Abstract
We evaluated the feasibility and acceptability of self-sampling for human papillomavirus (HPV) testing and calculated the prevalence of and risk factors for high-risk (hr) HPV infections in a community-based sample of American Indian women. To this end, we recruited 329 Hopi women aged 21-65 years to self-collect vaginal samples for hrHPV testing. Samples were tested by polymerase chain reaction for 14 hrHPV genotypes. We used chi-square tests to identify correlates of preference for clinician Pap testing versus HPV self-sampling, and age-adjusted Poisson regression to evaluate correlates of hrHPV prevalence. We found that satisfaction with HPV self-sampling was high, with 96% of women reporting that the sample was easy to collect and 87% reporting no discomfort. The majority (62%) indicated that they preferred HPV self-sampling to receiving a Pap test from a clinician. Preference for Pap testing over HPV self-sampling was positively associated with adherence to Pap screening and employment outside the home. All samples evaluated were satisfactory for HPV testing, and 22% were positive for hrHPV. HrHPV prevalence peaked in the late 20s and declined with increasing age. HrHPV positivity was inversely associated with having children living the household. In conclusion, HPV self-sampling is feasible and acceptable to Hopi women, and could be effective in increasing rates of cervical cancer screening in Hopi communities. HrHPV prevalence was similar to estimates in the general United States population.
Keywords: human papillomavirus (HPV), American Indian/Alaska Native (AI/AN), self-sampling, prevalence, acceptability
Introduction
Nearly all cervical cancers are linked to infection with high-risk (hr) types of human papillomavirus (HPV), with HPV types 16 and 18 contributing to ~70% of cervical cancers [1]. In the United States, despite the success of Pap screening programs in reducing disease burden over the past 60 years, ~12,000 new cases of cervical cancer are diagnosed annually [2]. More than half of new cases are diagnosed in women who were never or rarely screened [3]. Variations in screening uptake across racial and ethnic groups likely drive observed disparities in cervical cancer incidence. Notably, cervical cancer incidence and mortality rates are higher in American Indian women than in non-Hispanic White women [4]. Given these disparities, it is not surprising that uptake of Pap screening is lower in American Indians than in non-Hispanic Whites [2].
Reasons for non-participation in Pap screening are multifactorial, and include lack of insurance, inconvenience, difficulty finding childcare or taking time off work, lack of transportation, fear or embarrassment related to receiving a pelvic exam or an abnormal result, limited knowledge of cervical cancer, cultural attitudes, and negative experiences with medical care [5, 6, 7, 8]. Many of these barriers could be addressed by offering opportunities for non-clinic-based cervical cancer screening. Interest is growing in self-sampling as a strategy to increase screening participation, with referral to clinic-based diagnostic follow-up for women with positive results. Guidelines already endorse HPV testing as an adjunct to clinic-based Pap screening [9], and previous studies indicate that self-collected samples are as sensitive as clinician-collected samples for detecting HPV infections [10], particularly when polymerase chain reaction (PCR)-based assays are used for HPV testing [11, 12]. Studies also consistently show that self-sampling for HPV infection is feasible and acceptable [13], and that it increases participation in cervical cancer screening [14]. Yet no study has evaluated self-sampling in an American Indian population.
Our study goals were 1) to assess the feasibility and acceptability of self-sampling for HPV testing among American Indian women aged 21-65 years who are enrolled members of the Hopi Tribe, and 2) to describe the prevalence and correlates of hrHPV infection. Previous studies suggest that the epidemiology of HPV infection may differ between American Indian and non-Hispanic White women [15, 16, 17], but data on American Indian populations are scarce, and no study to date has focused specifically on Hopi women. Defining the epidemiology of hrHPV in a range of American Indian populations is essential for evaluating whether disparities in cervical cancer incidence result from differences in the prevalence of hrHPV infections.
Materials and Methods
From April 2013 to June 2014, we recruited Hopi women to self-collect vaginal samples for HPV testing and to complete a written survey of demographic data, health and sexual history, and attitudes toward HPV self-sampling. This cross-sectional, community-based study was a collaborative effort of the University of Washington, Cornell University, and the Hopi Tribe. The study protocol was developed with input from tribal partners, local project staff, and community advisors, and it was reviewed and approved by the Hopi Tribal Council and by the institutional review boards of the University of Washington and Cornell University.
Study Population and Setting
The Hopi Reservation encompasses approximately 1.6 million acres in northeastern Arizona. According to 2009 tribal records, more than 5,000 women are enrolled members of the Hopi Tribe. Approximately 75% of tribal members live on the Hopi Reservation in 12 rural villages situated on or below 3 adjoining mesas. In 2009, 32% of occupied housing units had no telephone service, and 25% of units had no vehicle (Lorencita Joshweseoma, personal communication).
The Hopi Office of Prevention and Intervention Cancer Support Services (HCSS) coordinates cervical cancer screening for women living on or near the Hopi Reservation through the Hopi Women's Health Program, which is part of the National Breast and Cervical CancerEarly Detection Program (NBCCEDP) of the Centers for Disease Control and Prevention. Pap tests are provided at no cost at two federally-funded Indian Health Service facilities: the Hopi Health Care Center (Polacca, AZ) and the Tuba City Regional Health Care Corporation (Tuba City, AZ). From July 2008 to June 2013, 2,040 Hopi women were served by NBCCEDP, and 1,106 of them (54%) received Pap tests (April 2014 submission of NBCCEDP Minimum Data Elements).
Study Procedures
Recruitment, eligibility screening, and data collection were coordinated by two local project staff at the HCSS office (Kykotsmovi, AZ). To reach women residing both on and off the reservation, we developed a multi-faceted recruitment strategy. Flyers and informational brochures were posted in public places (including post offices, community centers, health centers, and local businesses) and distributed face-to-face during community events (including community meetings, school parent-teacher meetings, and health fairs) and door-to-door health education campaigns sponsored by the HCSS. An electronic version of the flyer was distributed by email to tribal listservs and published in tribal newsletters, and public service announcements were aired on the tribal radio station.
