Abstract
Management of MRI-negative patients with intractable focal epilepsy after failed surgery is particularly challenging. In this study, we aim to investigate whether MRI post-processing could identify relevant targets for the re-evaluation of MRI-negative patients who failed the initial resective surgery. We examined a consecutive series of 56 MRI-negative patients who underwent resective surgery and had recurring seizures at 1-year follow-up. T1-weighted volumetric sequence from the pre-surgical MRI was used for voxel-based MRI post-processing which was implemented in a morphometric analysis program (MAP). MAP was positive in 15 of the 56 patients included in this study. In 5 patients, the MAP+ regions were fully resected. In 10 patients, the MAP+ regions were not or partially resected: two out of the 10 patients had a second surgery including the unresected MAP+ region, and both became seizure-free; the remaining 8 patients did not undergo further surgery, but the unresected MAP+ regions were concordant with more than one noninvasive modality in 7. In the 8 patients who had unresected MAP+ regions and intracranial-EEG before the previous surgery, the unresected MAP+ regions were concordant with ictal onset in 6. Our data suggest that scrutiny of the presurgical MRI guided by MRI post-processing may reveal relevant targets for reoperation in nonlesional epilepsies. MAP findings, when concordant with the patient's other noninvasive data, should be considered when planning invasive evaluation/reoperation for this most challenging group of patients.
Keywords: Epilepsy, Presurgical evaluation, MRI-negative, Voxel-based morphometry, Focal cortical dysplasia, Failed surgery
Introduction
Focal resective surgery is the most effective treatment for medically intractable epilepsy, especially in patients with a visible focal lesion identified on magnetic resonance imaging (MRI). The lack of a lesion on MRI has been shown as a predictor for surgical failure [1–3]. Evaluation and management of patients with nonlesional epilepsy after failed surgery is even more challenging, and can be strongly influenced by the collective expertise and experience in clinical, neurophysiological and imaging data interpretation [4]. Failure of the first surgery may have many complicated reasons: a wrong initial hypothesis, discordant MRI and electroclinical findings, spatial sampling bias during intracranial evaluation, limited resection due to overlap with functional cortex, multiple seizure foci, and finally, reorganization of the epileptic network [5, 6]. Moreover, new challenges present such as breach effect from the postoperative skull defects, which can severely distort scalp-EEG localization of epileptic events. At this moment, studies reported seizure-free rates for reoperation vary quite considerably from 9.5 to 57 % with considerable room for improvement [5, 7]. In devising the reoperation plan for the failed patients, any novel noninvasive technique which can add to the evaluation test battery is highly valued.
Focal cortical dysplasia (FCD) is the most common underlying pathology in epilepsies with apparently normal MRI [8]. MRI post-processing has been demonstrated to be effective in identifying subtle focal cortical dysplasia lesions in patients whose MRI scans were read as normal by visual analysis [9–12]. In this study, we aim to investigate whether MRI post-processing (performed on the pre-operative scans) could also help identify relevant targets for the presurgical evaluation of patients with previously failed surgery. We utilized a voxel-based MRI morphometric analysis program (MAP) to model gray–white matter junction blurring, a characteristic MRI feature of FCD [13]. The program provides a gray–white junction z score feature map, which guides the MRI reader's attention to suspicious brain regions. We then evaluated the epileptogenic relevance of the MAP-detected abnormalities, based on surgery (if available), electrophysiological and/or functional neuroimaging data.
Patients and methods
Study design and patient selection
This retrospective study was approved by the Cleveland Clinic institutional review board. We reviewed our surgical database over a 10-year period (2002–2011) and identified all MRI-negative patients who failed the first surgery. Inclusion criteria were: (1) patient had recurring seizures within 12 months of follow-up; (2) MRI was considered negative by radiology report, i.e., after being reviewed prospectively by dedicated epilepsy neuroradiologists; and (3) 1.5- or 3-Tesla MRI before the first surgery was available and included a T1-weighted Magnetization Prepared Rapid Acquisition with Gradient Echo (MPRAGE) sequence. Patients were excluded if they were less than 5 years old or had poor MRI quality.
MRI Post-processing
MAP was not previously available and therefore did not influence the preoperative hypothesis/surgical decision-making. MAP was carried out in SPM12 (Wellcome Department of Cognitive Neurology, London, UK) in MATLAB 2015a (MathWorks, Natick, Massachusetts) [9, 10]. MAP was performed on T1-weighted MPRAGE images acquired before the initial failed surgery. We used a 1.5T and 3T average normal database of 150 subjects (70 females, 80 males; mean age at MRI = 30.9 years, range = 15–77 years), with MRIs acquired on 5 different MRI scanners, which was kindly provided along with the MAP program [10]. Within MAP, segmentation was based on the former SPM5 algorithms for unified segmentation (3-class).
The computed output consists of three volumetric statistical maps, the junction, extension, and thickness files. All three files highlight brain structures in each individual patient which deviate from the average normal brain based on high z scores, and may therefore correlate with the presence of subtle features of FCD on the corresponding MRI. The junction file is sensitive to blurring of the gray-white matter junction. The extension file is sensitive to abnormal gyration and extension of gray matter into white matter. The thickness file is sensitive to abnormal cortical thickness.
