Abstract
Protein ubiquitination is a versatile protein modification that regulates virtually all cellular processes. This versatility originates from polyubiquitin chains, which can be linked in eight distinct ways. The combinatorial complexity of eight linkage types in homotypic (one chain type per polymer) and heterotypic (multiple linkage types per polymer) chains poses significant problems for biochemical analysis. Here we describe UbiCRest, in which substrates (ubiquitinated proteins or polyubiquitin chains) are treated with a panel of linkage-specific deubiquitinating enzymes (DUBs) in parallel reactions, followed by gel-based analysis. UbiCRest can be used to show that a protein is ubiquitinated, to identify which linkage type(s) are present on polyubiquitinated proteins and to assess the architecture of heterotypic polyubiquitin chains. DUBs used in UbiCRest can be obtained commercially; however, we include details for generating a toolkit of purified DUBs and for profiling their linkage preferences in vitro. UbiCRest is a qualitative method that yields insights into ubiquitin chain linkage types and architecture within hours, and it can be performed on western blotting quantities of endogenously ubiquitinated proteins.
Keywords: ubiquitin, deubiquitinating enzyme, polyubiquitin chain type, branched ubiquitin, mixed linkage chain
Introduction
Protein ubiquitination is the attachment of the 76 amino-acid protein ubiquitin to Lys residues in substrates1. Ubiquitination most commonly targets proteins for degradation, and it is estimated that the majority of cellular proteins are modified during their lifetime. However, ubiquitin also serves many other roles in cells, including the activation of signaling cascades, the shuttling of proteins to and from membranes and the assembly of protein complexes for example at sites of DNA damage. In addition, ubiquitination regulates replication, transcription, splicing and translation of proteins. Many of these roles do not involve protein degradation, but utilize adaptor functions of ubiquitin2.
The versatility of ubiquitin to perform such distinct functions is generated by its ability to assemble a large variety of signals. Proteins can be monoubiquitinated at one or multiple sites, but most importantly, the ubiquitin molecule itself can be ubiquitinated at eight distinct positions2,3. This gives rise to eight types of homotypic ubiquitin chains (which are chains that exclusively contain a single linkage type), but also a mind-boggling number of possibilities for heterotypic mixed and branched ubiquitin chains (also referred to as forked4 or hybrid5 chains). In heterotypic ubiquitin chains, a homotypic chain is either extended by a second chain type such that each ubiquitin still only has one Lys modified (mixed chains), or a ubiquitin molecule in the chain is modified at multiple positions (branched chains). While some functions of heterotypic ubiquitin chains have emerged recently5,6, their abundance, architectural diversity, and importance are largely unclear3.
Existing methods for analyzing ubiquitin chains
Numerous methods have been developed to assess which ubiquitin chain types are present on substrates. Ubiquitin mutants in which one or several Lys residues are mutated to Arg have been instrumental in revealing the diversity of the ubiquitin signal2,7. These mutants can be used in vitro to understand the specificity of E3 ligase systems, or in vivo where they can be used in addition to endogenous ubiquitin, or upon ubiquitin knockdown in ubiquitin replacement strategies8. However, mutation of ubiquitin can lead to changes in affinity of binding partners, or to changes in polyubiquitin structure and/or dynamics9,with the effect that chains are no longer assembled or disassembled10,11.
Linkage-specific antibodies that specifically recognize Lys11, Lys48, Lys63, or linear/Met1-linked chains have been developed and are excellent reagents to understand the involvement of these linkage types in different pathways12–14. Similar questions can be addressed with recently developed ubiquitin chain sensors based on linkage-specific ubiquitin binding domains15.
Advances in mass spectrometry (MS)-based methods to study ubiquitin sites in substrate proteins and to analyze the linkage types present in samples have revolutionized the field of ubiquitin chain research in recent years16–19. These are usually bottom-up approaches where proteins are trypsin-digested into peptides and then subjected to liquid chromatography-tandem MS (LC-MS/MS) for identification. Ubiquitination sites on substrates can be identified because trypsin cleavage results in a dipeptide (Gly-Gly) remnant with a monoisotopic mass of 114.043 Da on peptides that were ubiquitinated20. Absolute quantitation techniques allow assessment of relative abundance of various ubiquitin linkages16, and enrichment techniques such as the development of antibodies against tryptic ubiquitination-site remnants allow analysis of ubiquitination at a global proteomic scale17,19. However, although these techniques can help to identify particular ubiquitin linkage types on a substrate, they do not reveal details on chain architecture, which is difficult to assess using current technologies21. Xu and Peng22 described a method of middle-down MS that allows the characterization of chain length and linkage. In this method, ubiquitin is partially trypsin digested in optimized native conditions, resulting in a single cleavage event at Arg74 of ubiquitin, and the resulting monoubiquitin remnants could in theory also be investigated for presence of heterotypic chains.
The problem: what's in a smear
Gel electrophoresis-based methods can also be used to study ubiquitination. However, ubiquitinated proteins or even purified ubiquitin chains often do not migrate according to their molecular weight but rather according to their molecular shape. This is most obvious when purified di-, tri- and tetraubiquitin species are compared23. These ubiquitin chains differ only with regards to their linkage-type while being composed of identical mass and charge, but nonetheless run at distinct positions on denaturing SDS-PAGE gels, indicating that ubiquitin is not fully unfolded. This size difference has been used analytically23.
In cells, the vast majority of proteins are post-translationally modified at some point during their life cycle, often resulting in a fuzzy appearance on gels, which could be due to phosphorylation, glycosylation, due to modification by ubiquitin-like modifiers such as SUMO, and/or due to ubiquitination. Indeed, despite the addition of modifications of a defined size (8.5 kDa in the case of adding ubiquitin), cellular ubiquitinated proteins usually appear as high-molecular weight ‘smears’ rather than as defined species, for multiple reasons. Firstly, the protein may be heterogeneously ubiquitinated at multiple sites, in the simplest case with monoubiquitin, which would lead to heterogeneous running behavior. Secondly, when ubiquitin chains are attached, the chain types may differ, leading to distinct motilities. Hence, even if a protein has an identical number of ubiquitin molecules, its gel-appearance may be fuzzy due to distinct running behavior of individual molecules. But most importantly, differences in polyubiquitin chain length lead to heterogeneous samples. It is possible that many proteins, in particular when destined for proteasomal degradation, comprise multiple heterogeneous polyubiquitin modifications.
In addition, ubiquitin signaling is highly dynamic, and proteins may be ubiquitinated with distinct chain types at different points in their life cycles. This was demonstrated elegantly by using Lys63- and Lys48-linkage-specific antibodies in a study of RIP1, a well-studied ubiquitin target in cytokine signaling12. The apparent co-occurrence of ‘signaling’ chain types (Lys63, Met1) and degradative (Lys48, Lys11) chain types on proteins may simply reflect different pools of proteins at different stages of their life cycle.
Overview of UbiCRest: using DUBs to assess ubiquitin chain type and architecture
To design a qualitative and quick method to assess ubiquitin chain type and architecture, we exploited the intrinsic linkage-specificity of DUBs. Advances in biochemistry and chemical biology have enabled a comprehensive profiling of DUB linkage preference, and confirmed the specificity of e.g. the Lys63-specific DUB AMSH, and of the Lys48-specific DUB OTUB1, which is now understood in molecular detail24–26. We have contributed specificity analysis of the human ovarian tumor (OTU) DUB family, and we have discovered that OTU enzymes have defined linkage preferences, identifying enzymes with relative specificity for each of the eight ubiquitin linkage types27 (e.g., see Fig. 1). This is interesting and requires further studies, as it suggests independent roles for each chain type in the regulation of cellular signaling processes.
Figure 1. Qualitative DUB specificity analysis.
Purified OTU DUBs were incubated in parallel with all eight diubiquitin substrates for indicated times. Reactions were resolved on SDS-PAGE gradient gels and silver stained. Enzyme concentration is as indicated. (a) OTUB1 full-length (aa 1-271) shows a remarkable specificity for Lys48 linkages. (b) OTUD3 (aa 52-209) preferentially cleaves Lys6- and Lys11-linked diubiquitin.
In addition, we realized that the availability of linkage-specific DUBs enables their use as tools to address questions in ubiquitin chain research27 (Fig. 2 and Table 1; find a generalized purification protocol in Box 1). Once the linkage specificity of each DUB at a given working concentration has been established (Fig. 1 and Box 2), a panel of DUBs with complementary specificity can be used to treat polyubiquitin or polyubiquitinated samples (Fig. 2) to assess (i) the types of ubiquitin linkages present in the sample (Fig. 3), and (ii) the architecture of linkages within the chains (Fig. 4).