Printed recruitment materials and radio announcements invited interested women to telephone the HCSS project coordinators for additional information and eligibility screening. At in-person recruitment events, interested women were asked to fill out cards with their names and telephone numbers for subsequent contact and eligibility screening by telephone. Eligibility screening also occurred occasionally at community recruitment events, when sufficient time was available. Women also had the option of presenting for in-person eligibility screening at the HCSS office. After providing further information about the study, project coordinators answered questions from potential participants and obtained verbal permission to conduct a survey to determine eligibility. Inclusion criteria included age 21-65 years, enrollment in the Hopi Tribe, no current pregnancy, and no childbirth in the past 6 weeks.
Eligible women who verbally agreed to participate were given an informational brochure on HPV, a consent form for study participation, and a release-of-information form to ascertain compliance with national Pap screening recommendations. To promote participation in cancer screening, the consent form asked women if they would like their HPV test results and contact information to be shared with the Hopi Women's Health Program. The release-of-information form requested permission to allow the Hopi Health Care Center and the Tuba City Regional Health Care Corporation to release the date (but not the results or other details) of the most recent Pap test on record. Women were also asked to provide the names of any additional clinics or providers visited in the past 10 years for a Pap test, physical examination, or prenatal visit, so that additional Pap dates could be requested. Women were asked to read the consent and release-of-information forms carefully, and to contact a staff member if they had any questions. Signing the consent form was required for study participation, but completing the release-of-information form was optional. Women screened at the HCSS office were able to complete these forms in a private room; these women immediately received an HPV self-collection kit and a health assessment survey. For women screened by telephone, the informational brochure, consent form, and release-of-information form were mailed as part of packet that contained instructions for completing the forms, along with a pre-paid return mail envelope addressed to the HCSS office.
Women who provided written informed consent were enrolled and given a packet containing the self-collection kit, the survey, a flyer on interpreting HPV test results, and a pre-paid return mailing box. Women had the option of receiving the packet by mail or picking it up in person at the HCSS office. Women also had the option of completing the kit and survey at home or completing the kit in a private bathroom at the HCSS office and filling out the survey in a private room at the same location. Women who completed the kit and survey at home had the option of return mailing or hand-delivering the materials to the HCSS office, or telephoning to request pick-up from their homes by a community health representative or project coordinator. Each study participant received a $40 gift card as compensation for her time.
The HPV self-collection kit included two individually packaged 15.2-cm unscored Dacron-tipped swabs; a covered tube containing 1.5 ml of specimen transport medium (QIAGEN, Gaithersburg, MD); a pair of nitrile gloves; return shipping materials (a clear plastic bag, a biohazard bag, and a hard plastic soap dish); and a return mailing box addressed to the HCSS office with pre-paid priority mail postage. The kit also contained illustrated instructions explaining how to perform the self-collection and how to pack and ship the sample if it was collected at home. The instructions directed participants to insert two successive swabs into the vagina “as far as it will go without hurting, similar to how you would insert a tampon,” and to “gently turn it between your fingers for three full turns.” (Two sequential self-collected swabs increases sensitivity for HPV detection [18].) Other instructions asked participants to collect the sample at least two days after the end of the last menses and to refrain from vaginal intercourse and the use of “feminine products” for two days before sample collection.
Women were asked to complete the written survey after they used the self-collection kit. The survey captured information on demographics, health and sexual history, cultural practices, knowledge of HPV and cervical cancer, and attitudes toward self-collecting samples for HPV testing.
Self-collected samples were placed in a refrigerator on receipt at the HCSS office. All samples were batch-shipped by overnight FedEx at room temperature to the Molecular Diagnostics Laboratory of the University of Washington's Pathology Department (Seattle, WA) for HPV testing. DNA was isolated according to the QIAamp DNA Mini Kit vacuum protocol (QIAGEN, Gaithersburg, MD) by using 200 μL of the sample collected in the specimen transport medium. Final elution volume was 50 μL. HPV and HPRT1 polymerase chain reaction product was generated by amplification with HPV primers MY09 and MY11 (500 nm), HMB01 (100 nm), and HPRT1 forward and reverse primers (100 nm). Detection of 14 hrHPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68) [19] plus HPRT1 was performed by following a Liquid Bead Microarray assay protocol using 20 μL of the product; this protocol is described elsewhere [20]. Results were recorded as positive or negative for hrHPV DNA, or unsatisfactory if negative for HPRT1. HPV DNA testing was completed in May 2014. In accordance with an existing agreement with the Hopi Tribe, the samples were destroyed at the University of Washington in June 2014.
According to each participant's preference, HPV test results were communicated either by letter or by a telephone call from one of the project coordinators. To discuss their results, all participants were offered the opportunity to arrange either an in-person visit with one of the project coordinators or a telephone consultation with a study nurse based at the University of Washington.
Statistical Analyses
Women were classified as adherent to Pap screening guidelines if clinic records indicated receipt of a Pap test within the past three years, and as non-adherent if no Pap test appeared in the records or if the most recent Pap test occurred more than three years previously. In cases where no clinic records were requested for a participant, the categorical interval self-reported on the health survey was used to determine adherence or non-adherence. Women who reported a hysterectomy were not classified.
We used chi-square tests to examine the associations of test preference (Pap versus HPV self-test) with selected demographic characteristics, health history, cultural practices, knowledge of HPV and cervical cancer, and self-sampling location (at home versus HCSS office). At the request of our community partners, data on sexual history are excluded from this manuscript. Using qualitative methods, we identified themes among open-ended responses to a survey item that solicited reasons for preferring either Pap testing or HPV self-testing, and we summarized the prevalence of themes according to test preference. Two investigators (RLW and CJN) independently reviewed each response before reaching a consensus on thematic content. We also used chi-square tests to assess the associations of reported discomfort during vaginal sample collection with age, menopausal status, number of pregnancies, and Pap screening adherence.
We calculated the prevalence of any hrHPV infection, multiple-type hrHPV infection, and individual hrHPV types. We used age-adjusted Poisson regression to estimate the relative risk of any hrHPV detection associated with selected demographic factors, health history, cultural practices, and knowledge of HPV and cervical cancer [21]. All analyses were conducted by using Stata 12.1 (StataCorp LP, College Station, TX).