MAP was performed by ZIW who was blinded to the patient's clinical and imaging information. Candidate MAP+ abnormalities were those with significant deviation from the standard brain on the junction file (z score > 4). High z score areas caused by signal inhomogeneities due to technical reasons and nonspecific white matter lesions were excluded from review. With guidance from MAP findings, a neuroradiologist (SEJ) conducted a focused re-review of the pre-surgical clinical MRI (with T1-weighted MPRAGE, T2-weighted FLAIR and TSE sequences, imaging parameters as detailed elsewhere [14]), blinded to the patient's clinical and surgical information, in order to confirm or dismiss each candidate MAP high-z score area. If the neuroradiologist agreed that the conventional MRI showed subtle changes at these sites, the patient was regarded as MAP+. After the MAP+ positive regions were finalized, clinical and surgical information was unblinded. This methodology is consistent with our previous studies [12, 14] and literature [11, 15].
Comparing MAP+ regions and resection
In all the MAP+ patients, we used SPM12 to coregister the preoperative T1 and the MAP junction file with the post-operative MRI, which was obtained 6 months after the initial resective surgery. We then determined whether the location of the MAP+ regions were included in the resection cavity. MAP+ regions were categorized as: fully resected, partially resected or not resected.
Concordance with functional imaging, electrophysiology and pathology
We evaluated the concordance between the MAP+ regions and all the available functional imaging/electrophysiology data acquired at the previous or later investigations, including scalp-EEG, FDG-PET, ictal SPECT, Magnetoencephalography (MEG), and intracranial EEG (ICEEG).
Scalp-EEG monitoring was performed using the international 10–20 system of electrode placement, including anterior temporal electrodes and sphenoidal electrodes if appropriate. FDG-PET scans were performed on Siemens Biograph PET CT. Scans were coregistered to the volumetric MRI and visual analysis was performed by a nuclear medicine physician. SPECT images were acquired on a Siemens Symbia dual-head camera. The interictal image was subtracted from the ictal image and analyzed following methodology of subtraction ictal SPECT coregistered to MRI (SISCOM) [16]. MEG data was recorded from a 306-channel whole-head MEG system (Elekta, Helsinki, Finland), and individual spike source localization was performed on data segments containing visually identified epileptiform discharges using single equivalent current dipole model. For each patient, the strategy for ICEEG implantation and surgical resection was discussed at a multi-disciplinary patient management conference based on MRI, video-EEG, PET, and MEG. ICEEG studies were performed using a combination of subdural grid arrays and strip electrodes, or stereotactic-EEG (SEEG) depth electrodes.
Localization data of scalp-EEG, SPECT, PET, MEG and ICEEG were obtained from a comprehensive review of medical records. Tests were considered to be concordant with MAP if localization from both modalities had overlap in the same sublobar (lobar) region. Sublobar/lobar regions of the neocortex were defined using a common classification scheme [17]. Due to the retrospective nature of the study, none of the unresected MAP+ regions were directly targeted by ICEEG; we consider a unresected MAP+ region to be concordant with ICEEG, when the ICEEG interictal and/or ictal discharges were in the same lobe as the location of the MAP+ region. All available microscopic slides from surgical resections were reviewed in all cases (RAP) and FCD was classified following the Palmini classification [18].
Results
From 2002 to 2011, a total of 173 consecutive MRI-negative patients had resective epilepsy surgery; 69 patients had recurring seizures at 1-year follow-up. Excluding the 13 patients whose MRI were not available or had severe artifacts, a total of 56 patients fulfilled the inclusion criteria and underwent MAP processing. Mean age at time of surgery was 29.4 years (median 28.5, SD ± 14.5, range 9–65), and mean duration of epilepsy 14.3 years (median 13, SD ± 11.1, range 0.5–53). Twenty-one patients (38 %) were female. Forty-eight (86 %) were right-handed.
MAP+ Regions and resection
MAP was positive in 15 (27 %) of the 56 patients. The MAP+ regions were resected only partially or had not been included in the resection in 10 patients (Supplementary Table 1). In the other 5 patients, the MAP+ regions had been fully resected (Supplementary Table 2).
Patients with MAP+ regions not or partially resected
Of the 10 patients whose MAP+ regions were not or partially resected, 8 had invasive monitoring as part of their evaluation for the initial surgery, including 6 subdural grids/depth and 2 SEEG implantations. Seizures recurred at an average of 1.8 months (range 1 day–5 months) after resective surgery. Eight patients (80 %) had relatively early seizure recurrence before 3 months after surgery. The remaining 2 patients (20 %) had seizure recurrence between 3 and 6 months after surgery. Surgical pathology included FCD type IA in 5, type IIA in one and gliosis in 4.
Three of the 10 patients had a single MAP+ region that was not/partially resected. The remaining 7 had multiple MAP+ regions; in 2 patients none of the multiple MAP+ regions were resected and in 5 patients only one of the multiple MAP+ regions was resected. Locations of the unresected MAP+ region in relation to the initial resection were: 5 in the same lobe, 1 in a different lobe on the ipsilateral side, 1 on the contralateral side, 2 multilobar on the ipsilateral side and 1 multilobar bi-hemispheric. In the 8 patients who had ICEEG, the unresected MAP+ regions were concordant with ICEEG ictal onset in 6 (P1, P4, P5, P6, P8, P9).
