Abstract
Incubation temperature has an immediate and long-term influence on the embryonic development in birds. DNA methylation as an important environment-induced mechanism could serve as a potential link between embryos’ phenotypic variability and temperature variation, which reprogrammed by DNA (cytosine-5)-methyltransferases (DNMTS) and Methyl-CpG binding domain proteins (MBPS) 3&5 (MBD3&5). Five genes in DNMTS and MBPS gene families were selected as target genes, given their important role in epigenetic modification. In this study, we aimed to test whether raising incubation temperature from 37.8°C to 38.8°C between embryonic days (ED) 1–10, ED10–20 and ED20–27 have effect on DNA methylation and whether DNMTS, MBPS play roles in thermal epigenetic regulation of early development in duck. Real-time quantitative PCR analysis showed that increased incubation temperature by 1°C has remarkably dynamic effect on gene expression levels of DNMTS and MBPS. Slight changes in incubation temperature significantly increased mRNA levels of target genes in breast muscle tissue during ED1–10, especially for DNMT1, DNMT3A and MBD5. In addition, higher temperature significantly increased enzyme activities of DNMT1 in leg muscle during ED10–20, liver tissue during ED1–10, ED20–27 and DNMT3A in leg muscle and breast muscle tissue during ED10–20. These results suggest that incubation temperature has an extended effect on gene expression levels and enzyme activities of DNMTS and MBPS, which provides evidence that incubation temperature may influence DNA methylation in duck during early developmental stages. Our data indicated that DNMTS and MBPS may involved in thermal epigenetice regulation of embryos during the early development in duck. The potential links between embryonic temperature and epigenetic modification need further investigation
Keywords: duck, incubation temperature, DNA methylation, embryonic period
INTRODUCTION
The complete description of developmental series of avian embryos can be traced back to the end of the 19th century (Duval, 1889; Keibel, 1900). What's more, Hamburger and Hamilton estabilished a system of normal 42 stages of embryonic development available for birds, which represent a general developmental pattern (Hamburger and Hamilton, 1951). Numerous studies have well documented the detailed processes of early growth and development of the embryos based on morphological characters in avian (Romanoff, 1960; Starck and Ricklefs, 1998; Bellairs and Osmond, 2005; Patten, 2008). Furthermore, the complex physiological processes of incubation were previously described (Olsen, 1942; Sturkie, 1965; Freeman and Vince, 1974; Whittow, 1999). All of these studies indicate that the incubation stages is important for embryonic development and organogenesis.
The embryonic stages is apt to be influenced by surrounding physical factors, which consequently could lead to changes in particular phenotypes and eventially may obtain environmental adaptability (Feil and Fraga, 2012; Jablonka, 2013; LaFreniere and MacDonald, 2013; Schlichting and Wund, 2014). Together, these environmental factors influence phenotypes in one generation, which could also affect phenotypes in subsequent generations through epigenetic mechanisms. In avians, incubation temperature as one of the most important environmental factors can affect embryonic development (Deeming, 2002; Oviedo-Rondón et al., 2008; Barri et al., 2011; Shim and Pesti, 2011), metabolism (Lourens et al., 2006; Willemsen et al., 2010) and even the development of birds during post-hatch stages (Piestun et al., 2009; Piestun et al., 2013). Similarly, a previous study reported that raising the incubation temperature by 1°C during ED5–8 results in an increase in semitendinosus muscle fibre amount at 16 days after hatching in turkeys (Maltby et al., 2004). In addition, Hammond et al. demonstrated that incubation temperature raised by 1°C for 3 days at early stages in chick, led to an increase in the growth rate of muscle and embryo activity (Hammond et al., 2007). Our initial data demonstrated that raising incubation temperature by 1°C during ED 11–24 has an inhibiting effect on immune function at the middle embryonic stages and has significant increased effects on lipid metabolism in ducklings during post-hatch stages (Liu et al., 2013; Wang et al., 2014). Therefore, it is fully confirmed that thermal manipulation during incubation stages has an extensive influence on embryonic development, adult phenotypes even their offspring phenotypes. (Piestun et al., 2008; Shinder et al., 2011; Renaudeau et al., 2012; Frésard et al., 2013)
Epigenetic modifications could be the potential link between the response of embryo phenotypic variability and certain environmental stimuli (Jaenisch and Bird, 2003). A previous study demonstrated that ducklings at slightly cooler incubation temperature experienced weaker immunity due to reduced response to new antigens; meanwhile, they predicted that thermal manipulation would induce phenotype variations that are characteristic of epigenetic modifications including variation in growth rate, body condition and immune response (DuRant et al., 2012).