Results
Among 353 enrolled participants, 329 returned an HPV self-sampling kit, for a response rate of 93.2%. Analyses were restricted to these 329 women. Within this group, 322 (97.9%) also returned the survey. Among women included in analyses, the mean age was 43 years (standard deviation 13). As shown in Table 1, most women had a history of pregnancy (91%), had some level of post-secondary education (68%), and were married or living with a partner (51%). Most women had heard of HPV (61%) and were aware of its association with cervical cancer (56%). Twenty-one percent of women collected the sample at the HCSS office; the rest (79%) performed the collection at home. Within the latter group, 53% (i.e., 42% of all women in the analyses) hand-delivered the home-collected sample to HCSS, 33% requested pick-up from their homes, and 14% returned the sample by mail.
Table 1.
Demographics, HPV knowledge, cultural characteristics, and health history of study participants (n=329) in 2013-2014
| Characteristic | Na | % |
|---|---|---|
| Demographics | ||
| Age (years) | ||
| 21-24 | 34 | 10 |
| 25-29 | 35 | 11 |
| 30-39 | 59 | 18 |
| 40-49 | 65 | 20 |
| 50-65 | 136 | 41 |
| Education | ||
| Less than high school | 34 | 11 |
| High school graduate or GED | 66 | 21 |
| Some college or technical school | 163 | 52 |
| College associates degree or higher | 49 | 16 |
| Employment status | ||
| Employed full- or part-time | 141 | 46 |
| Unemployed or laid off | 31 | 10 |
| Keeping house or raising children | 98 | 32 |
| Other | 37 | 12 |
| Marital status | ||
| Divorced, separated, widowed, or never been married | 152 | 49 |
| Married or living with a partner | 161 | 51 |
| Children < 18 years old living in household | ||
| No | 78 | 26 |
| Yes | 220 | 74 |
| HPV knowledge | ||
| Heard of HPV | ||
| No | 124 | 39 |
| Yes | 196 | 61 |
| Aware that HPV can cause cervical cancer | ||
| No | 141 | 44 |
| Yes | 178 | 56 |
| Aware that HPV is spread by sexual contact | ||
| No | 163 | 51 |
| Yes | 155 | 49 |
| Culture | ||
| Ability to speak the Hopi or Tewa language | ||
| Very well | 79 | 25 |
| Moderately well | 88 | 28 |
| A little but not very well | 115 | 37 |
| I don't speak the Hopi or Tewa language | 32 | 10 |
| Language usually spoken at home | ||
| English | 200 | 66 |
| Hopi or Tewa | 102 | 34 |
| Health history | ||
| General health | ||
| Very good or Excellent | 139 | 43 |
| Good | 141 | 44 |
| Fair or Poor | 41 | 13 |
| Smoking status | ||
| Never | 208 | 65 |
| Former | 51 | 16 |
| Current | 59 | 19 |
| Ever been pregnant | ||
| No | 28 | 9 |
| Yes | 291 | 91 |
| History of HPV vaccination | ||
| No | 246 | 79 |
| Yes | 65 | 21 |
| Number of shots received by participants who reported a history of HPV vaccination (n=65) | ||
| 1 | 37 | 57 |
| 2 | 13 | 20 |
| 3 | 15 | 23 |
| Time since most recent Pap test, b by self-report | ||
| Within the last year | 112 | 39 |
| More than 1 year ago but within the last 3 years | 125 | 43 |
| More than 3 years ago but within the last 5 years | 21 | 7 |
| More than 5 years ago | 32 | 11 |
| Time since most recent Pap test, b according to clinic records | ||
| Within the last year | 70 | 26 |
| More than 1 year ago but within the last 3 years | 129 | 48 |
| More than 3 years ago but within the last 5 years | 23 | 8 |
| More than 5 years ago | 49 | 18 |
Numbers might not sum to 329 because of missing data.
Hysterectomized women were excluded.
Pap dates from medical records were received for 306 women (93.0% of women in the analyses). The agreement between medical record data and self-reported survey data for measuring Pap screening adherence was 80.2%. Using a composite of medical record and self-reported data to classify Pap screening adherence (whereby self-reported data was used only when medical record data were unavailable), 73.4% of women were determined to be adherent (i.e., received a Pap test within the past three years).
Most women were very satisfied with their experience using the HPV self-sampling kit (Table 2). Almost all (99%) reported that the instructions were easy to understand and follow, and 96% reported that the vaginal sample was easy to collect. A minority (13%) reported discomfort collecting the sample. Age and Pap screening adherence were not associated with reported discomfort (data not shown). Almost all (97%) would recommend the kit to a friend or relative, and 62% preferred self-sampling for HPV, whether at home or in a clinic, to a Pap test done by a clinician. Preference for Pap testing over HPV self-sampling was positively associated with Pap screening adherence and being employed full- or part-time (Table 3). Accuracy and professionalism were the most common reasons for preferring Pap testing (65%), whereas privacy, reduction in embarrassment, and dislike of receiving Pap tests from a clinician (reported by 68%) and convenience and ease (reported by 52%) were the most common reasons for preferring HPV self-testing (Table 4).
Table 2.
Attitudes about human papillomavirus (HPV) self-testing (n=329)
| Attitude | N a | % |
|---|---|---|
| Instructions for HPV self-test were easy to understand and follow | ||
| Yes | 319 | 99 |
| No | 2 | 1 |
| Easy to collect vaginal sample using HPV self-test | ||
| Yes | 308 | 96 |
| No | 12 | 4 |
| Discomfort collecting vaginal sample | ||
| Yes | 43 | 13 |
| No | 277 | 87 |
| Would recommend HPV self-test to friend or relative | ||
| Yes | 308 | 97 |
| No | 10 | 3 |
| Preference for Pap test or HPV self-test | ||
| Pap test done by doctor or nurse | 117 | 38 |
| HPV self-test | 189 | 62 |
Numbers might not sum to 329 because of missing data.
Table 3.
