Abstract
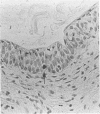
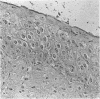
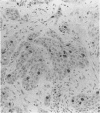
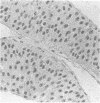
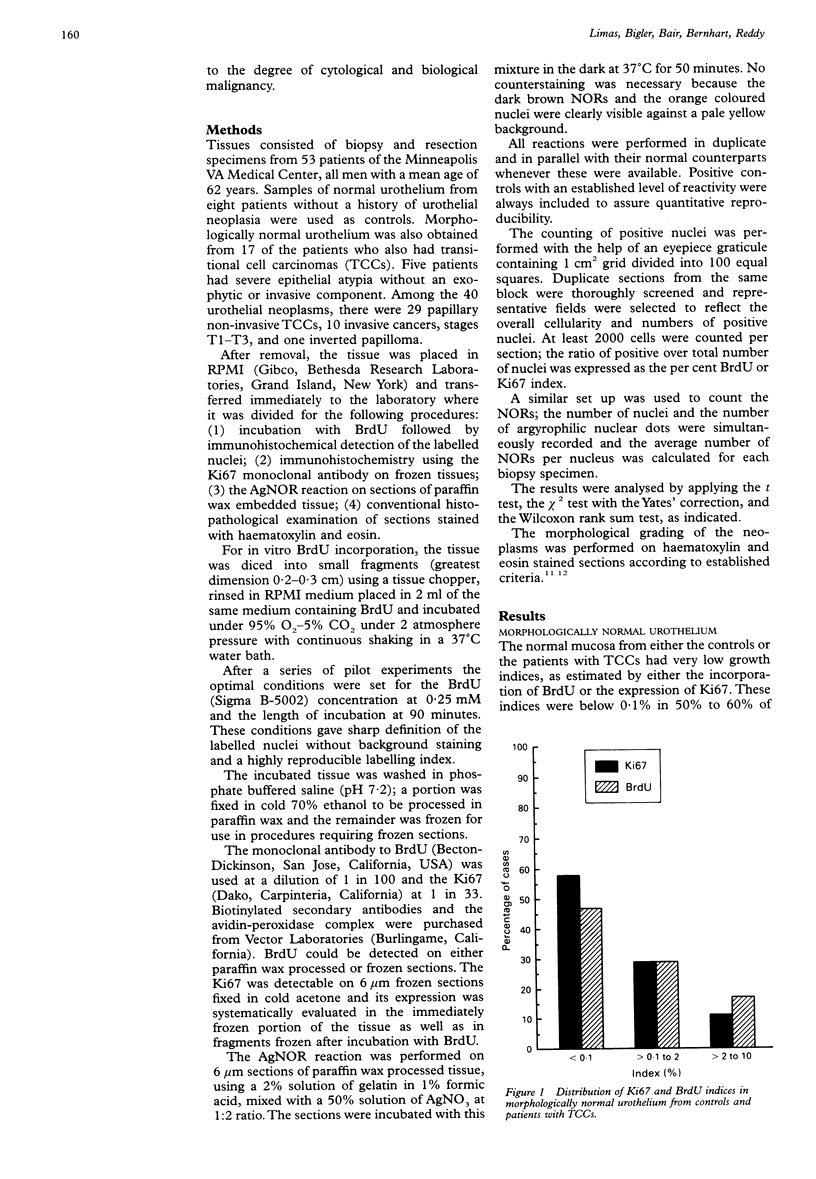
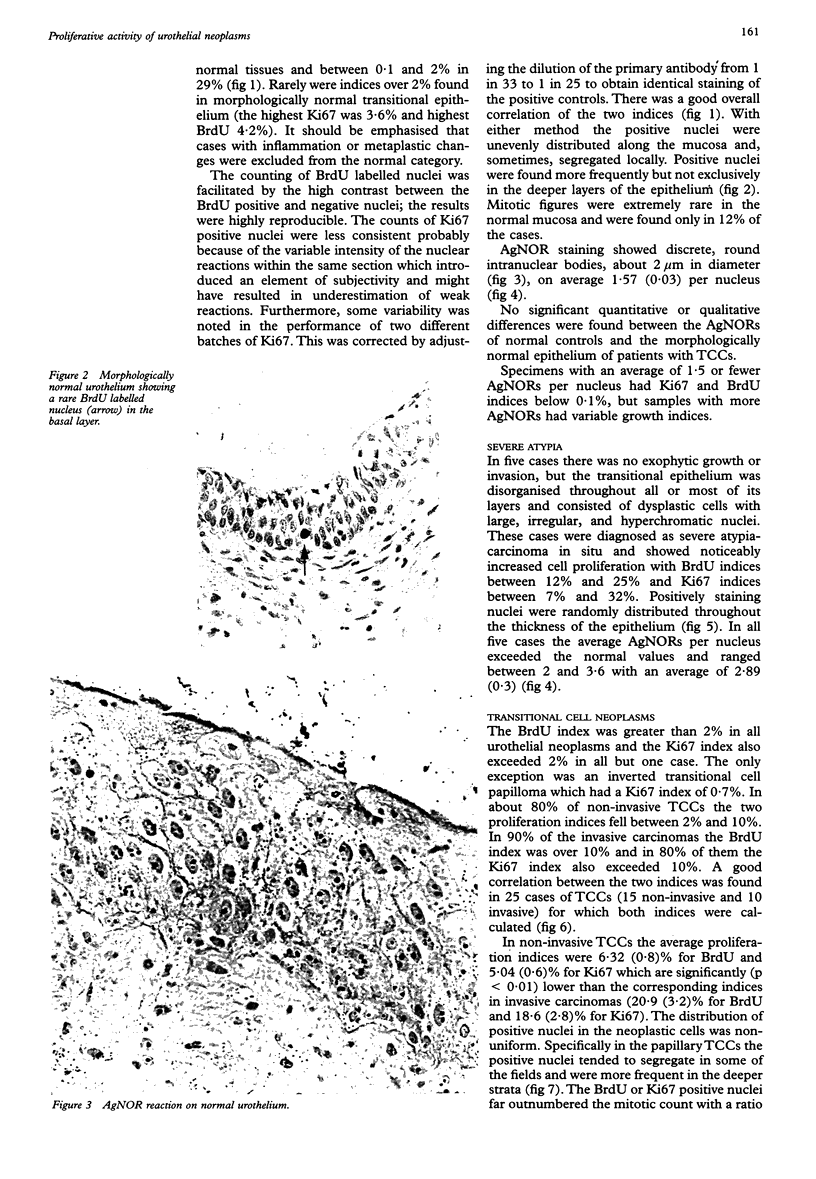
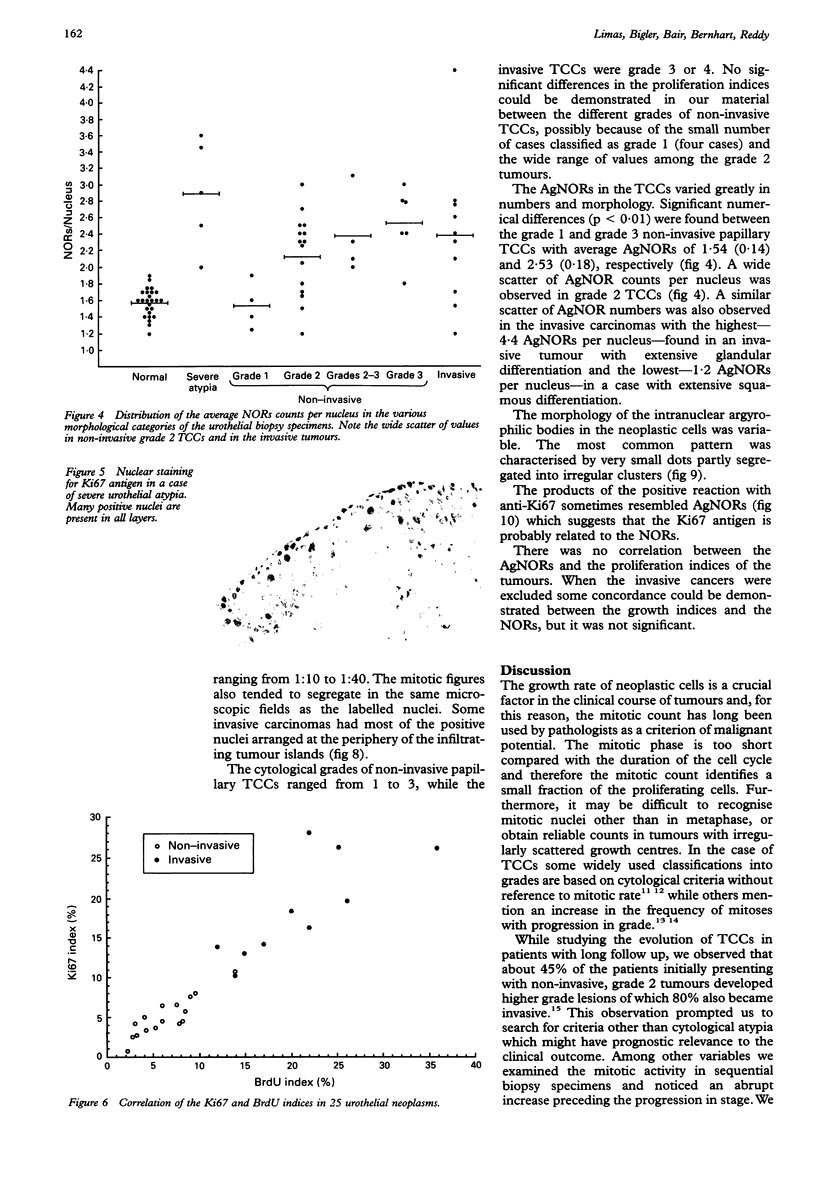
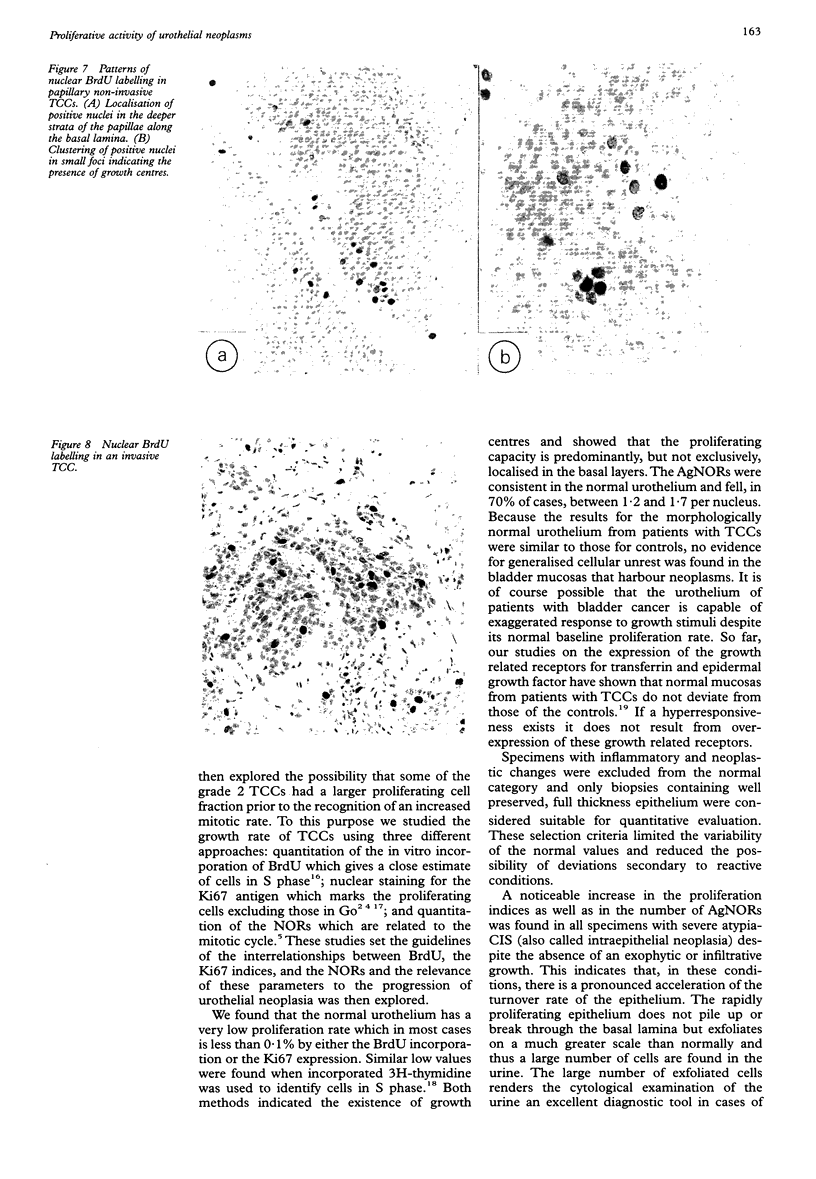
AIMS: To evaluate the proliferative activity of urothelial neoplasms, compare it with that of the normal urinary tract epithelium, and determine its relation to morphological grade and presence of invasion. METHODS: Multiple biopsy specimens from 53 individuals--eight normal controls, five patients with severe urothelial atypia, and 40 with transitional cell carcinomas (TCCs)--were studied using in vitro bromodeoxyuridine (BrdU) incorporation, Ki67 antigen expression, and quantitation of the nucleolar organiser regions (NORs). RESULTS: The percentage of nuclei labelled by BrdU (BrdU index) correlated well with the percentage of nuclei expressing the Ki67 antigen (Ki67 index). These proliferation indices were very low (less than 0.1% in 60% of samples) in the urothelium of normal controls and the morphologically unremarkable epithelium of patients with TCCs. Non-invasive TCCs had increased proliferation (BrdU index 6.32 (SD 0.8)%, Ki67 