Abstract
The aim of the study was to evaluate quantitatively ictal and interictal phonophobia in episodic migraine (EM). We included subjects with EM and age- and gender-matched controls. Sound stimuli were pure tones at frequencies of 1000, 4000 and 8000 Hz. Sound aversion thresholds (SATs) were determined as the minimal sound intensity perceived as unpleasant or painful. Migraineurs were examined both between and during attacks. We compared interictal SATs in migraineurs with those in controls. We also compared ictal and interictal SATs in migraineurs. Sixty migraineurs and 52 controls were included. Interictal mean SAT of migraineurs, averaged for the three frequencies, was significantly lower than that of controls [90.4 (0.8) dB vs. 105.9 (1.1) dB, respectively, P < 0.0001]. In migraineurs, mean ictal SAT, averaged for the three frequencies, was significantly lower than interictal SAT [76.0 (0.9) dB vs. 91.0 (0.8) dB, respectively, P < 0.0001]. Patients with EM exhibit increased sound aversion between attacks that is further augmented during an acute attack.
Keywords: Migraine, phonophobia, sound aversion threshold
Introduction
Migraine is a neurovascular disorder, characterized by repeated attacks of headache, autonomic dysfunction and gastrointestinal symptoms (1). Many migraine patients report on increased aversion to various sensory stimuli during an acute attack. These include aversion to light (photophobia), sound (phonophobia), odours (osmophobia) and mechanical or thermal stimuli to the skin (cutaneous allodynia) (2–4). Considerable data have been published on cutaneous allodynia in migraine (2, 5). Less is known about the characteristics of aversion to other sensory stimuli in migraine.
Phonophobia occurs in 70–80% of migraine patients during an acute attack (6, 7). The International Headache Society (IHS) lists phonophobia (along with photophobia) during an attack as one of the diagnostic criteria of migraine (8). However, the IHS does not provide a quantitative definition of this symptom. In clinical practice, assessment of phonophobia is based on a subjective report by the patient regarding the presence and degree of aversion to sound.
Despite its common occurrence, few studies have been done to assess phonophobia quantitatively in migraine patients, with inconsistent results (3, 7, 9). These previous studies have several limitations: only a single frequency was used as the sound stimulus; some studies included subjects who were using migraine-preventive drugs, potentially affecting sound aversion; and a standardized audiogram was not performed before inclusion of subjects in the studies. Data from auditory evoked potential studies are more consistent, showing abnormal processing of auditory stimuli in migraine subjects (10, 11).
We developed a method to evaluate aversion to sound quantitatively in human subjects and used this method to examine sound aversion in migraineurs, both between and within attacks, and to compare it with that of healthy controls, using a range of sound stimuli at frequencies commonly encountered in daily life.
Methods
Subjects
Subjects were prospectively recruited from the out-patient clinic of Jefferson Headache Center and from the population of the Philadelphia area through advertising. Subjects were adult women or men, aged 18–65 years, with a diagnosis of episodic migraine with or without aura, as defined by the IHS Headache Classification Committee (8). Included subjects were required to have at least one migraine attack per month, but < 15 headache days per month, for 6 months prior to enrolment, and to have a normal audiogram [defined as a hearing threshold of ≤ 20 dB at the tested sound frequencies; dB refers to decibels sound pressure level (dB SPL)]. Exclusion criteria were: any other headache diagnosis except for episodic tension-type headache (ETTH) as defined by the IHS; the use of any medication, vitamin or supplement considered or suspected to be a headache preventive (e.g. antidepressants, anticonvulsants, β-adrenergic blockers, calcium channel blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, serotonin antagonists, non-steroidal anti-inflammatory drugs, magnesium, riboflavin, coenzyme Q 10, Petadolex®, botulinum toxin) within 90 days prior to enrolment; and treatment with occipital nerve block or any other nerve blocks in the head or neck area within 30 days prior to enrolment. Control subjects were adult women or men, 18–65 years old, who did not suffer from headaches, except for IHS-defined infrequent ETTH (i.e. fewer than one tension-type headache day per month). They were also required to have a normal audiogram and not to use any drugs considered or suspected to be a headache preventive. Controls were recruited from the Thomas Jefferson University Hospital staff, and from the population of the Philadelphia area through advertising. Participants were recruited and examined from August 2006 to August 2007. The study was approved by the Thomas Jefferson University Institutional Review Board for Human Research. All participants signed an informed consent form prior to enrolment.