Significance of unresected MAP+ regions supported by reoperation
Patients 4 and 5
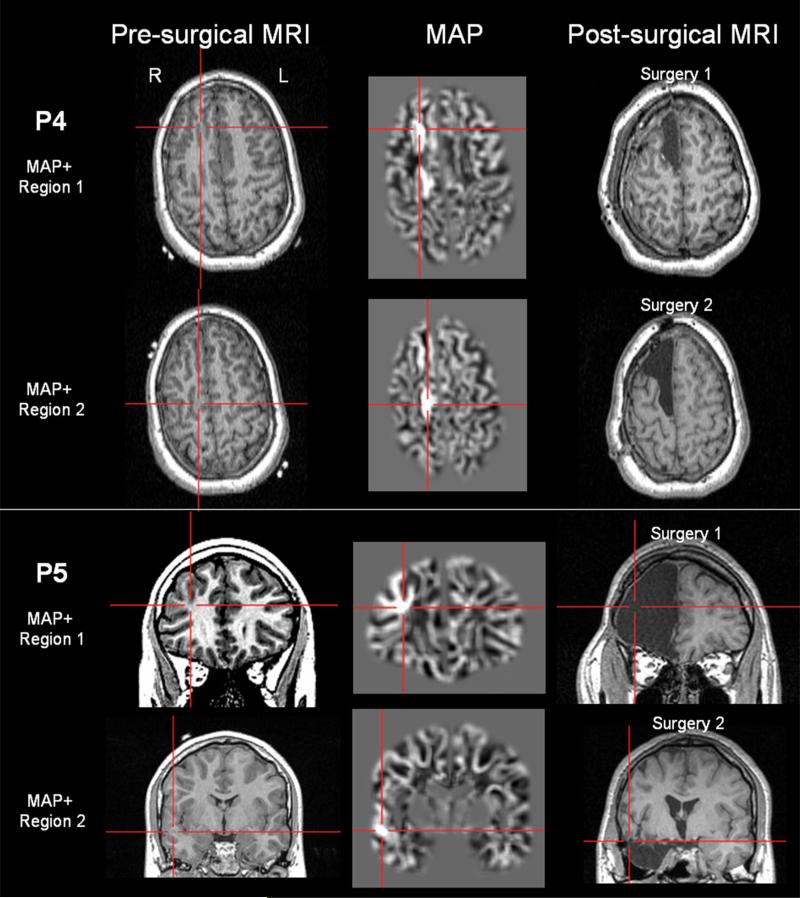
As shown in Fig. 1, P4 and P5 both had two MAP+ regions on their preoperative MRI. They underwent a first surgery which included one of the two regions, and had recurring seizures immediately after surgery. Both of them underwent a second surgery which included the other MAP+ region, and both patients became seizure free.
Fig. 1.
Unresected MAP+ regions supported by reoperation, exemplified in P4 (upper two rows) and P5 (lower two rows). Both patients had two MAP+ regions. For both patients, the first surgery included one of the two MAP+ regions, and seizures recurred immediately after surgery. Both patients underwent a second surgery which included the other MAP+ region, and became seizure-free. The left column shows the T1-MPRAGE images that the MAP processing was based on; the middle column shows the MAP finding; the right column shows the post-operative MRI which confirms resection of the MAP+ areas. In this and the following Figures, MAP+ regions were pinpointed by the crosshairs
Significance of unresected MAP+ region supported by noninvasive and invasive findings
The majority of the included patients (8/10) did not undergo reoperation; therefore, we assessed the epileptogenic relevance of the MAP+ regions by their correlation with all the available preoperative modalities, noninvasive and invasive (detailed in Supplementary Table 3). In 7 of the 8 patients, the unresected MAP+ regions were concordant with more than one noninvasive modality; in 4 patients, the unresected MAP+ regions had lobar concordance with invasive findings.
Patient 6
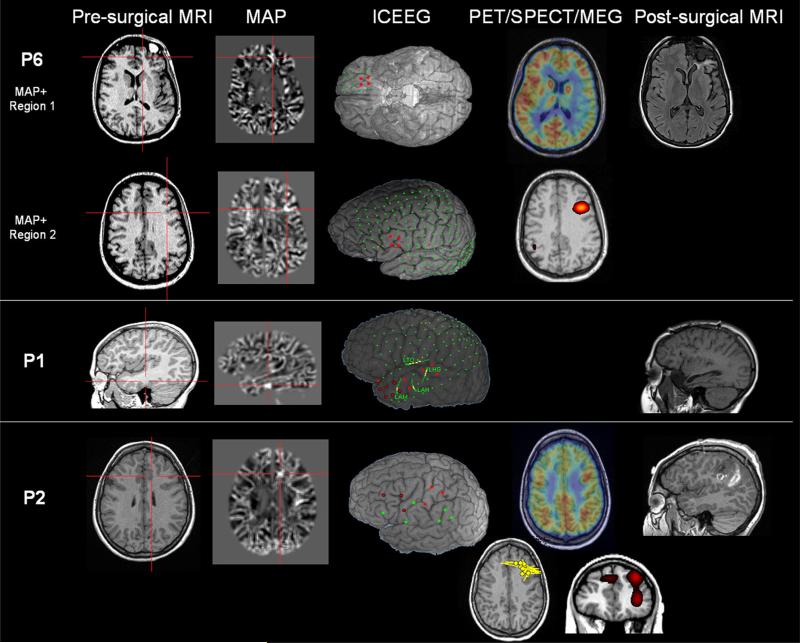
As shown in Fig. 2, P6 had two MAP+ regions: region 1 was in the orbital frontal area which was resected, and region 2 was in the inferior lateral frontal area which was not resected. SISCOM was concordant with the second region. PET showed broad hypometabolism concordant with both regions. During the ICEEG evaluation, the majority of the seizures were recorded from the orbital frontal area, concordant with region 1. However, there was one seizure recorded from the lateral frontal area, concordant with region 2. Similar to the preoperative scalp EEG, postoperative scalp EEG also showed frontotemporal interictal spikes which was concordant with the unresected MAP+ region.