DNA methylation is an essential epigenetic modification acquired in early life that stores epigenetic memory and therefore has an underlying effect on phenotypes of one generation or even the subsequent generations (Daxinger and Whitelaw, 2010). DNA (cytosine 5)-methyltransferases (DNMTS) and Methyl-CpG binding domain proteins (MBPS) are important components of the DNA methylation system, which involves the establishing and maintenance of DNA methylation patterns (Klose and Bird, 2006; Geiman and Muegge, 2010). Campos et al. reported that increasing embryonic temperature range in fish from 23–27°C to 23–31°C had a remarkable dynamic effect on gene expression of DNMT3A, which is a key gene regulating the de novo methylation process (Campos et al., 2012). In contrast, little is known about the effects of thermal stimuli on methylation of genomic DNA in birds.
Therefore, the aim of our present study was to test whether alteration of incubation temperature could affect DNA methylation and whether DNMTS and MBPS play roles in thermal epigenetic regulation of early development in duck. To conduct this, we artificially incubated duck eggs at different thermal treatment conditions, and then, DNMTS and MBD3&5 genes expression levels and DNMTS enzyme activities were examined. These data prove the incubation temperature manipulation affect epigenetic modification of duck embryos and may provide clues for the research that related to the long-term temperature adaptability acquisition when birds exposed to the changing environmental temperature condition.
MATERIALS AND METHODS
Birds and Tissues
A total of 100 eggs from breeders aged between 30 and 50 weeks. All eggs used in the study were obtained from Peking ducks (Anas platyrhynchos domestica). Peking duck eggs were selected from Sichuan Agricultural University Waterfowl Breeding Experiment Farm. Fresh eggs were artificially collected from nest boxes and storaged at the following environmental conditions no more than 7 days: room temperature of 15°C–18°C, relative humidity of 70–75%. These eggs were numbered and weighted individually at the range of 84.0 ± 2.0g and divided into two groups randomly. Eggs in the control group were transferred into the same incubation equipment controled by computer with fuzzy control system (Haijiang, Beijing, China) and maintained at a temperature of 37.8°C and relative humidity of 60–65% from ED1 to ED27; Meanwhile, eggs in the treatment group were incubated at 38.8°C, whereas other conditions remained identical with the control group. These eggs were turned once per hour during ED1–27. At ED10, all eggs were quality tested by candling to identify infertile eggs and dead embryos, then, unqualified eggs were removed from the subsequent experiment. The treatment group with 15 qualified eggs incubated at 38.8°C and the other conditions remained the same as the control group, during ED10–20. At ED20, 15qualified eggs were transferred from control group to treatment group and incubated at 38.8°C until the end of incubation. The incubation temperature design are listed in Figure 1. We divided the incubation stages into three phases, early embryonic stages (ED1–10), middle embryonic stages (ED10–20), later embryonic stages (ED20–27), which based on previous studies in birds (Collin et al., 2007; Hammond et al., 2007; Al-Musawi et al., 2012). Six ducklings were slaughtered by rapid bloodletting at ED10, 20 and 27. The leg muscle tissue, breast muscle tissue, heart tissue and liver tissue were isolated and frozen in liquid nitrogen for RNA extraction.
Figure 1.

The basic treatment process. CG, TG mean control group and treatment group. Treatment was comprised of increasing the incubation temperature by 1°C during ED1–10, ED10–20, ED20–27. means at ED10, more than 15 qualified eggs were transferred from control group to treatment group and incubated at 38.8°C during ED10–20, while eggs in control group still incubated at 37.8°C. means at ED20, more than 15 qualified eggs were transferred from control group to treatment group and incubated at 38.8°C during ED20–27, while eggs in control group still incubated at 37.8°C. Samples were taken at ED10, 20 and 27.
RNA Isolation and Real-Time Quantitative PCR
Total RNA was extracted from the leg muscle tissue, breast muscle tissue, heart tissue and liver tissue by using Trizol reagent (Invitrogen, CA, USA) following the instructions of the manufacturer. Electrophoresis on 1% (w/v) agarose gel was used to assess RNA quality. For real-time PCR analysis, the total RNA from all samples was treated with DNase I for 10 min and then quantified by spectrophotometric absorbance at 260 nm and 280 nm. Absorbance ratios were greater than 1.9, which indicated high purity RNA. For genes unrecorded in the NCBI database, RT-PCR was used to obtain their sequences. The detailed information of RT-PCR primers and the real-time PCR primers are listed respectively in Table 1 and Table 2. Primers were designed using the primer 5 software (Primer Biosoft International, USA) and were synthesised by BGI (Beijing, China). 96-well iCyclerIQ5 (USA) and TaKaRa Ex Taq RT-PCR kit (Takara, Dalian, China) were used to measure the relative mRNA expression of genes. The real-time PCR reaction contained 1 μL of cDNA template, 12.5 μL of SYBR Premix Ex Taq, 10.5 μL of sterile water, and 0.5 μL of primer. Thermal cycling parameters were one cycle of initial denaturation at 95°C for 30 s, 40 cycles of 95°C for 10 s and 60°C for 40 s. The reactions of each sample were repeated three times. The relative mRNA expression levels of DNMTS and MBD3&5 were calculated by the “normalized relative quantification’’ method followed by 2−ΔΔCT (Livak and Schmittgen, 2001).