Study protocol
An audiogram was performed at the Jefferson Center for Balance and Hearing, when subjects were not experiencing an acute headache. Eligible subjects with a normal audiogram proceeded with the study protocol. Other than the audiogram, all study procedures took place at the Jefferson Headache Center. Migraine subjects were examined when they were not experiencing an acute attack (nor within 72 h of the time they had had an attack) (visit 1), and when they were experiencing an acute untreated attack (visit 2). During visit 1, migraine subjects were interviewed using a structured questionnaire to obtain demographic data and data on migraine characteristics (frequency and duration of attacks, head pain severity during an attack, and number of years they had suffered from migraine). Subjects were then tested for sound aversion. Control subjects who had a normal audiogram were interviewed to obtain demographic data and were then tested for sound aversion. Control subjects were also tested for sound aversion 4 weeks after their first visit. This was done to assess for a possible learning effect that may have modified the results of phonophobia testing at visit 2. The 4-week time point was chosen because this was the average time interval between the first and second visits in migraineurs.
Phonophobia testing
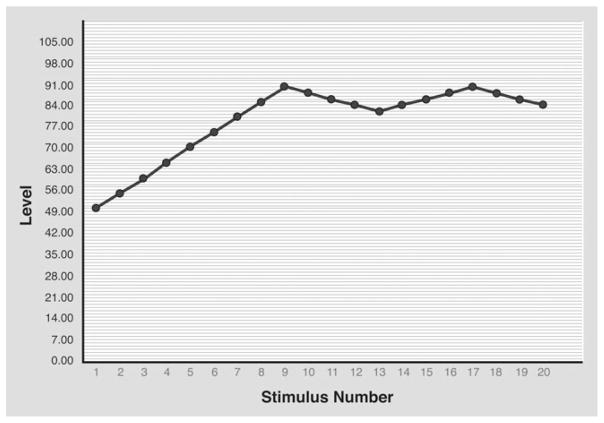
Phonophobia testing was performed with the subject sitting in a sound-proof booth (Eckel Industries, Morrisburg, Ontario, Canada). We used a RP2.1 enhanced real-time processor (Tucker-Davis Technologies, Alachua, FL, USA) and PSYCHRP psychoacoustic testing software (Tucker-Davis Technologies) for the test. Sound stimuli were pure tones at frequencies of 1000, 4000 and 8000 Hz, delivered simultaneously to both ears using ER3 insert earphones (Etymotic Research, Elk Grove Village, IL, USA). The rationale for using these sound frequencies as the stimulus is based on the fact that the human auditory system is most sensitive to sounds at around 4000 Hz (12). Each sound stimulus lasted 1.5 s and the interstimulus onset interval was 3 s. Initial sound intensity was 50 dB and was increased gradually, by increments of 5 dB, until an unpleasant/painful intensity was reached, at which time the subject pressed and held a response-box button, causing the sound intensity to decrease gradually. Once a tolerable sound intensity was reached, the subject released the response-box button, causing the sound intensity to increase again until an unpleasant/painful intensity was reached, repeating the testing cycle once more (Fig. 1). A sound aversion threshold (SAT) (measured in dB) was determined as the average of the unpleasant or painful sound intensities at the two cycles. For safety reasons, at no time did the delivered sound intensity exceed 110 dB. Each subject was tested for sound sensitivity at the three different frequencies, starting at 1000 Hz, then at 4000 Hz and finally at 8000 Hz. There was a 10-min between-test interval for the different frequencies for each subject. We adhered to the limits of sound exposure safety, as recommended by the Occupational Safety and Health Administration Noise Exposure Standards (13).
