related quality of life during pharmacotherapy and after reaching remission. This aspect offers unique opportunities to bridge symptomatic treatments with preventive medicine, and has implications for public health by addressing risk factors and burden of chronic diseases [302, 303]. Lastly, once the drug-device combination product is approved by a regulatory agency, merging copyrights of mHealth software with natural products will afford new proprietary products containing natural products currently existing in public domain. Engaging patients with their therapies using mHealth-delivered behavioral inter- ventions will improve their experience with medical treatment. Taken together, combining the clinical efficacy of natural products and pharmaceutical-based treatments with self-care will advance patient-centered health care.
Table 3.
Pleiotropic activities of selected natural products studied in humans. Studies and mechanism of enhancing positive mood and activating the brain reward system are also summarized.
| Natural Product | Preclinical Data on Antidepressant Activities and Interactions with the Reward System | Refs. |
|---|---|---|
| Curcumin | Decreased serum corticosterone levels Increases serotonin by inhibition of monoamine oxidases |
[14, 102-105] |
| Reduce immobility time and stressed-induced neurodegeneration | [88, 91] | |
| n-3 PUFA | Stabilizes/increases levels of brain serotonin, affects serotonergic transmission | [12] |
| Resveratrol | Increased serotonin levels in the brain Inhibition of monoamine oxidases (MAO-A) |
[106] |
| L-acetylcarnitine | Enhances dopamine release Upregulation of mGlu2 Receptors |
[107-109] |
| L-Theanine | Increases dopamine levels in the brain Increased levels of BDNF |
[110] [31] |
ACKNOWLEDGEMENTS
GB acknowledges past support from the National Institute of Health Grant U01 NS 066991 during this work.
ABBREVIATIONS
- BDNF
brain-derived neurotropic factor
- CVD
cardiovascular disease
- DHA
docosahexanoic acid
- EDA
electrodermal activity
- EEG
electroencephalograph
- EPA
eicosapentaenoic acid
- 5-FU
5-fluorouracil
- LA
α-lipoic acid
- MES
maximum electroshock
- mHealth
mobile health technologies
- MPR
medication possession ratio
- NSAIDs
nonsteroidal anti-inflammatory drugs
- PPARs
peroxisome proliferator-activated receptors
- PTZ
pentylenetetrazol
- n-3 PUFA
omega-3 polyunsaturated fatty acids
- RCT
randomized controlled trial
CONFLICT OF INTEREST
GB is a cofounder of Epicadence PBC, Public Benefit Corporation, a company developing mobile software as medical devices for treating chronic medical conditions.
REFERENCES
- 1.Grady P.A., Gough L.L. Self-management: a comprehensive approach to management of chronic conditions. Am. J. Public Health. 2014;104(8):e25–e31. doi: 10.2105/AJPH.2014.302041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison C. Patenting natural products just got harder. Nat. Biotechnol. 2014;32(5):403–404. doi: 10.1038/nbt0514-403a. [DOI] [PubMed] [Google Scholar]
- 3.Bulaj G. Combining non-pharmacological treatments with pharma- cotherapies for neurological disorders: a unique interface of the brain, drug-device, and intellectual property. Front. Neurol. 2014;5:126. doi: 10.3389/fneur.2014.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yates C.M., Calder P.C., Ed Rainger G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014;141(3):272–282. doi: 10.1016/j.pharmthera.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira A.S., Sousa E., Vasconcelos M.H., Pinto M. Curcumin: A Natural Lead for Potential New Drug Candidates. Curr. Med. Chem. 2015;22(36):4196–4232. doi: 10.2174/0929867322666151029104611. [DOI] [PubMed] [Google Scholar]
- 6.Moura F.A., de Andrade K.Q., dos Santos J.C., Goulart M.O. Lipoic Acid: its antioxidant and anti-inflammatory role and clinical applications. Curr. Top. Med. Chem. 2015;15(5):458–483. doi: 10.2174/1568026615666150114161358. [DOI] [PubMed] [Google Scholar]
- 7.Park S., Karunakaran U., Jeoung N.H., Jeon J.H., Lee I.K. Physiological effect and therapeutic application of alpha lipoic acid. Curr. Med. Chem. 2014;21(32):3636–3645. doi: 10.2174/0929867321666140706141806. [DOI] [PubMed] [Google Scholar]
- 8.Park E.J., Pezzuto J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta. 2015;1852(6):1071–1113. doi: 10.1016/j.bbadis.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Tome-Carneiro J., Larrosa M., Gonzalez-Sarrias A., Tomas-Barberan F.A., Garcia-Conesa M.T., Espin J.C. Resveratrol and clinical trials: the crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013;19(34):6064–6093. doi: 10.2174/13816128113199990407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirani A., Okuda D.T., Stuve O. Therapeutic Advances and Future Prospects in Progressive Forms of Multiple Sclerosis. Neurotherapeutics. 2016;13(1):58–69. doi: 10.1007/s13311-015-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tizabi Y., Hurley L.L., Qualls Z., Akinfiresoye L. Relevance of the anti-inflammatory properties of curcumin in neurodegenerative diseases and depression. Molecules. 2014;19(12):20864–20879. doi: 10.3390/molecules191220864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman M.P., Hibbeln J.R., Wisner K.L., et al. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. J. Clin. Psychiatry. 2006;67(12):1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- 13.Singer P., Shapiro H., Theilla M., Anbar R., Singer J., Cohen J. Anti-inflammatory properties of omega-3 fatty acids in critical illness: novel mechanisms and an integrative perspective. Intensive Care Med. 2008;34(9):1580–1592. doi: 10.1007/s00134-008-1142-4. [DOI] [PubMed] [Google Scholar]
- 14.Lopresti A.L., Hood S.D., Drummond P.D. Multiple antidepressant potential modes of action of curcumin: a review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. J. Psychopharmacol. 2012;26(12):1512–1524. doi: 10.1177/0269881112458732. [DOI] [PubMed] [Google Scholar]
- 15.Calder P.C. Fatty acids and inflammation: the cutting edge between food and pharma. Eur. J. Pharmacol. 2011;668(Suppl. 1):S50–S58. doi: 10.1016/j.ejphar.2011.05.085. [DOI] [PubMed] [Google Scholar]
- 16.Freeman M.P., Rapaport M.H. Omega-3 fatty acids and depression: from cellular mechanisms to clinical care. J. Clin. Psychiatry. 2011;72(2):258–259. doi: 10.4088/JCP.11ac06830. [DOI] [PubMed] [Google Scholar]
- 17.McMurray D.N., Bonilla D.L., Chapkin R.S. n-3 Fatty acids uniquely affect anti-microbial resistance and immune cell plasma membrane organization. Chem. Phys. Lipids. 2011;164(7):626–635. doi: 10.1016/j.chemphyslip.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luthra P.M., Lal N. Prospective of curcumin, a pleiotropic signalling molecule from Curcuma longa in the treatment of Glioblastoma. Eur. J. Med. Chem. 2015;109:23–35. doi: 10.1016/j.ejmech.2015.11.049. [DOI] [PubMed] [Google Scholar]
- 19.Sinclair D.A., Guarente L. Small-molecule allosteric activators of sirtuins. Annu. Rev. Pharmacol. Toxicol. 2014;54:363–380. doi: 10.1146/annurev-pharmtox-010611-134657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alayev A., Berger S.M., Holz M.K. Resveratrol as a novel treatment for diseases with mTOR pathway hyperactivation. Ann. N. Y. Acad. Sci. 2015;1348(1):116–123. doi: 10.1111/nyas.12829. [DOI] [PubMed] [Google Scholar]
- 21.Kakoti B.B., Hernandez-Ontiveros D.G., Kataki M.S., Shah K., Pathak Y., Panguluri S.K. Resveratrol and Omega-3 Fatty Acid: Its Implications in Cardiovascular Diseases. Front Cardiovasc Med. 2015;2:38. doi: 10.3389/fcvm.2015.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulkarni S.S., Canto C. The molecular targets of resveratrol. Biochim. Biophys. Acta. 2015;1852(6):1114–1123. doi: 10.1016/j.bbadis.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Britton R.G., Kovoor C., Brown K. Direct molecular targets of resveratrol: identifying key interactions to unlock complex mechanisms. Ann. N. Y. Acad. Sci. 2015;1348(1):124–133. doi: 10.1111/nyas.12796. [DOI] [PubMed] [Google Scholar]
- 24.Gupta S.C., Tyagi A.K., Deshmukh-Taskar P., Hinojosa M., Prasad S., Aggarwal B.B. Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols. Arch. Biochem. Biophys. 2014;559:91–99. doi: 10.1016/j.abb.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal B.B., Gupta S.C., Sung B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013;169(8):1672–1692. doi: 10.1111/bph.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang J.X., Weylandt K.H. Modulation of inflammatory cytokines by omega-3 fatty acids. Subcell. Biochem. 2008;49:133–143. doi: 10.1007/978-1-4020-8831-5_5. [DOI] [PubMed] [Google Scholar]
- 27.Khalili M., Azimi A., Izadi V., et al. Does lipoic acid consumption affect the cytokine profile in multiple sclerosis patients: a double-blind, placebo-controlled, randomized clinical trial. Neuroimmunomodulation. 2014;21(6):291–296. doi: 10.1159/000356145. [DOI] [PubMed] [Google Scholar]
- 28.Lyon M.R., Kapoor M.P., Juneja L.R. The effects of L-theanine (Suntheanine(R)) on objective sleep quality in boys with attention deficit hyperactivity disorder (ADHD): a randomized, double-blind, placebo-controlled clinical trial. Altern. Med. Rev. 2011;16(4):348–354. [PubMed] [Google Scholar]
