Abstract
Purpose of review
The objective of this article is to examine the safety of prostate biopsy and discuss the emerging role of MRI - ultrasound fusion technology in improving diagnostic accuracy.
Recent findings
Men undergoing prostate biopsy frequently experience minor complications, including hematospermia, hematuria, and infection. Quinolone-resistant bacteria are a growing concern; thus transperineal access or modification of antibiotic prophylaxis based on local antibiograms is now used to avoid infectious complications.
Multiparametric MRI allows visualization of many prostate cancers, and by fusing MRI with realtime ultrasound, a biopsy needle can be directed by a urologist into suspicious regions of interest. Using this new method, detection of clinically significant prostate cancer (csCaP) has increased and the incidence of falsely negative biopsies has decreased.
Summary
Prostate biopsy is generally a safe procedure, and with attention to local patterns of antibiotic resistance, infectious complications can be minimized. MRI - ultrasound fusion has significantly improved the accuracy of prostate biopsy, allowing tracking and targeting not previously possible.
Keywords: MRI - ultrasound fusion, clinically significant prostate cancer (csCaP), prostate biopsy
Introduction
Prostate cancer diagnosis is based on histologic examination of tissue routinely obtained via needle biopsy. (1) An estimated one million prostate biopsies are performed annually in the United States. (2) While typically considered a safe procedure, prostate biopsy infrequently results in severe complications. (3) In recent years, the incidence of infectious complications has increased significantly, which reflects the high prevalence of quinolone-resistant strains of Escherichia coli. (2–4) Identifying high-risk men prior to biopsy, and subsequently tailoring the selection of antibiotic prophylaxis has been shown to decrease the rate of infectious complications. (3,5)
Transrectal ultrasound (TRUS) - guided prostate biopsy has for three decades been the standard for obtaining tissue for histological diagnosis, but several limitations of this method are now apparent. (6,7) These limitations include frequent diagnosis of clinically insignificant prostate cancer and imprecise sampling, which misclassifies up to 50% of cases compared to radical prostatectomy specimen, and high-grade tumors missed in as many as 30% of cases. (6–8)
Over the past 6-8 years, multiparametric Magnetic Resonance Imaging (mpMRI) has gained widespread use to detect prostate cancer and guide prostate biopsy. (9) This new method provides a major improvement in detection of clinically significant prostate cancer (csCaP), and decreased detection of clinically insignificant prostate cancer. (7,10–16) The objective of the present review is to explore the safety of prostate biopsy, examine current methods for prevention of complications, and discuss the role of MRI – guided prostate biopsy in improving diagnostic accuracy.
Discussion
Safety of prostate biopsy
Prostate biopsy is generally safe with few major, but frequent minor complications. (17,18) Common complications include hematuria, infection, rectal bleeding, and hematospermia. (17,18) In a European study of more than 7,000 prostate biopsies, the most frequent complication was hematospermia, with an incidence of 53.8%. (19) Hematuria (24.3%), significant pain (4.8%), fever (4.1%), and hospital admission (0.7%) were less frequent. (19)
In recent years, there has been a significant increase in the number of infectious complications requiring hospital admission after prostate biopsy. (2) The significant increase in incidence of infectious complications has been attributed to an emergence of quinolone-resistant bacteria. (20) Rates of E. coli resistance to quinolones have been reported to be 12%, thus necessitating appropriate antibiotic selection in men undergoing prostate biopsy, especially in those at higher risk for infection. (2,21)
Use of antibiograms to select antibiotic prophylaxis
Local antibiograms should be considered when selecting the appropriate prophylaxis because of the prevalent regional variation seen with antibiotic resistance profiles. (22) In our experience at the University of California at Los Angeles (UCLA), men with no significant risk factors for infection receive antibiotic prophylaxis with ciprofloxacin and ceftriaxone. Our current practice is based on the American Urological Association (AUA) best practice policy statement, which recommends fluoroquinolone prophylaxis in men undergoing prostate biopsy. (23) In men identified as high-risk (e.g. immunosuppression, recent antibiotic exposure, diabetes mellitus, or hospitalization), we administer both ciprofloxacin and ertapenem as antibiotic prophylaxis.
The antibiogram at UCLA reports bacteria susceptibilities from urine isolates within the local patient population. The five most common urine isolates are shown in the 2015 antibiogram (Table 1). E.coli, which is only 78% susceptible to ciprofloxacin, is 93% susceptible to ceftriaxone and 99% susceptible to ertapenem. Following the dictates of the most current antibiogram allows us to modify antibiotic prophylaxis for high-risk patients on a contemporaneous basis.
Table 1.
Ronald Regan UCLA Hospital Outpatient Antibiogram: Adults (>21 y.o.) Gram-negative Bacteria – Urine Isolates, % Susceptible
| Organism | No. Isolates | Ampicillin | Cefazolin | Cefepime | Ceftriaxone | Ertapenem | Imipenem | Meropenem | Gentamicin | Ciprofloxacin | Nitrofurantoin | Trimethoprim-sulfamethoxazole |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Enterobacter cloacae | 75 | R | R | 96 | - | 89 | 97 | 99 | 97 | 92 | 12 | 85 |
| Escherichia coli | 5379 | 53 | 89 | 96 | 93 | 99 | 99 | 99 | 90 | 78 | 93 | 78 |
| Klebsiella pneumonia | 523 | R | 91 | 98 | 93 | 99 | 99 | 99 | 97 | 95 | 29 | 86 |
| Proteus mirabilis | 253 | 81 | 94 | 99 | 96 | 98 | 10 | 99 | 92 | 80 | R | 79 |
| Pseudomonas aeruginosa | 118 | R | R | 88 | R | R | 77 | 85 | 96 | 72 | R | R |
Antibiotic prophylaxis for prostate biopsy
Liss et al. recently described an increased risk of infection (OR 3.98, p<0.001) and hospital admission (OR 4.77, p<0.001) after transrectal biopsy, in men with fluoroquinolone-resistant positive rectal culture. (24) Targeted antibiotic prophylaxis, based on rectal swab culture results, has been shown to decrease the rate of infection after biopsy. (5) Taylor et al. reported their experience with 22 men found to have positive rectal swab cultures prior to biopsy. (25) All men received targeted antibiotic prophylaxis; there were no infectious complications after biopsy. (25) At UCLA, we augment antibiotic prophylaxis in every patient considered high-risk, based on the above guidelines, thus allowing us to avoid the extra measure of rectal swab cultures.