Figure 2. Schematic description of UbiCRest analysis.
(a,b) A panel of DUBs with different linkage specificities and preferences (a) is used as a toolkit to study ubiquitin chain composition of ubiquitinated substrates (b). These can be either generated in vitro by using a defined ubiquitin chain assembly system or by immunoprecipitating a ubiquitinated protein of interest from cell lysate. The color of the circles representing ubiquitin moieties indicates the Lys residue it is attached to. Substrate-bound and free ubiquitin is shown in white.
Table 1. DUBs used in UbiCRest.
| Linkage type | DUB | Useful final [conc] (1x) | Comments on the DUB | Ref. |
|---|---|---|---|---|
| All eight linkages including proximal ubiquitin | USP21 or USP2 | 1-5 µM (USP21) | Positive control. | 28 |
| All linkages excluding Met1 and most proximal ubiquitin | CCHFV viral OTU (vOTU) | 0.5-3 µM | Positive control, does not cleave Met1 linkages. | 38 |
| Lys6 | OTUD3 | 1-20 µM | Cleaves Lys11 chains equally well. Targets other isopeptide linkages at high concentrations (Lys63 better (>) than the others, not Met1). | 27,31 |
| Lys11 | Cezanne | 0.1-2 µM | Very active, non-specific at very high concentrations (Lys63 > Lys48 > others, not Met1). | 27,39 |
| Lys27 | OTUD2 | 1-20 µM | Also cleaves Lys11, Lys29, Lys33, prefers longer Lys11 chains. Non-specific at high concentrations (Lys48 > others, not Met1). | 27 |
| Lys29 | TRABID | 0.5-10 µM | Cleaves Lys 29 and Lys33 equally well, and Lys63 with lower activity. Might target other linkages at higher concentrations (not Met1). Low yields from bacterial expression. | 40 |
| Lys33 | ||||
| Lys48 | OTUB1 | 1-20 µM | Highly Lys48-specific. Not very active. Can be used at high concentrations. | 27,41 |
| Lys63 | OTUD1 | 0.1-2 µM | Very active, non-specific at high concentrations (Lys48 > others, not Met1). | 27 |
| Lys63 | AMSH / AMSH-LP | 1-20 μM | Highly Lys63-specific. Not very active against long chains. Can be used at high concentrations. | 5,23 |
| Met1/linear | OTULIN | Any (10 nM – 10 μM) | Very active and specific, can be used at high concentrations. | 32 |
Box 1. Expression and purification of DUBs TIMING 4-14 d.
Procedure
Transform E. coli strain Rosetta2 (DE3) (pLacI) with the appropriate DUB-encoding plasmid (see Table 2) using standard methods. Plate transformed bacteria onto agar plates with appropriate antibiotics and incubate overnight at 37 ºC.
Inoculate a 50 mL overnight culture of LB or 2xTY medium supplemented with appropriate antibiotics with a single colony and incubate overnight at 37 °C whilst shaking at 180-220 rpm.
Inoculate 2-4 L of LB or 2xTY medium containing appropriate antibiotics with 10 mL overnight culture per 1 L. Incubate at 37 °C whilst shaking at 180-220 rpm until the OD600 reaches 0.6-0.8, which usually takes around 2-5 h depending on type of cells, medium, antibiotics and type of flasks.
Induce expression of GST-DUBs with 0.5-1.0 mM IPTG (final concentration) for 16-20 h at 18°C.
-
Harvest the bacterial cells by centrifugation at 5,000 g for 15 min at 4 ºC, collect the pellet and store at -80 ºC.
PAUSE POINT Pellets can be stored at -80 ºC for at least 1 year.
Resuspend pellet in ~30-50 mL of GST buffer 1 supplemented with 1 EDTA free Complete protease inhibitor tablet, 1 mg/mL lysozyme and 0.1 mg/mL DNaseI.
Lyse the cells by sonication on ice (10 s bursts at 10 s intervals with an amplitude of 40-50 W for 4-6 min).
Clarify the lysate by centrifugation at 50,000 g for 30 min at 4 °C.
Filter the cleared lysate using a 0.45 μm syringe filter.
Pre-equilibrate 1.5-3 mL of Glutathione Sepharose 4B resin with 20 ml GST buffer 1 in a gravity flow column setup, let the buffer run through and close the bottom of the column.
Apply the cleared lysate, close the top of the column and incubate for 1 h at 4 °C on a roller mixer.
Wash the beads with 1-2 L of GST buffer 2 followed by 1-2 L of GST buffer 3.
To cleave the GST-tag from the fusion protein, add 50 µg of GST-tagged PreScission protease to the beads in 5 mL of GST buffer 3 and incubate at 4°C overnight.
The following day, collect the flow-through containing the cleaved protein from the beads. Wash the beads three times with 5 mL GST buffer 3 and collect each flow-through fraction.
Analyze the PreScission-cleaved protein on a SDS-PAGE gel (NuPAGE 4-12% Bis-Tris Protein Gels).
-
Concentrate the sample using an Amicon Ultra-15 centrifugal filter unit (MW cutoff 3 kDa or 10 kDa) and determine the protein concentration using your method of choice.
PAUSE POINT Purified DUBs can be flash-frozen and stored at -80 °C for at least 1 year.
TROUBLESHOOTING
Further purification of DUBs (optional)
-
17.
Subject the eluted protein to anion exchange chromatography (Resource Q 1 mL) on an ÄKTA system (GE Healthcare) in GST buffer 3 and GST buffer 2 where the DUB should elute as a single peak using a NaCl gradient from 50-500 mM.
-
18.
Pool the peak fractions, concentrate the protein sample using Amicon Ultra-15 centrifugal filters.
-
19.
Perform size exclusion chromatography (HiLoad 16/60 Superdex 75) on an ÄKTA system (GE Healthcare) in GST buffer 1.
-
20.
Concentrate the peak fractions and determine the protein concentration using your method of choice.
PAUSE POINT Purified DUBs can be flash-frozen and stored at -80 °C for at least 1 year.
Box 2. Qualitative in vitro DUB assay TIMING ~4 h.
The linkage-specificity of DUBs can be limited to certain chain types, and this may vary with DUB family. It is advised to characterize the linkage specificity for each DUB when purified for the first time. In addition to establish linkage specificity, the assay also reveals at which concentrations the particular DUB batch should be used. It may not be necessary to repeat the entire DUB specificity panel for highly specific DUBs for each purification, but for less specific DUBs batch-to-batch variation in activity may affect the working concentration.
Procedure
Prepare 2x concentrated ubiquitin stocks for all eight diubiquitin substrates containing 10 µM ubiquitin in DUB reaction buffer (5 µM final concentration in reactions). Diubiquitin concentrations should be frequently readjusted in order to ensure uniform staining results.
-
Dilute DUBs (prepared as described in Box 1 or obtained from commercial sources) in DUB dilution buffer to 2x final concentrations (Table 1) and pre-incubate at room temperature (22 ºC) for 10-15 min.
CRITICAL STEP Pre-incubation of enzymes with catalytic Cys residues in a high-DTT buffer (here: DUB dilution buffer) is often required to achieve full activity through reduction of oxidized species.
Start the reaction by mixing 10 µL of each of the diubiquitin solutions (from step 1) and enzyme stock (from step 2) and incubate at 37 ºC.
-
Remove 5 µL aliquots at required time points and immediately mix with 5 µL of 4x LDS sample buffer to stop the reaction. We suggest a time course of 0, 5 and 30 min. If required, adjust the DUB concentration in subsequent runs in order to observe incomplete cleavage of the preferred substrate after 5 min and, ideally, complete hydrolysis after 30 min. For the ‘0 min’ time point, mix 2.5 µL of both substrate and DUB solution directly in 4x LDS sample buffer one after the other.
PAUSE POINT Once samples are mixed with LDS sample buffer they can be stored at -20 °C for up to 1 year and analyzed by SDS-PAGE at a later time.
-
Resolve samples by SDS-PAGE on 4-12% gradient gels run with MES buffer (Fig. 1).
CRITICAL STEP Do not boil samples containing ubiquitin chains as this may cause degradation and smearing of bands on SDS-PAGE gels.