- 29.Nathan P.J., Lu K., Gray M., Oliver C. The neuropharmacology of L-theanine(N-ethyl-L-glutamine): a possible neuroprotective and cognitive enhancing agent. J. Herb. Pharmacother. 2006;6(2):21–30. [PubMed] [Google Scholar]
- 30.Lardner A.L. Neurobiological effects of the green tea constituent theanine and its potential role in the treatment of psychiatric and neurodegenerative disorders. Nutr. Neurosci. 2014;17(4):145–155. doi: 10.1179/1476830513Y.0000000079. [DOI] [PubMed] [Google Scholar]
- 31.Wakabayashi C., Numakawa T., Ninomiya M., Chiba S., Kunugi H. Behavioral and molecular evidence for psychotropic effects in L-theanine. Psychopharmacology (Berl.) 2012;219(4):1099–1109. doi: 10.1007/s00213-011-2440-z. [DOI] [PubMed] [Google Scholar]
- 32.Kakuda T. Neuroprotective effects of theanine and its preventive effects on cognitive dysfunction. Pharmacol. Res. 2011;64(2):162–168. doi: 10.1016/j.phrs.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Hopkins A.L. Network pharmacology: the next paradigm in drug discovery. Nat. Chem. Biol. 2008;4(11):682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 34.Kibble M., Saarinen N., Tang J., Wennerberg K., Makela S., Aittokallio T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat. Prod. Rep. 2015;32(8):1249–1266. doi: 10.1039/c5np00005j. [DOI] [PubMed] [Google Scholar]
- 35.Anighoro A., Bajorath J., Rastelli G. Polypharmacology: challenges and opportunities in drug discovery. J. Med. Chem. 2014;57(19):7874–7887. doi: 10.1021/jm5006463. [DOI] [PubMed] [Google Scholar]
- 36.Abete P., Testa G., Galizia G., Della-Morte D., Cacciatore F., Rengo F. PUFA for human health: diet or supplementation? Curr. Pharm. Des. 2009;15(36):4186–4190. doi: 10.2174/138161209789909665. [DOI] [PubMed] [Google Scholar]
- 37.Samsami-Kor M., Daryani N.E., Asl P.R., Hekmatdoost A. Anti-Inflammatory Effects of Resveratrol in Patients with Ulcerative Colitis: A Randomized, Double-Blind, Placebo-controlled Pilot Study. Arch. Med. Res. 2015;46(4):280–285. doi: 10.1016/j.arcmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Cottart C.H., Nivet-Antoine V., Beaudeux J.L. Review of recent data on the metabolism, biological effects, and toxicity of resveratrol in humans. Mol. Nutr. Food Res. 2014;58(1):7–21. doi: 10.1002/mnfr.201200589. [DOI] [PubMed] [Google Scholar]
- 39.Kanai M., Otsuka Y., Otsuka K., et al. A phase I study investigating the safety and pharmacokinetics of highly bioavailable curcumin (Theracurmin) in cancer patients. Cancer Chemother. Pharmacol. 2013;71(6):1521–1530. doi: 10.1007/s00280-013-2151-8. [DOI] [PubMed] [Google Scholar]
- 40.Sanmukhani J., Satodia V., Trivedi J., et al. Efficacy and safety of curcumin in major depressive disorder: a randomized controlled trial. Phytother. Res. 2014;28(4):579–585. doi: 10.1002/ptr.5025. [DOI] [PubMed] [Google Scholar]
- 41.Shay K.P., Moreau R.F., Smith E.J., Smith A.R., Hagen T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta. 2009;1790(10):1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegazy S.K., Tolba O.A., Mostafa T.M., Eid M.A., El-Afify D.R. Alpha-lipoic acid improves subclinical left ventricular dysfunction in asymptomatic patients with type 1 diabetes. Rev. Diabet. Stud. 2013;10(1):58–67. doi: 10.1900/RDS.2013.10.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hager K., Kenklies M., McAfoose J., Engel J., Munch G. Alpha-lipoic acid as a new treatment option for Alzheimer's disease--a 48 months follow-up analysis. J. Neural Transm. Suppl. 2007;(72):189–193. doi: 10.1007/978-3-211-73574-9_24. [DOI] [PubMed] [Google Scholar]
- 44.Shinto L., Quinn J., Montine T., et al. A randomized placebo-controlled pilot trial of omega-3 fatty acids and alpha lipoic acid in Alzheimer's disease. J. Alzheimers Dis. 2014;38(1):111–120. doi: 10.3233/JAD-130722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yadav V., Marracci G., Lovera J., et al. Lipoic acid in multiple sclerosis: a pilot study. Mult. Scler. 2005;11(2):159–165. doi: 10.1191/1352458505ms1143oa. [DOI] [PubMed] [Google Scholar]
- 46.Ota M., Wakabayashi C., Sato N., et al. Effect of L-theanine on glutamatergic function in patients with schizophrenia. Acta Neuropsychiatr. 2015;27(5):291–296. doi: 10.1017/neu.2015.22. [DOI] [PubMed] [Google Scholar]
- 47.Oliver C., Watson H. Omega-3 fatty acids for cystic fibrosis. Cochrane Database Syst. Rev. 2016;1:CD002201. doi: 10.1002/14651858.CD002201.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A., Mastana S.S., Lindley M.R. n-3 Fatty acids and asthma. Nutr. Res. Rev. 2016:1–16. doi: 10.1017/S0954422415000116. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 49.Albert B.B., Derraik J.G., Brennan C.M., et al. Supplementation with a blend of krill and salmon oil is associated with increased metabolic risk in overweight men. Am. J. Clin. Nutr. 2015;102(1):49–57. doi: 10.3945/ajcn.114.103028. [DOI] [PubMed] [Google Scholar]
- 50.Brasky T.M., Darke A.K., Song X., et al. Plasma phospholipid fatty acids and prostate cancer risk in the SELECT trial. J. Natl. Cancer Inst. 2013;105(15):1132–1141. doi: 10.1093/jnci/djt174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belcaro G., Cesarone M.R., Dugall M., et al. Efficacy and safety of Meriva(R), a curcumin-phosphatidylcholine complex, during extended administration in osteoarthritis patients. Altern. Med. Rev. 2010;15(4):337–344. [PubMed] [Google Scholar]
- 52.Belcaro G., Dugall M., Luzzi R., et al. Meriva(R)+Glucosamine versus Condroitin+Glucosamine in patients with knee osteoarthritis: an observational study. Eur. Rev. Med. Pharmacol. Sci. 2014;18(24):3959–3963. [PubMed] [Google Scholar]
- 53.Nakagawa Y., Mukai S., Yamada S., et al. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J. Orthop. Sci. 2014;19(6):933–939. doi: 10.1007/s00776-014-0633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hill C.L., March L.M., Aitken D., et al. Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Ann. Rheum. Dis. 2016;75(1):23–29. doi: 10.1136/annrheumdis-2014-207169. [DOI] [PubMed] [Google Scholar]
- 55.Chandran B., Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother. Res. 2012;26(11):1719–1725. doi: 10.1002/ptr.4639. [DOI] [PubMed] [Google Scholar]
- 56.Lopez-D'alessandro E., Escovich L. Combination of alpha lipoic acid and gabapentin, its efficacy in the treatment of Burning Mouth Syndrome: a randomized, double-blind, placebo controlled trial. Med. Oral Patol. Oral Cir. Bucal. 2011;16(5):e635–e640. doi: 10.4317/medoral.16942. [DOI] [PubMed] [Google Scholar]
- 57.Hartrick C.T., Pestano C., Carlson N., Hartrick S. Capsaicin instillation for postoperative pain following total knee arthroplasty: a preliminary report of a randomized, double-blind, parallel-group, placebo-controlled, multicentre trial. Clin. Drug Investig. 2011;31(12):877–882. doi: 10.1007/BF03256925. [DOI] [PubMed] [Google Scholar]
- 58.Letizia Mauro G., Cataldo P., Barbera G., Sanfilippo A. alpha-Lipoic acid and superoxide dismutase in the management of chronic neck pain: a prospective randomized study. Drugs R D. 2014;14(1):1–7. doi: 10.1007/s40268-013-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reda D.M., Abd-El-Fatah N.K., Omar Tel S., Darwish O.A. Fish Oil Intake and Seizure Control in Children with Medically Resistant Epilepsy. N. Am. J. Med. Sci. 2015;7(7):317–321. doi: 10.4103/1947-2714.161248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeGiorgio C.M., Miller P.R., Harper R., et al. Fish oil (n-3 fatty acids) in drug resistant epilepsy: a randomised placebo-controlled crossover study. J. Neurol. Neurosurg. Psychiatry. 2015;86(1):65–70. doi: 10.1136/jnnp-2014-307749. [DOI] [PubMed] [Google Scholar]
- 61.DeGiorgio C.M., Miller P., Meymandi S., Gornbein J.A. n-3 Fatty acids (fish oil) for epilepsy, cardiac risk factors, and risk of SUDEP: Clues from a pilot, double-blind, exploratory study. Epilepsy Behav. 2008;13(4):681–684. doi: 10.1016/j.yebeh.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Bromfield E., Dworetzky B., Hurwitz S., et al. A randomized trial of polyunsaturated fatty acids for refractory epilepsy. Epilepsy Behav. 2008;12(1):187–190. doi: 10.1016/j.yebeh.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 63.Kanai M., Yoshimura K., Asada M., et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011;68(1):157–164. doi: 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- 64.Mahammedi H., Planchat E., Pouget M., et al. The New Combination Docetaxel, Prednisone and Curcumin in Patients with Castration-Resistant Prostate Cancer: A Pilot Phase II Study. Oncology. 2016;90(2):69–78. doi: 10.1159/000441148. [DOI] [PubMed] [Google Scholar]
- 65.Ghalaut V.S., Sangwan L., Dahiya K., Ghalaut P.S., Dhankhar R., Saharan R. Effect of imatinib therapy with and without turmeric powder on nitric oxide levels in chronic myeloid leukemia. J. Oncol. Pharm. Pract. 2012;18(2):186–190. doi: 10.1177/1078155211416530. [DOI] [PubMed] [Google Scholar]
- 66.Bougnoux P., Hajjaji N., Ferrasson M.N., Giraudeau B., Couet C., Le Floch O. Improving outcome of chemotherapy of metastatic breast cancer by docosahexaenoic acid: a phase II trial. Br. J. Cancer. 2009;101(12):1978–1985. doi: 10.1038/sj.bjc.6605441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy R.A., Mourtzakis M., Chu Q.S., Baracos V.E., Reiman T., Mazurak V.C. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer. 2011;117(16):3774–3780. doi: 10.1002/cncr.25933. [DOI] [PubMed] [Google Scholar]