Cervical cancer screening test preference according to demographics, HPV knowledge, cultural characteristics, and health history (n= 306)a
| Characteristic |
Test preference b
|
P-value | |||
|---|---|---|---|---|---|
| Pap test done by doctor or nurse |
HPV self-test |
||||
| N | Row % | N | Row % | ||
| Demographic | |||||
| Age (years) | 0.30 | ||||
| 21-24 | 10 | 29 | 24 | 71 | |
| 25-29 | 14 | 40 | 21 | 60 | |
| 30-39 | 26 | 50 | 26 | 50 | |
| 40-49 | 20 | 33 | 40 | 67 | |
| 50-65 | 47 | 38 | 78 | 62 | |
| Education | 0.32 | ||||
| Less than high school | 12 | 35 | 22 | 65 | |
| High school graduate or GED | 30 | 47 | 34 | 53 | |
| Some college or technical school | 53 | 34 | 101 | 66 | |
| College associates degree or higher | 19 | 43 | 25 | 57 | |
| Employment status | 0.04 | ||||
| Employed full- or part-time | 58 | 45 | 70 | 55 | |
| Unemployed or laid off | 15 | 48 | 16 | 52 | |
| Keeping house or raising children | 28 | 29 | 68 | 71 | |
| Other | 11 | 31 | 25 | 69 | |
| Marital status | 0.06 | ||||
| Divorced, separated, widowed, or never been married | 62 | 44 | 80 | 56 | |
| Married or living with partner | 51 | 33 | 104 | 67 | |
| Children < 18 years old living in household | 0.23 | ||||
| No | 34 | 45 | 42 | 55 | |
| Yes | 76 | 37 | 130 | 63 | |
| HPV knowledge | |||||
| Heard of HPV | 0.93 | ||||
| No | 45 | 38 | 72 | 62 | |
| Yes | 71 | 38 | 116 | 62 | |
| Knew that HPV can cause cervical cancer | 0.19 | ||||
| No | 45 | 34 | 87 | 66 | |
| Yes | 71 | 42 | 100 | 58 | |
| Knew that HPV is spread by sexual contact | 0.39 | ||||
| No | 55 | 36 | 99 | 64 | |
| Yes | 60 | 41 | 88 | 59 | |
| Culture | |||||
| Ability to speak the Hopi or Tewa language | 0.65 | ||||
| Very well | 31 | 41 | 45 | 59 | |
| Moderately well | 33 | 40 | 50 | 60 | |
| A little but not very well | 36 | 34 | 71 | 66 | |
| I don't speak the Hopi or Tewa language | 14 | 44 | 18 | 56 | |
| Language usually spoken at home | 0.46 | ||||
| English | 74 | 39 | 115 | 61 | |
| Hopi or Tewa | 34 | 35 | 64 | 65 | |
| Health history | |||||
| General health | 0.93 | ||||
| Very good or excellent | 50 | 38 | 81 | 62 | |
| Good | 51 | 38 | 84 | 62 | |
| Fair or poor | 16 | 41 | 23 | 59 | |
| Ever been pregnant | 0.16 | ||||
| No | 14 | 50 | 14 | 50 | |
| Yes | 100 | 36 | 175 | 64 | |
| Received a Pap test within the past 3 yearsc | 0.02 | ||||
| No | 20 | 27 | 54 | 73 | |
| Yes | 86 | 42 | 117 | 58 | |
Twenty-three women did not respond to the question on test preference.
The question on test preference was: “In the future, if you could choose between a Pap test done by a doctor or nurse and an HPV test that you could do at home by yourself (as you did today), which would you prefer?”
Based on available clinic records (93%) and self-report (7%). Hysterectomized women were excluded.
Table 4.
Reasons for cervical cancer screening test preference a
| Reason | N | % | Example comments |
|---|---|---|---|
| Women who preferred Pap test done by doctor or nurse (n=81 provided comments)b | |||
| More accurate or professional | 53 | 65 | “A Pap test in the doctor's office will ensure that the test procedure was administered properly and in a sterile environment.” |
| Opportunity to receive other care or have questions answered at clinic visit | 16 | 20 | “The doctor's office is better for me because it checks for everything and not one specific thing.” |
| “Because if I have any questions to ask they can be answered. Or if something is noticeable then I can be told right away.” | |||
| Difficulty or physical discomfort with HPV self-test | 8 | 10 | “Kind of had a hard time inserting swab.” |
| Women who preferred HPV self-test (n=155 provided comments)c | |||
| Physically more comfortable | 13 | 8 | “Because doing it myself didn't cause as much pain like when the doctor does it.” |
| Convenience, ease | 80 | 52 | “It is much easier to do and doesn't require a drive to the hospital and loss of wages from work” |
| “Only because with our daily lives and schedules not everyone has the time for doctors’ appointments. The test was fast and easy!” | |||
| Privacy, less embarrassment, dislike receiving Pap test from a clinician | 106 | 68 | “This method of collecting (sample) is more acceptable to me because I do get privacy – less tension or anxiety, etc.” |
| “Because I hate to go into a doctor's office and have someone (even though I have been to the same doctor for 4 years) do the test for me.” | |||
Reasons were identified in open-ended responses and are not mutually exclusive.
Thirty-six of 117 women (31%) did not provide a reason for preferring the Pap test.
Thirty-four of 189 women (18%) did not provide a reason for preferring the HPV self-test.
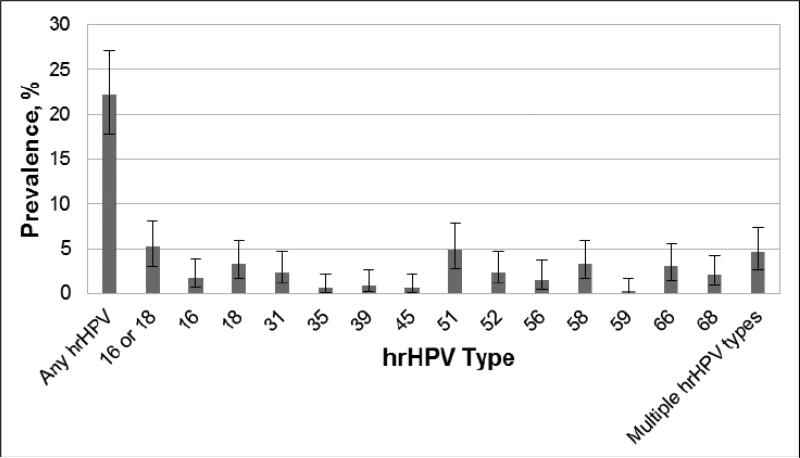
All samples tested were satisfactory for HPV DNA testing. Overall, 73 women (22.2%) tested positive for at least one hrHPV type, while 15 (4.6%) tested positive for more than one hrHPV type (Figure 1). The most prevalent types were HPV-51 (4.9%), HPV-18 (3.3%), HPV-58 (3.3%), and HPV-66 (3.0%). Among the 73 hrHPV positive women, 23.2% were positive for types HPV-16 or HPV-18. HrHPV prevalence was negatively associated with age, as women younger than 30 years had a higher prevalence than women aged 30 or older (Table 5). In an age-adjusted analysis, women who reported having children younger than 18 years living the household were less likely to have hrHPV than women who reported not having children living in the household.