TROUBLESHOOTING
-
Perform silver staining, e.g. by using the SilverStain Plus kit (Bio-Rad) according to the manufacturer’s instructions. Stop the staining reaction as soon as a satisfactory level of staining has been achieved.
CRITICAL STEP Untimely stopping results in uneven staining or a strong background.
Figure 3. UbiCRest analysis to determine ubiquitin linkage types on substrates.
(a) A model substrate with Lys63 linkages was generated by the autoubiquitination of GST-tagged NEDD4 HECT domain with UBE2L3. The substrate was treated with a panel of DUBs in parallel reactions for 15 min at 37 ºC, resolved on SDS-PAGE gradient gels and silver stained. M, marker; Control, model substrate without DUB treatment; dollar ($) marks enzyme bands; asterisks (*) indicate impurities in DUB preparations. (b) Ubiquitin di-, tri- and tetramers of all currently available linkage types (Lys6, Lys11, Lys48, Lys63 and Met1) show distinct electrophoretic mobilities on a SDS-PAGE gel. (c) OTUD2-released polyubiquitin species from autoubiquitinated GST-NEDD4 (a) compared to free Lys63-linked polyubiquitin. Numbers of ubiquitin moieties in ubiquitin chains are indicated. (d) The TNF receptor complex (TNF-RSC) was isolated from HEK 293T cells after stimulation with Flag-tagged TNFα for 10 min. Following a Flag IP, the beads were washed, and subsequently treated with the same panel of DUBs as in (a) in parallel reactions for 30 min at 37 ºC. Ubiquitin chains on RIP1 were detected by western blot analysis using a RIP1 antibody. Control, TNF-RSC IP without DUB treatment. An asterisk (*) indicates a non-specific band. See also ref. 28.
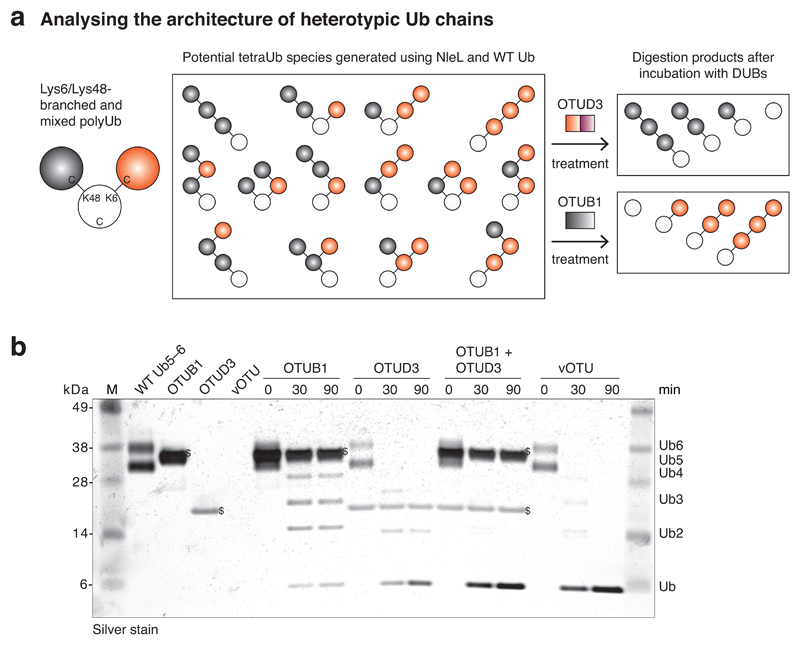
Figure 4. UbiCRest analysis to test for heterotypic polyubiquitin.
(a) UbiCRest can be used to analyze the architecture of heterotypic ubiquitin chains. Schematic showing the potential tetramer complexity in NleL-assembled WT ubiquitin products (middle) and cleavage products after treatment with OTUD3 and OTUB1, respectively (right). The C-terminus of ubiquitin can be linked either to Lys6 (red) or Lys48 (gray) resulting in 14 distinct species. A white circle indicates ubiquitin with a free C-terminus. (b) SDS-PAGE analysis of purified penta/hexaubiquitin (assembly B in Step 1A) assembled from WT ubiquitin (WT Ub5-6) treated with 4.7 µM OTUB1 full-length (aa 1-271), 5.5 µM OTUD3 OTU (52-209), a combination of both or 6.1 nM vOTU (1-217). Dollar ($) marks enzyme bands; M, marker. Neither OTUD3 nor OTUB1 alone cleave all chains, indicating the presence of two distinct linkage types (see discussion in Anticipated Results). Reproduced with permission from ref. 32, Nature Publishing Group.
Overview of the procedure
The starting point for UbiCRest is faithfully reproducible ubiquitinated protein that can be visualized by a PAGE-based method. Once visualization of polyubiquitin is optimized, the protein preparation is transferred into DUB-compatible buffer and split to yield samples with equal amounts of protein. Next, a positive control is established in which a nonspecific DUB removes all polyubiquitin from the protein and generates monoubiquitin. We use the highly active DUB USP21 (ref. 28) for this purpose, but other USP DUBs, such as USP2, are equally suitable. The remaining aliquots are then incubated with DUBs of different linkage specificity (Table 1 and Fig. 2a). Variables that can be adjusted are incubation time, temperature, and DUB concentration. We usually perform short assays (15-30 min) at low and high DUB concentrations, whereby signs of activity at low concentrations indicate the presence of the tested chain type, while at higher concentration cleavage of the preferred linkage is complete and may indicate whether other chains are still on the substrate (Fig. 3a, d). Alternatively, longer incubation times can be beneficial to test completeness of the reaction. To reveal the sometimes small changes in polyubiquitination by DUB treatment, it is important that all samples have equal amounts of protein, and that they are run on a single gel side-by-side. This often limits the number of time points or DUB concentrations that can be used in a single assay (Fig. 3a, d).
Advantages and applications of UbiCRest
UbiCRest analysis allows for quick and easy identification of protein ubiquitination (as opposed to, e.g. SUMOylation), the linkage types present in polyubiquitinated substrates and chain architecture of heterotypic chains. The advantages of UbiCRest are the easy set-up that does not require special equipment, and the speed of the assay, which takes merely hours to run once DUBs are available (depending on the method of detection).
With UbiCRest, most linkage types can be distinguished but the assay is particularly useful for detection of Lys11, Lys48, Lys63 and Met1-linked chains, since highly specific DUBs are available for these chain types. Similarly specific DUBs for the remaining chain types are unknown to date, but would be highly beneficial for this purpose. So far, only a subset of DUBs have shown preference for different ubiquitin linkage types27. We expect that in some cases, the activity or specificity of the used DUBs could be enhanced by mutations or fusion to binding partners. For example, an OTUB1-E2 fusion protein should enhance the activity of this enzyme26,29. In addition, it is possible that non-human OTU DUBs have distinct linkage preferences and have evolved missing activities of, e.g., a Lys6-specific DUB. Hence it is always worthwhile to test the linkage specificity of unstudied enzymes. Moreover, while most members of the USP family show broad specificity30 (and are substrate- rather than linkage-targeted DUBs), linkage-specific members of this DUB family may well exist. One example is the ubiquitin-specific protease CYLD, which prefers Lys63- and Met1-linkages over Lys48-linked chains23
It is worthwhile noting that linkage-specific DUBs may have many applications beyond UbiCRest, such as in ubiquitin proteomics and global ubiquitin linkage profiling. Also, for the identification of ubiquitination sites on proteins, it may be beneficial to decrease the complexity of the modification. This could be achieved by using DUBs to remove ubiquitin chains from the substrate in such a way that only the last attached, proximal ubiquitin on the substrate is preserved. Moreover, inactivation of linkage specific enzymes by mutation of the catalytic Cys can generate high-affinity, linkage-specific ubiquitin binding domains that could be used in other methods e.g. as ubiquitin chain sensors. These potential applications would help address many current questions and problems in ubiquitin research.
Limitations of UbiCRest
As with any biochemical method, there are a number of caveats that must be considered when using UbiCRest. Firstly, several of the enzymes may become non-specific, and target other than the expected ubiquitin chain types when used at high concentrations. Exceptions to this are the highly specific DUBs OTULIN, OTUB1 and AMSH, which can be used at very high concentrations without showing signs of cross-specificity31,32 (Fig. 3a, d). DUB specificity has so far only been comprehensively tested against diubiquitin, and it may be different for longer chains. A good example is OTUD2, which contains an additional ubiquitin binding site that targets the enzyme to longer Lys11-linked ubiquitin chains27. These issues are exacerbated by the fact that it is often difficult to estimate the amount of substrate, and the length of individual ubiquitin chains on the substrate, e.g., for material from immunoprecipitations.