- 68.Ghoreishi Z., Esfahani A., Djazayeri A., et al. Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo controlled trial. BMC Cancer. 2012;12:355. doi: 10.1186/1471-2407-12-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Magbanua M.J., Roy R., Sosa E.V., et al. Gene expression and biological pathways in tissue of men with prostate cancer in a randomized clinical trial of lycopene and fish oil supplementation. PLoS One. 2011;6(9):e24004. doi: 10.1371/journal.pone.0024004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Miranda Torrinhas R.S., Santana R., Garcia T., et al. Parenteral fish oil as a pharmacological agent to modulate post-operative immune response: a randomized, double-blind, and controlled clinical trial in patients with gastrointestinal cancer. Clin Nutr (Edinburgh, Scotland) 2013;32(4):503–510. doi: 10.1016/j.clnu.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Brasnyo P., Molnar G.A., Mohas M., et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011;106(3):383–389. doi: 10.1017/S0007114511000316. [DOI] [PubMed] [Google Scholar]
- 72.Wickenberg J., Ingemansson S.L., Hlebowicz J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr. J. 2010;9:43. doi: 10.1186/1475-2891-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang D.Q., Li M.X., Ma Y.J., Wang Y., Wang Y. Efficacy and safety of prostaglandin E1 plus lipoic acid combination therapy versus monotherapy for patients with diabetic peripheral neuropathy. J. Clin. Neurosci. 2016;27:8–16. doi: 10.1016/j.jocn.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 74.Arora V., Kuhad A., Tiwari V., Chopra K. Curcumin ameliorates reserpine-induced pain-depression dyad: behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology. 2011;36(10):1570–1581. doi: 10.1016/j.psyneuen.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 75.Yu J.J., Pei L.B., Zhang Y., Wen Z.Y., Yang J.L. Chronic Supplementation of Curcumin Enhances the Efficacy of Antidepressants in Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Clin. Psychopharmacol. 2015;35(4):406–410. doi: 10.1097/JCP.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 76.Lyoo I.K., Yoon S., Kim T.S., et al. A randomized, double-blind placebo-controlled trial of oral creatine monohydrate augmentation for enhanced response to a selective serotonin reuptake inhibitor in women with major depressive disorder. Am. J. Psychiatry. 2012;169(9):937–945. doi: 10.1176/appi.ajp.2012.12010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kraguljac N.V., Montori V.M., Pavuluri M., Chai H.S., Wilson B.S., Unal S.S. Efficacy of omega-3 fatty acids in mood disorders - a systematic review and metaanalysis. Psychopharmacol. Bull. 2009;42(3):39–54. [PubMed] [Google Scholar]
- 78.Miodownik C., Maayan R., Ratner Y., et al. Serum levels of brain-derived neurotrophic factor and cortisol to sulfate of dehydroepiandrosterone molar ratio associated with clinical response to L-theanine as augmentation of antipsychotic therapy in schizophrenia and schizoaffective disorder patients. Clin. Neuropharmacol. 2011;34(4):155–160. doi: 10.1097/WNF.0b013e318220d8c6. [DOI] [PubMed] [Google Scholar]
- 79.Ritsner M.S., Miodownik C., Ratner Y., et al. L-theanine relieves positive, activation, and anxiety symptoms in patients with schizophrenia and schizoaffective disorder: an 8-week, randomized, double-blind, placebo-controlled, 2-center study. J. Clin. Psychiatry. 2011;72(1):34–42. doi: 10.4088/JCP.09m05324gre. [DOI] [PubMed] [Google Scholar]
- 80.Block K.I., Gyllenhaal C., Lowe L., et al. Designing a broad-spectrum integrative approach for cancer prevention and treatment. Semin. Cancer Biol. 2015;35(Suppl.):S276–S304. doi: 10.1016/j.semcancer.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eom D.W., Lee J.H., Kim Y.J., et al. Synergistic effect of curcumin on epigallocatechin gallate-induced anticancer action in PC3 prostate cancer cells. BMB Rep. 2015;48(8):461–466. doi: 10.5483/BMBRep.2015.48.8.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chung S.S., Vadgama J.V. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NFkappaB signaling. Anticancer Res. 2015;35(1):39–46. [PMC free article] [PubMed] [Google Scholar]
- 83.Suganuma M., Saha A., Fujiki H. New cancer treatment strategy using combination of green tea catechins and anticancer drugs. Cancer Sci. 2011;102(2):317–323. doi: 10.1111/j.1349-7006.2010.01805.x. [DOI] [PubMed] [Google Scholar]
- 84.Samadi A.K., Bilsland A., Georgakilas A.G., et al. A multi-targeted approach to suppress tumor-promoting inflammation. Semin. Cancer Biol. 2015;35(Suppl.):S151–S184. doi: 10.1016/j.semcancer.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Z., Dabrosin C., Yin X., et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin. Cancer Biol. 2015;35(Suppl.):S224–S243. doi: 10.1016/j.semcancer.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Anderson G., Maes M. Oxidative/nitrosative stress and immuno-inflammatory pathways in depression: treatment implications. Curr. Pharm. Des. 2014;20(23):3812–3847. doi: 10.2174/13816128113196660738. [DOI] [PubMed] [Google Scholar]
- 87.Su K.P., Lai H.C., Yang H.T., et al. Omega-3 fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol. Psychiatry. 2014;76(7):559–566. doi: 10.1016/j.biopsych.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 88.Sanmukhani J., Anovadiya A., Tripathi C.B. Evaluation of antidepressant like activity of curcumin and its combination with fluoxetine and imipramine: an acute and chronic study. Acta Pol. Pharm. 2011;68(5):769–775. [PubMed] [Google Scholar]
- 89.Reeta K.H., Mehla J., Pahuja M., Gupta Y.K. Pharmacokinetic and pharmacodynamic interactions of valproate, phenytoin, phenobarbitone and carbamazepine with curcumin in experimental models of epilepsy in rats. Pharmacol. Biochem. Behav. 2011;99(3):399–407. doi: 10.1016/j.pbb.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 90.Pages N., Maurois P., Delplanque B., Bac P., Vamecq J. Brain anticonvulsant protection of mice given chronic carbamazepine under various fatty acid and magnesium diet conditions. Prostaglandins Leukot. Essent. Fatty Acids. 2012;87(2-3):63–70. doi: 10.1016/j.plefa.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 91.Huang Z., Zhong X.M., Li Z.Y., Feng C.R., Pan A.J., Mao Q.Q. Curcumin reverses corticosterone-induced depressive-like behavior and decrease in brain BDNF levels in rats. Neurosci. Lett. 2011;493(3):145–148. doi: 10.1016/j.neulet.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 92.Laino C.H., Fonseca C., Sterin-Speziale N., Slobodianik N., Reines A. Potentiation of omega-3 fatty acid antidepressant-like effects with low non-antidepressant doses of fluoxetine and mirtazapine. Eur. J. Pharmacol. 2010;648(1-3):117–126. doi: 10.1016/j.ejphar.2010.08.047. [DOI] [PubMed] [Google Scholar]
- 93.Laino C.H., Garcia P., Podesta M.F., Hocht C., Slobodianik N., Reines A. Fluoxetine potentiation of omega-3 fatty acid antidepressant effect: evaluating pharmacokinetic and brain fatty acid-related aspects in rodents. J. Pharm. Sci. 2014;103(10):3316–3325. doi: 10.1002/jps.24123. [DOI] [PubMed] [Google Scholar]
- 94.Yoon M.H., Kim K.S., Lee H.G., et al. Synergistic interaction between intrathecal ginsenosides and morphine on formalin-induced nociception in rats. J. Pain. 2011;12(7):774–781. doi: 10.1016/j.jpain.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 95.Escudero G.E., Romanuk C.B., Toledo M.E., Olivera M.E., Manzo R.H., Laino C.H. Analgesia enhancement and prevention of tolerance to morphine: beneficial effects of combined therapy with omega-3 fatty acids. J. Pharm. Pharmacol. 2015;67(9):1251–1262. doi: 10.1111/jphp.12416. [DOI] [PubMed] [Google Scholar]
- 96.Kunnumakkara A.B., Diagaradjane P., Anand P., et al. Curcumin sensitizes human colorectal cancer to capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int. J. Cancer. 2009;125(9):2187–2197. doi: 10.1002/ijc.24593. [DOI] [PubMed] [Google Scholar]