Figure 1.
Prevalence of type-specific high-risk (hr) human papillomavirus (HPV) infection (n=329). Error bars represent 95% confidence intervals. No samples tested positive for hrHPV type 33.
Table 5.
Relative risk for any high-risk (hr) human papillomavirus (HPV) infection according to demographic characteristics and health history (n=329)
| Any hrHPV infection | ||||||
|---|---|---|---|---|---|---|
| No |
Yes |
|||||
| Characteristic | N | Row % | N | Row % | Age-adjusteda relative risk | 95% CI |
| Demographic | ||||||
| Age (years) | ||||||
| 21-24 | 21 | 62 | 13 | 38 | Ref | |
| 25-29 | 20 | 57 | 15 | 43 | 1.1 | 0.5-2.4 |
| 30-39 | 46 | 78 | 13 | 22 | 0.6 | 0.3-1.2 |
| 40-49 | 52 | 80 | 13 | 20 | 0.5 | 0.2-1.1 |
| 50+ | 117 | 86 | 19 | 14 | 0.4 | 0.2-0.7 |
| Education | ||||||
| Less than high school | 25 | 74 | 9 | 26 | Ref | |
| High school graduate or GED | 50 | 76 | 16 | 24 | 0.9 | 0.4-2.1 |
| Some college or technical school | 126 | 77 | 37 | 23 | 0.9 | 0.4-1.9 |
| College associate degree or higher | 41 | 84 | 8 | 16 | 0.9 | 0.3-2.3 |
| Employment status | ||||||
| Employed full- or part-time | 111 | 79 | 30 | 21 | Ref | |
| Unemployed or laid off | 21 | 68 | 10 | 32 | 1.3 | 0.7-2.7 |
| Keeping house or raising children | 75 | 77 | 23 | 23 | 0.9 | 0.5-1.6 |
| Other | 31 | 84 | 6 | 16 | 0.9 | 0.4-2.1 |
| Marital status | ||||||
| Divorced, separated, widowed, or never been married | 114 | 75 | 38 | 25 | Ref | |
| Married or living with partner | 128 | 80 | 33 | 21 | 0.7 | 0.4-1.1 |
| Children < 18 years old living in household | ||||||
| No | 52 | 67 | 26 | 33 | Ref | |
| Yes | 175 | 80 | 45 | 20 | 0.5 | 0.3-0.8 |
| Health | ||||||
| General health | ||||||
| Very good or excellent | 107 | 77 | 32 | 23 | Ref | |
| Good | 110 | 78 | 31 | 22 | 1.0 | 0.6-1.7 |
| Fair or poor | 32 | 78 | 9 | 22 | 1.1 | 0.5-2.4 |
| Smoking status | ||||||
| Never | 166 | 80 | 42 | 20 | Ref | |
| Former | 42 | 82 | 9 | 18 | 0.9 | 0.4-1.9 |
| Current | 39 | 66 | 20 | 34 | 1.5 | 0.9-2.6 |
| Ever been pregnant | ||||||
| No | 15 | 54 | 13 | 46 | Ref | |
| Yes | 232 | 80 | 59 | 20 | 0.6 | 0.3-1.0 |
| Received ≥ 1 HPV vaccineb | ||||||
| No | 234 | 95 | 12 | 5 | Ref | |
| Yes | 60 | 92 | 5 | 8 | 1.0 | 0.3-3.2 |
| Received a Pap test within the past 3 yearsc | ||||||
| No | 62 | 78 | 17 | 22 | Ref | |
| Yes | 169 | 76 | 52 | 24 | 1.1 | 0.7-2.0 |
CI = confidence interval
All analyses were adjusted for continuous age, except for the analysis of the association between categorical age and hrHPV prevalence.
Analysis was restricted to HPV types 16 and 18.
Based on available clinic records (93%) and self-report (7%). Hysterectomized women were excluded.
DISCUSSION
Since 2012, guidelines in the United States have recommended Pap/HPV co-testing as a strategy for cervical cancer screening in women aged 30 years and older [9]. In 2014, the first HPV test was FDA-approved for primary HPV screening (whereby a Pap test or colposcopy is performed only if the HPV test is positive), and guidelines on primary HPV screening for women aged 25 years and older were issued in 2015 [22]. With the expanded use of HPV testing in clinical practice, it is conceivable that self-collected samples, which are as sensitive as clinician-collected samples for detecting HPV infections [10], could soon be a guideline-acceptable option for improving uptake of cervical cancer screening. In our study population, the percentage of screening-eligible women categorized as adherent to screening (73.4%) was comparable to national estimates in the United States (73.2% in 2010 [23]), but considerably higher than the estimate of 54% for Hopi women served by the NBCCEDP between 2008 and 2013. This discrepancy is likely due to self-selection bias among participants in our study. Nonetheless, our data confirm that, as in the United States general population, a significant proportion of Hopi women are underscreened. Our results suggest that offering self-sampling for HPV testing could be effective in overcoming several barriers to cervical cancer screening encountered on the Hopi Reservation.
Ninety-three percent of participants self-collected a sample for HPV testing, either at home or in the HCSS office, and all samples had sufficient DNA for HPV testing. This result demonstrates the feasibility of self-collection among Hopi women. As in other studies, most women reported positive attitudes toward self-sampling [6, 8, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40], with almost all reporting that the test was easy to use, and that they would recommend it to a friend or relative. A minority reported discomfort. Associated comments indicated that discomfort tended to be minor, with each of the following reported by a few women: dryness, bleeding, cramping, and pinching.