Another issue that we have observed repeatedly relates to immunoprecipitations and the amount of substrate on beads. Usually, one would minimize the bead volume to increase concentration of polyubiquitinated material. We have found that the concentration of ubiquitinated protein on beads e.g. in an immunoprecipitate, can be too high, and we suspect that ubiquitin forms partly unfolded aggregates or superstructures that become uncleavable. Boiling of samples can also result in uncleavable material, and other harsh conditions during the isolation of the protein may also lead to material that is inert to DUB treatment. This can be overcome by careful handling of polyubiquitinated samples and by reducing the amount of polyubiquitinated protein on beads.
Even when kept intact, ubiquitin chain structures may pose problems for DUB cleavage11,28. Morgan and colleagues elegantly showed that many DUBs were unable or severely impaired to hydrolyze long (n>4) Lys48-linked chains, which may well be due to the compact structures adopted by these polymers 11.
What is not clear at this stage is whether the DUBs used are equally active against complex heterotypic (mixed or branched) polyubiquitin chains compared to homotypic chains. It is conceivable that more complicated branched structures pose a significant problem for DUBs that have to recognize two ubiquitin molecules in a linkage-specific fashion. However, so far, marked differences could not be detected when heterotypic Lys6/Lys48, Met1/Lys63 and Lys48/Lys63 polymers were analyzed5,31,33. Taken together, while false positive results may arise from enzyme cross-specificity, negative results from UbiCRest analysis may be due to reasons other than the absence of particular chain linkages in the substrates. It is highly advisable to use UbiCRest in conjunction with complementary, orthogonal technique such as mass-spectrometry, or ubiquitin mutants. However, due to the speed and sensitivity of UbiCRest, our method may be a good starting point for ubiquitin chain type analysis.
Experimental Design
Deubiquitinases
Linkage-specific DUBs are able to hydrolyze specific ubiquitin chain types on substrates, making them useful biochemical reagents. DUBs from the OTU and JAMM (JAB1/MPN/Mov34 metalloenzyme) family can be expressed in E. coli and can be purified with few chromatographic steps to high purity. In most cases, expression as a cleavable glutathione-S transferase (GST)-fusion enables the generation of milligram-quantities (see protocol in Box 1). Alternatively, most DUBs are now available from commercial sources, and a UbiCRest kit can be purchased that contains the key enzymes for the experiments detailed below. Table 1 lists the DUBs utilized in UbiCRest.
We have found that the activity of individual DUBs varies greatly27. Although some are highly active (OTULIN, vOTU (viral OTU), USP21, OTUD1, Cezanne) and should be used at low concentrations, others require significantly higher concentrations to show activity (OTUB1, OTUD2; Table 1). Box 2 outlines an assay for determining the linkage specificity of DUBs over a range of concentrations in order to determine the optimal working concentration of each DUB for UbiCRest analysis. OTU DUBs are Cys proteases and prone to (reversible) oxidation of their catalytic residue34, which needs attention during their purification.
Samples
Polyubiquitin is the substrate for UbiCRest analysis. In vitro-assembled and in vitro-purified polyubiquitin of unknown composition and architecture, generated, e.g., by an uncharacterized E2 or E3 ligase, can be examined using UbiCRest regardless whether the polyubiquitin is unattached (described in Step 1A, ‘assembly A’ of the PROCEDURE, using the example of NleL-assembled chains), attached to the ligase (autoubiquitination, described in Step 1A, ‘assembly B’ of the PROCEDURE, using E3 ubiquitin-protein ligase NEDD4 (NEDD4) as an example) or attached to a substrate In vivo-ubiquitinated substrates can be examined by UbiCRest for linkage composition and architecture when polyubiquitin can be faithfully and reproducibly detected (as described in Step 1B of the PROCEDURE, using the example of RIP1); in these applications, western blotting against proteins or tags is essential due to the high background of ubiquitinated proteins in cells (see ‘Visualizing the sample’). In addition to the experimental samples, purified ubiquitin chains of defined linkage composition are important to establish DUB preference and working concentration using the assay described in Box 2; these substrates can be obtained commercially.
Visualizing the sample
Perhaps the most important prerequisite for this protocol is that polyubiquitin or a polyubiquitinated protein can be faithfully visualized. This can be achieved as follows:
Silver, SyPro or Coomassie staining: If large amounts of protein is available (e.g., from in vitro reactions) samples can be separated by SDS-PAGE and visualized by standard Silver or Coomassie staining protocols. If a polyubiquitin smear is visible, this is ideal for UbiCRest, and generates the highest amount of information. We have found that Coomassie staining often leads to poor staining of polyubiquitin, in contrast to silver staining, which seems to visualize the high molecular weight polyubiquitin much better (e.g., Fig. 3a)
Western blotting against ubiquitin: This method uses either anti-ubiquitin antibodies, which examine the entire ubiquitin content of the sample, or when a tagged ubiquitin (such as FLAG-ubiquitin, hemagglutinin (HA)-ubiquitin etc.) is used, an antibody against the tag. Standard protocols to visualize a polyubiquitinated protein in a cell lysate usually include an initial immunoprecipitation step to enrich the protein of interest in its modified and unmodified form, and the ubiquitinated forms are then visualized by detecting the ubiquitin. UbiCRest can be used on immunoprecipitated material whilst still immobilized.
Western blotting using linkage-specific antibodies: As discussed above, linkage specific antibodies are available for 4 of the eight linkage types12–14, and can be used to visualize individual chain types in samples, and investigate their disappearance with UbiCRest.
Western blotting against the protein of interest: This method is the most direct way to test which chain types are present on a protein of interest. In some cases, antibodies directed against a protein of interest can visualize the modified forms of the protein; this depends on the quality of the antibody and on the fraction of protein that is modified. A good example is RIP1, which shows strong polyubiquitination after e.g. TNFα stimulation of cells12 (Fig. 3d), and MyD88 and IRAK that are ubiquitinated after IL-1 treatment5.
In essence, researchers that are able to visualize polyubiquitinated proteins by whatever means necessary, should be able to perform UbiCRest on such samples. Since the question about the ubiquitin chain type usually arises from having seen the modified form of the protein, this step is usually already established. We have found that the visualization protocols for polyubiquitin detection vary greatly from project to project and from lab to lab, and have often undergone significant optimization – hence every visualization protocol is different. Nonetheless, the described UbiCRest protocol should be useful for most if not all cases.
Controls
UbiCRest is intrinsically controlled, as numerous DUBs are used to treat a polyubiquitinated sample. Most important is the establishment of a positive control, whereby USP21 or another non-specific DUB should remove all polyubiquitin in the reaction. An untreated and a USP21 treated sample establish the window in which differences can be detected.
In some cases, it can be beneficial to include a catalytically inactive or inactivated DUB as a control. Met1-linked ubiquitin chains can be attached to pre-existing Lys63-linked chains, and may account for only a small fraction of the entire polyubiquitin content5. In the case of UbiCRest on RIP2, running reactions with OTULIN and inactive OTULIN side-by-side revealed clear differences showing that high-molecular weight ubiquitin chains on RIP2 contained Met1-linkages35. The easiest way to inactivate DUBs is to boil the enzymes for 5 min prior to using them in UbiCRest.
Materials
Reagents
DUBs: plasmids for expression and purification of DUBs (Box 1) of DUBs are available on request from the Komander laboratory or from Addgene (Table 2). Purified DUBs can be purchased from Boston Biochem or similar reagent providers. A UbiCRest kit with a panel of DUBs is also available (Boston Biochem, cat. no. K-400).