- 97.Zhou X., Wang W., Li P., et al. Curcumin Enhances the Effects of 5-Fluorouracil and Oxaliplatin in Inducing Gastric Cancer Cell Apoptosis Both In Vitro and In Vivo. Oncol. Res. 2016;23(1):29–34. doi: 10.3727/096504015X14452563486011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dun J., Chen X., Gao H., Zhang Y., Zhang H., Zhang Y. Resveratrol synergistically augments anti-tumor effect of 5-FU in vitro and in vivo by increasing S-phase arrest and tumor apoptosis. Exp. Biol. Med. (Maywood) 2015;240(12):1672–1681. doi: 10.1177/1535370215573396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Davidson E.P., Holmes A., Coppey L.J., Yorek M.A. Effect of combination therapy consisting of enalapril, alpha-lipoic acid, and menhaden oil on diabetic neuropathy in a high fat/low dose streptozotocin treated rat. Eur. J. Pharmacol. 2015;765:258–267. doi: 10.1016/j.ejphar.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 100.Wu M.H., Huang C.C., Chio C.C., et al. Inhibition of Peripheral TNF-alpha and Downregulation of Microglial Activation by Alpha-Lipoic Acid and Etanercept Protect Rat Brain Against Ischemic Stroke. Mol. Neurobiol. 2015 doi: 10.1007/s12035-015-9418-5. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 101.Vasconcelos G.S., Ximenes N.C., de Sousa C.N., et al. Alpha-lipoic acid alone and combined with clozapine reverses schizophrenia-like symptoms induced by ketamine in mice: Participation of antioxidant, nitrergic and neurotrophic mechanisms. Schizophr. Res. 2015;165(2-3):163–170. doi: 10.1016/j.schres.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 102.Li Y-C., Wang F-M., Pan Y., et al. Antidepressant-like effects of curcumin on serotonergic receptor-coupled AC-cAMP pathway in chronic unpredictable mild stress of rats. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(3):435–449. doi: 10.1016/j.pnpbp.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 103.Kulkarni S.K., Bhutani M.K., Bishnoi M. Antidepressant activity of curcumin: involvement of serotonin and dopamine system. Psychopharmacology (Berl.) 2008;201(3):435–442. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- 104.Xia X., Cheng G., Pan Y., Xia Z.H., Kong L.D. Behavioral, neurochemical and neuroendocrine effects of the ethanolic extract from Curcuma longa L. in the mouse forced swimming test. J. Ethnopharmacol. 2007;110(2):356–363. doi: 10.1016/j.jep.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 105.Yu Z.F., Kong L.D., Chen Y. Antidepressant activity of aqueous extracts of Curcuma longa in mice. J. Ethnopharmacol. 2002;83(1-2):161–165. doi: 10.1016/s0378-8741(02)00211-8. [DOI] [PubMed] [Google Scholar]
- 106.Yu Y., Wang R., Chen C., et al. Antidepressant-like effect of trans-resveratrol in chronic stress model: behavioral and neurochemical evidences. J. Psychiatr. Res. 2013;47(3):315–322. doi: 10.1016/j.jpsychires.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 107.Harsing L.G., Jr, Sershen H., Toth E., Hashim A., Ramacci M.T., Lajtha A. Acetyl-L-carnitine releases dopamine in rat corpus striatum: an in vivo microdialysis study. Eur. J. Pharmacol. 1992;218(1):117–121. doi: 10.1016/0014-2999(92)90154-v. [DOI] [PubMed] [Google Scholar]
- 108.Nasca C., Xenos D., Barone Y., et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc. Natl. Acad. Sci. USA. 2013;110(12):4804–4809. doi: 10.1073/pnas.1216100110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bersani G., Meco G., Denaro A., et al. L-Acetylcarnitine in dysthymic disorder in elderly patients: a double-blind, multicenter, controlled randomized study vs. fluoxetine. Eur. Neuropsychopharmacol. 2013;23(10):1219–1225. doi: 10.1016/j.euroneuro.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 110.Yamada T., Terashima T., Kawano S., et al. Theanine, gamma-glutamylethylamide, a unique amino acid in tea leaves, modulates neurotransmitter concentrations in the brain striatum interstitium in conscious rats. Amino Acids. 2009;36(1):21–27. doi: 10.1007/s00726-007-0020-7. [DOI] [PubMed] [Google Scholar]
- 111.Sabaté E. Adherence to long-term therapies: evidence for action. Switzerland: World Health Organization; 2003. [Google Scholar]
- 112.Osterberg L., Blaschke T. Adherence to medication. N. Engl. J. Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 113.Thomas F., Katrina F. Estimated annual pharmaceutical revenue loss due to medication non-adherence. Capgemini Consulting; 2012. [Google Scholar]
- 114.Ettinger A.B., Manjunath R., Candrilli S.D., Davis K.L. Prevalence and cost of nonadherence to antiepileptic drugs in elderly patients with epilepsy. Epilepsy Behav. 2009;14(2):324–329. doi: 10.1016/j.yebeh.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 115.Manjunath R., Davis K.L., Candrilli S.D., Ettinger A.B. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009;14(2):372–378. doi: 10.1016/j.yebeh.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 116.Gharibian D., Polzin J.K., Rho J.P. Compliance and persistence of antidepressants versus anticonvulsants in patients with neuropathic pain during the first year of therapy. Clin. J. Pain. 2013;29(5):377–381. doi: 10.1097/AJP.0b013e31825e45d9. [DOI] [PubMed] [Google Scholar]
- 117.McLaughlin T., Hogue S.L., Stang P.E. Once-daily bupropion associated with improved patient adherence compared with twice-daily bupropion in treatment of depression. Am. J. Ther. 2007;14(2):221–225. doi: 10.1097/01.mjt.0000208273.80496.3f. [DOI] [PubMed] [Google Scholar]
- 118.Chowdhury R., Khan H., Heydon E., et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur. Heart J. 2013;34(38):2940–2948. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 119.Wigertz A., Ahlgren J., Holmqvist M., et al. Adherence and discontinuation of adjuvant hormonal therapy in breast cancer patients: a population-based study. Breast Cancer Res. Treat. 2012;133(1):367–373. doi: 10.1007/s10549-012-1961-4. [DOI] [PubMed] [Google Scholar]
- 120.Hertz R.P., Unger A.N., Lustik M.B. Adherence with pharmacotherapy for type 2 diabetes: a retrospective cohort study of adults with employer-sponsored health insurance. Clin. Ther. 2005;27(7):1064–1073. doi: 10.1016/j.clinthera.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 121.Herndon J.B., Mattke S., Evans Cuellar A., Hong S.Y., Shenkman E.A. Anti-inflammatory medication adherence, healthcare utilization and expenditures among Medicaid and children's health insurance program enrollees with asthma. Pharmacoeconomics. 2012;30(5):397–412. doi: 10.2165/11586660-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 122.Hugtenburg J.G., Timmers L., Elders P.J., Vervloet M., van Dijk L. Definitions, variants, and causes of nonadherence with medication: a challenge for tailored interventions. Patient Prefer. Adherence. 2013;7:675–682. doi: 10.2147/PPA.S29549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kardas P., Lewek P., Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front. Pharmacol. 2013;4:91. doi: 10.3389/fphar.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Davis L., Brunetti L., Lee E.K., Yoon N., Cho S.H., Suh D.C. Effects of computerized physician order entry on medication turnaround time and orders requiring pharmacist intervention. Res. Social Adm. Pharm. 2014;10(5):756–767. doi: 10.1016/j.sapharm.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 125.Brown M.T., Bussell J.K. Medication adherence: WHO cares? Mayo Clin. Proc. 2011;86(4):304–314. doi: 10.4065/mcp.2010.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Medic G., Higashi K., Littlewood K.J., Diez T., Granstrom O., Kahn R.S. Dosing frequency and adherence in chronic psychiatric disease: systematic review and meta-analysis. Neuropsychiatr. Dis. Treat. 2013;9:119–131. doi: 10.2147/NDT.S39303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Davis K.L., Tangirala M., Meyers J.L., Wei W. Real-world comparative outcomes of US type 2 diabetes patients initiating analog basal insulin therapy. Curr. Med. Res. Opin. 2013;29(9):1083–1091. doi: 10.1185/03007995.2013.811403. [DOI] [PubMed] [Google Scholar]
- 128.Korhonen M.J., Halonen J.I., Brookhart M.A., et al. Childhood adversity as a predictor of non-adherence to statin therapy in adulthood. PLoS One. 2015;10(5):e0127638. doi: 10.1371/journal.pone.0127638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.DiMatteo M.R., Lepper H.S., Croghan T.W. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch. Intern. Med. 2000;160(14):2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 130.Bet P.M., Penninx B.W., van Laer S.D., Hoogendijk W.J., Hugtenburg J.G. Current and remitted depression and anxiety disorders as risk factors for medication nonadherence. J. Clin. Psychiatry. 2015;76(9):e1114–e1121. doi: 10.4088/JCP.14m09001. [DOI] [PubMed] [Google Scholar]
- 131.Demonceau J., Ruppar T., Kristanto P., et al. Identification and assessment of adherence-enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta-analysis. Drugs. 2013;73(6):545–562. doi: 10.1007/s40265-013-0041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Deci E.L., Koestner R., Ryan R.M. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol. Bull. 1999;125(6):627–668. doi: 10.1037/0033-2909.125.6.627. [DOI] [PubMed] [Google Scholar]
- 133.Szmukler G. Ethical problems with paying patients to improve adherence to treatment. BMJ. 2013;347:f6837. doi: 10.1136/bmj.f6837. [DOI] [PubMed] [Google Scholar]
- 134.DeFulio A., Silverman K. The use of incentives to reinforce medication adherence. Prev. Med. 2012;55(Suppl.):S86–S94. doi: 10.1016/j.ypmed.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sen A.P., Sewell T.B., Riley E.B., et al. Financial incentives for home-based health monitoring: a randomized controlled trial. J. Gen. Intern. Med. 2014;29(5):770–777. doi: 10.1007/s11606-014-2778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Blondon K., Klasnja P., Coleman K., Pratt W. An exploration of attitudes toward the use of patient incentives to support diabetes self-management. Psychol. Health. 2014;29(5):552–563. doi: 10.1080/08870446.2013.867346. [DOI] [PubMed] [Google Scholar]