Fig. 2.
Unresected MAP+ region supported by noninvasive and invasive findings, exemplified in P6, P1 and P2. P6 had two MAP+ regions with only one of them resected; P1 and P2 had one MAP+ region (both were not resected). In this and the following Figures, the first column shows the T1-MPRAGE images that the MAP processing was based on; the second column shows the MAP+ regions; columns 3 and 4 show ICEEG/PET/SPECT/MEG findings (whenever available and relevant); the last column shows the post-operative MRI which confirms that the MAP+ areas were not resected. ICEEG localization was shown by overlaying the electrodes (reconstructed from CT and MRI coregistration) on top of a cortical surface generated by the pre-surgical MRI. P6 had subdural grid implantation; ictal onset was shown by red contacts. P1 had subdural grid and additional depth electrodes (marked with LTO, LHG, LAM, LAH). Ictal onset was shown by red contacts on the grid, and ictal onset on the depth electrodes was shown by yellow. For P1, three seizures were recorded during the invasive evaluation, each with slightly different evolution. Seizure 1 started from anterior temporal area (denoted by red outlined contacts). Seizure 2 started from the lateral contacts of two depth electrodes LHG and LTO (denoted by the yellow contacts). Seizure 3 started from anterior and lateral temporal areas (denoted by all the red contacts) as well as lateral contacts of LAM and LAH (denoted by yellow contacts). P2 had stereotactic EEG (SEEG) implantation (for best projection effects, only entry points are shown) and the seizure onset involved the most lateral contacts of the SEEG electrodes. Seizure onset was seen in left post-central and precentral (red contacts), sometimes left SMA regions (red outlined contacts). These contacts also showed interictal discharges. SISCOM was performed using z = 2. For P2, the yellow dipoles showed magnetic source imaging localization based on individual interictal spikes; each dipole represents a single interictal spike captured at different time points of the MEG recording
Patient 1
P1 (Fig. 2) had a single MAP+ region in the left posterior basal temporal area which was concordant with the interictal and ictal scalp-EEG. The location of the MAP+ region fits with the seizure onset zone on ICEEG on a lobar level. Surgical resection was restricted to the anterior temporal area and did not include the MAP+ region. Postoperative scalp EEG also showed interictal spikes in the left temporal region, indicating remnant of epileptic pathology.
Patient 2
P2 (Fig. 2) had a MAP+ region in the left inferior mesial frontal area. This patient has complicated interictal activities in bilateral frontal and vertex regions, and ictal EEG was nonlocalizable (compatible with a mesial frontal focus). MEG showed lobar concordance with the MAP+ finding. PET showed bilateral frontal hypometabolism, which was also compatible with the MAP+ finding. Although the SEEG evaluation showed widespread ictal onset, it was felt that the left parietal lobe was atrophic after re-review of the MRI and a left post-central resection was performed with the understanding that the seizure-free likelihood would be low. Since the patient had immediate seizure recurrence, a postoperative SPECT was performed and showed hyperperfusion in the left insula and left anterior dorsal lateral frontal region, which was concordant with the MAP+ finding. Overall, the unresected MAP+ finding was concordant with MEG, SISCOM and PET, and could explain the widespread ictal SEEG finding.
Patient 8
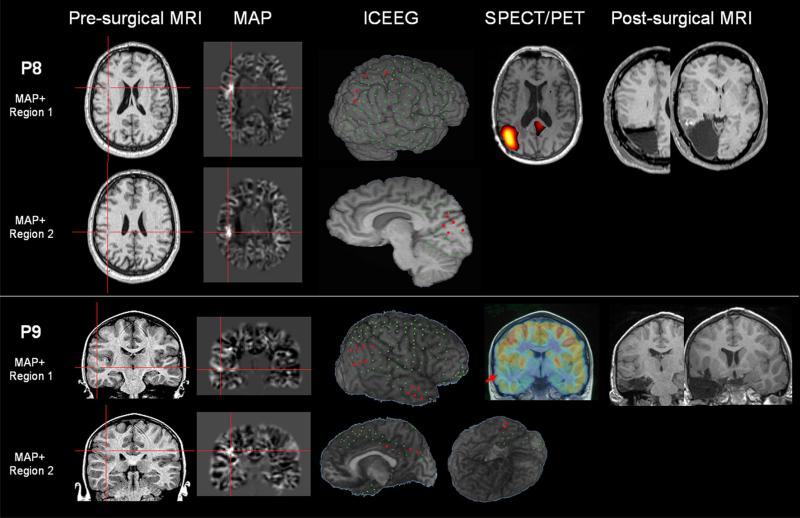
P8 (Fig. 3) had two MAP+ regions and neither was included in the resection. The parietal MAP+ region could fit with the interictal and ictal scalp-EEG localization in the right parieto-occipital region. The right parietal hyperperfusion shown by SISCOM was included in the surgical resection, and the localization had lobar concordance with the parietal MAP+ region. ICEEG showed widespread onset over the right mesial and lateral parieto-occipital cortex, which was also concordant with the parietal MAP+ region.
Fig. 3.