Table 1.
Primers for PCR analysis
| Gene | Primer sequence (5′-3′) | Product length(bp) | Tm(°C) | |
|---|---|---|---|---|
| dnmt1 | F | 5′-GCTATGTCGCCCTGGATTTC-3′ | 507 | 64.1 |
| R | 5′-CACAGGACTCCATACCCAAGAA-3′ | |||
| dnmt3a | F | 5′-GGAGCACCCTTTGTTTATCG-3′ | 481 | 56.8 |
| R | 5′-TTCGGAGGCAATGTAGCG-3′ | |||
| dnmt3b | F | 5′-CCACTACACCGACGTTTCCA-3′ | 443 | 60.3 |
| R | 5′-GCCTCCACCACTTTCTCCTC-3′ | |||
| mbd3 | F | 5′-TGGACCTCAGCACTTTCGAC-3′ | 387 | 61.6 |
| R | 5′-GCAGAGCACTAGCGATAGCA-3′ | |||
| mbd5 | F | 5′-GCTATGTCGCCCTGGATTTC-3′ | 525 | 63.5 |
| R | 5′-CACAGGACTCCATACCCAAGAA-3′ |
Notes: F, forward primer; R, reverse primer.
Table 2.
Primers for real-time PCR
| Gene | Primer sequence (5′-3′) | Product length(bp) | Tm(°C) | |
|---|---|---|---|---|
| β-actin | F | 5′-GCTATGTCGCCCTGGATTTC-3′ | 168 | 60 |
| R | 5′-CACAGGACTCCATACCCAAGAA-3′ | |||
| GADPH | F | 5′-AAGGCTGAGAATGGGAAAC-3′ | 254 | 60 |
| R | 5′-TTCAGGGACTTGTCATACTTC-3′ | |||
| 18S rRNA | F | 5′-TTGGTGGAGCGATTTGTC-3′ | 129 | 60 |
| R | 5′-ATCTCGGGTGGCTGAACG-3′ | |||
| dnmt1 | F | 5′-GAAATCGACGGTCGTCTCCTC-3′ | 149 | 60 |
| R | 5′-TCAGCAACGGCAAGCCTAAC-3′ | |||
| dnmt3a | F | 5′-GAGGCAATGTAGCGATCCACC-3′ | 160 | 60 |
| R | 5′-CCAACAACCACGACCAGGAGT-3′ | |||
| dnmt3b | F | 5′-ACAGGCAAAGTAATCCTTGAGCG-3′ | 110 | 60 |
| R | 5′-CCGACGTTTCCAACATCAGC-3′ | |||
| mbd3 | F | 5′-ACCTTATTGCTGGGATGGTTTGT-3′ | 175 | 60 |
| R | 5′-GCACGGGAAAGATGTTGATGAG-3′ | |||
| mbd5 | F | 5′-GTTTCCATAGCCACCTCCTCC-3′ | 120 | 60 |
| R | 5′-TGTTTCTGCCATTGACACCACT-3′ |
Notes: F, forward primer; R, reverse primer.
Elisa
DNMTS concentration of samples was measured by using DNMTS ELISA Kits (Jijin, Shanghai, China) following the manufacturer's instructions. The standard curve was generated by standard samples used for determining the concentration of unknown samples. All standard curves had r2 values > 0.9900 (Figure 2).
Figure 2.
Standard curve is used to determine DNMTS concentration in each sample. A. Standard curve of enzyme DNMT1 activity, B. Standard curve of enzyme DNMT3A activity, C. Standard curve of enzyme DNMT3B activity.
Statistical Analysis
All data were subjected to M.S. Excel program and performed in SAS V.8.0 (SAS Institute Inc., Cary, NC, USA). All data were listed as the format of mean±S.D. For the gene mRNA analyses, n = 6; For the enzyme activities measurement, n = 6. One-way analysis of variance was used to determine the statistical significance between the groups. The significance of data was recognised at level of P < 0.05.
RESULTS
Gene Expression Levels of DNMTS and MBD3&5
To investigate the response to temperature manipulation during embryonic periods, gene expression levels of DNMTS and MBD3&5 were detected by using real-time PCR in leg muscle tissue, breast muscle tissue, heart tissue and liver tissue. Data are listed in Figure 3. These results showed significantly stage-specific responses.
Figure 3.
The relative mRNA expression levels of DNMTS and MBD3&5 in treatment and control groups (n = 6) were confirmed by real-time RCR. A. Relative gene expression of DNMT1, B. Relative gene expression of DNMT3A, C. Relative gene expression of DNMT3B, D. Relative gene expression of MBD3, E. Relative gene expression of MBD5. Label * on the bar means a significant difference at the level P < 0.05.
