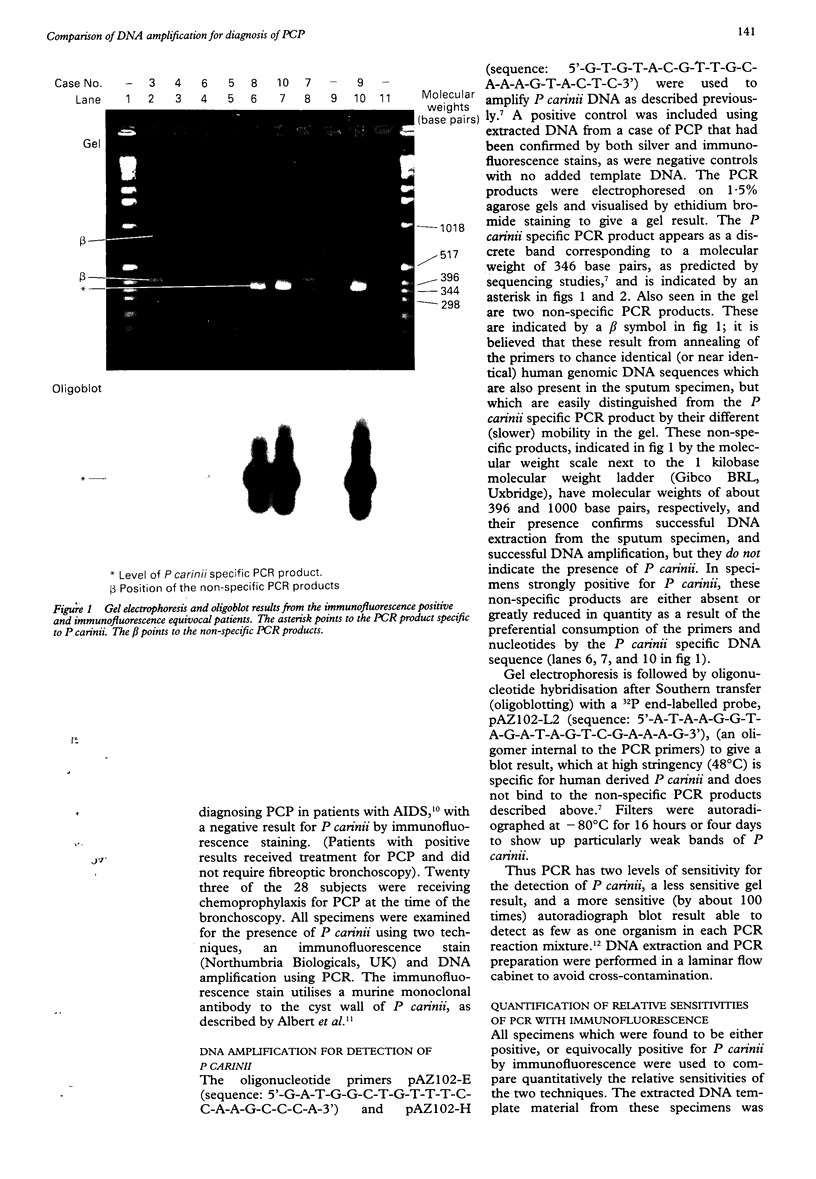
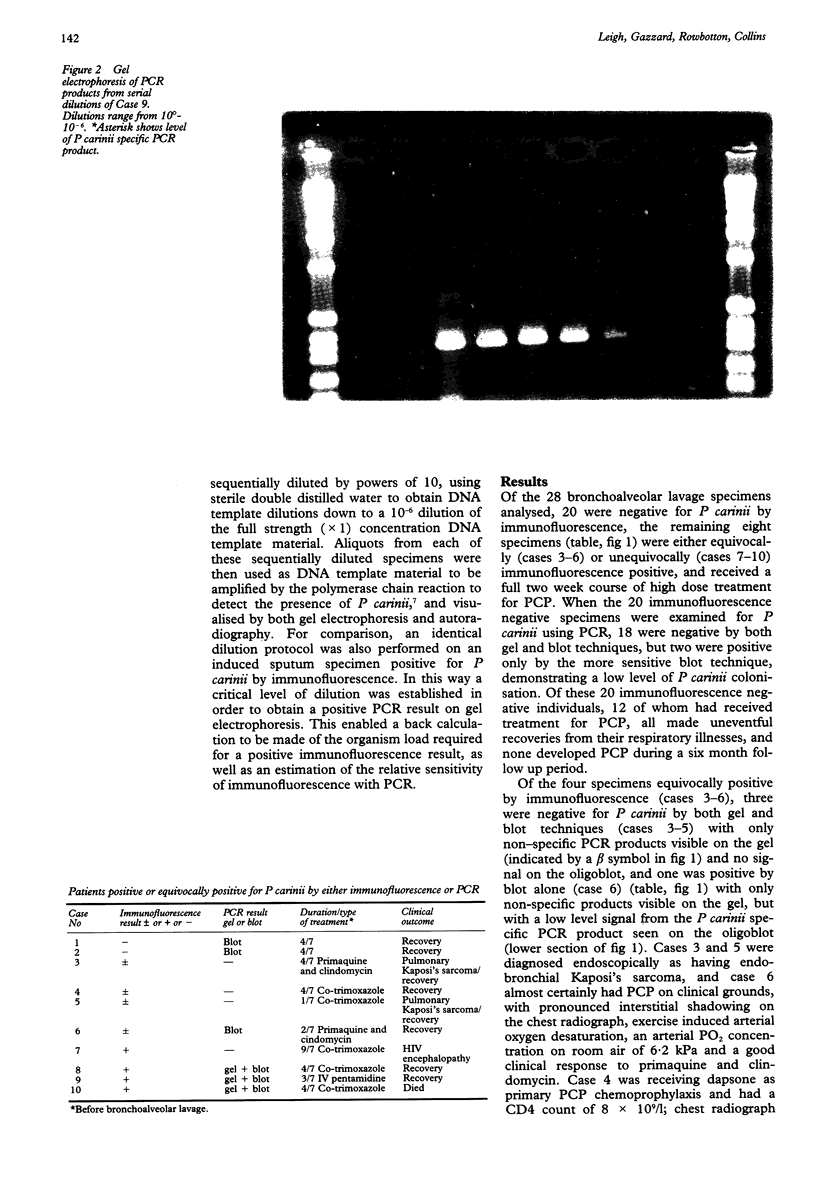
Abstract
AIM: To compare the results of DNA amplification by the polymerase chain reaction (PCR) with immunofluorescence staining for detecting Pneumocystis carinii in bronchoalveolar lavage specimens taken from symptomatic HIV seropositive patients with suspected P carinii pneumonia (PCP). METHODS: Bronchoalveolar lavage specimens were obtained from 28 symptomatic HIV seropositive patients. Specimens were examined for P carinii using immunofluorescence, and by DNA amplification with PCR to obtain results on gel electrophoresis (gel) and a more sensitive Southern hybridisation (blot) technique. Specimens positive by immunofluorescence and gel electrophoresis were serially diluted to a 10(-6) concentration and each dilution strength tested for P carinii using PCR to compare quantitatively immunofluorescence with PCR. RESULTS: Of the 28 specimens analysed, 18 were negative for P carinii by both immunofluorescence and PCR, two were positive only by the blot technique of PCR, four were equivocally positive and four unequivocally positive by immunofluorescence. Three of the four equivocally positive patients tested by immunofluorescence were negative for P carinii by PCR, although one was positive by PCR (blot) technique. This patient had clinically confirmed PCP. Of the four unequivocally positive patients tested by immunofluorescence, three were gel and blot positive by PCR and had PCP clinically, but one was negative by both gel and blot techniques, although the patient certainly had PCP on clinical grounds. This patient had received nine days of treatment with high dose co-trimoxazole before bronchoalveolar lavage specimens were obtained. The three specimens positive by gel and blot techniques remained gel positive down to dilutions of between 10(-4) and 10(-6). CONCLUSIONS: PCR results may become negative soon after starting treatment for PCP. Specimens should therefore be taken before, or soon after, starting treatment. PCR seems to be between 10(4) and 10(6) times more sensitive than immunofluorescence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett C. L., Gertler P., Guze P. A., Garfinkle J. B., Kanouse D. E., Greenfield S. The relation between resource use and in-hospital mortality for patients with acquired immunodeficiency syndrome-related Pneumocystis carinii pneumonia. Arch Intern Med. 1990 Jul;150(7):1447–1452. [PubMed] [Google Scholar]
- Currie D. C., Higgs E., Metcalfe S., Roberts D. E., Cole P. J. Simple method of monitoring colonising microbial load in chronic bronchial sepsis: pilot comparison of reduction in colonising microbial load with antibiotics given intermittently and continuously. J Clin Pathol. 1987 Aug;40(8):830–836. doi: 10.1136/jcp.40.8.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham-McGregor S. M., Powell C. A., Walker S. P., Himes J. H. Nutritional supplementation, psychosocial stimulation, and mental development of stunted children: the Jamaican Study. Lancet. 1991 Jul 6;338(8758):1–5. doi: 10.1016/0140-6736(91)90001-6. [DOI] [PubMed] [Google Scholar]
- Leigh T. R., Parsons P., Hume C., Husain O. A., Gazzard B., Collins J. V. Sputum induction for diagnosis of Pneumocystis carinii pneumonia. Lancet. 1989 Jul 22;2(8656):205–206. doi: 10.1016/s0140-6736(89)90382-6. [DOI] [PubMed] [Google Scholar]
- Leigh T. R., Wakefield A. E., Peters S. E., Hopkin J. M., Collins J. V. Comparison of DNA amplification and immunofluorescence for detecting Pneumocystis carinii in patients receiving immunosuppressive therapy. Transplantation. 1992 Sep;54(3):468–470. doi: 10.1097/00007890-199209000-00016. [DOI] [PubMed] [Google Scholar]