Escherichia coli strain Rosetta2 DE3 (pLacI or pLysS) (Novagen, cat. no. 71404 (pLacI) and 71403 (pLysS))
Isopropyl-β-D-thiogalactoside (IPTG; EMD Millipore, cat. no. 420322)
LB or 2xTY medium, agar plates and antibiotics (user-specific)
Complete protease inhibitors, EDTA-free (Roche Diagnostics, cat. no. 04 693 132 001)
Lysozyme (Sigma-Aldrich, cat. no. L6876)
Deoxyribonuclease I (DNaseI; Sigma-Aldrich, cat. no. DN25)
Trizma base (Sigma-Aldrich, cat. no. T1503)
Hydrochloric Acid 5M (HCl, Fisher Scientific, cat. no. 10695872)
Sodium chloride (Fisher Scientific, cat. no. 10356340)
Dithiothreitol (DTT; Melford Biolaboratories, cat. no. MB1015)
Instant Blue coomassie based gel stain (Expedeon, cat. no. ISB1L)
Glutathione Sepharose beads 4B (GE Healthcare, cat. no. 17-0756-05)
PreScission protease (GE Healthcare, cat. no. 27-0843-01)
Silver Stain Plus Kit (Bio-Rad, cat. no. 161-0449)
Acetic acid (VWR, cat. no. 20104.334)
-
Methanol (Fisher Scientific, cat. no. 10284580)
CAUTION Methanol is toxic; avoid exposure.
NuPAGE Novex LDS Sample Buffer (Life Technologies, cat. no. NP0008)
NuPAGE Novex MES SDS Running Buffer (20X) (Life Technologies, cat. no. NP0002)
NuPAGE Novex MOPS SDS Running Buffer (20X) (Life Technologies, cat. no. NP0001)
NuPAGE Novex 4-12% Bis-Tris Protein Gels, 1.0 mm (Life Technologies, cat. no. NP0323BOX (15 well), cat. no. WG1403BOX (26 well))
MilliQ water
Diubiquitin chains, e.g. from UbiQ (cat. no. UbiQ-013 (Lys6), UbiQ-014 (Lys11), UbiQ-015 (Lys27), UbiQ-016 (Lys29), UbiQ-017 (Lys33), UbiQ-033 (Lys48), UbiQ-034 (Lys63), UbiQ-070 (Met1)), Boston Biochem (cat. no. UC-61 (Lys27), UC-81 (Lys29), UC-101 (Lys33), UC-200 (Lys48), UC-300 (Lys63), UC-700 (Met1)) or similar.
Ubiquitin (Sigma-Aldrich, cat. no. U6253)
ATP (Sigma-Aldrich, cat. no. A7699)
Apyrase (Sigma-Aldrich, cat. no. A2230)
HEK 293T cells (ATCC, cat. no. CRL-3216)
Cell culture medium, inactivated fetal bovine serum and antibiotics (user-specific)
anti-FLAG M2 affinity gel (Sigma-Aldrich, cat. no. A2220)
TNF-α, FLAG-tagged (Enzo, cat. no. ALX-522-008-C050)
Anti-RIP1 antibody (BD Biosciences, cat. no. 610458)
PVDF transfer membrane (Millipore, cat. no. IPVH00010)
-
NaF (Sigma-Aldrich, cat. no. 201154)
CAUTION NaF is toxic; avoid exposure.
MgCl2 (Fisher Scientific, cat. no. BP214-500)
Triton X-100 (Sigma-Aldrich, cat. no. X100)
-
Iodoacetamide (Sigma-Aldrich, cat. no. I1149)
CAUTION Iodoacetamide is toxic; avoid exposure.
-
N-ethylmaleimide (Sigma-Aldrich, cat. no. 04259)
CAUTION N-ethylmaleimide is very toxic; avoid exposure.
Table 2.
Expression plasmids available from the Komander lab or from Addgene
| DUB | Construct | Vector | Species |
|---|---|---|---|
| USP21 | 196-565 | pOPIN-S | Human |
| vOTU | 1-183 | pOPIN-K | Crimean-Congo hemorrhagic fever virus |
| OTUD3 | 52-209 | pOPIN-K | Human |
| Cezanne | 53-446 | pOPIN-K | Human |
| OTUD2 | 1-348 | pOPIN-K | Human |
| TRABID | 245-697 | pOPIN-K | Human |
| OTUB1 | 1-271 | pOPIN-K | Human |
| OTUD1 | 287-481 | pOPIN-K | Human |
| AMSH | 1-424 | pGEX6P1 | Human |
| OTULIN | 1-352 | pOPIN-B | Human |
Equipment
Gravity flow column; Econo-Column Chromatography Columns (Biorad, cat. no. 737-1512)
Benchtop centrifuge (e.g., Eppendorf 5810R)
Sonicator (Misonix; Cole-Parmer, cat. no. EW-04711-81)
Amicon Ultra-15 centrifugal filter unit with Ultracel-3 or -10 membrane (Millipore, cat. no. UFC900324 (Ultracel-3) UFC901024 (Ultracel-10))
XCell SureLock Mini-Cell Electrophoresis System (Novex Life Technologies, cat. no. EI0001)
ÄKTA systems (GE Healthcare; e.g., ÄKTA Explorer; ÄKTA Micro)
Resource Q column, 1 mL (GE Healthcare, cat. no. 17-1177-01)
HiLoad 16/60 Superdex 75 prep grade column (GE Healthcare, cat. no. 17-1068-01)
Superdex 75 PC 3.2/30 column (GE Healthcare, cat. no. 17-0771-01)
Roller mixer (e.g., Stuart roller mixer SRT6)
Rotating wheel
Dry heat block (e.g., Grant QBD2)
Syringe filters (e.g., Elkay Laboratory Products, cat. no. E25-PV45-50S) and syringes
Timer
Cell culture hood (user specific)
Western blotting transfer unit (e.g., TE 77 PWR Semi-Dry Transfer Unit, GE Healthcare, cat. no. 11-0013-42)
X-ray films (e.g., Fujifilm, Super RX)
Reagent Setup
GST buffer 1 (50 mM Tris (pH 8-8.5), 200 mM NaCl and 5 mM DTT). For lysis and size exclusion chromatography. For 1 liter, dissolve 6.1 g Trizma base, 11.7 g NaCl and 0.77 g DTT in ddH2O, adjust pH with HCl to 8-8.5 and add ddH2O to 1 liter. Filter (0.2 µM) and degas all buffers before using ÄKTA systems. Prepare fresh and keep at 4 °C for up to 1 day.
GST buffer 2 (50 mM Tris (pH 8-8.5), 500 mM NaCl and 5 mM DTT). High salt GST buffer for anion exchange chromatography. For 1 liter, dissolve 6.1 g Trizma base, 29.2 g NaCl and 0.77 g DTT in ddH2O, adjust pH with HCl to 8-8.5 and add ddH2O to 1 liter. Filter (0.2 µM) and degas. Prepare fresh and keep at 4 °C for up to 1 day.
GST buffer 3 (50 mM Tris (pH 8-8.5), 50 mM NaCl and 5 mM DTT). Low salt GST buffer for anion exchange chromatography. For 1 liter, dissolve 6.1 g Trizma base, 2.9 g NaCl and 0.77 g DTT in ddH2O, adjust pH with HCl to 8-8.5 and add ddH2O to 1 liter. Filter (0.2 µM) and degas. Prepare fresh and keep at 4 °C for up to 1 day.
DUB dilution buffer (25 mM Tris (pH 7.5), 150 mM NaCl and 10 mM DTT). For 500 mL, dissolve 1.5 g Trizma base, 4.4 g NaCl and 0.77 g DTT in ddH2O, adjust pH with HCl to 7.5 and add ddH2O to 500 mL. Store this buffer in aliquots of 1 mL at -20 ºC for at least 1 year.
10x DUB reaction buffer (500 mM Tris (pH 7.5), 500 mM NaCl and 50 mM DTT). For 100 mL, dissolve 6.1 g Trizma base, 2.9 g NaCl and 0.77 g DTT in ddH2O, adjust pH with HCl to 7.5 and add ddH2O to 100 mL. This buffer can be stored in small aliquots of 0.5 mL at -20 ºC for at least 1 year.
20x Ubiquitin ligation buffer (800 mM Tris (pH 7.5), 200 mM MgCl2 and 12 mM DTT). For 100 mL, dissolve 9.7 g Trizma base, 1.2 g NaCl and 0.19 g DTT in ddH2O, adjust pH with HCl to 7.5 and add ddH2O to 100 mL. This buffer can be stored in small aliquots of 0.5 mL at -20 ºC for at least 1 year.
IP lysis buffer. For immunoprecipitation of in vivo substrates, prepare IP lysis buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, 2.5 mM EDTA, 1% (v/v) Triton X100, EDTA free Complete protease inhibitor, 100 mM NaF, 5 mM N-ethylmaleimide (NEM) and 10 mM iodoacetamide. This buffer should be freshly prepared.