Relative expression patterns were markedly dynamic in DNMTS during the entire embryonic phases; during the early embryonic stages we found gene expression levels of DNMTS in leg muscle tissue was significantly lower under thermal treatment conditions (P < 0.05) (Figure 3). Gene expression level of DNMT1 and DNMT3A in breast muscle tissue were up-regulated in the treatment group with significant change (P < 0.05) (Figure 3A and B). Additionally, mRNA expression levels of DNMT1 in heart tissue were up-regulated in the treatment group and exhibited a significant difference (P < 0.05) (Figure 3A), however, DNMT3A showed an opposite gene expression trend with significant change (P < 0.05) (Figure 3B). The gene expression levels of DNMT3A and DNMT3B in liver tissue were significantly lower under thermal treatment conditions (P < 0.05) (Figure 3B and C); During the middle embryonic stages, the mRNA levels of DNMT1 in liver tissue was significantly increased in the treatment group (P < 0.05), and mRNA levels of DNMT3A in leg muscle tissue significantly decreased in the treatment group (P < 0.05) (Figure 3A and B). During the later embryonic stages, gene expressions levels of DNMT1, DNMT3A in breast muscle tissue and DNMT1 in heart tissue was significantly down-regulated in the treatment group (P < 0.05) (Figure 3A and B).
Gene expression trends of MBD3&5 were similar during the developmental stages, especially during the middle and later period, with MBD3&5 having higher gene expression levels in heart tissue and MBD5 in liver tissue in the treatment group with significant changes during the early embryonic stages (P < 0.05) (Figure 3D and E). During the middle embryonic stages, gene expression levels of MBD3 in leg muscle tissue (Figure 3D), heart tissue, MBD5 in leg muscle tissue, breast muscle tissue (Figure 3E) was down-regulated in the treatment group and exhibited a significant difference (P < 0.05). Additionally, gene expression levels of MBD3 in heart tissue (Figure 3D), and MBD5 in leg muscle tissue, breast muscle tissue, heart tissue (Figure 3E) were significantly down-regulated under thermal treatment conditions during the later embryonic stages (P < 0.05).
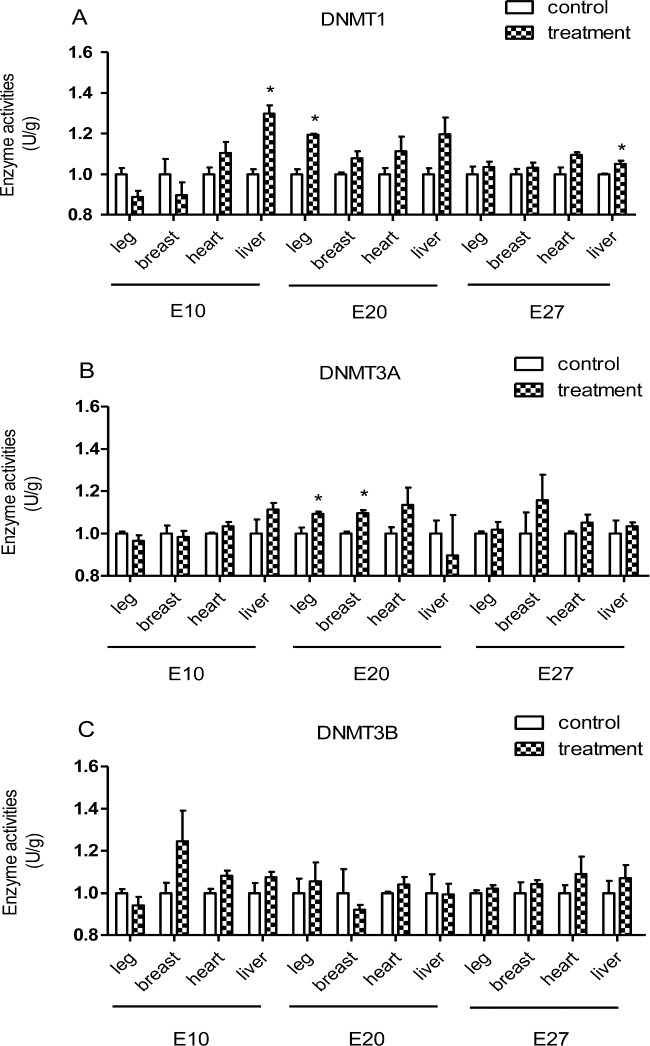
Enzyme Activities of DNMTS
To further test the relationship between incubation temperature and DNA methylation, enzyme activities of DNMTS were measured in the current study. The ELISA data in Figure 4 showed that the enzymatic activity of DNMT1 in liver tissue during the middle and later embryonic stages and of DNMT1 in leg muscle tissue (Figure 4A) during the middle embryonic stages were significantly enhanced by thermal manipulation (P < 0.05). The enzyme activities of DNMT3A (Figure 4B) during the middle embryonic stages show significant difference between the two groups in leg muscle tissue and breast muscle tissue (P < 0.05). For the other tissues during the entire embryonic phases measured, few significant differences were found.
Figure 4.
The enzyme activities of DNMTS in treatment and control groups (n = 6) were confirmed by DNMTS ELISA Kits. A. Determination of DNMT1 enzyme activity, B. Determination of DNMT3A enzyme activity, C. Determination of DNMT3B enzyme activity. Label * on the bar means a significant difference at the level P < 0.05.