- Masur H., Lane H. C., Kovacs J. A., Allegra C. J., Edman J. C. NIH conference. Pneumocystis pneumonia: from bench to clinic. Ann Intern Med. 1989 Nov 15;111(10):813–826. doi: 10.7326/0003-4819-111-10-813. [DOI] [PubMed] [Google Scholar]
- Midgley J., Parsons P., Leigh T. R., Collins J. V., Shanson D. C., Husain O. A., Harcourt-Webster J. N. Increased sensitivity of immunofluorescence for detection of Pneumocystis carinii. Lancet. 1989 Dec 23;2(8678-8679):1523–1523. doi: 10.1016/s0140-6736(89)92964-4. [DOI] [PubMed] [Google Scholar]
- Murray J. F., Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part II. Am Rev Respir Dis. 1990 Jun;141(6):1582–1598. doi: 10.1164/ajrccm/141.6.1582. [DOI] [PubMed] [Google Scholar]
- Peters S. E., Wakefield A. E., Banerji S., Hopkin J. M. Quantification of the detection of Pneumocystis carinii by DNA amplification. Mol Cell Probes. 1992 Apr;6(2):115–117. doi: 10.1016/0890-8508(92)90055-3. [DOI] [PubMed] [Google Scholar]
- Shelhamer J. H., Ognibene F. P., Macher A. M., Tuazon C., Steiss R., Longo D., Kovacs J. A., Parker M. M., Natanson C., Lane H. C. Persistence of Pneumocystis carinii in lung tissue of acquired immunodeficiency syndrome patients treated for pneumocystis pneumonia. Am Rev Respir Dis. 1984 Dec;130(6):1161–1165. doi: 10.1164/arrd.1984.130.6.1161. [DOI] [PubMed] [Google Scholar]
- Wakefield A. E., Pixley F. J., Banerji S., Sinclair K., Miller R. F., Moxon E. R., Hopkin J. M. Amplification of mitochondrial ribosomal RNA sequences from Pneumocystis carinii DNA of rat and human origin. Mol Biochem Parasitol. 1990 Nov;43(1):69–76. doi: 10.1016/0166-6851(90)90131-5. [DOI] [PubMed] [Google Scholar]
- Wakefield A. E., Pixley F. J., Banerji S., Sinclair K., Miller R. F., Moxon E. R., Hopkin J. M. Detection of Pneumocystis carinii with DNA amplification. Lancet. 1990 Aug 25;336(8713):451–453. doi: 10.1016/0140-6736(90)92008-6. [DOI] [PubMed] [Google Scholar]