- 137.Petry N.M., Cengiz E., Wagner J.A., Hood K.K., Carria L., Tamborlane W.V. Incentivizing behaviour change to improve diabetes care. Diabetes Obes. Metab. 2013;15(12):1071–1076. doi: 10.1111/dom.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Arsand E., Froisland D.H., Skrovseth S.O., et al. Mobile health applications to assist patients with diabetes: lessons learned and design implications. J. Diabetes Sci. Technol. 2012;6(5):1197–1206. doi: 10.1177/193229681200600525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cameron J., Banko K.M., Pierce W.D. Pervasive negative effects of rewards on intrinsic motivation: The myth continues. Behav. Anal. 2001;24(1):1–44. doi: 10.1007/BF03392017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Patall E.A., Cooper H., Robinson J.C. The effects of choice on intrinsic motivation and related outcomes: a meta-analysis of research findings. Psychol. Bull. 2008;134(2):270–300. doi: 10.1037/0033-2909.134.2.270. [DOI] [PubMed] [Google Scholar]
- 141.Hain D.J., Sandy D. Partners in care: patient empowerment through shared decision-making. Nephrol. Nurs. J. 2013;40(2):153–157. [PubMed] [Google Scholar]
- 142.Baars J.E., Markus T., Kuipers E.J., van der Woude C.J. Patients' preferences regarding shared decision-making in the treatment of inflammatory bowel disease: results from a patient-empowerment study. Digestion. 2010;81(2):113–119. doi: 10.1159/000253862. [DOI] [PubMed] [Google Scholar]
- 143.Clayman M.L., Bylund C.L., Chewning B., Makoul G. The Impact of Patient Participation in Health Decisions Within Medical Encounters: A Systematic Review. Med. Decis. Making. 2016;36(4):427–452. doi: 10.1177/0272989X15613530. [DOI] [PubMed] [Google Scholar]
- 144.Nota I., Drossaert C.H., Taal E., Vonkeman H.E., van de Laar M.A. Patient participation in decisions about disease modifying anti-rheumatic drugs: a cross-sectional survey. BMC Musculoskelet. Disord. 2014;15:333. doi: 10.1186/1471-2474-15-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bellon M., Pfeiffer W., Maurici V. Choice and control: how involved are people with epilepsy and their families in the management of their epilepsy? Results from an Australian survey in the disability sector. Epilepsy Behav. 2014;37:227–232. doi: 10.1016/j.yebeh.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 146.Bruggers C.S., Altizer R.A., Kessler R.R., et al. Patient-empowerment interactive technologies. Sci. Transl. Med. 2012;4(152):152ps16. doi: 10.1126/scitranslmed.3004009. [DOI] [PubMed] [Google Scholar]
- 147.Bradway M., Arsand E., Grottland A. Mobile Health: empowering patients and driving change. Trends Endocrinol. Metab. 2015;26(3):114–117. doi: 10.1016/j.tem.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 148.Govender M., Bowen R.C., German M.L., Bulaj G., Bruggers C.S. Clinical and Neurobiological Perspectives of Empowering Pediatric Cancer Patients Using Videogames. Games Health J. 2015;4(5):362–374. doi: 10.1089/g4h.2015.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wildevuur S.E., Simonse L.W. Information and communication technology-enabled person-centered care for the “big five” chronic conditions: scoping review. J. Med. Internet Res. 2015;17(3):e77. doi: 10.2196/jmir.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Charlier N., Zupancic N., Fieuws S., Denhaerynck K., Zaman B., Moons P. Serious Games for improving knowledge and self-management in young people with chronic conditions: A systematic review and meta-analysis. J. Am. Med. Inform. Assoc. 2016;23(1):230–239. doi: 10.1093/jamia/ocv100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Theng Y.L., Lee J.W., Patinadan P.V., Foo S.S. The Use of Videogames, Gamification, and Virtual Environments in the Self-Management of Diabetes: A Systematic Review of Evidence. Games Health J. 2015;4(5):352–361. doi: 10.1089/g4h.2014.0114. [DOI] [PubMed] [Google Scholar]
- 152.Miller A.S., Cafazzo J.A., Seto E. A game plan: Gamification design principles in mHealth applications for chronic disease management. Health Informatics J. 2016;22(2):184–193. doi: 10.1177/1460458214537511. [DOI] [PubMed] [Google Scholar]
- 153.Zhao S., Iyengar R. Systems pharmacology: network analysis to identify multiscale mechanisms of drug action. Annu. Rev. Pharmacol. Toxicol. 2012;52:505–521. doi: 10.1146/annurev-pharmtox-010611-134520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Knight Z.A., Lin H., Shokat K.M. Targeting the cancer kinome through polypharmacology. Nat. Rev. Cancer. 2010;10(2):130–137. doi: 10.1038/nrc2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kummar S., Chen H.X., Wright J., et al. Utilizing targeted cancer therapeutic agents in combination: novel approaches and urgent requirements. Nat. Rev. Drug Discov. 2010;9(11):843–856. doi: 10.1038/nrd3216. [DOI] [PubMed] [Google Scholar]
- 156.Chaparro L.E., Wiffen P.J., Moore R.A., Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst. Rev. 2012;7:CD008943. doi: 10.1002/14651858.CD008943.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Romano CL, Romano D, Lacerenza M. Antineuropathic and antinociceptive drugs combination in patients with chronic low back pain: a systematic review. 2012. [DOI] [PMC free article] [PubMed]
- 158.Margineanu D.G. Systems biology, complexity, and the impact on antiepileptic drug discovery. Epilepsy Behav. 2014;38C:131–142. doi: 10.1016/j.yebeh.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 159.Millan M.J. On 'polypharmacy' and multi-target agents, complementary strategies for improving the treatment of depression: a comparative appraisal. Int. J. Neuropsychopharmacol. 2014;17(7):1009–1037. doi: 10.1017/S1461145712001496. [DOI] [PubMed] [Google Scholar]
- 160.Barnett A.H. Complementing insulin therapy to achieve glycemic control. Adv. Ther. 2013;30(6):557–576. doi: 10.1007/s12325-013-0039-y. [DOI] [PubMed] [Google Scholar]
- 161.Holst J.J., Vilsboll T. Combining GLP-1 receptor agonists with insulin: therapeutic rationales and clinical findings. Diabetes Obes. Metab. 2013;15(1):3–14. doi: 10.1111/j.1463-1326.2012.01628.x. [DOI] [PubMed] [Google Scholar]
- 162.Benedetti F. Mechanisms of placebo and placebo-related effects across diseases and treatments. Annu. Rev. Pharmacol. Toxicol. 2008;48:33–60. doi: 10.1146/annurev.pharmtox.48.113006.094711. [DOI] [PubMed] [Google Scholar]
- 163.Benedetti F. Placebo effects: from the neurobiological paradigm to translational implications. Neuron. 2014;84(3):623–637. doi: 10.1016/j.neuron.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 164.Jackson C.F., Makin S.M., Marson A.G., Kerr M. Non-pharmacological interventions for people with epilepsy and intellectual disabilities. Cochrane Database Syst. Rev. 2015;9:CD005502. doi: 10.1002/14651858.CD005502.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kwan P., Brodie M.J. Epilepsy after the first drug fails: substitution or add-on? Seizure. 2000;9(7):464–468. doi: 10.1053/seiz.2000.0442. [DOI] [PubMed] [Google Scholar]
- 166.Kwan P., Brodie M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 167.Brodie M.J., Barry S.J., Bamagous G.A., Kwan P. Effect of dosage failed of first antiepileptic drug on subsequent outcome. Epilepsia. 2013;54(1):194–198. doi: 10.1111/j.1528-1167.2012.03722.x. [DOI] [PubMed] [Google Scholar]
- 168.Brodie M.J., Barry S.J., Bamagous G.A., Norrie J.D., Kwan P. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78(20):1548–1554. doi: 10.1212/WNL.0b013e3182563b19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kanner A.M. Management of psychiatric and neurological comorbidities in epilepsy. Nat. Rev. Neurol. 2016;12:106–6. doi: 10.1038/nrneurol.2015.243. [DOI] [PubMed] [Google Scholar]
- 170.Tejada S., Martorell M., Capo X., Tur J.A., Pons A., Sureda A. Omega-3 Fatty Acids in the Management of Epilepsy. Curr. Top. Med. Chem. 2016;16(17):1897–1905. doi: 10.2174/1568026616666160204123107. [DOI] [PubMed] [Google Scholar]
- 171.Yuen A.W., Flugel D., Poepel A., Bell G.S., Peacock J.L., Sander J.W. Non-randomized open trial of eicosapentaenoic acid (EPA), an omega-3 fatty acid, in ten people with chronic epilepsy. Epilepsy Behav. 2012;23(3):370–372. doi: 10.1016/j.yebeh.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 172.Yuen A.W., Sander J.W., Fluegel D., et al. Omega-3 fatty acid supplementation in patients with chronic epilepsy: a randomized trial. Epilepsy Behav. 2005;7(2):253–258. doi: 10.1016/j.yebeh.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 173.Kim W., McMurray D.N., Chapkin R.S. n-3 polyunsaturated fatty acids--physiological relevance of dose. Prostaglandins Leukot. Essent. Fatty Acids. 2010;82(4-6):155–158. doi: 10.1016/j.plefa.2010.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Trepanier M.O., Kwong K.M., Domenichiello A.F., Chen C.T., Bazinet R.P., Burnham W.M. Intravenous infusion of docosahexaenoic acid increases serum concentrations in a dose-dependent manner and increases seizure latency in the maximal PTZ model. Epilepsy Behav. 2015;50:71–76. doi: 10.1016/j.yebeh.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 175.Vreugdenhil M., Bruehl C., Voskuyl R.A., Kang J.X., Leaf A., Wadman W.J. Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc. Natl. Acad. Sci. USA. 1996;93(22):12559–12563. doi: 10.1073/pnas.93.22.12559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Xiao Y., Li X. Polyunsaturated fatty acids modify mouse hippocampal neuronal excitability during excitotoxic or convulsant stimulation. Brain Res. 1999;846(1):112–121. doi: 10.1016/s0006-8993(99)01997-6. [DOI] [PubMed] [Google Scholar]
- 177.Trepanier MO, Hopperton KE, Orr SK, Bazinet RP. 2015.