DISCUSSION
Incubation period is very important for embryonic development and organogenesis, and related studies have been conducted in avian to signify the importance of incubation stages. (Yılmaz et al., 2011; Al-Musawi et al., 2012; Schwabl et al., 2012; Zhang et al., 2012; Chen et al., 2013; Kim et al., 2013; Vince, 2013). Previous studies in Montastraea faveolata (Voolstra et al., 2009) and Atlantic cod embryos (Skjaerven et al., 2014) showed that increasing incubation temperature during the embryonic stages could influence gene expression. In the current studies, DNMTS and MBD3&5 gene expressions changed significantly in different tissues at embryonic stages. Our results are consistent with these studies and provide available evidence that embryonic stage is crucial for gene expression.
Environmental factors, i.e., temperature (Nichelmann, 2004), chemicals (Vandegehuchte et al., 2010), or nutrition (Anderson et al., 2012) affect phenotypes during early developmental stages. The changes of DNA methylation may prove that environmental factors could cause the phenotypic variability. Change in incubation temperature is one of the most significant stressors for bird, so the current study was conducted to determine whether altering incubation temperature could influence methylation of genomic DNA. DNA methylation during embryonic stages is a dynamic mechanism, which plays a crucial role in vertebrate development. DNMTS are responsible for regulating DNA methylation in the genome and they are essential for this process (Kamei et al., 2010). In mammals, the DNMTS gene family consists of DNMT1, DNMT3A, and DNMT3B, which are grouped by the differences of structure and function (Goll and Bestor, 2005). DNMTS have been confirmed have catalytic activity in vivo (La Salle and Trasler, 2006). It has been reported that DNMT1 is regarded as the major maintenance methyltransferase, whereas, DNMT3A and DNMT3B have novo methylation activity and act as important methylation repair enzymes (Chen et al., 2003). On the other hand, MBD3&5 are members of the Methyl-CpG binding domain protein (MBPS) family, which have crucial roles in transcriptional regulation and development (Fournier et al., 2012). These genes were selected in the current study due to their critical role in epigenetic modification. We first examined gene expressions since previous studies have reported that a single physical factor like temperature can influence gene expression levels. We show here that embryos studied after thermal manipulation have stage-specific changes in mRNA levels for target genes; meanwhile, mRNA expression profiles of DNMTS and MBD3&5 were highly dynamic during the embryonic development, showing their essential function during the early developmental stages. For the DNMTS gene family, gene expression levels in leg muscle tissue and liver tissue were significantly down-regulated under thermal manipulation conditions during ED1–10 (P < 0.05) except DNMT1. We also found that DNMTS gene expression levels in breast muscle tissue were up-regulated by a higher incubation temperature during ED1–10. This effect showed that the expression profile of the de novo and maintenance methyltransferases is tissue-specific during embryonic developmental stages (Li et al., 1992). Gene expressions levels of MBD3&5 were found to be more dynamic than DNMTS. Relative expression patterns of MBD3&5 were similar in different tissues during all embryonic stage investigated, but significant differences between incubation temperatures were observed during ED20–27. The results showed that the gene expression levels of MBD3&5 in leg muscle tissue, breast muscle tissue, heart tissue were down-regulated (P < 0.05) in the treatment group. While during ED1–10, gene expression levels of MBD3 in heart tissue and MBD5 in breast muscle tissue, heart tissue, liver tissue were significantly up-regulated (P < 0.05) under thermal manipulation conditions, the opposite expression trend was observed in liver tissue. It seems that higher incubation temperature represses gene expression, particularly for MBD3&5 at later embryonic stages. For the DNMTS gene family, the treatment incubation temperature has a complex effect on expression levels during all embryonic stages, especially in the early developmental period, except for DNMT3B. The different gene expressions of dnmts and MBD3&5 levels under slightly higher embryonic incubating temperature suggest that they may have different roles in epigenetic gene regulation during embryonic development. In order to further validate our hypothesis that DNA methylation indeed can be influenced by temperature, enzyme activities of DNMTS were measured in the current study and significant differences were observed in the enzyme activity of DNMT1 in liver tissue during ED1–10, ED20–27, in leg muscle tissue during ED10–20. Enzyme activity of DNMT3A in leg muscle tissue and breast muscle tissue were significantly enhanced under thermal manipulation conditions. We show here that embryos researched after higher incubation temperature treatment also have a stage-specific increase in enzyme activities for DNMTS, both de novo and the maintenance methyltransferases that play an important role in embryonic development. We also demonstrate that higher thermal manipulation has an increased effect on enzyme activities of DNMTS. Previous work reported in fish that DNMT3A gene expressions increased during all developmental stages when incubation temperature was increased moderately (Campos et al., 2012). These results suggest that animals that are sensitive to incubation temperature may have similar epigenetic modifications related to DNA methylation. Collectively, our findings in duck indicate that incubation temperature stimuli have an extended effect on DNA methylation, which may provide evidence that links epigenetic modification to thermal manipulation during embryonic periods. Our findings may support the hypothesis that DNMTS and MBPS play roles in thermal epigenetic regulation of the early development in duck. To our present knowledge, this is the first evidence proving that tissue-specific and stage-specific DNA methylation is caused by environmental stimuli in avian species. However, more work is required in the future to ascertain if these expression changes induce modifications in the methylation status of key genes is beneficial to development of the embryos.