index 5.04 (0.6)% but lagged behind the invasive tumours (BrdU 20.9 (3.2)%, Ki67 18.6 (2.8)%). The average NOR count was 1.57 (0.03) in morphological normal epithelium, which increased progressively with grade in non-invasive TCCs, but varied greatly in invasive tumours and did not correlate with the proliferation indices. The spectrum of values for both proliferation indices and NORs was particularly wide in grade 2 TCCs. Severe atypias without exophytic growth had an increase in BrdU and Ki67 indices comparable with that found in grade 3-4 invasive TCCs; these also had the highest NORs per nucleus. CONCLUSIONS: The growth potential of urothelial neoplasms is an important indicator of their aggressive course. In particular, growth indices over 10% are strongly associated with the presence of invasion. Papillary grade 2 TCCs show heterogeneity in their growth characteristics which may relate to their diverse clinical course. The mitotic count underestimates the growth potential of papillary TCCs and the addition of proliferation indices such as BrdU incorporation or the Ki67 index may enhance the prognostic accuracy of conventional morphological grading.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown D. C., Gatter K. C. Monoclonal antibody Ki-67: its use in histopathology. Histopathology. 1990 Dec;17(6):489–503. doi: 10.1111/j.1365-2559.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Crocker J., Nar P. Nucleolar organizer regions in lymphomas. J Pathol. 1987 Feb;151(2):111–118. doi: 10.1002/path.1711510203. [DOI] [PubMed] [Google Scholar]
- Friedell G. H., Parija G. C., Nagy G. K., Soto E. A. The pathology of human bladder cancer. Cancer. 1980 Apr 15;45(7 Suppl):1823–1831. [PubMed] [Google Scholar]
- Gerdes J., Dallenbach F., Lennert K., Lemke H., Stein H. Growth fractions in malignant non-Hodgkin's lymphomas (NHL) as determined in situ with the monoclonal antibody Ki-67. Hematol Oncol. 1984 Oct-Dec;2(4):365–371. doi: 10.1002/hon.2900020406. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Goodpasture C., Bloom S. E. Visualization of nucleolar organizer regions im mammalian chromosomes using silver staining. Chromosoma. 1975 Nov 20;53(1):37–50. doi: 10.1007/BF00329389. [DOI] [PubMed] [Google Scholar]
- Gratzner H. G. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982 Oct 29;218(4571):474–475. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- Howell W. M., Black D. A. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia. 1980 Aug 15;36(8):1014–1015. doi: 10.1007/BF01953855. [DOI] [PubMed] [Google Scholar]