Transperineal biopsy
Transperineal biopsy is an alternative to the transrectal route, especially because of fewer infections. (26,27) In a recent systematic review of 165 articles on the subject, transrectal biopsy was associated with higher rates of hospitalization (1.1% vs. 0.9%) and sepsis (0.8% vs. 0.1%) compared to transperineal biopsy, while urinary retention was more common using the transperineal approach (4.2% vs 0.9%). (28) Transperineal biopsies are poorly tolerated in a clinic setting, thus general anesthesia is customarily used, which limits the widespread use of this approach.
Repeat biopsies
Bokhorst et al. recently evaluated the risk of complications from serial prostate biopsies in men on active surveillance. (29) The number of previous biopsies did not significantly predict the risk of infection (OR 1.04). (29) The type of antibiotic prophylaxis used was the only significant predictor of infection after biopsy. (29)
Transrectal ultrasound – guided prostate biopsy
Transrectal ultrasound (TRUS) – guided prostate biopsy has for more than three decades been the method for diagnosing prostate cancer. (6) Hodge et al. originally compared the use of TRUS - guided biopsy in a directed versus random systematic manner, ultimately leading to the widespread use of this technique. (30) Several limitations of TRUS - guided biopsy have since been described, including underestimation of tumor grade, inadequate sampling of tumor (e.g. anterior zone), and over-detection of low-risk prostate cancer. (6,7,31)
When whole organs are studied, Gleason score upgrading beyond biopsy findings is common. (8,32) Cohen et al. evaluated radical prostatectomy specimens of 2,890 men diagnosed with prostate cancer via TRUS-guided biopsy. (33) Of those men, 36% of radical prostatectomy specimens revealed a higher grade than previously diagnosed on initial biopsy. (33)
Use of MRI to detect prostate cancer
Since the 1980's, magnetic resonance imaging (MRI) has been used to image the prostate gland. (34) Advances in technology have led to the development of multiparametric MRI (mpMRI), thus allowing enhanced detection of clinically significant prostate cancer (csCaP). (34,35) Multiparametric MRI combines anatomic T2-weighted images with functional and physiological assessments, including diffusion weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MRI. (34,36,37)
In 2012, the Prostate Imaging – Reporting and Data System (PI-RADS) was introduced to standardize the reporting of mpMRI results. (37) PI-RADS utilizes a five-grade scoring system based on the likelihood that mpMRI findings correlate with csCaP within the prostate. (34,37) Grade 1 lesions are “highly unlikely” to contain csCaP, whereas grade 5 lesions are considered “highly likely” to represent csCaP. (34,37)
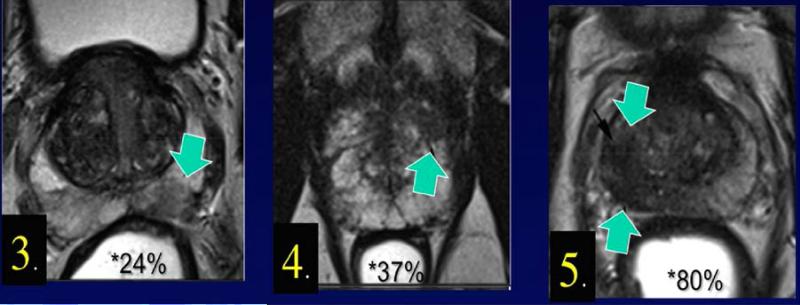
Using a similar scoring system, the likelihood of detecting csCaP in 825 men with grade ≥ 3 regions of interest (ROIs) at UCLA is shown in Figure 1. (10) Men with grade 3 ROIs on mpMRI carry a 24% chance of csCaP on prostate biopsy, compared to an 80% chance of csCaP in those men with grade 5 ROIs. (10)
Figure 1.
Percent likelihood of clinically significant prostate cancer (csCaP) based on region of interest (ROI) grade, (N=825). (10) Region of interest grade is shown at lower left corner of each section. Chance of csCaP is directly related to ROI grade.