Results from our current study may provide a novel insight into avian evolution. Because environmental factors have an important influence on DNA methylation during early developmental stages, epigenetic markers acquired in embryonic periods could influence adult phenotypes that could be inherited in future generations via heritable DNA methylation patterns (Renaudeau et al., 2012). Evidence from our study may also have agronomic value in animal breeding, taking into account the possibility that the demands for poultry meat will rise in the near future, and breeding workers must have a comprehensive understanding regarding high quality phenotypes that can be obtained by appropriately manipulating the environmental factors in breeding conditions (Frésard et al., 2013). However, it appear limited that in present study we focus on the effect of incubation temperature on DNA methylation, the underlying relation between embryonic temperature and epigenetic modification need further investigation
Acknowledgments
This work was supported by Chinese Agriculture Research Service (No.CARS-43-6), the National Natural Science Foundation of China (No.31301964), the Major Project of Sichuan Education Department (13ZA0252).
REFERENCES
- Al-Musawi S. L., Stickland N. C., Bayol S. A. In ovo temperature manipulation differentially influences limb musculoskeletal development in two lines of chick embryos selected for divergent growth rates. The Journal of experimental biology. 2012;215:1594–1604. doi: 10.1242/jeb.068791. [DOI] [PubMed] [Google Scholar]
- Anderson O. S., Sant K. E., Dolinoy D.C. Nutrition and epigenetics: an interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. The Journal of Nutritional Biochemistry. 2012;23:853–859. doi: 10.1016/j.jnutbio.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barri A., Honaker C., Sottosanti J., Hulet R., McElroy A. Effect of incubation temperature on nutrient transporters and small intestine morphology of broiler chickens. Poult. Sci. 2011;90:118–125. doi: 10.3382/ps.2010-00908. [DOI] [PubMed] [Google Scholar]
- Bellairs R., Osmond M. Atlas of chick development. Academic Press; 2005. [Google Scholar]
- Campos C., Valente L. M., Fernandes J. M. Molecular evolution of zebrafish dnmt3 genes and thermal plasticity of their expression during embryonic development. Gene. 2012;500:93–100. doi: 10.1016/j.gene.2012.03.041. [DOI] [PubMed] [Google Scholar]
- Chen W., Lv Y., Zhang H., Ruan D., Wang S., Lin Y. Developmental specificity in skeletal muscle of late-term avian embryos and its potential manipulation. Poult. Sci. 2013;92:2754–2764. doi: 10.3382/ps.2013-03099. [DOI] [PubMed] [Google Scholar]
- Chen T., Ueda Y., Dodge J. E., Wang Z., Li E. Establishment and Maintenance of Genomic Methylation Patterns in Mouse Embryonic Stem Cells by Dnmt3a and Dnmt3b. Molecular and Cellular Biology. 2003;23:5594–5605. doi: 10.1128/MCB.23.16.5594-5605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin A., Berri C., Tesseraud S., Rodon F. R., Skiba-Cassy S., Crochet S., Duclos M., Rideau N., Tona K., Buyse J. Effects of thermal manipulation during early and late embryogenesis on thermotolerance and breast muscle characteristics in broiler chickens. Poult. Sci. 2007;86:795–800. doi: 10.1093/ps/86.5.795. [DOI] [PubMed] [Google Scholar]
- Daxinger L., Whitelaw E. Transgenerational epigenetic inheritance: more questions than answers. Genome Research. 2010;20:1623–1628. doi: 10.1101/gr.106138.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeming C. Avian incubation: behaviour, environment and evolution. Oxford University Press; 2002. [Google Scholar]
- DuRant S. E., Hopkins W. A., Hawley D. M., Hepp G. R. Incubation temperature affects multiple measures of immunocompetence in young wood ducks (Aix Sponsa) Biology Letters. 2012;8:108–111. doi: 10.1098/rsbl.2011.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval M. Atlas d'embryologie. 1889. G. Masson.