- Isola J. J., Helin H. J., Helle M. J., Kallioniemi O. P. Evaluation of cell proliferation in breast carcinoma. Comparison of Ki-67 immunohistochemical study, DNA flow cytometric analysis, and mitotic count. Cancer. 1990 Mar 1;65(5):1180–1184. doi: 10.1002/1097-0142(19900301)65:5<1180::aid-cncr2820650525>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Limas C., Lange P., Fraley E. E., Vessella R. L. A, B, H antigens in transitional cell tumors of the urinary bladder: correlation with the clinical course. Cancer. 1979 Dec;44(6):2099–2107. doi: 10.1002/1097-0142(197912)44:6<2099::aid-cncr2820440621>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- MELAMED M. R., VOUTSA N. G., GRABSTALD H. NATURAL HISTORY AND CLINICAL BEHAVIOR OF IN SITU CARCINOMA OF THE HUMAN URINARY BLADDER. Cancer. 1964 Dec;17:1533–1545. doi: 10.1002/1097-0142(196412)17:12<1533::aid-cncr2820171205>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- MELICOW M. M., HOLLOWELL J. W. Intra-urothelial cancer: carcinoma in situ, Bowen's disease of the urinary system: discussion of thirty cases. J Urol. 1952 Oct;68(4):763–772. doi: 10.1016/S0022-5347(17)68279-X. [DOI] [PubMed] [Google Scholar]