Recently, Cash et al. reported cancer detection rates (CDRs) and their correlations with PI-RADS score in 408 patients undergoing prostate biopsy. (38) Men with PI-RADS grade 3, 4, and 5 lesions on mpMRI were reported as having clinically significant CDRs of 66% (19/29), 74% (70/94), and 95% (94/99), respectively. (38) Comparable results were reported by Radtke et al., with a positive predictive value of 68% and 81% for PI-RADS 4 and 5 lesions to harbor csCaP, respectively. (39)
The accuracy of PI-RADS version 1.0 has been validated in a meta-analysis with a combined area under the curve (AUC) of 0.82 in receiver operating characteristics curve analysis. (40) However, a learning curve for the PI-RADS scoring system occurs among radiologists for both PI-RADS version 1.0 and the recently updated version 2.0. (41,42) In addition, PI-RADS grading is not as accurate in the transition zone as it is in the peripheral zone. (42)
Applying targeted biopsy cores in MRI suspicious lesions can be prone to a variety of targeting errors. In a cohort of 120 men who underwent radical prostatectomy after MRI – ultrasound fusion biopsy, 8% of the targeted biopsies missed the MRI suspicious lesion. (14) Using rigid co-registration, Hadaschik and Simpfendörfer described a mean targeting error of 2-3mm. (13,43)
MRI – guided prostate biopsy
Suspicious lesions identified on mpMRI can be targeted during fusion biopsy. (44) Three methods of MRI targeted biopsy exist: cognitive fusion, in-bore, and MRI - ultrasound fusion. (44) Cognitive fusion biopsy requires the operator to infer the location of suspicious lesions on MRI using TRUS images. (45) In-bore biopsy is usually performed by a radiologist and involves targeting a suspicious lesion within the MRI tube. (46) Landmarks and targets for biopsy are defined using the preceding diagnostic mpMRI. (47) MRI - ultrasound fusion biopsy utilizes registration or fusion software to identify and target a lesion on MRI during TRUS - guided biopsy. (46)
Increased detection of significant prostate cancer
Use of MRI – ultrasound fusion to guide biopsy results in increased detection of clinically significant prostate cancer (csCaP). (7,15,48,49) In the largest series reported to date, Filson et al. recently evaluated the combined use of targeted and systematic biopsy in detecting csCaP in 1042 men. (10) Combining both techniques diagnosed more csCaP (n=289) than targeted biopsy (n=229) or systematic biopsy (n=199) alone. (10) ROI grade was the strongest predictor of csCaP on fusion biopsy. (10) When compared to men with a grade 3 ROI, those with a grade 5 ROI were found to have 9 times the odds of csCaP (OR, 9.05). (10) Table 2 shows the likelihood of cancer detection in biopsy naïve patients, depending on degree of suspicion on mpMRI. (10)
Table 2.
Risk of prostate cancer on fusion biopsy based on mpMRI findings in biopsy naïve patients. (10)
| Systematic Plus Targeted | ||||
|---|---|---|---|---|
| Maximum ROI grade | Negative | GS 3+3=6 | GS 3+4=7 | GS ≥ 4+3=7 |
| No lesion/grade 1-2 | 38 (68%) | 11 (20%) | 4 (7%) | 3 (5%) |
| Grade 3 | 60 (46%) | 27 (21%) | 32 (25%) | 10 (8%) |
| Grade 4 | 36 (33%) | 28 (26%) | 21 (19%) | 24 (22%) |
| Grade 5 | 1 (3%) | 5 (14%) | 8 (23%) | 21 (60%) |
Sonn et al. investigated the ability of MRI - ultrasound fusion biopsy to detect prostate cancer in 105 men with prior negative biopsy and persistently elevated prostate-specific antigen (PSA). (11) Targeted and systematic biopsies identified prostate cancer in 36 men (34%) altogether. (11) Using Gleason 3+4 or maximal core length (MCL) ≥ 4mm as the definition for clinical significance, 21 of the 23 men (91%) diagnosed with cancer on targeted biopsy had csCaP. (11) By contrast, 15 of the 28 men (54%) had csCaP on conventional systematic biopsy. (11) The combination provided greater sensitivity in detection of csCaP than either alone.
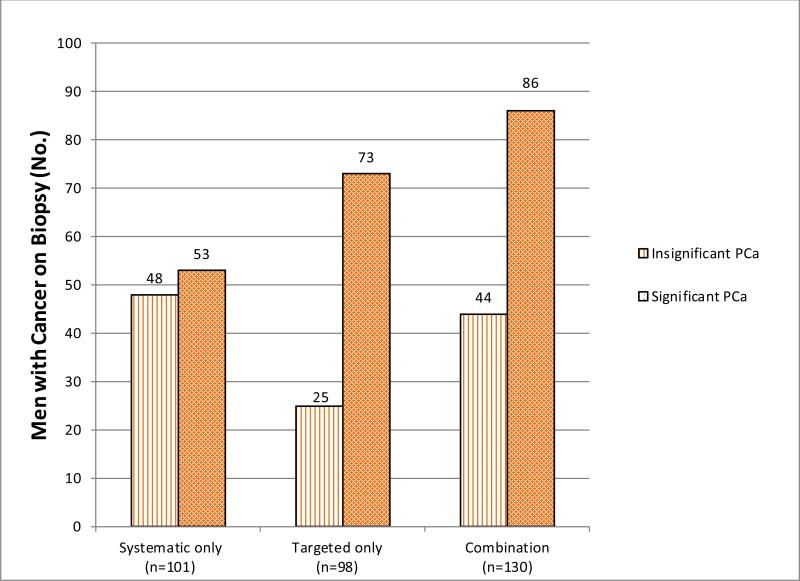
Figure 2 graphically displays current UCLA targeted and systematic biopsy data in 320 men with a prior negative biopsy and persistently elevated PSA undergoing initial MRI - ultrasound fusion biopsy. Targeted biopsy diagnosed csCaP in 73 men, compared to only 53 men diagnosed with csCaP using systematic biopsy alone. Combining the two methods diagnosed csCaP in 86 men.
Figure 2.
This graph shows the number of subjects with a prior negative biopsy and persistently elevated PSA diagnosed with significant and insignificant cancers depending on biopsy method. The combination of systematic and targeted biopsy results in detection of more csCaP than either alone.