Figure 1.
A graph showing the results of sound aversion testing in a migraine patient during an attack.
Data analysis
The primary analysis focused on testing for differences in SATs between migraineurs and controls at the three sound frequencies. We hypothesized that SATs in migraineurs would be significantly lower than those in controls. Demographic variables were summarized using medians and ranges (for continuous variables) and frequencies (for categorical variables). The Wilcoxon rank sum test was used to test for between-group differences of continuous variables. Pearson’s χ2 test was used to test for between-group differences of categorical variables. SATs had a maximum value of 110 dB, and some subjects reached this value. To account for this, a censored normal distribution was assumed for this outcome. A mixed-effects regression model was used to model jointly the distribution of SATs at each frequency. Terms were included to allow for estimation of the mean SAT score for each frequency for both migraineurs and controls. A random intercept was assumed to account for correlation among scores from the same subject. SATs in migraineurs and in controls were compared for each frequency within the overall model. Mixed-effects censored normal regression was used to assess for association between SATs and age or gender. The same model was used to determine whether SATs in migraineurs were associated with duration of disease, attack frequency or headache severity during an attack.
All analyses were performed using SAS Version 9.1.3 (SAS Institute, Cary, NC, USA). A P-value of < 0.05 was considered to reflect statistical significance.
Results
Subject characteristics
We recruited 112 subjects (60 migraineurs and 52 controls) to the study. There were no significant differences with respect to age or gender between the two groups (Table 1).
Table 1.
Demographic characteristics of the two groups
| Migraine (n = 60) | Control (n = 52) | Total (n = 112) | P-value | |
|---|---|---|---|---|
| Men, n (%) | 11 (18.3) | 12 (23.1) | 23 (20.5) | 0.535 |
| Women, n (%) | 49 (81.7) | 40 (76.9) | 89 (79.5) | |
| Age (years) median (min, max) | 25.5 (18, 51) | 26 (19, 57) | 26 (18, 57) | 0.447 |
In the migraine group, 17 (28%) subjects had migraine with aura and 43 (72%) migraine without aura. Median disease duration was 8.5 years (range 1–38 years), median attack frequency was 3.5 per month (range 1–9) and median head pain severity during an acute untreated attack was 8 (range 4–10) on a verbal scale of 0–10. None of the migraineurs in this study had medication overuse headache.
Interictal SATs in the migraine group vs. SATs in the control group
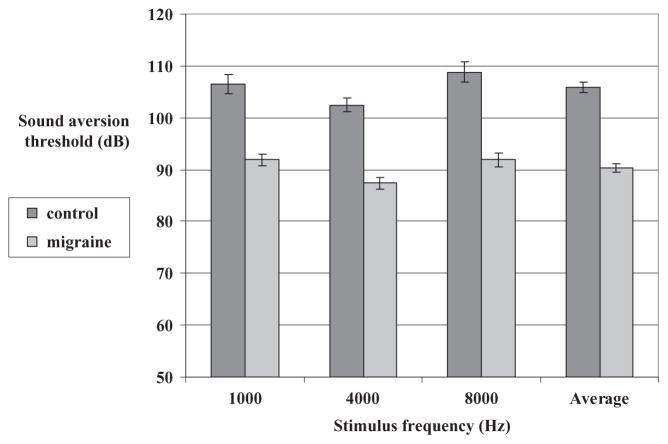
Migraine subjects, when tested between attacks, had significantly lower SATs compared with controls for all tested sound frequencies (Fig. 2). The difference [95% confidence interval (CI)] in mean SATs between migraineurs and controls, averaged for the three sound frequencies, was −15.5 dB (−18.3, −12.8) [−14.6 dB (−18.5, −10.8) at 1000 Hz, −15.1 dB (−18.5, −11.7) at 4000 Hz and −16.9 dB (−22.3, −11.6) at 8000 Hz] (P < 0.0001 for all frequencies and for the average).