Most women in our study preferred self-sampling over Pap testing by a clinician as a modality for HPV testing. Most previous studies have also reported that a majority of participating women preferred self-sampling [6, 8, 24, 25, 27, 29, 30, 31, 35, 36, 37, 38], although some have returned the opposite finding [26, 28, 34, 39]. In our study, a higher proportion of women who were not adherent to Pap screening, relative to adherent women, preferred self-testing over clinician testing. A similar trend has also been reported for rural women in El Salvador [35], suggesting that this strategy could be particularly effective for hard-to-reach populations. Whereas a handful of other studies have reported that preference for self-sampling was inversely related to educational level [35, 39] or cervical cancer knowledge [26], these factors were not associated with test preference in our analyses.
As in other study populations, common reasons cited for preferring HPV self-testing over Pap testing were enhanced privacy and reduced embarrassment [6, 8, 27, 29, 30, 33, 34, 35, 37] and ease and convenience (including not having to travel to a clinic or take time off work) [6, 8, 24, 25, 30, 31, 32, 35, 37, 39]. Nevertheless, almost two-thirds of participants in our study chose to visit the HCSS office either to perform self-sampling on site (21%) or to hand-deliver a sample collected at home (42%). Only 11% returned their samples by mail. In this context we note that residences on the Hopi reservation do not have private mailboxes, and that traveling to the post office is less convenient for some women than traveling to a clinic. Approximately one-fourth of participants requested that their samples be picked up from their homes by a community health representative or project coordinator.
Among the minority of participants who preferred Pap testing over HPV self-sampling, the primary reasons cited were clinicians’ professionalism and participants’ belief that the Pap test would be more accurate. The same themes have also been noted in previous studies [24, 25, 26, 27, 28, 29, 30, 31, 34, 35, 39]. If self-sampling is adopted in clinical practice, future efforts to educate patients might emphasize the accuracy of self-sampling for HPV testing. Furthermore, as in earlier studies [30, 31], several women cited opportunities to receive other health care and to obtain immediate answers to their questions as reasons for preferring clinic-based Pap testing.
To our knowledge, this is the first study to document hrHPV prevalence in Hopi women. We found that prevalence peaked in the 20s and declined with increasing age. This trend was similar to age-specific hrHPV prevalence patterns reported in 4,150 women who performed self-sampling from 2003 to 2006 in the population-based National Health and Nutrition Examination Survey (NHANES) in the United States. However, prevalence peaked in the early 20s in NHANES, but in the late 20s in our study [41]. We emphasize that differences in populations, sampling methods for HPV testing, and HPV testing assays (including the number of hrHPV types included in assays) complicate inter-study comparisons of absolute prevalence. Overall, 22% of women in our study were positive for hrHPV, including any of 14 established high-risk types. In comparison, hrHPV prevalence was 29% in NHANES (age range 14-59 years and testing for 23 types) [41], 30% in 235 American Indian women recruited from Indian Health Service clinics in the Northern Plains (age range 18-65 years and testing for 17 types) [16], and 33% in 291 American Indian women recruited from clinics (e.g., for family planning, primary care, or screening sexually transmitted disease) in 6 United States cities (age range 14-65 years and testing for 22 types) [17].
Twenty-three percent of women testing positive for hrHPV in our study were infected with either HPV-16 or HPV-18, equivalent to the proportion reported for American Indian and non-American Indian women recruited from urban health clinics in the United States during the pre-vaccine era (2003-2005) [17]. Although 21% of women in our study reported a history of prophylactic HPV vaccination, vaccination status was not associated with HPV-16 or HPV-18 infection after adjusting for age. To establish protection before sexual debut, the preferred age for HPV vaccination is 11-12 years, with catch-up vaccination recommended up to age 26 [42]. Given the age range of our study population, and the fact that HPV vaccines were not commercially available until 2006, most vaccinated women in our study were likely vaccinated after sexual debut. Therefore, the lack of association between vaccination status and prevalence of HPV-16 and HPV-18 infection is not surprising.
In an age-adjusted analysis, women who reported having children living in the household were less likely to have hrHPV infection than women who reported not having children living in the household. Other than age, no other risk factors evaluated were significantly associated with hrHPV infection. To our knowledge, only one other study has evaluated risk factors for prevalent HPV infection in American Indian women [15]. In that study, which assessed women in the Northern Plains, age and current smoking were the only risk factors independently associated with HPV infection, with age inversely associated and smoking positively associated.
Limitations of our study include a self-selected group of women willing to self-sample for HPV testing. As our study population represented less than 10% of adult Hopi women, our results might not be generalizable to other women in the tribe. Furthermore, given the diversity of American Indian tribes, our results among Hopi women might not be generalizable to women of other tribes. In addition, although our sample size was larger than most prior HPV prevalence studies in American Indians [16, 17, 43], it was smaller than most such studies in other racial groups, so we had limited power to evaluate risk factors for hrHPV infection. Furthermore, at the request of our community partners, sexual behavior variables (e.g. lifetime number of sex partners) were excluded from the risk factor analysis, thus limiting comparisons to other populations. Finally, our cross-sectional study design did not permit assessment of HPV incidence or natural history parameters that might shed light on differences in cervical cancer rates between American Indian women and women of other racial groups.
In sum, our results suggest that self-sampling for HPV testing is feasible and acceptable to Hopi women, and could be effective in improving cervical cancer screening coverage in this population. Future studies among the Hopi should explore preferences for home-based versus clinic-based sampling, as well as preferred strategies for follow-up on positive HPV results. Our data do not suggest any notable differences in the epidemiology of hrHPV infections in Hopi women that might contribute to observed disparities in cervical cancer incidence.
Acknowledgments
Funding: This research was performed under the auspices of the Collaborative to Improve Native Cancer Outcomes, a P50 program project sponsored by the National Cancer Institute (grant no. 1P50CA148110). The National Cancer Institute had no involvement in the study design; collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
We are grateful to the women who participated in this project, and to the Hopi Tribal Council and Lorencita Joshweseoma for their support. We thank our local project coordinators, Olivia Dennis and Lorene Vicente, for their coordination efforts, and our community advisors, Carrie Watahomagie, Lisa Lomavaya, and Marilyn Fredericks, for their input and advice. We also thank Odile Lallemand, John Lin, and Lisa Vu at the University of Washington for their assistance with project coordination, and we thank Raymond Harris at the University of Washington for editing the final manuscript.