Siddiqui et al. recently compared the diagnostic accuracy of conventional TRUS - guided biopsy, with MRI - ultrasound fusion prostate biopsy. (7) In their study, targeted biopsy diagnosed 30% more high-risk prostate cancers (173 vs. 122 cases, p<.001) and 17% fewer low-risk prostate cancers (213 vs. 258 cases, p=. 002). (7) Thus, use of targeted biopsy clearly yields an improved detection of clinically significant prostate cancer when compared to conventional systematic biopsy. (7,11)
Screening for active surveillance
Active surveillance is a favorable management strategy for many men with low-risk prostate cancer. (50) In 1994, the Epstein histological criteria were introduced to define clinically insignificant prostate cancer and determine eligibility for active surveillance. (51) Based on their results, clinically insignificant prostate cancer had no Gleason 4 disease, no more than 2 cores involved, and no core with >50% involvement. (51,52) The Epstein histological criteria were determined using biopsy specimens obtained on conventional TRUS biopsy. (51)
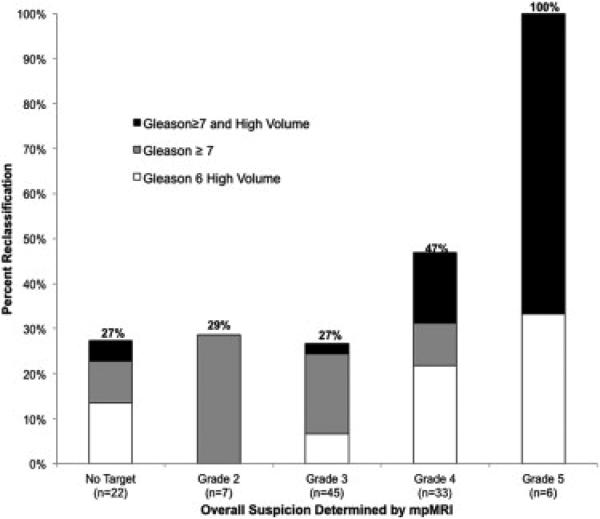
Hu et al. recently applied MRI – guided biopsy to men fulfilling the Epstein criteria on conventional biopsy. (52) In this study, 113 men enrolled in the UCLA active surveillance program who met Epstein criteria at initial diagnosis, subsequently underwent confirmatory biopsy using MRI - ultrasound fusion. (52) Targeted biopsy resulted in reclassification in 41 men (36%) beyond the Epstein criteria. (52) On further analysis of reclassified men, 26 (23%) were reclassified due to Gleason grade ≥ 7 and 15 (13.3%) were reclassified due to higher volume Gleason 6 disease. (52) Men with mpMRI ROI grade 4 or 5 were reclassified more often than those men with mpMRI ROI grade 2 or 3 (OR 3.2, p=0.006). (52) Figure 3 details these results graphically. (52)
Figure 3.
Effect of MRI grade on reclassification beyond Epstein criteria using mpMRI-US biopsy. (52) The higher the suspicion grade (UCLA scoring system), the greater the chance of reclassification beyond traditional Epstein criteria.
Using the Prostate Cancer Research International Active Surveillance (PRIAS) protocol, data from the Heidelberg group report similar results. (53) Men whose active surveillance was based on an initial TRUS – guided biopsy had a significantly higher probability of upgrading due to pathological progression on MRI – ultrasound fusion confirmatory biopsy, compared to men whose active surveillance was based on initial MRI – ultrasound fusion biopsy. (53) Targeting suspicious lesions on mpMRI may decrease sampling error seen with conventional biopsy, thus allowing accurate detection of clinically insignificant prostate cancer in men on active surveillance. (52)
Tracking of tumors
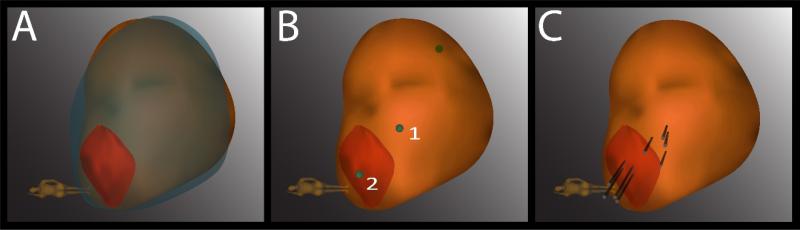
Sonn et al. investigated the use of an MRI - ultrasound fusion device (Artemis) to electronically monitor specific sites of prostate cancer in men on active surveillance. (54) In this study, 53 men enrolled in an active surveillance program underwent an initial MRI - ultrasound fusion biopsy, followed by a repeat tracking biopsy of prostate cancer specific sites. (54) On initial biopsy, all positive biopsy sites were mapped and tracked to enable specific resampling on subsequent biopsy. (54) Figure 4 illustrates the method of resampling prior positive sites using the MRI - ultrasound fusion device.
Figure 4.
Example of resampling of prior positive biopsy sites using the MRI - ultrasound fusion (Artemis) device. (A) A 3-dimensional model of the prostate from a second biopsy (brown) is superimposed on the model from a first biopsy (blue), revealing a close match in shape and size. The superimposed model is created in real time at second biopsy. An MRI target (red) is displayed in the model. (B) The location of prior positive sites (1 and 2) is mapped by the Artemis device. Site 1 is a systematic site and site 2 is from the MRI-targeted core. (C) A total of 4 cores (black cylinders) are taken from each site.
Repeat tracking biopsy revealed prostate cancer in 29 sites (39%), of which 14 (49%) had csCaP. (54) Cancer detection rate was directly related to cancer core length (CCL) and presence of tumor within an mpMRI ROI at initial biopsy. (54) On repeat biopsy, there was a 71% CDR when CCL was ≥ 4mm, compared to a 14% CDR when CCL was < 1mm. (54) When prostate cancer specific sites were located within an mpMRI ROI and the initial CCL was > 4mm, 5 of 6 (83%) revealed cancer on repeat biopsy. MRI ROI grade was the strongest predictor of prostate cancer on repeat biopsy (OR 1.48). (54) In men on active surveillance, electronic tracking of specific tumor sites may improve monitoring.