Unresected MAP+ region supported by noninvasive and invasive findings, exemplified in P8 and P9. Both patients had two MAP+ regions, neither of which was resected. Ictal onset was shown by red contacts on the grid. In P8, SISCOM showed multi-lobar localization, involving most prominently left temporal cortex, followed by right anterior frontal and right parietal; only the parietal hyperperfusion is shown. In this Figure and Fig. 4, arrows on the PET images show hypometabolism corresponding to the location of unresected MAP+ regions
Patient 9
P9 (Fig. 3) had two MAP+ regions in the right superior temporal and right inferior parietal areas; neither was included in the resection. Scalp EEG, both interictal and ictal, localized to the right centro-parietal area, which was concordant with the right parietal MAP+ region. PET showed anterior and middle temporal lobe hypometabolism which fit with the right temporal MAP+ region. The ICEEG ictal onset showed repetitive spiking at the anterior temporal lobe with lower amplitude discharges simultaneously at the temporal parietal area both laterally and mesially, which would be compatible with both MAP+ regions.
Significance of unresected MAP+ region supported by noninvasive findings only
In 2 patients, the unresected MAP+ region was supported by noninvasive findings only. In P3, invasive implantation was on the hemisphere contralateral to the MAP+ region; in P7, no invasive evaluation was performed.
Patient 3
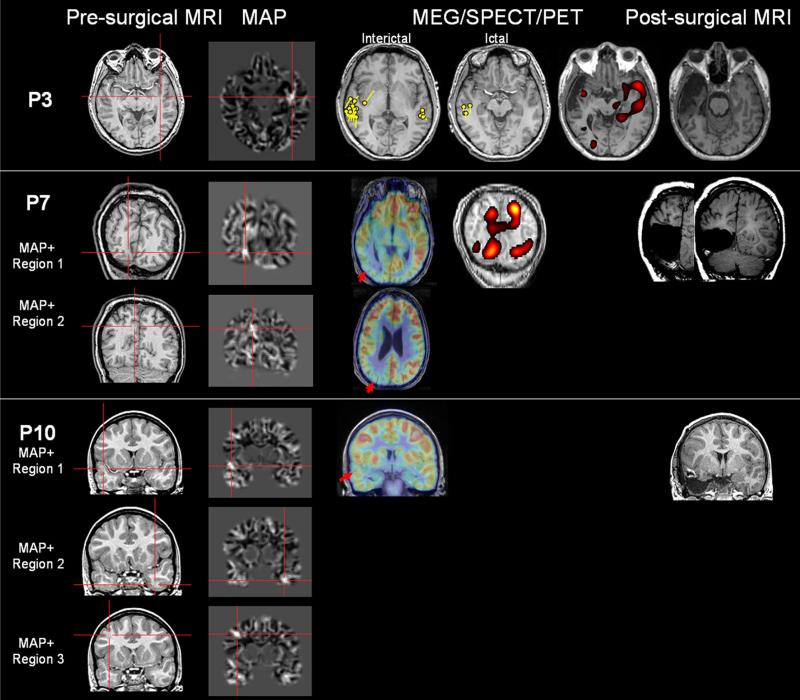
P3 (Fig. 4) had one MAP+ region in the left middle temporal gyrus, but the other preoperative data favored a right temporal localization leading to an unsuccessful right temporal lobectomy. The patient had two different seizure types from different hemispheres as indicated by ictal scalp EEG. Pre-operative interictal and ictal MEG showed the majority of the epileptic activities were from the right temporal area, but there was also a MEG cluster from the left temporal area. Post-operative ictal scalp EEG was lateralized to the left side, and post-operative SISCOM showed activation in the left temporal area. Overall, in P3, the MAP+ region could be relevant based on the preoperative MEG, postoperative SISCOM and postoperative scalp EEG findings, supporting the hypothesis of bi-temporal lobe epilepsy.
Fig. 4.
Unresected MAP+ region supported by noninvasive findings only (P3 and P7), or with unclear relevance (P10). P3 had one MAP+ region which was not resected. P7 had two MAP+ regions, one of which was resected. P10 had three MAP+ regions; only the right temporal one was included in the resection. P3 had both interictal and ictal MEG the yellow dipoles were modeled at the peak of interictal/ictal spikes. For P7, SISCOM was performed using z = 1.5
Patient 7
P7 (Fig. 4) had two MAP+ regions, both in the right occipital lobe, with the more inferior MAP+ region fully resected and the more superior one left alone. Interictal scalp EEG was localized to the right occipito parietal area. Both regions had concordant hypometabolism on PET. SISCOM showed multilobar hyperperfusion in the right temporo-occipital areas (z = 2). With a lowered z score of 1.5 [8], the hyperperfused areas overlapped with both MAP+ regions, with a complex hour-glass pattern, suggesting that ictal activities could have come from these regions. Overall, the preoperative scalp EEG, PET and SISCOM were concordant with both MAP+ regions.
Unresected MAP+ region with unclear significance
Patient 10
P10 (Fig. 4) had three MAP+ regions in the right temporal, left temporal area and right frontal areas. Only the right temporal MAP+ region was included in the surgical resection. Scalp EEG interictal and ictal activities were either generalized or multiregional left and right. PET showed right superior frontal and right temporal hypometabolism; the latter was concordant with the right temporal MAP+ region. No ICEEG was performed. The epileptogenic relevance of the unresected MAP+ regions is unclear.
Patients with MAP+ regions fully resected
Five patients had MAP+ regions fully resected but seizures still recurred, although in 4 patients seizure frequency was reduced. In this subgroup, seizures recurred at an average of 3.3 months (range 10 days–8 months) after resective surgery. Three patients (60 %) had relatively early seizure recurrence before 3 months after surgery. The remaining 2 patients (40 %) had seizure recurrence between 6 and 12 months and postoperatively. Surgical pathology included FCD type IA in 2, FCD type IA with HS in 1 and gliosis in 2. In this group, four patients underwent temporal lobectomy (3 on nondominant side and 1 on dominant side) and the remaining patient underwent frontal lobe resection with electrocorticography. All 5 patients had a single MAP+ region which was included in the resection cavity. In all patients, the MAP+ regions showed concordance with at least one noninvasive modality. In P15 who had SEEG evaluation, the MAP+ region showed lobar concordance with the SEEG ictal finding. The relationship between MAP+ regions and all the available preoperative noninvasive and invasive data are detailed in Supplementary Table 4.