- 178.De Almeida A.C., dos Santos H.L., Rodrigues A.M., et al. Non-synaptic mechanisms that could be responsible for potential antiepileptic effects of omega-3 fatty acids. Epilepsy Behav. 2012;25(1):138–140. doi: 10.1016/j.yebeh.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 179.Xu X.P., Erichsen D., Borjesson S.I., Dahlin M., Amark P., Elinder F. Polyunsaturated fatty acids and cerebrospinal fluid from children on the ketogenic diet open a voltage-gated K channel: a putative mechanism of antiseizure action. Epilepsy Res. 2008;80(1):57–66. doi: 10.1016/j.eplepsyres.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 180.Yazdi S., Stein M., Elinder F., Andersson M., Lindahl E. The Molecular Basis of Polyunsaturated Fatty Acid Interactions with the Shaker Voltage-Gated Potassium Channel. PLOS Comput. Biol. 2016;12(1):e1004704. doi: 10.1371/journal.pcbi.1004704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chang P., Terbach N., Plant N., Chen P.E., Walker M.C., Williams R.S. Seizure control by ketogenic diet-associated medium chain fatty acids. Neuropharmacology. 2013;69:105–114. doi: 10.1016/j.neuropharm.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wlaz P., Socala K., Nieoczym D., et al. Acute anticonvulsant effects of capric acid in seizure tests in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;57:110–116. doi: 10.1016/j.pnpbp.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 183.Chang P., Augustin K., Boddum K., et al. Seizure control by decanoic acid through direct AMPA receptor inhibition. Brain. 2016;139(Pt 2):431–443. doi: 10.1093/brain/awv325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Choudhary K.M., Mishra A., Poroikov V.V., Goel R.K. Ameliorative effect of Curcumin on seizure severity, depression like behavior, learning and memory deficit in post-pentylenetetrazole-kindled mice. Eur. J. Pharmacol. 2013;704(1-3):33–40. doi: 10.1016/j.ejphar.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 185.Kaur H., Patro I., Tikoo K., Sandhir R. Curcumin attenuates inflammatory response and cognitive deficits in experimental model of chronic epilepsy. Neurochem. Int. 2015;89:40–50. doi: 10.1016/j.neuint.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 186.Jiang Z., Guo M., Shi C., et al. Protection against cognitive impairment and modification of epileptogenesis with curcumin in a post-status epilepticus model of temporal lobe epilepsy. Neuroscience. 2015;310:362–371. doi: 10.1016/j.neuroscience.2015.09.058. [DOI] [PubMed] [Google Scholar]
- 187.Kiasalari Z., Roghani M., Khalili M., Rahmati B., Baluchnejadmojarad T. Antiepileptogenic effect of curcumin on kainate-induced model of temporal lobe epilepsy. Pharm. Biol. 2013;51(12):1572–1578. doi: 10.3109/13880209.2013.803128. [DOI] [PubMed] [Google Scholar]
- 188.Mishra V., Shuai B., Kodali M., et al. Resveratrol Treatment after Status Epilepticus Restrains Neurodegeneration and Abnormal Neurogenesis with Suppression of Oxidative Stress and Inflammation. Sci. Rep. 2015;5:17807. doi: 10.1038/srep17807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Saha L., Chakrabarti A. Understanding the anti-kindling role and its mechanism of Resveratrol in Pentylenetetrazole induced-kindling in a rat model. Pharmacol. Biochem. Behav. 2014;120:57–64. doi: 10.1016/j.pbb.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 190.Wang S.J., Bo Q.Y., Zhao X.H., Yang X., Chi Z.F., Liu X.W. Resveratrol pre-treatment reduces early inflammatory responses induced by status epilepticus via mTOR signaling. Brain Res. 2013;1492:122–129. doi: 10.1016/j.brainres.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 191.Pallas M., Ortuno-Sahagun D., Benito-Andres P., Ponce-Regalado M.D., Rojas-Mayorquin A.E. Resveratrol in epilepsy: preventive or treatment opportunities? Front. Biosci. (Landmark Ed.) 2014;19:1057–1064. doi: 10.2741/4267. [DOI] [PubMed] [Google Scholar]
- 192.Shetty A.K. Promise of resveratrol for easing status epilepticus and epilepsy. Pharmacol. Ther. 2011;131(3):269–286. doi: 10.1016/j.pharmthera.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bedada S.K., Nearati P. Effect of resveratrol on the phar- macokinetics of carbamazepine in healthy human volunteers. Phytother. Res. 2015;29(5):701–706. doi: 10.1002/ptr.5302. [DOI] [PubMed] [Google Scholar]
- 194.Yuen A.W., Sander J.W., Flugel D., et al. Erythrocyte and plasma fatty acid profiles in patients with epilepsy: does carbamazepine affect omega-3 fatty acid concentrations? Epilepsy Behav. 2008;12(2):317–323. doi: 10.1016/j.yebeh.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 195.Shegog R., Bamps Y.A., Patel A., et al. Managing Epilepsy Well: Emerging e-Tools for epilepsy self-management. Epilepsy Behav. 2013;29(1):133–140. doi: 10.1016/j.yebeh.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 196.Pandher P.S., Bhullar K.K. Smartphone applications for seizure management. Health Informatics J. 2014;22(2):209–220. doi: 10.1177/1460458214540906. [DOI] [PubMed] [Google Scholar]
- 197.Jory C., Shankar R., Coker D., McLean B., Hanna J., Newman C. Safe and sound? A systematic literature review of seizure detection methods for personal use. Seizure. 2016;36:4–15. doi: 10.1016/j.seizure.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 198.Tseng K.C., Lin B.S., Wong A.M., Lin B.S. Design of a mobile brain computer interface-based smart multimedia controller. Sensors (Basel) 2015;15(3):5518–5530. doi: 10.3390/s150305518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ramirez R., Palencia-Lefler M., Giraldo S., Vamvakousis Z. Musical neurofeedback for treating depression in elderly people. Front. Neurosci. 2015;9:354. doi: 10.3389/fnins.2015.00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu X., Wang R., Zhou D., Hong Z. Feasibility and acceptability of smartphone applications for seizure self-management in China: Questionnaire study among people with epilepsy. Epilepsy Behav. 2015;55:57–61. doi: 10.1016/j.yebeh.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 201.Gillham R.A. Refractory epilepsy: an evaluation of psychological methods in outpatient management. Epilepsia. 1990;31(4):427–432. doi: 10.1111/j.1528-1157.1990.tb05498.x. [DOI] [PubMed] [Google Scholar]
- 202.Tieffenberg J.A., Wood E.I., Alonso A., Tossutti M.S., Vicente M.F. A randomized field trial of ACINDES: a child-centered training model for children with chronic illnesses (asthma and epilepsy). J. Urban Health. 2000;77(2):280–297. doi: 10.1007/BF02390539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.May T.W., Pfafflin M. The efficacy of an educational treatment program for patients with epilepsy (MOSES): results of a controlled, randomized study. Modular Service Package Epilepsy. Epilepsia. 2002;43(5):539–549. doi: 10.1046/j.1528-1157.2002.23801.x. [DOI] [PubMed] [Google Scholar]
- 204.Lundgren T., Dahl J., Melin L., Kies B. Evaluation of acceptance and commitment therapy for drug refractory epilepsy: a randomized controlled trial in South Africa--a pilot study. Epilepsia. 2006;47(12):2173–2179. doi: 10.1111/j.1528-1167.2006.00892.x. [DOI] [PubMed] [Google Scholar]
- 205.Lundgren T., Dahl J., Yardi N., Melin L. Acceptance and Commitment Therapy and yoga for drug-refractory epilepsy: a randomized controlled trial. Epilepsy Behav. 2008;13(1):102–108. doi: 10.1016/j.yebeh.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 206.McLaughlin D.P., McFarland K. A randomized trial of a group based cognitive behavior therapy program for older adults with epilepsy: the impact on seizure frequency, depression and psychosocial well-being. J. Behav. Med. 2011;34(3):201–207. doi: 10.1007/s10865-010-9299-z. [DOI] [PubMed] [Google Scholar]
- 207.Coppola G., Toro A., Operto F.F., et al. Mozart's music in children with drug-refractory epileptic encephalopathies. Epilepsy Behav. 2015;50:18–22. doi: 10.1016/j.yebeh.2015.05.038. [DOI] [PubMed] [Google Scholar]
- 208.Lin L.C., Lee W.T., Wang C.H., et al. Mozart K.448 acts as a potential add-on therapy in children with refractory epilepsy. Epilepsy Behav. 2011;20(3):490–493. doi: 10.1016/j.yebeh.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 209.Dastgheib S.S., Layegh P., Sadeghi R., Foroughipur M., Shoeibi A., Gorji A. The effects of Mozart's music on interictal activity in epileptic patients: systematic review and meta-analysis of the literature. Curr. Neurol. Neurosci. Rep. 2014;14(1):420. doi: 10.1007/s11910-013-0420-x. [DOI] [PubMed] [Google Scholar]
- 210.Fleeman N., Bradley P.M., Lindsay B. Care delivery and self management strategies for children with epilepsy. Cochrane Database Syst. Rev. 2015;12:CD006245. doi: 10.1002/14651858.CD006245.pub3. [DOI] [PubMed] [Google Scholar]
- 211.Roepke A.M., Jaffee S.R., Riffle O.M., McGonigal J., Broome R., Maxwell B. Randomized Controlled Trial of SuperBetter, a Smartphone-Based/Internet-Based Self-Help Tool to Reduce Depressive Symptoms. Games Health J. 2015;4(3):235–246. doi: 10.1089/g4h.2014.0046. [DOI] [PubMed] [Google Scholar]
- 212.Ebert D.D., Zarski A.C., Christensen H., et al. Internet and computer-based cognitive behavioral therapy for anxiety and depression in youth: a meta-analysis of randomized controlled outcome trials. PLoS One. 2015;10(3):e0119895. doi: 10.1371/journal.pone.0119895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Birney A.J., Gunn R., Russell J.K., Ary D.V. MoodHacker Mobile Web App With Email for Adults to Self-Manage Mild-to-Moderate Depression: Randomized Controlled Trial. JMIR Mhealth Uhealth. 2016;4(1):e8. doi: 10.2196/mhealth.4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Goldenholz D.M., Goldenholz S.R. Response to placebo in clinical epilepsy trials-Old ideas and new insights. Epilepsy Res. 2016;122:15–25. doi: 10.1016/j.eplepsyres.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ross D, Armen B, Anita C, Anusuya C, Kyu K, Soojung K, Kevin K. An Unhealthy America: The Economic Burden of Chronic Disease -- Charting a New Course to Save Lives and Increase Productivity and Economic Growth. 2007 [Google Scholar]
- 216.Goodman R.A., Posner S.F., Huang E.S., Parekh A.K., Koh H.K. Defining and Measuring Chronic Conditions: Imperatives for Research, Policy, Program, and Practice. Prev. Chronic Dis. 2013;10:E66. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.CDC report. The Power of Prevention. Chronic disease... the public health challenge of the 21st century. Web site.http://www.cdc.gov/chronicdisease/pdf/2009-power-ofprevention.pdf. 2009. pp. 1–16.