Improved reflection of final pathology
Gleason score concordance from conventional systematic biopsy to radical prostatectomy has been described as weak. (33) Shaw et al. reported a misclassification rate of up to 50% on conventional TRUS – guided prostate biopsy compared to radical prostatectomy specimen. (8) More recently, Le et al. investigated the use of MRI - ultrasound fusion biopsy as a predictor of final pathology on radical prostatectomy specimen in 54 men. (55)
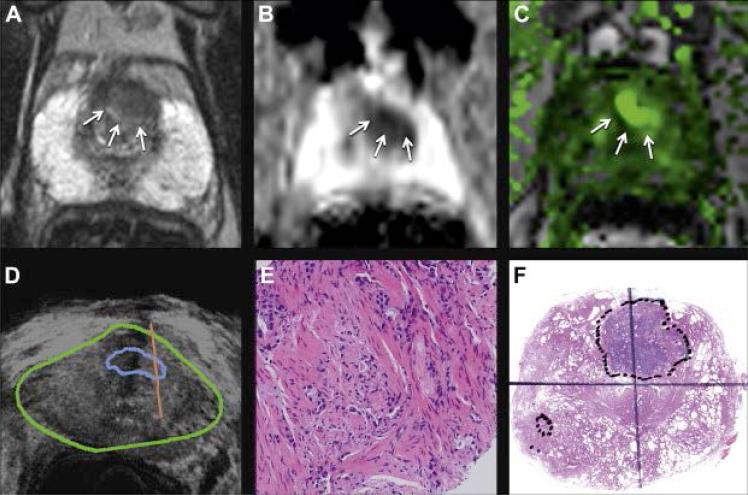
Figure 5 depicts a case scenario of a patient enrolled in the study. MRI - ultrasound fusion included systematic mapping biopsy and targeted biopsy. (55) Each method alone revealed a concordance rate of 54%. (55) In contrast, both modalities combined resulted in a concordance rate of 81%. (55) Combining both modalities significantly improved the predictive accuracy of prostate biopsy. (55)
Figure 5.
68 year old man with PSA 8.3 ng/ml underwent mp-MRI, including T2-weighted (A), diffusion weighted (B) and DCE (C) imaging, followed by fusion biopsy (D). Mapping biopsy revealed Gleason 3+3 but targeted biopsy (E) revealed Gleason 4+5 disease (reduced from ×20). GS on whole mount prostatectomy specimen was Gleason 4+3 with tertiary pattern 5 (F, reduced from ×1). Fusion biopsy, which included MRI targeted ROI, predicted highest Gleason grade at final pathology. (55)
Radtke et al. recently combined a transperineal saturation biopsy with MRI – ultrasound fusion biopsy. (14) Compared to radical prostatectomy specimen, the combined approach detected 96% of index lesions and 97% of significant multifocal lesions. (14)
Conclusion
Biopsy currently remains the only method to confirm a diagnosis of prostate cancer. (1,56) Common complications of prostate biopsy include hematuria, rectal bleeding, hematospermia, and infection, but these are generally self-limiting. (9,18) In recent years, infectious complications have increased significantly, largely due to an emergence of quinolone-resistant bacteria. (2,20) Judicious selection of antibiotic prophylaxis, based on local antibiograms will decrease the risk of infectious complications. (22,23)
Diagnostic accuracy of prostate biopsy has improved with the use of MRI - ultrasound fusion. (7,10,11,38) When combined with conventional biopsy, the detection of csCaP is significantly enhanced. (7,10) Targeted biopsy also enables more accurate sampling of the highest grade tumor component, which may provide an improvement in selection of candidates for active surveillance. (55) MRI - ultrasound fusion biopsy improves accuracy of prostate cancer detection in several specific applications; however, further research is necessary to define its overall advantage, cost-effectiveness, and decrease target errors. (7,10,11,14,15,38,57)
Key points.
Prostate biopsy safely diagnoses prostate cancer with few major, but frequent minor complications.
Local antibiograms should be considered when selecting antibiotic prophylaxis.
Use of targeted prostate biopsy results in detection of more clinically significant prostate cancer (csCaP) than conventional biopsy.
Combining targeted and systematic biopsy via MRI- ultrasound fusion results in greater sensitivity for detection of csCaP than either alone.
Acknowledgements
The project described was supported by Award Number R01CA158627 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the NIH. Additional support was provided by the Beckman Coulter Foundation, the Jean Perkins Foundation and the Steven C. Gordon Family Foundation. BH is grateful for funding from the German Research Foundation.
Footnotes
There are no conflicts of interest to report.