Discussion
The first step of re-evaluating patients with previously “failed” surgery usually starts with a revisit of clinical, MRI, and EEG findings obtained before the first surgery. Our findings highlight that scrutinizing the pre-surgical MRI in such patients, preferably with guidance from MRI post-processing, may shed light on the unresected epileptogenic lesions causing insufficient seizure control. Our study describes this process in a consecutive series of 56 nonlesional patients from our center who failed the initial resective surgery. Because of the difficulty to manage and perform second surgery in these patients, only 2 patients in the cohort had histological confirmation. Nevertheless, our approach could still give an opportunity to guide the reevaluation of these patients, given the high concordance of the MAP+ regions with noninvasive and invasive data.
Among the many reasons for the failure of previous surgery, a missed epileptogenic zone is the most intriguing, as it is tempting to hypothesize that the site of reoperation can be re-discovered after utilizing newer techniques for lesion detection and demarcation. The development of high-resolution MRI and postprocessing techniques in the past decade can be a major player in detecting subtle epileptic pathologies. The majority of the patients in our study had relatively early seizure recurrence, suggesting that the predominant seizure focus has not been removed (i.e., a missed epileptogenic zone) [6, 19]. It is likely that subtle epileptogenic lesions were indeed there on the pre-operative MRI, but escaped detection by visual inspection. These missed lesions are likely FCD, which has been demonstrated as the most common pathology underlying MR-negative epilepsies [20]. MAP is especially sensitive to subtle gray-white matter junction blurring associated with FCD [13] and therefore can be especially suited to pick out these missed lesions. Another advantage of MAP (and other post-processing techniques) is that it operates in 3D and allows the simultaneous consideration of information from consecutive slices, whereas conventional visual analysis examines the brain volume one slice at a time. Studies have reported additional diagnostic yield of MAP in epilepsy patients who are MRI-negative by visual analysis [9, 11, 14]. In the 10 patients included in this study who had unresected MAP+ regions, two patients had a second surgery including the unresected MAP+ region, and both became seizure-free, providing strong evidence of the epileptogenicity of the unresected MAP+ regions. In 7 of the remaining 8 patients who did not get surgery, the unresected MAP+ region was concordant with at more than one noninvasive modality. Although the epileptogenicity of the MAP+ regions in these 7 patients remains to be confirmed, their concordance with noninvasive modalities can serve as evidence for further exploration.
It is plausible that had the MAP findings be used pre-operatively they would have resulted in more electrodes implanted. Therefore, in retrospect, the MAP results could have made a difference in seizure outcomes. It will be intriguing to use the unresected MAP+ foci as new targets for exploration if the patients decide to come back for re-evaluation.
Another potential benefit of our study is the contribution of MRI postprocessing in the re-evaluation of pediatric patients who failed the initial surgery. Although this study was not designed to be a pediatric study and that our normal control database mainly consists of adults, out of the 10 patients in whom we discussed in detail the relevance of the unresected MAP+ regions, 7 were children, ranging from 9 to 15 years of age. As indicated by previous studies, current approaches in pediatric epilepsy surgery reflected a preference for noninvasive modalities, especially improved neuroimaging techniques, to invasive evaluation in the planning of resections [7, 21, 22]. Overall, our findings suggest MAP can be particularly helpful in pediatric patients with FCD-associated epilepsies. We are in the process of acquiring MRI from younger normal controls to improve the performance of MAP in this patient population.
Seven of the 15 MAP+ patients had multiple MAP+ regions. The locations of the MAP+ regions can be in the same lobe, in a different lobe on the ipsilateral side, or on the contralateral side. Previous studies have similarly documented the multifocal presence of MAP+ areas [23]. Theses findings perhaps challenge the assumption of uni-focality of FCD at least in a subset of patients. Although beyond the scope of the current study, such patients need to be carefully identified, systematically characterized and followed up over a long period of time.
In the 5 patients whose MAP+ regions were fully resected but still had recurring seizures, surgical specimens in the majority of patients contained type I FCD, which is known to be associated with less favorable surgical outcomes [13, 24, 25]. Our findings suggest that in these patients, MAP may not be able to accurately delineate the full extent and boundaries of the underlying pathology, therefore, resection containing merely the visible lesion may not have included the entire area of cortical disruption. In these cases, invasive evaluation is essential to map the extent of the cortical abnormalities. The capability of MAP may be improved by incorporating other sequences into the processing such as T2w images [26] and FLAIR images [27], modeling other features of FCD including signal intensity and cortical thickness [28], and using automated statistical thresholding.
The main limitations that should be noted about our study include the following:
Lack of histological validation. Out of the 10 patients who had unresected MAP+ regions, only 2 underwent a second surgery with the validity of MAP+ finding confirmed. For those patients who did not have a second surgery, the epileptogenic relevance of the MAP+ regions can only be inferred at this stage, based on noninvasive and invasive findings. Whether the MAP+ regions are true positives can only be known when the patients have further surgery. Future prospective study using the MAP methodology will add validity to our current study.