IP wash buffer. For immunoprecipitation of in vivo substrates, prepare IP wash buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, 2.5 mM EDTA, 1% (v/v) Triton X100 and EDTA free Complete protease inhibitor. This buffer should be freshly prepared.
Procedure
Generation of substrates for UbiCRest TIMING 1-6 d
-
Substrates for UbiCRest analysis can be generated in a number of ways (see ‘Experimental design’ section). Here we provide details for in vitro generation of polyubiquitin (Step 1A) and immunoprecipitation of polyubiquitinated protein from cell lysate (Step 1B). In Step 1A, we provide details for generating the model substrates used in Figures 3a and 4b. In Step 1B, we use immunoprecipitation of the TNF receptor complex (TNF-RSC) as an example, the UbiCRest analysis of which is shown in Figure 3d.
CRITICAL STEP: Any appropriate in vitro assembly system can be used to generate substrates for UbiCRest analysis. Established assembly systems with known ubiquitin chain composition can serve as positive controls.
(A) Generation of in vitro substrates for UbiCRest analysis TIMING ~1 d
- To assemble Lys63-linked chains through autoubiquitination of GST-tagged NEDD4 (assembly A) or free Lys6/Lys48-branched and mixed polyubiquitin chains (assembly B), set up the reaction tabulated below and incubate for 1 h (assembly A) or 3 h (assembly B) at 37 ºC.
Component (stock concentration) Amount per assembly (µL) Final concentration Assembly A Assembly B Ubiquitin (5 mM) 2 100 50 µM / 2.5 mM E1 (10 µM) 2 2 100 nM UBE2L3 (450 µM) 1 1 2.3 µM GST-NEDD4 (100 µM) 20 — 10 µM NleL (60 µM) — 30 9 µM ATP (100 mM) 20 20 10 mM Ubiquitin ligation buffer (20x) 10 10 1x MilliQ water 145 37 Total 200 200 -
Stop the reaction by adding 2 mU apyrase. Check the quality of the assembled substrate on a 4-12% SDS-PAGE gradient gel.
TROUBLESHOOTING
PAUSE POINT Samples can be flash-frozen and stored at -80 °C for at least 1 year.
-
(Optional) If significant amounts of monoubiquitin remain in the assembly reaction (assembly A) or a specific length of polyubiquitin chains is required (assembly B), a subsequent purification of the substrate is recommended to enable clearer visualization of subsequent UbiCRest analysis. Subject the sample to size exclusion chromatography using GST buffer 1 (assembly A: Superdex 75 PC 3.2/30 on an ÄKTA Micro system; assembly B: HiLoad 16/60 Superdex 75 on an ÄKTA Explorer system (GE Healthcare)). Collect peak fractions and confirm the quality on a 4-12% SDS-PAGE gradient gel.
PAUSE POINT Samples can be flash-frozen and stored at -80 °C for at least 1 year.
(B) Immunoprecipitation of ubiquitinated substrates for UbiCRest analysis TIMING ~6 d
CRITICAL STEP This option describes how to generate and detect an in vivo ubiquitinated protein, using RIP1 as an example. Cell lines, cell culture techniques, stimulations, detection methods, affinity tags, antibodies etc. will vary for other proteins under investigation. The key aim is to obtain a sample that generates a detectable polyubiquitin smear in western blot analysis and that is compatible with DUB cleavage. Steps 1B(ix-xi) needs to be optimized for each different substrate in subsequent UbiCRest analysis.
Seed HEK 293T cells at a density of 5x106 cells/dish into five 15 cm dishes and culture in the presence of 20 mL cell culture medium per 15 cm dish until they reach confluence. This will take 2-3 days.
Replace with fresh medium.
-
Scrape cells off the 15 cm dishes and transfer to 50 mL Falcon tubes.
CRITICAL STEP Do not trypsinize the cells, as surface receptors would get destroyed.
-
Stimulate cells by adding FLAG-tagged TNF-α to a final concentration of 100 ng/mL for 10 min at 37 °C.
CRITICAL STEP Stimulate cells for an appropriate amount of time to observe optimal levels of substrate ubiquitination. In the given example, cells should not be stimulated for longer than 10 min for optimal RIP1 ubiquitination.
Centrifuge cells at 300 g for 2 min at 4 °C. Remove supernatant and wash the cells once with 2 mL of ice cold PBS.
Remove PBS, add IP lysis buffer and lyse cells on ice for 30 min.
-
Centrifuge lysates at 16,000 g for 30 min at 4 °C.
CRITICAL STEP Include protease/DUB inhibitors (complete protease inhibitors, EDTA-free (Roche), 5 mM N-ethylmaleimide (NEM), 10 mM iodoacetamide) in the lysis buffer to prevent cleavage of polyubiquitinated species by cellular DUBs present in the lysate.
To the clarified lysate, add 200 µL of anti-FLAG M2 affinity gel (40 µL per 15 cm dish) which was equilibrated by washing and centrifuging three times in IP lysis buffer and incubate on a rotating wheel at 4 °C overnight.
-
The following day, wash the beads five times. This is done by centrifugation of the beads at 1000 g for 3 min, removing supernatant and adding 1 mL IP wash buffer at 4 ºC. Resuspend beads in DUB dilution buffer in the final (fifth) step.
CRITICAL STEP The volume of the final sample in DUB dilution buffer depends both on how many DUB incubations need to be carried out and the concentration of the polyubiquitinated substrate present after the IP. If the substrate concentration is too low, the amount of starting cells can be increased. If the substrate concentration is too high, the sample can be further diluted using DUB dilution buffer. Whether the substrate concentration is appropriate can only be judged by performing the UbiCRest analysis and analyzing the reactions by western blot.
Aliquot beads into separate tubes for subsequent DUB incubations. For the analysis of polyubiquitinated RIP1 (Fig. 3d), 10 µL of beads were aliquoted per reaction.
-
Analyse the experiment by western blot using an antibody specific to the substrate protein under investigation; we used a RIP1-specific antibody (Fig. 3d).
TROUBLESHOOTING
UbiCRest analysis TIMING 5 – 18 h
-
2.
Prepare 2x and/or 5x concentrated DUB stocks (depending on reaction set-up, see step 3 below) on ice by diluting enzymes using DUB dilution buffer and pre-incubate at room temperature (22 ºC) for 10-15 min. Note, OTUD3, Cezanne, OTUD2, TRABID and OTUD1 are used at two different final enzyme concentrations (Table 1).
CRITICAL STEP Pre-incubation of enzymes with catalytic Cys residues in a high-DTT (here: DUB dilution buffer) buffer is often required to achieve full activity through reduction of oxidized species.
-
3.
Depending on the substrate and aim of the experiment, set up the UbiCRest reaction as follows: to determine ubiquitin chain composition of in vitro assembled polyubiquitin (from Step 1A), set up reaction A (Fig. 3a); to determine ubiquitin chain composition of immunoprecipitated polyubiquitinated protein (from Step 1B), set up reaction B (Fig. 3d); and to analyze ubiquitin chain architecture of in vitro assembled heterotypic polyubiquitin (from Step 1A), set up reaction C (Fig. 4b). For each UbiCRest experiment, the number and type of DUBs can be attuned to the given scientific question.
CRITICAL STEP Reaction volumes depend on the experimental set-up and number of time points. Amount of input substrate depends on the minimal amount from which the polyubiquitin content can be faithfully and conveniently visualized.Component Amount per reaction Final Reaction A Reaction B Reaction C In vitro ubiquitinated substrate (from Step 1A) 4 µL — — * Immunoprecipitated substrate (from Step 1B) — 10 µL — * Heterotypic substrate (from Step 1A) — — 2-4 µg 2-4 µg 2x or 5x DUB (from Step 2) 5 µL of 2x 5 µL of 5x 20 µL of 2x 1x 10x DUB reaction buffer 1 µL 2.5 µL 4 µL 1x MilliQ water — 7.5 µL To 40 µL FINAL 10 µL 25 µL 40 µL *Note: Actual concentration of these substrates is not known. The used amount is what is needed for clear visualization. -
4.
Place samples on dry heat block at 37 °C. For reactions A and B stop the reaction after 15-30 min by adding 5-10 µL 4x LDS sample buffer. For reaction C, take a 5 µL sample for each time point and mix with 5 µL 4x LDS sample buffer. Typical time course: 0, 5, 30, 60, 90 min.