References
- 1.Van Der Kwast T, Bubendorf L, Mazerolles C, et al. Guidelines on processing and reporting of prostate biopsies: The 2013 update of the pathology committee of the European Randomized Study of Screening for Prostate Cancer (ERSPC). Virchows Arch. 2013;463(3):367–77. doi: 10.1007/s00428-013-1466-5. [DOI] [PubMed] [Google Scholar]
- 2.Loeb S, Carter HB, Berndt SI, et al. Complications after prostate biopsy: Data from SEER-Medicare. J Urol. 2011;186(5):1830–4. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Lee SJ. Infection after transrectal ultrasound-guided prostate biopsy. Korean Journal of Urology. 2015:346–50. doi: 10.4111/kju.2015.56.5.346. [This article highlights the increasing incidence of infection after prostate biopsy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lundstrom KJ, Drevin L, Carlsson S, et al. Nationwide population based study of infections after transrectal ultrasound guided prostate biopsy. J Urol. 2014;192:1116–22. doi: 10.1016/j.juro.2014.04.098. [DOI] [PubMed] [Google Scholar]
- 5*.Cussans A, Somani BK, Basarab A, Dudderidge T. The role of targeted prophylactic antimicrobial therapy prior to transrectal ultrasound (TRUS) guided prostate biopsy in reducing infection rates: a systematic review. BJU Int [Internet] 2015 doi: 10.1111/bju.13402. Available from: http://doi.wiley.com/10.1111/bju.13402. [This article discusses the importance of targeted prophylaxis in decreasing infection rates.] [DOI] [PubMed]
- 6.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. Part 1: Screening, diagnosis, and local treatment with curative intent - Update 2013. Eur Urol. 2014;65(1):124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 7**.Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/Ultrasound Fusion–Guided Biopsy With Ultrasound-Guided Biopsy for the Diagnosis of Prostate Cancer. 2016;1210(4):390–7. doi: 10.1001/jama.2014.17942. [This study describes the increased sensitivity of fusion biopsy compared to conventional biopsy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw GL, Thomas BC, Dawson SN, et al. Identification of pathologically insignificant prostate cancer is not accurate in unscreened men. Br J Cancer [Internet] 2014;110(10):2405–11. doi: 10.1038/bjc.2014.192. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24722183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Bjurlin MA, Wysock JS, Taneja SS. Optimization of prostate biopsy: Review of technique and complications. Urologic Clinics of North America. 2014:299–313. doi: 10.1016/j.ucl.2014.01.011. [This article highlights several common complications of prostate biopsy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Filson CP, Natarajan S, Margolis DJA, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer. 2016:1–9. doi: 10.1002/cncr.29874. [This is the largest series to date compaing conventional and fusion biopsy. This study suggests an advantage to combining both methods.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65(4):809–15. doi: 10.1016/j.eururo.2013.03.025. [This study describes the increased detection of clinically significant prostate cancer using fusion biopsy in men who have had previous negative biopsies.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Le JD, Tan N, Shkolyar E, et al. Multifocality and Prostate Cancer Detection by Multiparametric Magnetic Resonance Imaging: Correlation with Whole-mount Histopathology. Eur Urol [Internet] 2014;67(3):1–8. doi: 10.1016/j.eururo.2014.08.079. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25257029. [This study reports that tumor size and grade on MRI are important predictors of tumor detection.] [DOI] [PubMed] [Google Scholar]
- 13.Hadaschik BA, Kuru TH, Tulea C, et al. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. J Urol. 2011;186(6):2214–20. doi: 10.1016/j.juro.2011.07.102. [DOI] [PubMed] [Google Scholar]
- 14*.Radtke JP, Schwab C, Wolf MB, et al. Multiparametric Magnetic Resonance Imaging (MRI) and MRI-Transrectal Ultrasound Fusion Biopsy for Index Tumor Detection: Correlation with Radical Prostatectomy Specimen. [2016 Feb 4];Eur Urol [Internet] 2016 Jan 19; doi: 10.1016/j.eururo.2015.12.052. Available from: http://www.sciencedirect.com/science/article/pii/S0302283816000105. [This study reports on dectection rates of targeted and systematic biopsies, and the combination of both for index tumor lesions compared to whole mount sections on radical prostatectomy specimen.] [DOI] [PubMed]
- 15*.Baco E, Ukimura O, Rud E, et al. Magnetic resonance imaging-transectal ultrasound image-fusion biopsies accurately characterize the index tumor: Correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol. 2015;67(4):787–94. doi: 10.1016/j.eururo.2014.08.077. [This study revealed that fusion biopsy reliably identified the location and primary of the index tumor lesion in greater than 90% of patients.] [DOI] [PubMed] [Google Scholar]
- 16.Siddiqui MM, Rais-Bahrami S, Truong H, Stamatakis L, Vourganti S, Nix J, et al. Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol. 2013;64(5):713–9. doi: 10.1016/j.eururo.2013.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez LV, Terris Mk. Risks And Complications Of Transrectal Ultrasound Guided Prostate Needle Biopsy: A Prospective Study And Review Of The Literature. J Urol [Internet] 1998;160(6):2115–20. doi: 10.1097/00005392-199812010-00045. Available from: http://www.sciencedirect.com/science/article/pii/S0022534701622559. [DOI] [PubMed] [Google Scholar]
- 18.Berger AP, Gozzi C, Steiner H, et al. Complication rate of transrectal ultrasound guided prostate biopsy: a comparison among. 3 protocols with 6, 10 and. 15 cores. [Internet]. The Journal of urology. 2004:1478–80. doi: 10.1097/01.ju.0000116449.01186.f7. discussion 1480–1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15017202. [DOI] [PubMed]