Currently examination of MAP and MRI was based on visual inspection alone without using automated detection algorithms, and expert radiologist's review still constitutes an important part of the methodology. Overall, there is a need for an automated statistical thresholding method to improve the sensitivity, specificity, and consistency of MAP.
Last but not least, the patients presented in this study are a selected cohort (all MRI-negative surgical failures) and do not represent the entire population of patients who are epilepsy surgical candidates. One should use caution when generalizing the results from our study.
Conclusions
Our findings suggest that MAP can serve as a valuable noninvasive tool to provide additional complementary information for the re-evaluation of patients who failed the initial surgery. MAP findings, when concordant with the patient's other noninvasive data, should be considered when planning invasive evaluation/reoperation for this most challenging group of patients.
Supplementary Material
Acknowledgments
Supported by the Epilepsy Foundation Postdoctoral Fellowship Grant (Z.I.W.), NIH NINDS grant R01-NS074980 (Z.I.W., S.E.J.). Z.I.W and S.E.J received grant support from NINDS (R01). S.E.J. and J.A.G-M received grant support from CURE. B.K. received grant support from NINDS (R03). Z.I.W. and B.K. received support from Epilepsy Foundation Postdoctoral Fellowship Grant.
Abbreviations
- FLAIR
Fluid-attenuated inversion recovery
- FCD
Focal cortical dysplasia
- MAP
Morphometric analysis program
- MRI
Magnetic resonance imaging
- PFE
Pharmacoresistant focal epilepsy
- ICEEG
Intracranial EEG
- SEEG
Stereotactic EEG
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00415-016-8171-7) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflicts of interest AV.A.: editorial board, Epileptic Disorders. I.M.N.: speaker's bureau, UCB.
Ethical standards This study has been approved by the Cleveland Clinic Institutional Review Board and have, therefore, been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
References
- 1.Bien CG, Szinay M, Wagner J, Clusmann H, Becker AJ, Urbach H. Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging-negative epilepsies. Arch Neurol. 2009;66(12):1491–1499. doi: 10.1001/archneurol.2009.283. [DOI] [PubMed] [Google Scholar]
- 2.Jeha LE, Najm I, Bingaman W, Dinner D, Widdess-Walsh P, Luders H. Surgical outcome and prognostic factors of frontal lobe epilepsy surgery. Brain. 2007;130(Pt 2):574–584. doi: 10.1093/brain/awl364. [DOI] [PubMed] [Google Scholar]
- 3.Tellez-Zenteno JF, Hernandez Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89(2–3):310–318. doi: 10.1016/j.eplepsyres.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Jayakar P, Dunoyer C, Dean P, Ragheb J, Resnick T, Morrison G, Bhatia S, Duchowny M. Epilepsy surgery in patients with normal or nonfocal MRI scans: integrative strategies offer long-term seizure relief. Epilepsia. 2008;49(5):758–764. doi: 10.1111/j.1528-1167.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 5.Surges R, Elger CE. Reoperation after failed resective epilepsy surgery. Seizure. 2013;22(7):493–501. doi: 10.1016/j.seizure.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Najm I, Jehi L, Palmini A, Gonzalez-Martinez J, Paglioli E, Bingaman W. Temporal patterns and mechanisms of epilepsy surgery failure. Epilepsia. 2013;54(5):772–782. doi: 10.1111/epi.12152. [DOI] [PubMed] [Google Scholar]
- 7.Ramantani G, Strobl K, Stathi A, Brandt A, Schubert-Bast S, Wiegand G, Korinthenberg R, Stephani U, van Velthoven V, Zentner J, Schulze-Bonhage A, Bast T. Reoperation for refractory epilepsy in childhood: a second chance for selected patients. Neurosurgery. 2013;73(4):695–704. doi: 10.1227/NEU.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 8.Newey CR, Wong C, Wang ZI, Chen X, Wu G, Alexopoulos AV. Optimizing SPECT SISCOM analysis to localize seizure-onset zone by using varying z scores. Epilepsia. 2013;54(5):793–800. doi: 10.1111/epi.12139. [DOI] [PubMed] [Google Scholar]
- 9.Huppertz HJ, Grimm C, Fauser S, Kassubek J, Mader I, Hochmuth A, Spreer J, Schulze-Bonhage A. Enhanced visualization of blurred gray-white matter junctions in focal cortical dysplasia by voxel-based 3D MRI analysis. Epilepsy Res. 2005;67(1–2):35–50. doi: 10.1016/j.eplepsyres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Huppertz HJ, Wellmer J, Staack AM, Altenmuller DM, Urbach H, Kroll J. Voxel-based 3D MRI analysis helps to detect subtle forms of subcortical band heterotopia. Epilepsia. 2008;49(5):772–785. doi: 10.1111/j.1528-1167.2007.01436.x. [DOI] [PubMed] [Google Scholar]