Figure 2.
Mean interictal sound aversion thresholds (SATs) in the migraine group vs. SATs in the control group.
Ictal vs. interictal SATs in the migraine group
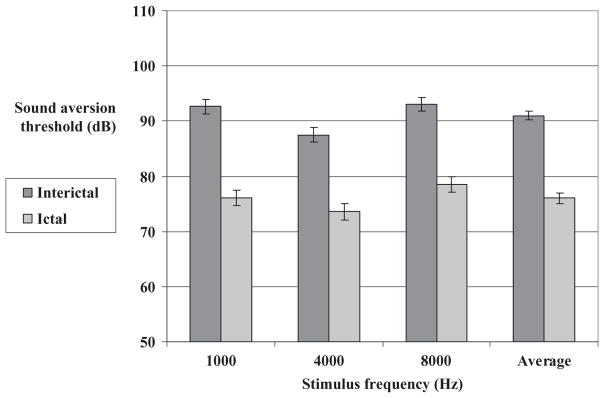
Thirty-eight of the 60 migraineurs (63.3%) were tested both between attacks and during an acute migraine attack. There were no significant differences between migraineurs who completed the two visits and those who did not, with respect to age, gender or migraine characteristics (disease duration, attack frequency and headache severity during an attack). In migraineurs, SATs during an acute attack were significantly lower than SATs between attacks for all tested sound frequencies (Fig. 3). The difference (95% CI) between ictal and interictal mean SATs, averaged for the three sound frequencies, was −15.0 dB (−17.1, −12.8) [−16.6 dB (−20.1, −13.0) at 1000 Hz, −13.9 dB (−17.5, −10.4) at 4000 Hz and −14.4 dB (−18.0, −10.8) at 8000 Hz] (P < 0.0001 for all frequencies and for the average). In the control group, there were no significant differences in mean SATs between visits 1 and 2 (data not shown).
Figure 3.
Mean interictal and ictal sound aversion thresholds in the migraine group.
SATs and demographic data in the two groups
In the migraine group, women had significantly lower SATs compared with men, both between attacks and during an acute attack (Table 2). The mean between-gender difference (95% CI) in SATs (women–men), averaged for the three sound frequencies, was −17.8 dB (−22.2, −13.4) between attacks (P < 0.0001) and −11.9 dB (−15.7, −8.1) during attacks (P < 0.0001). In the control group, mean SATs, averaged for the three sound frequencies, were also significantly lower in women compared with men, with a between-gender difference (95% CI) of −5.1 dB (−9.0, −1.1) (P = 0.01) (Table 2).
Table 2.
Mean (S.E.M.) sound aversion thresholds (SATs), averaged for the three sound frequencies, vs. gender in the two groups (W, women, M, men)
| SAT (dB): women | SAT (dB): men | SAT difference (women–men) (95% CI) | P-value | |
|---|---|---|---|---|
| Migraine—interictal (n = 60, W 49, M 11) | 85.1 (0.7) | 102.9 (2.1) | −17.8 (−22.2, −13.4) | < 0.0001 |
| Migraine—ictal (n = 38, W 30, M 8) | 73.7 (1.3) | 85.6 (1.4) | −11.9 (−15.7, −8.1) | < 0.0001 |
| Control (n = 52, W 40, M 12) | 105.5 (1.2) | 110.6 (1.7) | −5.1 (−9.0, −1.1) | 0.01 |
In the control group, there was no significant association between age and SATs.
In the migraine group, older subjects had higher mean SATs compared with younger ones. However, when adjusted for disease duration, there was no significant association between age and SATs in this group either.
SATs and migraine characteristics
There was no significant association between SATs and migraine attack frequency or headache severity during an attack. There was a trend for within-attack (but not between-attack) SATs to decrease with longer disease duration, but this trend did not reach statistical significance (P = 0.07).
Discussion
We found that migraine subjects had significantly increased between-attack sound aversion, as demonstrated by lower SATs, compared with controls. This sound aversion in migraineurs was further augmented during an acute attack.