- 19.Van Den Heuvel S, Loeb S, Zhu X, et al. 825 Complications of initial prostate biopsy in a European randomized screening trial. Eur Urol Suppl. 2012;11(1):e825–e825a. [PMC free article] [PubMed] [Google Scholar]
- 20.Feliciano J, Teper E, Ferrandino M, et al. The Incidence of Fluoroquinolone Resistant Infections After Prostate Biopsy-Are Fluoroquinolones Still Effective Prophylaxis? J Urol. 2008;179(3):952–5. doi: 10.1016/j.juro.2007.10.071. [DOI] [PubMed] [Google Scholar]
- 21.Al-Hasan MN, Lahr BD, Eckel-Passow JE, Baddour LM. Antimicrobial resistance trends of Escherichia coli bloodstream isolates: A population-based study, 1998-2007. J Antimicrob Chemother. 2009;64(1):169–74. doi: 10.1093/jac/dkp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22*.Averch T, Tessier C, Clemens JQ, et al. AUA Quality Improvement Summit 2014: Conference Proceedings on Infectious Complications of Transrectal Prostate Needle Biopsy. Urol Pract. 2015;2(4):172–80. doi: 10.1016/j.urpr.2014.10.011. [This article highlights the importance of utilizing antibiograms to select appropriate antibiotic prophylaxis.] [DOI] [PubMed] [Google Scholar]
- 23.Wolf JS, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol [Internet] 2008;179(4):1379–90. doi: 10.1016/j.juro.2008.01.068. Available from: http://www.sciencedirect.com/science/article/pii/S0022534708001766. [DOI] [PubMed] [Google Scholar]
- 24**.Liss MA, Taylor SA, Batura D, et al. Fluoroquinolone resistant rectal colonization predicts risk of infectious complications after transrectal prostate biopsy. J Urol. 2014;192(6):1673–8. doi: 10.1016/j.juro.2014.06.005. [This article describes an association between quinolone-resistance and infectious complications after biopsy.] [DOI] [PubMed] [Google Scholar]
- 25.Taylor AK, Zembower TR, Nadler RB, et al. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol. 2012;187(4):1275–9. doi: 10.1016/j.juro.2011.11.115. [DOI] [PubMed] [Google Scholar]
- 26.Williamson DA, Barrett LK, Rogers BA. Infectious complications following transrectal ultrasound-guided prostate biopsy: New challenges in the era of multidrug-resistant escherichia coli. Clin Infect Dis. 2013;57(2):267–74. doi: 10.1093/cid/cit193. [DOI] [PubMed] [Google Scholar]
- 27.Grummet JP, Weerakoon M, Huang S, et al. Sepsis and “superbugs”: Should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int. 2014;114(3):384–8. doi: 10.1111/bju.12536. [DOI] [PubMed] [Google Scholar]
- 28**.Bennett HY, Roberts MJ, Doi SAR, Gardiner RA. The global burden of major infectious complications following prostate biopsy. Epidemiol Infect [Internet] 2015:1–8. doi: 10.1017/S0950268815002885. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26645476. [This article highlights higher rates of hospitalization and sepsis with transrectal biopsy compared to transperineal prostate biopsy.] [DOI] [PMC free article] [PubMed]
- 29*.Bokhorst LP, Lepistö I, Kakehi Y, et al. Complications after prostate biopsies in men on active surveillance and its effect on receiving further biopsies in the Prostate cancer Research International: Active Surveillance (PRIAS) study. BJU Int [Internet] 2016 doi: 10.1111/bju.13410. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26765682. [This study evaluates complications of repeat biopsies in men on active surveillance. There was no increase in complications with repeat biopsies.] [DOI] [PubMed]
- 30.Hodge KK, McNeal JE, Terris MK, Stamey T a. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142:71–4. doi: 10.1016/s0022-5347(17)38664-0. discussion 74–5. [DOI] [PubMed] [Google Scholar]
- 31.Louie-Johnsun M, Neill M, Treurnicht K, et al. Final outcomes of patients with low-risk prostate cancer suitable for active surveillance but treated surgically. BJU Int. 2009;104(10):1501–4. doi: 10.1111/j.1464-410X.2009.08597.x. [DOI] [PubMed] [Google Scholar]
- 32.Epstein JI, Sanderson H, Carter HB, Scharfstein DO. Utility of saturation biopsy to predict insignificant cancer at radical prostatectomy. Urology. 2005;66(2):356–60. doi: 10.1016/j.urology.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Cohen MS, Hanley RS, Kurteva T, et al. Comparing the Gleason Prostate Biopsy and Gleason Prostatectomy Grading System: The Lahey Clinic Medical Center Experience and an International Meta-Analysis. Eur Urol. 2008;54(2):371–81. doi: 10.1016/j.eururo.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 34*.Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. European Urology. 2015 doi: 10.1016/j.eururo.2015.08.052. [This article describes the updated version of PI-RADS used to report suspicious lesions seen on MRI.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turkbey B, Mani H, Shah V, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: Histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011;186(5):1818–24. doi: 10.1016/j.juro.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Vargas HA, Hötker AM, Goldman DA, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol. 2015 doi: 10.1007/s00330-015-4015-6. [This study evaluates the ability of the PI-RADS scoring system to accurately detect prostate cancer based on whole-mount pathology results. PI-RADS detected the majority of prostate cancer foci.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22(4):746–57. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Cash H, Maxeiner A, Stephan C, et al. The detection of significant prostate cancer is correlated with the Prostate Imaging Reporting and Data System (PI-RADS) in MRI/transrectal ultrasound fusion biopsy. World J Urol [Internet] 2015 doi: 10.1007/s00345-015-1671-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26293117. [This study highlights the correlation between PI-RADS score and detection of clinically significant prostate cancer on biopsy.] [DOI] [PubMed]
- 39**.Radtke JP, Kuru TH, Boxler S, et al. J Urol. 1. Vol. 193. Elsevier Ltd; 2015. Comparative Analysis of Transperineal Template Saturation Prostate Biopsy Versus Magnetic Resonance Imaging Targeted Biopsy with Magnetic Resonance Imaging-Ultrasound Fusion Guidance. pp. 87–94. [This study reported the combination of systematic and targeted biopsy as the gold standard for prostate cancer detection.] [DOI] [PubMed] [Google Scholar]