- 218.Bodenheimer T., Lorig K., Holman H., Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- 219.Brenk-Franz K., Strauss B., Tiesler F., et al. The Influence of Adult Attachment on Patient Self-Management in Primary Care - The Need for a Personalized Approach and Patient-Centred Care. PLoS One. 2015;10(9):e0136723. doi: 10.1371/journal.pone.0136723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Iversen M.D., Brawerman M., Iversen C.N. Recommendations and the state of the evidence for physical activity interventions for adults with rheumatoid arthritis: 2007 to present. Int. J. Clin. Rheumatol. 2012;7(5):489–503. doi: 10.2217/ijr.12.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.O'Grady M., Fletcher J., Ortiz S. Therapeutic and physical fitness exercise prescription for older adults with joint disease: an evidence-based approach. Rheum. Dis. Clin. North Am. 2000;26(3):617–646. doi: 10.1016/s0889-857x(05)70159-9. [DOI] [PubMed] [Google Scholar]
- 222.Mura G., Moro M.F., Patten S.B., Carta M.G. Exercise as an add-on strategy for the treatment of major depressive disorder: a systematic review. CNS Spectr. 2014;19(6):496–508. doi: 10.1017/S1092852913000953. [DOI] [PubMed] [Google Scholar]
- 223.Tomlinson C.L., Patel S., Meek C., et al. Physiotherapy versus placebo or no intervention in Parkinson's disease. Cochrane Database Syst. Rev. 2012;(7):CD002817. doi: 10.1002/14651858.CD002817.pub3. [DOI] [PubMed] [Google Scholar]
- 224.Dobson J.L., McMillan J., Li L. Benefits of exercise intervention in reducing neuropathic pain. Front. Cell. Neurosci. 2014;8:102. doi: 10.3389/fncel.2014.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Baumann F.T., Bloch W., Beulertz J. Clinical exercise interventions in pediatric oncology: a systematic review. Pediatr. Res. 2013;74(4):366–374. doi: 10.1038/pr.2013.123. [DOI] [PubMed] [Google Scholar]
- 226.Hoogeboom T.J., Dronkers J.J., Hulzebos E.H., van Meeteren N.L. Merits of exercise therapy before and after major surgery. Curr. Opin. Anaesthesiol. 2014;27(2):161–166. doi: 10.1097/ACO.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Durcan L., Wilson F., Cunnane G. The effect of exercise on sleep and fatigue in rheumatoid arthritis: a randomized controlled study. J. Rheumatol. 2014;41(10):1966–1973. doi: 10.3899/jrheum.131282. [DOI] [PubMed] [Google Scholar]
- 228.Cramer H., Lauche R., Haller H., Dobos G. A systematic review and meta-analysis of yoga for low back pain. Clin. J. Pain. 2013;29(5):450–460. doi: 10.1097/AJP.0b013e31825e1492. [DOI] [PubMed] [Google Scholar]
- 229.Cramer H., Lauche R., Hohmann C., Langhorst J., Dobos G. Yoga for chronic neck pain: a 12-month follow-up. Pain Med. 2013;14(4):541–548. doi: 10.1111/pme.12053. [DOI] [PubMed] [Google Scholar]
- 230.Cramer H., Lauche R., Hohmann C., et al. Randomized-controlled trial comparing yoga and home-based exercise for chronic neck pain. Clin. J. Pain. 2013;29(3):216–223. doi: 10.1097/AJP.0b013e318251026c. [DOI] [PubMed] [Google Scholar]
- 231.Kiecolt-Glaser J.K., Bennett J.M., Andridge R., et al. Yoga's impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J. Clin. Oncol. 2014;32(10):1040–1049. doi: 10.1200/JCO.2013.51.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Taso C.J., Lin H.S., Lin W.L., Chen S.M., Huang W.T., Chen S.W. The effect of yoga exercise on improving depression, anxiety, and fatigue in women with breast cancer: a randomized controlled trial. J. Nurs. Res. 2014;22(3):155–164. doi: 10.1097/jnr.0000000000000044. [DOI] [PubMed] [Google Scholar]
- 233.Ebnezar J., Nagarathna R., Yogitha B., Nagendra H.R. Effects of an integrated approach of hatha yoga therapy on functional disability, pain, and flexibility in osteoarthritis of the knee joint: a randomized controlled study. J. Altern. Complement. Med. 2012;18(5):463–472. doi: 10.1089/acm.2010.0320. [DOI] [PubMed] [Google Scholar]
- 234.Ebnezar J., Nagarathna R., Yogitha B., Nagendra H.R. Effect of integrated yoga therapy on pain, morning stiffness and anxiety in osteoarthritis of the knee joint: A randomized control study. Int. J. Yoga. 2012;5(1):28–36. doi: 10.4103/0973-6131.91708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Cheung C., Wyman J.F., Resnick B., Savik K. Yoga for managing knee osteoarthritis in older women: a pilot randomized controlled trial. BMC Complement. Altern. Med. 2014;14:160. doi: 10.1186/1472-6882-14-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Panebianco M., Sridharan K., Ramaratnam S. Yoga for epilepsy. Cochrane Database Syst. Rev. 2015;5:CD001524. doi: 10.1002/14651858.CD001524.pub2. [DOI] [PubMed] [Google Scholar]
- 237.Vempati R., Bijlani R.L., Deepak K.K. The efficacy of a comprehensive lifestyle modification programme based on yoga in the management of bronchial asthma: a randomized controlled trial. BMC Pulm. Med. 2009;9:37. doi: 10.1186/1471-2466-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Innes KE, Selfe TK. 2016.
- 239.van der Kolk B.A., Stone L., West J., et al. Yoga as an adjunctive treatment for posttraumatic stress disorder: a randomized controlled trial. J. Clin. Psychiatry. 2014;75(6):e559–e565. doi: 10.4088/JCP.13m08561. [DOI] [PubMed] [Google Scholar]
- 240.Najafi Ghezeljeh T., Mohades Ardebili F., Rafii F., Haghani H. The Effects of Music Intervention on Background Pain and Anxiety in Burn Patients: Randomized Controlled Clinical Trial. J. Burn Care Res. 2015 doi: 10.1097/BCR.0000000000000266. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 241.Shirani Bidabadi S., Mehryar A. Music therapy as an adjunct to standard treatment for obsessive compulsive disorder and co-morbid anxiety and depression: A randomized clinical trial. J. Affect. Disord. 2015;184:13–17. doi: 10.1016/j.jad.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 242.Gruhlke L.C., Patricio M.C., Moreira D.M. Mozart, but not the Beatles, reduces systolic blood pressure in patients with myocardial infarction. Acta Cardiol. 2015;70(6):703–706. doi: 10.2143/AC.70.6.3120183. [DOI] [PubMed] [Google Scholar]
- 243.Koelsch S. Brain correlates of music-evoked emotions. Nat. Rev. Neurosci. 2014;15(3):170–180. doi: 10.1038/nrn3666. [DOI] [PubMed] [Google Scholar]
- 244.Chanda M.L., Levitin D.J. The neurochemistry of music. Trends Cogn. Sci. 2013;17(4):179–193. doi: 10.1016/j.tics.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 245.Fancourt D., Ockelford A., Belai A. The psychoneuroimmunological effects of music: A systematic review and a new model. Brain Behav. Immun. 2014;36:15–26. doi: 10.1016/j.bbi.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 246.Koepp M.J., Gunn R.N., Lawrence A.D., et al. Evidence for striatal dopamine release during a video game. Nature. 1998;393(6682):266–268. doi: 10.1038/30498. [DOI] [PubMed] [Google Scholar]
- 247.Gold B.P., Frank M.J., Bogert B., Brattico E. Pleasurable music affects reinforcement learning according to the listener. Front. Psychol. 2013;4:541. doi: 10.3389/fpsyg.2013.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Salimpoor V.N., Benovoy M., Larcher K., Dagher A., Zatorre R.J. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat. Neurosci. 2011;14(2):257–262. doi: 10.1038/nn.2726. [DOI] [PubMed] [Google Scholar]
- 249.Salimpoor V.N., van den Bosch I., Kovacevic N., McIntosh A.R., Dagher A., Zatorre R.J. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science. 2013;340(6129):216–219. doi: 10.1126/science.1231059. [DOI] [PubMed] [Google Scholar]
- 250.Katsyri J., Hari R., Ravaja N., Nummenmaa L. Just watching the game ain't enough: striatal fMRI reward responses to successes and failures in a video game during active and vicarious playing. Front. Hum. Neurosci. 2013;7:278. doi: 10.3389/fnhum.2013.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Cole S.W., Yoo D.J., Knutson B. Interactivity and reward-related neural activation during a serious videogame. PLoS One. 2012;7(3):e33909. doi: 10.1371/journal.pone.0033909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Xing Y., Chen W., Wang Y., et al. Music exposure improves spatial cognition by enhancing the BDNF level of dorsal hippocampal subregions in the developing rats. Brain Res. Bull. 2016;121:131–137. doi: 10.1016/j.brainresbull.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 253.Xing Y., Xia Y., Kendrick K., et al. Mozart, Mozart Rhythm and Retrograde Mozart Effects: Evidences from Behaviours and Neurobiology Bases. Sci. Rep. 2016;6:18744. doi: 10.1038/srep18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Freeman J.M., Vining E.P., Kossoff E.H., Pyzik P.L., Ye X., Goodman S.N. A blinded, crossover study of the efficacy of the ketogenic diet. Epilepsia. 2009;50(2):322–325. doi: 10.1111/j.1528-1167.2008.01740.x. [DOI] [PubMed] [Google Scholar]
- 255.Neal E.G., Chaffe H., Schwartz R.H., et al. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7(6):500–506. doi: 10.1016/S1474-4422(08)70092-9. [DOI] [PubMed] [Google Scholar]
- 256.Klein P., Tyrlikova I., Mathews G.C. Dietary treatment in adults with refractory epilepsy: a review. Neurology. 2014;83(21):1978–1985. doi: 10.1212/WNL.0000000000001004. [DOI] [PubMed] [Google Scholar]
- 257.Kverneland M., Selmer K.K., Nakken K.O., Iversen P.O., Tauboll E. A prospective study of the modified Atkins diet for adults with idiopathic generalized epilepsy. Epilepsy Behav. 2015;53:197–201. doi: 10.1016/j.yebeh.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 258.Kverneland M., Tauboll E., Selmer K.K., Iversen P.O., Nakken K.O. Modified Atkins diet may reduce serum concentrations of antiepileptic drugs. Acta Neurol. Scand. 2015;131(3):187–190. doi: 10.1111/ane.12330. [DOI] [PubMed] [Google Scholar]
- 259.Widmer R.J., Flammer A.J., Lerman L.O., Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am. J. Med. 2015;128(3):229–238. doi: 10.1016/j.amjmed.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Lasa A., Miranda J., Bullo M., et al. Comparative effect of two Mediterranean diets versus a low-fat diet on glycaemic control in individuals with type 2 diabetes. Eur. J. Clin. Nutr. 2014;68(7):767–772. doi: 10.1038/ejcn.2014.1. [DOI] [PubMed] [Google Scholar]
- 261.Messier S.P., Mihalko S.L., Legault C., et al. Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. JAMA. 2013;310(12):1263–1273. doi: 10.1001/jama.2013.277669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Beltran A., Li R., Ater J., et al. Adapting a Videogame to the Needs of Pediatric Cancer Patients and Survivors. Games Health J. 2013;2(4):213–221. doi: 10.1089/g4h.2013.0018. [DOI] [PubMed] [Google Scholar]
- 263.Duncan M., Vandelanotte C., Kolt G.S., et al. Effectiveness of a web- and mobile phone-based intervention to promote physical activity and healthy eating in middle-aged males: randomized controlled trial of the ManUp study. J. Med. Internet Res. 2014;16(6):e136. doi: 10.2196/jmir.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Du H., Venkatakrishnan A., Youngblood G.M., Ram A., Pirolli P. A Group-Based Mobile Application to Increase Adherence in Exercise and Nutrition Programs: A Factorial Design Feasibility Study. JMIR Mhealth Uhealth. 2016;4(1):e4. doi: 10.2196/mhealth.4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Institute of Medicine (US) Committee on Health and Behavior 2001.