PAUSE POINT Once samples are mixed with LDS sample buffer they can be stored at -20 °C for up to 1 year and analysed by SDS-PAGE at a later time.
-
5.
Load 1-10 µL of each sample on SDS-PAGE gels and visualize protein by silver staining or western blotting using standard methods.
CRITICAL STEP Do not boil samples containing ubiquitin chains as this may cause degradation and smearing of bands on SDS-PAGE gels.
CRITICAL STEP Use of MES versus MOPS SDS-PAGE running buffer determines how well monoubiquitin and short ubiquitin chains can be visualized as compared to polyubiquitinated proteins. Fig. 3a was performed using MES running buffer, while in Fig. 3d, MOPS buffer was used.
TROUBLESHOOTING
-
6.
If required, UbiCRest analysis (Steps 2-5) can be repeated using different enzyme and/or substrate concentrations. Additionally, the time course can be changed e.g. including more points for analysis.
TROUBLESHOOTING
Troubleshooting advice can be found in Table 3.
Table 3. Troubleshooting.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 1A(ii) | No polyubiquitinated product or free polyubiquitin chains after in vitro assembly | Old stock of E1, E2, E3 enzymes | Express and purify new enzyme stocks (or purchase commercially) |
| Incubation time too short | Perform a longer time course | ||
| Ubiquitin input concentration too low | Adjust the amount of monoubiquitin present in the assembly reaction | ||
| 1B(xi) | Little or no immunoprecipitated ubiquitinated substrate detected | Not enough cells used for pull-down | Increase number of dishes |
| Inefficient pull-down | Optimize pull-down conditions with fresh antibody/affinity gel | ||
| 5 | No ubiquitin chain hydrolysis detected on SDS-PAGE gel | Old stock of DUB | Express and purify new stocks of DUB |
| Enzyme concentration too low | Optimize enzyme concentration; can try several in parallel | ||
| Incubation time too short | Increase incubation time, perform a longer initial time course | ||
| Substrate concentration too high | Dilute substrate and repeat, can try several concentrations in parallel | ||
| Box 1, step16 | No DUB can be detected on SDS-PAGE gels | Poor protein expression | Use fresh aliquot of competent bacterial cells, optimize expression conditions (IPTG concentration, induction times and growth temperature). |
| No protein present in sample after elution from beads | Old aliquots of Prescission protease | Replace PreScission protease batch with a fresh one | |
| Inefficient cleavage | Optimize cleavage conditions (incubation time and buffer composition) | ||
| Eluted protein is not pure | Insufficient washing of beads | Wash with 2 L of GST buffer 2 and GST buffer 1 | |
| Used too much Glutathione Sepharose 4B resin | Optimize the amount of resin used | ||
| Box 2, step 5 | No ubiquitin chain hydrolysis detected on SDS-PAGE gel | Old stock of DUB | Express and purify new stocks of DUB |
| Enzyme concentration too low | Optimize enzyme concentration; can try several in parallel | ||
| Incubation time too short | Increase incubation time, perform a longer initial time course |
TIMING
Step 1A, generation of in vitro substrates for UbiCRest analysis: ~1 d
Step 1B, immunoprecipitation of ubiquitinated substrates for UbiCRest analysis: ~6 d
Steps 2-6, UbiCRest analysis: 5-18h (depends on the method of visualization: silver staining versus blotting)
Box 1, expression and purification of DUBs: 4-14 d (Depends on how many DUBs are required. Multiple DUBs can be purified simultaneously.)
Box 2, qualitative in vitro DUB assay: ~4 h
Anticipated Results
Interpreting the results
Ubiquitin chain restriction analysis can reveal a lot of information, depending on the subsequent detection of the reaction products. For example, in Figure 3a, autoubiquitinated NEDD4 was purified and treated, in solution, with the indicated DUBs. The resulting reaction mixture was then directly loaded onto an SDS-PAGE 4-12% (wt/vol) gradient gel and bands were visualized by silver staining. The disappearance of the polyubiquitin smear is the key result as this indicates that the given DUB has worked on the substrate, and that the linkage in question is present (Fig. 3a). It is equally interesting to visualize the small molecular weight reaction products, and see whether monoubiquitin has been generated exclusively. This would provide evidence for chains of one chain type exclusively, or it would indicate whether short chains have been released (Fig. 3a and 4b). The latter could indicate mixed or branched heterotypic polyubiquitination (see below, Fig. 4). The distinct running behavior of differentially linked free ubiquitin chains often allow an identification of released small chains (Fig. 3b,c). In some cases, OTU DUBs are unable to remove the last, proximal ubiquitin of a chain, which remains on the substrate, leading to defined bands with a molecular weight higher than the unmodified substrate. This shift of the substrate can indicate how many ubiquitination sites are present on particular proteins, and targeted MS/MS analysis of such bands may allow identification of the modified site(s).
In Figure 3d, a variation of UbiCRest is shown, in which an endogenously modified protein, RIP1, is purified by TNF-receptor immunoprecipitation, and subjected to UbiCRest. The purified TNF-RSC consists of a large number of proteins many of which are ubiquitinated; this renders silver staining or detection by anti-ubiquitin less useful. Fortunately, the available antibody for RIP1 reveals modified forms of the protein (Fig. 3d). High concentration of OTUD1 reduces the smear as compared to the control, suggesting presence of Lys63-linked chains. OTUD2 also reduces the smear, however, this enzyme often releases intact chains, which cannot be visualized by western blotting against RIP1. Further examples of UbiCRest can be found in the literature5,27,35,36.
Characterization of mixed linkage chains
Branched ubiquitin chains represent a new frontier in ubiquitin research, and their abundance, function and relevance is currently unclear. We had shown that the bacterial effector E3 ligase NleL31 assembles free ubiquitin chains with Lys6 and Lys48 linkages, giving rise to large number of individual species37 (Fig. 4a). We used UbiCRest to show that both linkages can occur in the same polymer (Fig. 4b). For this, pentaubiquitin and hexaubiquitin were assembled using NleL and wild-type ubiquitin. The purified heterotypic sample was incubated with DUBs in four parallel reactions; OTUB1 (Lys48-specific), OTUD3 (prefers Lys6 over Lys48 chains), a mixture of OTUB1 and OTUD3, and vOTU (cleaves all isopeptide linkages). Samples were taken over a 90 min time course and analyzed on a silver-stained SDS-PAGE gel. The complete hydrolysis of purified penta- and hexaubiquitin to monoubiquitin by vOTU or the combination of OTUD3 and OTUB1 indicates that these enzymes can cleave all linkages present in these heterotypic ubiquitin chains (Fig. 4b). When the sample was treated with OTUB1 alone, the penta- and hexaubiquitin chains were hydrolyzed partially leaving behind a mixture of mono-, di-, tri- and tetraubiquitin. Similarly, OTUD3 incubation resulted in a distinct pattern of bands, with predominantly mono-, di- and a weak signal for triubiquitin (Fig. 4b). The resulting ubiquitin chains after OTUB1 and OTUD3 treatment represented different linkages as they exhibited distinct electrophoretic mobilities when analyzed on SDS-PAGE gels (compare Fig. 3b,c). These ubiquitin chains are Lys6-linked (after OTUB1 treatment) and Lys48-linked (after OTUD3 treatment). Therefore, NleL-assembled unanchored polyubiquitin chains comprise both Lys6 and Lys48 linkages in the same polymer as shown by UbiCRest analysis. Lys6 linkages seem to predominate over Lys48 linkages and are found in direct succession in longer stretches more often. Most ubiquitin polymers however contain at least one Lys48 linkage given that intermediate building blocks of the ubiquitin chain remain after OTUD3 treatment31. A similar analysis was recently performed for IL1β-induced ubiquitination of signaling proteins, which are modified by heterotypic Lys63- and Met1-linked chains in cells5. UbiCRest is hence a first method to evaluate whether heterotypic ubiquitin chains are present in ubiquitin polymers, and should help to understand their functional properties.
Acknowledgements
We would like to thank present and past members of the DK lab for reagents and critical comments on the manuscript. This work was supported by the Medical Research Council [U105192732], the European Research Council [309756], the Lister Institute for Preventive Medicine, the EMBO Young Investigator Program (all DK) and the Marie Curie Initial Training Network “UPStream” (to TETM).
Footnotes
Author Contributions
MKH, TETM and DK designed the method and wrote the manuscript.