Few studies have examined quantitatively sound aversion in migraine (3, 7, 9). Vanagaite Vingen et al. examined migraine patients for sound aversion by delivering a 200-Hz tone to migraineurs and to controls (7). Consistent with our results, they found that migraineurs had increased sound aversion compared with controls, both between attacks and during an attack. Similarly, Main et al., using a tone of 1000 Hz as the sound stimulus, reported an increased hearing discomfort in migraine subjects between attacks (3). Woodhouse and Drummond, however, did not find increased between-attack sound aversion in migraine, using an 8000-Hz tone as the sound stimulus (9). In the above studies, only one sound frequency was used as the stimulus. Here we show consistently lower SATs in migraine subjects in the interictal state compared with controls, at different sound frequencies encountered in daily life, and a significant further decrease of those SATs during an acute attack.
We did not investigate the impact of the interictal or ictal sound aversion of migraineurs on their daily life. However, we used sound stimuli at frequencies that are often encountered in daily life. Interictally, SATs in migraineurs were on average 15.5 dB lower than those of controls. Considering the fact that the decibel scale is logarithmic, this is a substantial difference (e.g. a decrease of 10 dB in SAT corresponds to a 10-fold decrease in the intensity of the aversive sound) (14, 15). Further studies are needed to evaluate the behavioural implications of our findings as regards migraineurs’ daily life.
The mechanism of phonophobia in migraine can not be determined based on our results. However, these findings suggest abnormal processing of auditory stimuli in migraine in the interictal state that is further altered during an attack. In auditory evoked potentials studies, migraine patients exhibited lack of habituation, and even potentiation, of the cortical response to repeated auditory stimuli in the interictal state (10). Migraine subjects also showed increased intensity dependence of the cortical response to auditory stimuli, as measured by the amplitude/stimulus intensity slope, in the interictal state (10, 11). These observations demonstrate abnormal processing of auditory stimuli in migraine patients between attacks, in line with our results (16). Interestingly, lack of habituation was also found interictally in migraineurs in response to visual and somatosensory stimuli (16–18).
We found no significant association between migraine parameters (attack frequency, headache severity and disease duration) and sound aversion thresholds. Therefore, we have no evidence to support the hypothesis of repeated migraine attacks as a contributing factor in the occurrence of phonophobia in migraine. However, our data on migraine characteristics were obtained retrospectively using a questionnaire, and therefore were subject to recall bias and should be taken with reservations.
Women exhibited lower SATs than men in both the migraine and control groups. The between-gender differences in SATs were more robust in migraineurs than in controls. Since our study was not powered to test specifically for between-gender SAT differences, and since the number of men in both groups was small, these results should be regarded as preliminary data only. Other limitations of this study include the following: (i) investigators examining the subjects for phonophobia were not blinded to the group to which subjects belonged (migraine or control). However, this probably had little effect on the results, since a computerized system recorded the sound intensity at which the subject reported the stimulus as aversive; (ii) sound aversion testing did not cover the entire frequency range of human hearing.
In summary, using a standardized quantitative method we have demonstrated increased aversion to sound in migraineurs between attacks, compared with controls. This sound aversion in migraineurs further increased during an acute migraine attack. These data can provide a basis for further studies on the mechanisms of phonophobia in migraine and on the effect of migraine drugs on sound aversion. Further studies are also needed to assess the possible association of phonophobia with aversion to other sensory stimuli in migraine.
Acknowledgments
This study was supported by Merck Inc., IISP grant no. 31402 to A.A. The authors thank Benjamin Leiby, PhD for his help with data analysis, James W. Shaw, PhD, MPH, PharmD for his helpful advice on study design and data analysis, and Mrs Lynne Kaiser for her skilful editing of the manuscript.