- 266.Maizes V., Rakel D., Niemiec C. Integrative medicine and patient-centered care. Explore (NY) 2009;5(5):277–289. doi: 10.1016/j.explore.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 267.Knowlton B.J., Mangels J.A., Squire L.R. A neostriatal habit learning system in humans. Science. 1996;273(5280):1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 268.Graybiel A.M. The basal ganglia: learning new tricks and loving it. Curr. Opin. Neurobiol. 2005;15(6):638–644. doi: 10.1016/j.conb.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 269.Cole S.W., Yoo D.J., Knutson B. Interactivity and reward-related neural activation during a serious videogame. PLoS One. 2012;7(3):e33909. doi: 10.1371/journal.pone.0033909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kato P.M., Cole S.W., Bradlyn A.S., Pollock B.H. A video game improves behavioral outcomes in adolescents and young adults with cancer: a randomized trial. Pediatrics. 2008;122(2):e305–e317. doi: 10.1542/peds.2007-3134. [DOI] [PubMed] [Google Scholar]
- 271.Baranowski T., Buday R., Thompson D., Lyons E.J., Lu A.S., Baranowski J. Developing Games for Health Behavior Change: Getting Started. Games Health J. 2013;2(4):183–190. doi: 10.1089/g4h.2013.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Finley J.W., Finley J.W., Ellwood K., Hoadley J. Launching a new food product or dietary supplement in the United States: industrial, regulatory, and nutritional considerations. Annu. Rev. Nutr. 2014;34:421–447. doi: 10.1146/annurev-nutr-071813-105817. [DOI] [PubMed] [Google Scholar]
- 273.Jayaraman K.S. US patent office withdraws patent on Indian herb. Nature. 1997;389:6646–6. doi: 10.1038/37838. [DOI] [PubMed] [Google Scholar]
- 274.Marshall E., Bagla P. India applauds U.S. patent reversal. Science. 1997;277:5331–1429. doi: 10.1126/science.277.5331.1429. [DOI] [PubMed] [Google Scholar]
- 275.Shakeri A., Sahebkar A. Optimized curcumin formulations for the treatment of Alzheimer's disease: A patent evaluation. J. Neurosci. Res. 2016;94(2):111–113. doi: 10.1002/jnr.23696. [DOI] [PubMed] [Google Scholar]
- 276.Bandyopadhyay D. Farmer to pharmacist: curcumin as an anti-invasive and antimetastatic agent for the treatment of cancer. Front Chem. 2014;2:113. doi: 10.3389/fchem.2014.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Pezzuto J.M., Kondratyuk T.P., Ogas T. Resveratrol derivatives: a patent review (2009 - 2012). Expert Opin. Ther. Pat. 2013;23(12):1529–1546. doi: 10.1517/13543776.2013.834888. [DOI] [PubMed] [Google Scholar]
- 278.Villalba J.M., de Cabo R., Alcain F.J. A patent review of sirtuin activators: an update. Expert Opin. Ther. Pat. 2012;22(4):355–367. doi: 10.1517/13543776.2012.669374. [DOI] [PubMed] [Google Scholar]
- 279.Shuren J. The FDA's role in the development of medical mobile applications. Clin. Pharmacol. Ther. 2014;95(5):485–488. doi: 10.1038/clpt.2014.45. [DOI] [PubMed] [Google Scholar]
- 280.Cortez N.G., Cohen I.G., Kesselheim A.S. FDA regulation of mobile health technologies. N. Engl. J. Med. 2014;371(4):372–379. doi: 10.1056/NEJMhle1403384. [DOI] [PubMed] [Google Scholar]
- 281.Engel JS, Thomas PR, Bailey RL, Thurn AL. MyDS: A Mobile Application to Track Dietary Supplements Use. 2011.
- 282.Kuehn B.M. Is there an app to solve app overload? JAMA. 2015;313(14):1405–1407. doi: 10.1001/jama.2015.2381. [DOI] [PubMed] [Google Scholar]
- 283.Steinhubl S.R., Muse E.D., Topol E.J. The emerging field of mobile health. Sci. Transl. Med. 2015;7(283):283rv3. doi: 10.1126/scitranslmed.aaa3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Hayes D.F., Markus H.S., Leslie R.D., Topol E.J. Personalized medicine: risk prediction, targeted therapies and mobile health technology. BMC Med. 2014;12:37. doi: 10.1186/1741-7015-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Daniel H., Sulmasy L.S., Health and Public Policy Committee of the American College of Physicians Policy Recommendations to Guide the Use of Telemedicine in Primary Care Settings: An American College of Physicians Position Paper. Ann. Intern. Med. 2015;163(10):787–789. doi: 10.7326/M15-0498. [DOI] [PubMed] [Google Scholar]
- 286.Benedetti F., Carlino E., Piedimonte A. Increasing uncertainty in CNS clinical trials: the role of placebo, nocebo, and Hawthorne effects. Lancet Neurol. 2016;15(7):736–747. doi: 10.1016/S1474-4422(16)00066-1. [DOI] [PubMed] [Google Scholar]
- 287.van Diepen R.M., Cohen M.X., Denys D., Mazaheri A. Attention and temporal expectations modulate power, not phase, of ongoing alpha oscillations. J. Cogn. Neurosci. 2015;27(8):1573–1586. doi: 10.1162/jocn_a_00803. [DOI] [PubMed] [Google Scholar]
- 288.Lappe C., Trainor L.J., Herholz S.C., Pantev C. Cortical plasticity induced by short-term multimodal musical rhythm training. PLoS One. 2011;6(6):e21493. doi: 10.1371/journal.pone.0021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Nozaradan S. Exploring how musical rhythm entrains brain activity with electroencephalogram frequency-tagging. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014;369(1658):20130393. doi: 10.1098/rstb.2013.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Bailes F., Dean R.T., Pearce M.T. Music cognition as mental time travel. Sci. Rep. 2013;3:2690. doi: 10.1038/srep02690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Enck P., Bingel U., Schedlowski M., Rief W. The placebo response in medicine: minimize, maximize or personalize? Nat. Rev. Drug Discov. 2013;12(3):191–204. doi: 10.1038/nrd3923. [DOI] [PubMed] [Google Scholar]
- 292.French J.A., Gil-Nagel A., Malerba S., Kramer L., Kumar D., Bagiella E. Time to prerandomization monthly seizure count in perampanel trials: A novel epilepsy endpoint. Neurology. 2015;84(20):2014–2020. doi: 10.1212/WNL.0000000000001585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Goldenholz D.M., Moss R., Scott J., Auh S., Theodore W.H. Confusing placebo effect with natural history in epilepsy: A big data approach. Ann. Neurol. 2015;78(3):329–336. doi: 10.1002/ana.24470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Ryvlin P., Cucherat M., Rheims S. Risk of sudden unexpected death in epilepsy in patients given adjunctive antiepileptic treatment for refractory seizures: a meta-analysis of placebo-controlled randomised trials. Lancet Neurol. 2011;10(11):961–968. doi: 10.1016/S1474-4422(11)70193-4. [DOI] [PubMed] [Google Scholar]
- 295.Epi P.M. A roadmap for precision medicine in the epilepsies. Lancet Neurol. 2015;14(12):1219–1228. doi: 10.1016/S1474-4422(15)00199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Walker L.E., Mirza N., Yip V.L., Marson A.G., Pirmohamed M. Personalized medicine approaches in epilepsy. J. Intern. Med. 2015;277(2):218–234. doi: 10.1111/joim.12322. [DOI] [PubMed] [Google Scholar]
- 297.Gerlag D.M., Norris J.M., Tak P.P. from risk factors and pathogenesis to prevention: Towards prevention of autoantibody-positive rheumatoid arthritis: from lifestyle modification to preventive treatment. Rheumatology (Oxford) 2016;55(4):607–614. doi: 10.1093/rheumatology/kev347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Schumann G., Binder E.B., Holte A., et al. Stratified medicine for mental disorders. Eur. Neuropsychopharmacol. 2014;24(1):5–50. doi: 10.1016/j.euroneuro.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 299.Wittchen H.U., Knappe S., Andersson G., et al. The need for a behavioural science focus in research on mental health and mental disorders. Int. J. Methods Psychiatr. Res. 2014;23(Suppl. 1):28–40. doi: 10.1002/mpr.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Landgrave-Gomez J., Mercado-Gomez O., Guevara-Guzman R. Epigenetic mechanisms in neurological and neurodegenerative diseases. Front. Cell. Neurosci. 2015;9:58. doi: 10.3389/fncel.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Neufer P.D., Bamman M.M., Muoio D.M., et al. Understanding the Cellular and Molecular Mechanisms of Physical Activity-Induced Health Benefits. Cell Metab. 2015;22(1):4–11. doi: 10.1016/j.cmet.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 302.Bauer U.E., Briss P.A., Goodman R.A., Bowman B.A. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52. doi: 10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- 303.Lim S.S., Vos T., Flaxman A.D., et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]