Competing Financial Interests
DK is part of the DUB Alliance that includes Cancer Research Technology and FORMA Therapeutics, and is a consultant for FORMA Therapeutics. A patent application has been filed for the described method. Boston Biochem / Biotechne distribute an enzyme kit for performing UbiCRest analysis.
References
- 1.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 2.Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 3.Kulathu Y, Komander D. Atypical ubiquitylation - the unexplored world of polyubiquitin beyond Lys48 and Lys63 linkages. Nat Rev Mol Cell Biol. 2012;13:508–523. doi: 10.1038/nrm3394. [DOI] [PubMed] [Google Scholar]
- 4.Kim HT, Kim KP, Uchiki T, Gygi SP, Goldberg AL. S5a promotes protein degradation by blocking synthesis of nondegradable forked ubiquitin chains. EMBO J. 2009;28:1867–1877. doi: 10.1038/emboj.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emmerich CH, et al. Activation of the canonical IKK complex by K63/M1-linked hybrid ubiquitin chains. Proceedings of the National Academy of Sciences. 2013;110:15247–15252. doi: 10.1073/pnas.1314715110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer H-J, Rape M. Enhanced protein degradation by branched ubiquitin chains. Cell. 2014;157:910–921. doi: 10.1016/j.cell.2014.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volk S, Wang M, Pickart CM. Chemical and genetic strategies for manipulating polyubiquitin chain structure. Meth Enzymol. 2005;399:3–20. doi: 10.1016/S0076-6879(05)99001-0. [DOI] [PubMed] [Google Scholar]
- 8.Xu M, Skaug B, Zeng W, Chen ZJ. A Ubiquitin Replacement Strategy in Human Cells Reveals Distinct Mechanisms of IKK Activation by TNFα and IL-1&beta. Mol Cell. 2009;36:302–314. doi: 10.1016/j.molcel.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ye Y, et al. Ubiquitin chain conformation regulates recognition and activity of interacting proteins. Nature. 2012;492:266–270. doi: 10.1038/nature11722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wickliffe KE, Lorenz S, Wemmer DE, Kuriyan J, Rape M. The mechanism of linkage-specific ubiquitin chain elongation by a single-subunit E2. Cell. 2011;144:769–781. doi: 10.1016/j.cell.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaefer JB, Morgan DO. Protein-linked ubiquitin chain structure restricts activity of deubiquitinating enzymes. J Biol Chem. 2011;286:45186–45196. doi: 10.1074/jbc.M111.310094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton K, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678. doi: 10.1016/j.cell.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto ML, et al. Engineering and structural characterization of a linear polyubiquitin-specific antibody. J Mol Biol. 2012;418:134–144. doi: 10.1016/j.jmb.2011.12.053. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto ML, et al. K11-linked polyubiquitination in cell cycle control revealed by a K11 linkage-specific antibody. Mol Cell. 2010;39:477–484. doi: 10.1016/j.molcel.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 15.van Wijk SJL, et al. Fluorescence-based sensors to monitor localization and functions of linear and K63-linked ubiquitin chains in cells. Mol Cell. 2012;47:797–809. doi: 10.1016/j.molcel.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkpatrick DS, et al. Quantitative analysis of in vitro ubiquitinated cyclin B1 reveals complex chain topology. Nature Cell Biology. 2006;8:700–710. doi: 10.1038/ncb1436. [DOI] [PubMed] [Google Scholar]
- 17.Kim W, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell. 2011;44:325–340. doi: 10.1016/j.molcel.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu P, et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–145. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wagner SA, et al. A proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Mol Cell Proteomics. 2011;10:M111.013284. doi: 10.1074/mcp.M111.013284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng J, et al. A proteomics approach to understanding protein ubiquitination. Nat Biotechnol. 2003;21:921–926. doi: 10.1038/nbt849. [DOI] [PubMed] [Google Scholar]
- 21.Dammer E, Peng J. At the crossroads of ubiquitin signaling and mass spectrometry. Expert Rev Proteomics. 2010;7:643–645. doi: 10.1586/epr.10.90. [DOI] [PubMed] [Google Scholar]
- 22.Xu P, Peng J. Characterization of polyubiquitin chain structure by middle-down mass spectrometry. Anal Chem. 2008;80:3438–3444. doi: 10.1021/ac800016w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komander D, et al. Molecular discrimination of structurally equivalent Lys 63-linked and linear polyubiquitin chains. EMBO Rep. 2009;10:466–473. doi: 10.1038/embor.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato Y, et al. Structural basis for specific cleavage of Lys 63-linked polyubiquitin chains. Nature. 2008;455:358–362. doi: 10.1038/nature07254. [DOI] [PubMed] [Google Scholar]
- 25.Wiener R, Zhang X, Wang T, Wolberger C. The mechanism of OTUB1-mediated inhibition of ubiquitination. Nature. 2012;483:618–622. doi: 10.1038/nature10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juang Y-C, et al. OTUB1 Co-opts Lys48-Linked Ubiquitin Recognition to Suppress E2 Enzyme Function. Mol Cell. 2012;45:384–397. doi: 10.1016/j.molcel.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mevissen TET, et al. OTU Deubiquitinases Reveal Mechanisms of Linkage Specificity and Enable Ubiquitin Chain Restriction Analysis. Cell. 2013;154:169–184. doi: 10.1016/j.cell.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye Y, et al. Polyubiquitin binding and cross-reactivity in the USP domain deubiquitinase USP21. EMBO Rep. 2011;12:350–357. doi: 10.1038/embor.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiener R, et al. E2 ubiquitin-conjugating enzymes regulate the deubiquitinating activity of OTUB1. Nat Struct Mol Biol. 2013;20:1033–1039. doi: 10.1038/nsmb.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faesen AC, et al. The differential modulation of USP activity by internal regulatory domains, interactors and eight ubiquitin chain types. Chem Biol. 2011;18:1550–1561. doi: 10.1016/j.chembiol.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 31.Hospenthal MK, Freund SMV, Komander D. Assembly, analysis and architecture of atypical ubiquitin chains. Nat Struct Mol Biol. 2013;20:555–565. doi: 10.1038/nsmb.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keusekotten K, et al. OTULIN antagonizes LUBAC signaling by specifically hydrolyzing Met1-linked polyubiquitin. Cell. 2013;153:1312–1326. doi: 10.1016/j.cell.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakasone MA, Livnat-Levanon N, Glickman MH, Cohen RE, Fushman D. Mixed-linkage ubiquitin chains send mixed messages. Structure. 2013;21:727–740. doi: 10.1016/j.str.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulathu Y, et al. Regulation of A20 and other OTU deubiquitinases by reversible oxidation. Nature Communications. 2013;4:1569. doi: 10.1038/ncomms2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiil BK, et al. OTULIN Restricts Met1-Linked Ubiquitination to Control Innate Immune Signaling. Mol Cell. 2013;50:818–830. doi: 10.1016/j.molcel.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Birsa N, et al. Lysine 27 ubiquitination of the mitochondrial transport protein Miro is dependent on serine 65 of the Parkin ubiquitin ligase. J Biol Chem. 2014;289:14569–14582. doi: 10.1074/jbc.M114.563031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin DY-W, Diao J, Zhou D, Chen J. Biochemical and Structural Studies of a HECT-like Ubiquitin Ligase from Escherichia coli O157:H7. J Biol Chem. 2011;286:441–449. doi: 10.1074/jbc.M110.167643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akutsu M, Ye Y, Virdee S, Chin JW, Komander D. Molecular basis for ubiquitin and ISG15 cross-reactivity in viral ovarian tumor domains. Proc Natl Acad Sci USA. 2011;108:2228–2233. doi: 10.1073/pnas.1015287108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bremm A, Freund SMV, Komander D. Lys11-linked ubiquitin chains adopt compact conformations and are preferentially hydrolyzed by the deubiquitinase Cezanne. Nat Struct Mol Biol. 2010;17:939–947. doi: 10.1038/nsmb.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Licchesi JDF, et al. An ankyrin-repeat ubiquitin-binding domain determines TRABID's specificity for atypical ubiquitin chains. Nat Struct Mol Biol. 2012;19:62–71. doi: 10.1038/nsmb.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang T, et al. Evidence for bidentate substrate binding as the basis for the K48 linkage specificity of otubain 1. J Mol Biol. 2009;386:1011–1023. doi: 10.1016/j.jmb.2008.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]