References
- 1.Goadsby PJ, Lipton RB, Ferrari MD. Migraine—current understanding and treatment. N Engl J Med. 2002;346:257–70. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 2.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–24. [PubMed] [Google Scholar]
- 3.Main A, Dowson A, Gross M. Photophobia and phonophobia in migraineurs between attacks. Headache. 1997;37:492–5. doi: 10.1046/j.1526-4610.1997.3708492.x. [DOI] [PubMed] [Google Scholar]
- 4.Zanchin G, Dainese F, Trucco M, Mainardi F, Mampreso E, Maggioni F. Osmophobia in migraine and tension-type headache and its clinical features in patients with migraine. Cephalalgia. 2007;27:1061–8. doi: 10.1111/j.1468-2982.2007.01421.x. [DOI] [PubMed] [Google Scholar]
- 5.Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the developing allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- 6.Kayan A, Hood JD. Neuro-otological manifestations of migraine. Brain. 1984;107:1123–42. doi: 10.1093/brain/107.4.1123. [DOI] [PubMed] [Google Scholar]
- 7.Vanagaite Vingen J, Pareja JA, Storen O, White LR, Stovner LJ. Phonophobia in migraine. Cephalalgia. 1998;18:243–9. doi: 10.1046/j.1468-2982.1998.1805243.x. [DOI] [PubMed] [Google Scholar]
- 8.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. Cephalalgia. (2) 2004;24(Suppl 1):1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 9.Woodhouse A, Drummond PD. Mechanisms of increased sensitivity to noise and light in migraine headache. Cephalalgia. 1993;13:417–21. doi: 10.1046/j.1468-2982.1993.1306417.x. [DOI] [PubMed] [Google Scholar]
- 10.Ambrosini A, Rossi P, De Pasqua V, Pierelli F, Schoenen J. Lack of habituation causes high intensity dependence of auditory evoked cortical potentials in migraine. Brain. 2003;126:2009–15. doi: 10.1093/brain/awg206. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Berthier MT, Schoenen J. Intensity dependence of auditory evoked potentials is pronounced in migraine: an indication of cortical potentiation and low serotonergic neurotransmission. Neurology. 1996;46:1404–9. doi: 10.1212/wnl.46.5.1404. [DOI] [PubMed] [Google Scholar]
- 12.Kinsler LE, Frey AR, Coppens AB, Sanders JV. Noise, signal detection, hearing, and speech. In: Kinsler LE, Frey AR, Coppens AB, Sanders JV, editors. Fundamentals of acoustics. 4. Hoboken, NJ: Wiley; 2000. pp. 302–32. [Google Scholar]
- 13.US Department of Labor. [Accessed 8 December 2008];Occupational noise exposure. 2007 [WWW document]. URL http://www.osha.gov.
- 14.Kinsler LE, Frey AR, Coppens AB, Sanders JV. The acoustic wave equation and simple solutions. In: Kinsler LE, Frey AR, Coppens AB, Sanders JV, editors. Fundamentals of acoustics. 4. Hoboken, NJ: Wiley; 2000. pp. 113–48. [Google Scholar]
- 15.Kinsler LE, Frey AR, Coppens AB, Sanders JV. Environmental acoustics. In: Kinsler LE, Frey AR, Coppens AB, Sanders JV, editors. Fundamentals of acoustics. 4. Hoboken, NJ: Wiley; 2000. pp. 359–89. [Google Scholar]
- 16.Ambrosini A, Schoenen J. The electrophysiology of migraine. Curr Opin Neurol. 2003;16:327–31. doi: 10.1097/01.wco.0000073945.19076.1f. [DOI] [PubMed] [Google Scholar]
- 17.Ozkul Y, Uckardes A. Median nerve somatosensory evoked potentials in migraine. Eur J Neurol. 2002;9:227–32. doi: 10.1046/j.1468-1331.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 18.Afra J, Proietti CA, Sandor PS, Schoenen J. Comparison of visual and auditory evoked cortical potentials in migraine patients between attacks. Clin Neurophysiol. 2000;111:1124–9. doi: 10.1016/s1388-2457(00)00271-6. [DOI] [PubMed] [Google Scholar]