- Feil R., Fraga M. F. Epigenetics and the environment: emerging patterns and implications. Nature Reviews Genetics. 2012;13:97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- Fournier A., Sasai N., Nakao M., Defossez P. A. The role of methyl-binding proteins in chromatin organization and epigenome maintenance. Briefings in Functional Genomics. 2012;11:251–264. doi: 10.1093/bfgp/elr040. [DOI] [PubMed] [Google Scholar]
- Frésard L., Morisson M., Brun J.-M., Collin A., Pain B., Minvielle F., Pitel F. Epigenetics and phenotypic variability: some interesting insights from birds. Genet. Sel. Evol. 2013;45:16. doi: 10.1186/1297-9686-45-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman B. M., Vince M. A. A Behavioural and Physiological Study. Chapman and Hall; 1974. Development of the avian embryo. [Google Scholar]
- Geiman T. M., Muegge K. DNA methylation in early development. Molecular Reproduction and Development. 2010;77:105–113. doi: 10.1002/mrd.21118. [DOI] [PubMed] [Google Scholar]
- Goll M. G., Bestor T. H. Eukaryotic cytosine methyltransferases. Annual Review of Biochemistry. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H. L. A series of normal stages in the development of the chick embryo. Journal of Morphology. 1951;88:49–92. [PubMed] [Google Scholar]
- Hammond C. L., Simbi B. H., Stickland N. C. In ovo temperature manipulation influences embryonic motility and growth of limb tissues in the chick (Gallus gallus) The Journal of Experimental Biology. 2007;210:2667–2675. doi: 10.1242/jeb.005751. [DOI] [PubMed] [Google Scholar]
- Jablonka E. Epigenetic inheritance and plasticity: the responsive germline. Progress in Biophysics and Molecular Biology. 2013;111:99–107. doi: 10.1016/j.pbiomolbio.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature Genetics. 2003;33 Suppl:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Kamei Y., Suganami T., Ehara T., Kanai S., Hayashi K., Yamamoto Y., Miura S., Ezaki O., Okano M., Ogawa Y. Increased expression of DNA methyltransferase 3a in obese adipose tissue: studies with transgenic mice. Obesity (Silver Spring) 2010;18:314–321. doi: 10.1038/oby.2009.246. [DOI] [PubMed] [Google Scholar]
- Keibel F. Normentafeln zur Entwicklungsgeschichte der Wirbelthiere: Des Huhnes (Gallus domesticus) 1900. G. Fischer. [Google Scholar]
- Kim M., Parvin R., Mushtaq M., Hwangbo J., Kim J., Na J., Kim D., Kang H., Kim C., Cho K. Influence of monochromatic light on quality traits, nutritional, fatty acid, and amino acid profiles of broiler chicken meat. Poult. Sci. 2013;92:2844–2852. doi: 10.3382/ps.2013-03159. [DOI] [PubMed] [Google Scholar]
- Klose R. J., Bird A. P. Genomic DNA methylation: the mark and its mediators. Trends in Biochemical Sciences. 2006;31:89–97. doi: 10.1016/j.tibs.2005.12.008. [DOI] [PubMed] [Google Scholar]
- LaFreniere P., MacDonald K. A post-genomic view of behavioral development and adaptation to the environment. Developmental Review. 2013;33:89–109. [Google Scholar]
- La Salle S., Trasler J. M. Dynamic expression of DNMT3a and DNMT3b isoforms during male germ cell development in the mouse. Developmental Biology. 2006;296:71–82. doi: 10.1016/j.ydbio.2006.04.436. [DOI] [PubMed] [Google Scholar]
- Li, et al. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Liu J., Yan X., Li Q., Wang G., Liu H., Wang J., Li L., Du X., Han C., He H. Thermal manipulation during the middle incubation stage has a repressive effect on the immune organ development of Peking ducklings. Journal of Thermal Biology. 2013;38:520–523. [Google Scholar]
- Livak K. J., Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lourens A., Van den Brand H., Heetkamp M., Meijerhof R., Kemp B. Metabolic responses of chick embryos to short-term temperature fluctuations. Poult. Sci. 2006;85:1081–1086. doi: 10.1093/ps/85.6.1081. [DOI] [PubMed] [Google Scholar]
- Maltby V., Somaiya A., French N. A., Stickland N. C. In ovo temperature manipulation influences post-hatch muscle growth in the turkey. British Poultry Science. 2004;45:491–498. doi: 10.1080/00071660412331286190. [DOI] [PubMed] [Google Scholar]
- Nichelmann M. Perinatal epigenetic temperature adaptation in avian species: comparison of turkey and Muscovy duck. Journal of Thermal Biology. 2004;29:613–619. [Google Scholar]
- Olsen M. W. Maturation, fertilization, and early cleavage in the hen's egg. Journal of Morphology. 1942;70:513–533. [Google Scholar]
- Oviedo-Rondón E., Small J., Wineland M., Christensen V., Mozdziak P., Koci M., Funderburk S., Ort D., Mann K. Broiler embryo bone development is influenced by incubator temperature, oxygen concentration and eggshell conductance at the plateau stage in oxygen consumption 1. British Poultry Science. 2008;49:666–676. doi: 10.1080/00071660802433149. [DOI] [PubMed] [Google Scholar]
- Patten B. M. Early embryology of the chick. Wildside Press LLC; 2008. [Google Scholar]