- Mellon K., Neal D. E., Robinson M. C., Marsh C., Wright C. Cell cycling in bladder carcinoma determined by monoclonal antibody Ki67. Br J Urol. 1990 Sep;66(3):281–285. doi: 10.1111/j.1464-410x.1990.tb14927.x. [DOI] [PubMed] [Google Scholar]
- Smith R., Crocker J. Evaluation of nucleolar organizer region-associated proteins in breast malignancy. Histopathology. 1988 Feb;12(2):113–125. doi: 10.1111/j.1365-2559.1988.tb01923.x. [DOI] [PubMed] [Google Scholar]
- Sufrin G., Meyer J. S., Martin S. A., Schechtman K. Proliferative activity of urothelium and tumors of renal pelvis, ureter, and urinary bladder evaluated by thymidine labeling. Urology. 1984 Mar;23(3 Suppl):15–22. doi: 10.1016/s0090-4295(84)80109-0. [DOI] [PubMed] [Google Scholar]
- Welberg J. W., de Vries E. G., Hardonk M. J., Mulder N. H., Harms G., Grond J., Zwart N., Koudstaal J., de Ley L., Kleibeuker J. H. Proliferation rate of colonic mucosa in normal subjects and patients with colonic neoplasms: a refined immunohistochemical method. J Clin Pathol. 1990 Jun;43(6):453–456. doi: 10.1136/jcp.43.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Huang G. S., Zhu X. S. Role of nucleolar organiser regions in differentiating malignant from benign tumours of the colon. J Clin Pathol. 1990 Mar;43(3):235–238. doi: 10.1136/jcp.43.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]