Footnotes
The Collaborative to Improve Native Cancer Outcomes includes D. Buchwald, D.R. Flum, E.M. Garroutte, A.A. Gonzales, J.A. Henderson, P. Nez Henderson, D.L. Patrick, S.P. Tu, and R.L. Winer.
Conflict of Interest: The authors declare that they have no conflict of interest.
REFERENCES
- 1.Bosch FX, Broker TR, Forman D, Moscicki AB, Gillison ML, Doorbar J, et al. Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31(Suppl 7):H1–31. doi: 10.1016/j.vaccine.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benard VB, Thomas CC, King J, Massetti GM, Doria-Rose VP, Saraiya M. Vital signs: cervical cancer incidence, mortality, and screening - United States, 2007-2012. MMWR Morb Mortal Wkly Rep. 2014;63(44):1004–1009. [PMC free article] [PubMed] [Google Scholar]
- 3.Leyden WA, Manos MM, Geiger AM, Weinmann S, Mouchawar J, Bischoff K, et al. Cervical cancer in women with comprehensive health care access: attributable factors in the screening process. J Natl Cancer Inst. 2005;97(9):675–683. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- 4.Watson M, Benard V, Thomas C, Brayboy A, Paisano R, Becker T. Cervical cancer incidence and mortality among American Indian and Alaska Native women, 1999-2009. Am J Public Health. 2014;104(Suppl 3):S415–422. doi: 10.2105/AJPH.2013.301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coe K, Martin L, Nuvayestewa L, Attakai A, Papenfuss M, De Zapien JG, et al. Predictors of Pap test use among women living on the Hopi reservation. Health Care Women Int. 2007;28(9):764–781. doi: 10.1080/07399330701562956. [DOI] [PubMed] [Google Scholar]
- 6.Catarino R, Jr., Vassilakos P, Stadali-Ullrich H, Royannez-Drevard I, Guillot C, Petignat P. Feasibility of at-home self-sampling for HPV testing as an appropriate screening strategy for nonparticipants in Switzerland: preliminary results of the DEPIST study. J Low Genit Tract Dis. 2015;19(1):27–34. doi: 10.1097/LGT.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 7.Waller J, Bartoszek M, Marlow L, Wardle J. Barriers to cervical cancer screening attendance in England: a population-based survey. J Med Screen. 2009;16(4):199–204. doi: 10.1258/jms.2009.009073. [DOI] [PubMed] [Google Scholar]
- 8.Sultana F, Mullins R, English DR, Simpson JA, Drennan KT, Heley S, et al. Women's experience with home-based self-sampling for human papillomavirus testing. BMC Cancer. 2015;15:849. doi: 10.1186/s12885-015-1804-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542. doi: 10.1309/AJCPTGD94EVRSJCG. [DOI] [PubMed] [Google Scholar]
- 10.Petignat P, Faltin DL, Bruchim I, Tramer MR, Franco EL, Coutlee F. Are self-collected samples comparable to physician-collected cervical specimens for human papillomavirus DNA testing? A systematic review and meta-analysis. Gynecol Oncol. 2007;105(2):530–535. doi: 10.1016/j.ygyno.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Arbyn M, Castle PE. Offering Self-Sampling Kits for HPV Testing to Reach Women Who Do Not Attend in the Regular Cervical Cancer Screening Program. Cancer Epidemiol Biomarkers Prev. 2015;24(5):769–772. doi: 10.1158/1055-9965.EPI-14-1417. [DOI] [PubMed] [Google Scholar]
- 12.Arbyn M, Verdoodt F, Snijders PJ, Verhoef VM, Suonio E, Dillner L, et al. Accuracy of human papillomavirus testing on self-collected versus clinician-collected samples: a meta-analysis. Lancet Oncol. 2014;15(2):172–183. doi: 10.1016/S1470-2045(13)70570-9. [DOI] [PubMed] [Google Scholar]
- 13.Schmeink CE, Bekkers RL, Massuger LF, Melchers WJ. The potential role of self-sampling for high-risk human papillomavirus detection in cervical cancer screening. Rev Med Virol. 2011;21(3):139–153. doi: 10.1002/rmv.686. [DOI] [PubMed] [Google Scholar]
- 14.Racey CS, Withrow DR, Gesink D. Self-collected HPV testing improves participation in cervical cancer screening: a systematic review and meta-analysis. Can J Public Health. 2013;104(2):e159–166. doi: 10.1007/BF03405681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell MC, Schmidt-Grimminger D, Jacobsen C, Chauhan SC, Maher DM, Buchwald DS. Risk factors for HPV infection among American Indian and white women in the Northern Plains. Gynecol Oncol. 2011;121(3):532–536. doi: 10.1016/j.ygyno.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt-Grimminger DC, Bell MC, Muller CJ, Maher DM, Chauhan SC, Buchwald DS. HPV infection among rural American Indian women and urban white women in South Dakota: an HPV prevalence study. BMC Infect Dis. 2011;11:252. doi: 10.1186/1471-2334-11-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alfonsi GA, Datta SD, Mickiewicz T, Koutsky LA, Ghanem K, Hagensee M, et al. Prevalence of high-risk HPV types and abnormal cervical cytology in American Indian/Alaska Native women, 2003-2005. Public Health Rep. 2011;126(3):330–337. doi: 10.1177/003335491112600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harper DM, Longacre MR, Noll WW, Belloni DR, Cole BF. Factors affecting the detection rate of human papillomavirus. Ann Fam Med. 2003;1(4):221–227. doi: 10.1370/afm.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10(4):321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 20.Cherne S, Popov V, Feng Q. Protocol for the detection and genotyping of human papillomaviruses using a liquid bead microarray assay. Methods Mol Biol. 2012;903:205–223. doi: 10.1007/978-1-61779-937-2_13. [DOI] [PubMed] [Google Scholar]