- Piestun Y., Druyan S., Brake J., Yahav S. Thermal treatments prior to and during the beginning of incubation affect phenotypic characteristics of broiler chickens posthatching. Poult. Sci. 2013;92:882–889. doi: 10.3382/ps.2012-02568. [DOI] [PubMed] [Google Scholar]
- Piestun Y., Harel M., Barak M., Yahav S., Halevy O. Thermal manipulations in late-term chick embryos have immediate and longer term effects on myoblast proliferation and skeletal muscle hypertrophy. Journal of Applied Physiology. 2009;106:233–240. doi: 10.1152/japplphysiol.91090.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piestun Y., Shinder D., Ruzal M., Halevy O., Brake J., Yahav S. Thermal manipulations during broiler embryogenesis: effect on the acquisition of thermotolerance. Poult. Sci. 2008;87:1516–1525. doi: 10.3382/ps.2008-00030. [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Collin A., Yahav S., de Basilio V., Gourdine J. L., Collier R. J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal : an International Journal of Animal Bioscience. 2012;6:707–728. doi: 10.1017/S1751731111002448. [DOI] [PubMed] [Google Scholar]
- Romanoff A. L. The avian embryo. Structural and functional development. 1960 The avian embryo. Structural and Functional Development. [Google Scholar]
- Schlichting C. D., Wund M. A. Phenotypic plasticity and epigenetic marking: an assessment of evidence for genetic accommodation. Evolution. 2014;68:656–672. doi: 10.1111/evo.12348. [DOI] [PubMed] [Google Scholar]
- Schwabl H., Holmes D., Strasser R., Scheuerlein A. Embryonic exposure to maternal testosterone influences age-specific mortality patterns in a captive passerine bird. Age. 2012;34:87–94. doi: 10.1007/s11357-011-9222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim M., Pesti G. Effects of incubation temperature on the bone development of broilers. Poult. Sci. 2011;90:1867–1877. doi: 10.3382/ps.2010-01242. [DOI] [PubMed] [Google Scholar]
- Shinder D., Ruzal M., Giloh M., Druyan S., Piestun Y., Yahav S. Improvement of cold resistance and performance of broilers by acute cold exposure during late embryogenesis. Poult. Sci. 2011;90:633–641. doi: 10.3382/ps.2010-01089. [DOI] [PubMed] [Google Scholar]
- Skjaerven K. H., Hamre K., Penglase S., Finn R. N., Olsvik P. A. Thermal stress alters expression of genes involved in one carbon and DNA methylation pathways in Atlantic cod embryos. Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology. 2014;173C:17–27. doi: 10.1016/j.cbpa.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Starck J. M., Ricklefs R. E. Avian growth and development: evolution within the altricial-precocial spectrum. Oxford University Press; 1998. [Google Scholar]
- Sturkie P. D. Avian Physiology Second Edition. Ithaca, New York: Cornell Univ. Press; 1965. [Google Scholar]
- Vandegehuchte M. B., Lemiere F., Vanhaecke L., Vanden Berghe W., Janssen C. R. Direct and transgenerational impact on Daphnia magna of chemicals with a known effect on DNA methylation. Comparative biochemistry and physiology. Toxicology & Pharmacology : CBP. 2010;151:278–285. doi: 10.1016/j.cbpc.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Vince M. A. Some environmental effects on the activity and development of the avian embryo. In: Gottlieb G., editor. Behavioral Embryology. 2013. pp. 285–323. [Google Scholar]
- Voolstra C. R., Schnetzer J., Peshkin L., Randall C. J., Szmant A. M., Medina M. Effects of temperature on gene expression in embryos of the coral Montastraea faveolata. BMC Genomics. 2009;10:627. doi: 10.1186/1471-2164-10-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Liu J., Xiang S., Yan X., Li Q., Cui C., Li L., Liu H. Influence of in ovo thermal manipulation on lipid metabolism in embryonic duck liver. Journal of Thermal Biology. 2014;43:40–45. doi: 10.1016/j.jtherbio.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Whittow G. C. Sturkie's avian physiology. Academic Press; 1999. [Google Scholar]
- Willemsen H., Kamers B., Dahlke F., Han H., Song Z., Pirsaraei Z. A., Tona K., Decuypere E., Everaert N. High-and low-temperature manipulation during late incubation: Effects on embryonic development, the hatching process, and metabolism in broilers. Poult. Sci. 2010;89:2678–2690. doi: 10.3382/ps.2010-00853. [DOI] [PubMed] [Google Scholar]
- Yılmaz A., Tepeli C., Garip M., Çağlayan T. The effects of incubation temperature on the sex of Japanese quail chicks. Poult. Sci. 2011;90:2402–2406. doi: 10.3382/ps.2011-01471. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang H., Qiao X., Yue H., Wu S., Yao J., Qi G. Effect of monochromatic light stimuli during embryogenesis on muscular growth, chemical composition, and meat quality of breast muscle in male broilers. Poult. Sci. 2012;91:1026–1031. doi: 10.3382/ps.2011-01899. [DOI] [PubMed] [Google Scholar]