- 11.Wagner J, Weber B, Urbach H, Elger C, Huppertz H. Morphometric MRI analysis improves detection of focal cortical dysplasia type II. Brain. 2011;134(10):2844–2854. doi: 10.1093/brain/awr204. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZI, Jones SE, Jaisani Z, Najm IM, Prayson RA, Burgess RC, Krishnan B, Ristic A, Wong CH, Bingaman W, Gonzalez-Martinez JA, Alexopoulos AV. Voxel-based morphometric magnetic resonance imaging (MRI) postprocessing in MRI-negative epilepsies. Ann Neurol. 2015;77(6):1060–1075. doi: 10.1002/ana.24407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krsek P, Maton B, Korman B, Pacheco-Jacome E, Jayakar P, Dunoyer C, Rey G, Morrison G, Ragheb J, Vinters HV, Resnick T, Duchowny M. Different features of histopathological subtypes of pediatric focal cortical dysplasia. Ann Neurol. 2008;63(6):758–769. doi: 10.1002/ana.21398. [DOI] [PubMed] [Google Scholar]
- 14.Wang ZI, Alexopoulos AV, Jones SE, Najm IM, Ristic A, Wong C, Prayson R, Schneider F, Kakisaka Y, Wang S, Bingaman W, Gonzalez-Martinez JA, Burgess RC. Linking MRI postprocessing with magnetic source imaging in MRI-negative epilepsy. Ann Neurol. 2014;75(5):759–770. doi: 10.1002/ana.24169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huppertz HJ, Kassubek J, Altenmuller DM, Breyer T, Fauser S. Automatic curvilinear reformatting of three-dimensional MRI data of the cerebral cortex. Neuroimage. 2008;39:80–86. doi: 10.1016/j.neuroimage.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien TJ, O'Connor MK, Mullan BP, Brinkmann BH, Hanson D, Jack CR, So EL. Subtraction ictal SPET co-registered to MRI in partial epilepsy: description and technical validation of the method with phantom and patient studies. Nucl Med Commun. 1998;19(1):31–45. doi: 10.1097/00006231-199801000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Schneider F, Alexopoulos AV, Wang Z, Almubarak S, Kakisaka Y, Jin K, Nair D, Mosher JC, Najm IM, Burgess RC. Magnetic source imaging in non-lesional neocortical epilepsy: additional value and comparison with ICEEG. Epilepsy Behav. 2012;24(2):234–240. doi: 10.1016/j.yebeh.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 18.Palmini A. Terminology and classification of the cortical dysplasias. Neurology. 2004;62:S2–S8. doi: 10.1212/01.wnl.0000114507.30388.7e. [DOI] [PubMed] [Google Scholar]
- 19.Jehi L, Sarkis R, Bingaman W, Kotagal P, Najm I. When is a postoperative seizure equivalent to “epilepsy recurrence” after epilepsy surgery? Epilepsia. 2010;51(6):994–1003. doi: 10.1111/j.1528-1167.2010.02556.x. [DOI] [PubMed] [Google Scholar]
- 20.Wang ZI, Ristic AJ, Wong CH, Jones SE, Najm IM, Schneider F, Wang S, Gonzalez-Martinez JA, Bingaman W, Alexopoulos AV. Neuroimaging characteristics of MRI-negative orbito-frontal epilepsy with focus on voxel-based morphometric MRI postprocessing. Epilepsia. 2013;54(12):2195–2203. doi: 10.1111/epi.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemb M, Velasco TR, Parnes MS, Wu JY, Lerner JT, Matsumoto JH, Yudovin S, Shields WD, Sankar R, Salamon N, Vinters HV, Mathern GW. Improved outcomes in pediatric epilepsy surgery: the UCLA experience, 1986-2008. Neurology. 2010;74(22):1768–1775. doi: 10.1212/WNL.0b013e3181e0f17a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramantani G, Kadish NE, Strobl K, Brandt A, Stathi A, Mayer H, Schubert-Bast S, Wiegand G, Korinthenberg R, Stephani U, van Velthoven V, Zentner J, Schulze-Bonhage A, Bast T. Seizure and cognitive outcomes of epilepsy surgery in infancy and early childhood. Eur J Paediatr Neurol. 2013;17(5):498–506. doi: 10.1016/j.ejpn.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Fauser S, Sisodiya SM, Martinian L, Thom M, Gumbinger C, Huppertz HJ, Hader C, Strobl K, Steinhoff BJ, Prinz M, Zentner J, Schulze-Bonhage A. Multi-focal occurrence of cortical dysplasia in epilepsy patients. Brain. 2009;132(Pt 8):2079–2090. doi: 10.1093/brain/awp145. [DOI] [PubMed] [Google Scholar]
- 24.Widdess-Walsh P, Diehl B, Najm I. Neuroimaging of focal cortical dysplasia. J Neuroimaging. 2006;16(3):185–196. doi: 10.1111/j.1552-6569.2006.00025.x. [DOI] [PubMed] [Google Scholar]
- 25.Krsek P, Pieper T, Karlmeier A, Hildebrandt M, Kolodziejczyk D, Winkler P, Pauli E, Blumcke I, Holthausen H. Different presurgical characteristics and seizure outcomes in children with focal cortical dysplasia type I or II. Epilepsia. 2009;50(1):125–137. doi: 10.1111/j.1528-1167.2008.01682.x. [DOI] [PubMed] [Google Scholar]
- 26.House PM, Lanz M, Holst B, Martens T, Stodieck S, Huppertz HJ. Comparison of morphometric analysis based on T1- and T2-weighted MRI data for visualization of focal cortical dysplasia. Epilepsy Res. 2013;106(3):403–409. doi: 10.1016/j.eplepsyres.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Focke NK, Bonelli SB, Yogarajah M, Scott C, Symms MR, Duncan JS. Automated normalized FLAIR imaging in MRI-negative patients with refractory focal epilepsy. Epilepsia. 2009;50(6):1484–1490. doi: 10.1111/j.1528-1167.2009.02022.x. [DOI] [PubMed] [Google Scholar]
- 28.Hong SJ, Kim H, Schrader D, Bernasconi N, Bernhardt BC, Bernasconi A. Automated detection of cortical dysplasia type II in MRI-negative epilepsy. Neurology. 2014;83(1):48–55. doi: 10.1212/WNL.0000000000000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.