- 21.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 22.Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: Interim clinical guidance. Gynecol Oncol. 2015;136(2):178–182. doi: 10.1016/j.ygyno.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 23.National Center for Health Statistics. Health, United States Table 84. Use of Pap smears among women aged 18 and over, by selected characteristics: United States, selected years 1987–2010. 2013 [Google Scholar]
- 24.Bansil P, Wittet S, Lim JL, Winkler JL, Paul P, Jeronimo J. Acceptability of self-collection sampling for HPV-DNA testing in low-resource settings: a mixed methods approach. BMC Public Health. 2014;14:596. doi: 10.1186/1471-2458-14-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbee L, Kobetz E, Menard J, Cook N, Blanco J, Barton B, et al. Assessing the acceptability of self-sampling for HPV among Haitian immigrant women: CBPR in action. Cancer Causes Control. 2010;21(3):421–431. doi: 10.1007/s10552-009-9474-0. [DOI] [PubMed] [Google Scholar]
- 26.Berner A, Hassel SB, Tebeu PM, Untiet S, Kengne-Fosso G, Navarria I, et al. Human papillomavirus self-sampling in Cameroon: women's uncertainties over the reliability of the method are barriers to acceptance. J Low Genit Tract Dis. 2013;17(3):235–241. doi: 10.1097/LGT.0b013e31826b7b51. [DOI] [PubMed] [Google Scholar]
- 27.Dzuba IG, Diaz EY, Allen B, Leonard YF, Lazcano Ponce EC, Shah KV, et al. The acceptability of self-collected samples for HPV testing vs. the pap test as alternatives in cervical cancer screening. J Womens Health Gend Based Med. 2002;11(3):265–275. doi: 10.1089/152460902753668466. [DOI] [PubMed] [Google Scholar]
- 28.Guan Y, Castle PE, Wang S, Li B, Feng C, Ci P, et al. A cross-sectional study on the acceptability of self-collection for HPV testing among women in rural China. Sex Transm Infect. 2012 doi: 10.1136/sextrans-2012-050477. [DOI] [PubMed] [Google Scholar]
- 29.Igidbashian S, Boveri S, Spolti N, Radice D, Sandri MT, Sideri M. Self-collected human papillomavirus testing acceptability: comparison of two self-sampling modalities. J Womens Health (Larchmt) 2011;20(3):397–402. doi: 10.1089/jwh.2010.2189. [DOI] [PubMed] [Google Scholar]
- 30.Jones HE, Brudney K, Sawo DJ, Lantigua R, Westhoff CL. The acceptability of a self-lavaging device compared to pelvic examination for cervical cancer screening among low-income women. J Womens Health (Larchmt) 2012;21(12):1275–1281. doi: 10.1089/jwh.2012.3512. [DOI] [PubMed] [Google Scholar]
- 31.Litton AG, Castle PE, Partridge EE, Scarinci IC. Cervical cancer screening preferences among African American women in the Mississippi Delta. J Health Care Poor Underserved. 2013;24(1):46–55. doi: 10.1353/hpu.2013.0017. [DOI] [PubMed] [Google Scholar]
- 32.Montealegre JR, Mullen PD, M, L. J.-W., Vargas Mendez MM, Scheurer ME. Feasibility of Cervical Cancer Screening Utilizing Self-sample Human Papillomavirus Testing Among Mexican Immigrant Women in Harris County. J Immigr Minor Health. A Pilot Study; Texas: 2014. [DOI] [PubMed] [Google Scholar]
- 33.Oranratanaphan S, Termrungruanglert W, Khemapech N. Acceptability of self-sampling HPV testing among Thai women for cervical cancer screening. Asian Pac J Cancer Prev. 2014;15(17):7437–7441. doi: 10.7314/apjcp.2014.15.17.7437. [DOI] [PubMed] [Google Scholar]
- 34.Quincy BL, Turbow DJ, Dabinett LN. Acceptability of self-collected human papillomavirus specimens as a primary screen for cervical cancer. J Obstet Gynaecol. 2012;32(1):87–91. doi: 10.3109/01443615.2011.625456. [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaum AJ, Gage JC, Alfaro KM, Ditzian LR, Maza M, Scarinci IC, et al. Acceptability of self-collected versus provider-collected sampling for HPV DNA testing among women in rural El Salvador. Int J Gynaecol Obstet. 2014;126(2):156–160. doi: 10.1016/j.ijgo.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Trope LA, Chumworathayi B, Blumenthal PD. Feasibility of community-based careHPV for cervical cancer prevention in rural Thailand. J Low Genit Tract Dis. 2013;17(3):315–319. doi: 10.1097/LGT.0b013e31826b7b70. [DOI] [PubMed] [Google Scholar]
- 37.Virtanen A, Nieminen P, Niironen M, Luostarinen T, Anttila A. Self-sampling experiences among non-attendees to cervical screening. Gynecol Oncol. 2014;135(3):487–494. doi: 10.1016/j.ygyno.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Waller J, McCaffery K, Forrest S, Szarewski A, Cadman L, Austin J, et al. Acceptability of unsupervised HPV self-sampling using written instructions. J Med Screen. 2006;13(4):208–213. doi: 10.1177/096914130601300409. [DOI] [PubMed] [Google Scholar]
- 39.Anhang R, Nelson JA, Telerant R, Chiasson MA, Wright TC., Jr. Acceptability of self-collection of specimens for HPV DNA testing in an urban population. J Womens Health (Larchmt) 2005;14(8):721–728. doi: 10.1089/jwh.2005.14.721. [DOI] [PubMed] [Google Scholar]
- 40.Penaranda E, Molokwu J, Flores S, Byrd T, Brown L, Shokar N. Women's Attitudes Toward Cervicovaginal Self-Sampling for High-Risk HPV Infection on the US-Mexico Border. J Low Genit Tract Dis. 2015;19(4):323–328. doi: 10.1097/LGT.0000000000000134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hariri S, Unger ER, Sternberg M, Dunne EF, Swan D, Patel S, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003-2006. J Infect Dis. 2011;204(4):566–573. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 42.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014;63(RR-05):1–30. [PubMed] [Google Scholar]
- 43.Bell MC, Schmidt-Grimminger D, Patrick S, Ryschon T, Linz L, Chauhan SC. There is a high prevalence of human papillomavirus infection in American Indian women of the Northern Plains. Gynecol Oncol. 2007;107(2):236–241. doi: 10.1016/j.ygyno.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]