- 40*.Hamoen EHJ, de Rooij M, Witjes JA, et al. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-analysis. Eur Urol [Internet] 2014;67(6):1112–21. doi: 10.1016/j.eururo.2014.10.033. Available from: http://www.sciencedirect.com/science/article/pii/S0302283814011221. [This study emphasizes the accuracy of PI-RADS scoring to detect prostate cancer.] [DOI] [PubMed] [Google Scholar]
- 41*.Muller BG, Shih JH, Sankineni S, et al. Prostate Cancer: Interobserver Agreement and Accuracy with the Revised Prostate Imaging Reporting and Data System at Multiparametric MR Imaging. Radiology [Internet] 2015:142818. doi: 10.1148/radiol.2015142818. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26098458. [This study reports that the updated version of PI-RADS yields moderately reproducible MR imaging scores.] [DOI] [PMC free article] [PubMed]
- 42.Rosenkrantz AB, Lim RP, Haghighi M, et al. Comparison of interreader reproducibility of the prostate imaging reporting and data system and likert scales for evaluation of multiparametric prostate MRI. Am J Roentgenol. 2013;201(4) doi: 10.2214/AJR.12.10173. [DOI] [PubMed] [Google Scholar]
- 43.Simpfendörfer T, Kuru TH, Steinemann S, et al. Trocar-Sharpened Needles for Image-Guided Prostate Biopsy Improve Sample Quality and Performance: First Clinical Results. J Endourol [Internet] 2014;28(11):1384–8. doi: 10.1089/end.2014.0121. Available from: http://online.liebertpub.com/doi/abs/10.1089/end.2014.0121. [DOI] [PubMed] [Google Scholar]
- 44.Puech P, Rouvière O, Renard-Penna R. Prostate Cancer Diagnosis : Multiparametric MR-targeted Biopsy with Cognitive and Transrectal US –MR Fusion Guidance versus Systematic Biopsy —. Radiology. 2013;268(2):461–9. doi: 10.1148/radiol.13121501. [DOI] [PubMed] [Google Scholar]
- 45*.Cool DW, Zhang X, Romagnoli C, et al. Evaluation of MRI-TRUS fusion versus cognitive registration accuracy for MRI-targeted, TRUS-guided prostate biopsy. American Journal of Roentgenology. 2015:83–91. doi: 10.2214/AJR.14.12681. [This study compares the accuracy of fusion biopsy to cognitive biopsy.] [DOI] [PubMed] [Google Scholar]
- 46.Moore CM, Robertson NL, Arsanious N, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: A systematic review. European Urology. 2013:125–40. doi: 10.1016/j.eururo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 47*.Garmer M, Busch M, Mateiescu S. Accuracy of MRI-Targeted in-Bore Prostate Biopsy According to the Gleason Score with Postprostatectomy Histopathologic Control-a Targeted Biopsy-Only Strategy with Limited Number of Cores. Acad Radiol. 2015;22(11):1409–18. doi: 10.1016/j.acra.2015.06.020. [This study evaluates whether MRI in-bore biopsy accurately identifies the Gleason score of prostate cancer in histopathologic correlation.] [DOI] [PubMed] [Google Scholar]
- 48*.Mozer P, Roupret M, Le Cossec C, et al. First round of targeted biopsies using magnetic resonance imaging/ultrasonography fusion compared with conventional transrectal ultrasonography-guided biopsies for the diagnosis of localised prostate cancer. BJU Int. 2015;115(1):50–7. doi: 10.1111/bju.12690. [This study highlights the increased detection of clinically significant prostate cancer using targeted prostate biopsy.] [DOI] [PubMed] [Google Scholar]
- 49.Kasivisvanathan V, Dufour R, Moore CM, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol. 2013;189(3):860–6. doi: 10.1016/j.juro.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Ehdaie B, Vertosick E, Spaliviero M, et al. The impact of repeat biopsies on infectious complications in men with prostate cancer on active surveillance. J Urol. 2014;191(3):660–4. doi: 10.1016/j.juro.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 51.Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994;271(5):368–74. [PubMed] [Google Scholar]
- 52*.Hu JC, Chang E, Natarajan S, et al. Targeted prostate biopsy in select men for active surveillance - Do the epstein criteria still apply? J Urol. 2014;192(2):385–90. doi: 10.1016/j.juro.2014.02.005. [This study describes upgrading of Gleason score beyond Epstein criteria with targeted biopsy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radtke JP, Kuru TH, Bonekamp D, et al. Further reduction of disqualification rates by additional MRI-targeted biopsy with transperineal saturation biopsy compared to standard 12-core systematic biopsies for selection of prostate cancer patients for active surveillance. Prostate Cancer Prostatic Dis. 2016 doi: 10.1038/pcan.2016.16. in press. [DOI] [PubMed] [Google Scholar]
- 54*.Sonn GA, Filson CP, Chang E, et al. Initial experience with electronic tracking of specific tumor sites in men undergoing active surveillance of prostate cancer. Urol Oncol Semin Orig Investig. 2014;32(7):952–7. doi: 10.1016/j.urolonc.2014.04.003. [This study evaluates the use of monitoring specific tumor sites in men on active surveillance.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55*.Le JD, Stephenson S, Brugger M, et al. Magnetic resonance imaging-ultrasound fusion biopsy for prediction of final prostate pathology. J Urol. 2014;192(5):1367–73. doi: 10.1016/j.juro.2014.04.094. [This study describes an increased concordance rate with combining conventional systematic and targeted biopsy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silletti JP, Gordon GJ, Bueno R, et al. Prostate Biopsy: Past, Present, and Future. Urology. 2007;69(3):413–6. doi: 10.1016/j.urology.2007.01.096. [DOI] [PubMed] [Google Scholar]
- 57*.Arsov C, Rabenalt R, Blondin D, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol. 2015;68(4):713–20. doi: 10.1016/j.eururo.2015.06.008. [This randomized controlled trial analyzed the detection rates of MRI in-bore and targeted fusion biopsies.] [DOI] [PubMed] [Google Scholar]