Abstract
[Purpose] The aim of this study was to investigate the effects of hypothyroidism on femoral cartilage thickness by using ultrasound, which has been found to be useful in the early diagnosis of knee osteoarthritis. [Subjects and Methods] Forty patients diagnosed with hypothyroidism and 30 age-, gender-, smoking status, physical activity-, and body mass index-matched healthy subjects were enrolled. The thickness of the femoral articular cartilage was measured using a 7- to 12-MHz linear probe. Three mid-point measurements were taken from each knee at the lateral condyle, intercondylar area, and medial condyle. [Results] Age, gender, body mass index, smoking status, and physical activity were similar between the groups, but patients with hypothyroidism had thinner femoral cartilage than the healthy controls at all measurement sites. Nonetheless, the differences were not statistically significant (except in the case of the left medial condyle). [Conclusion] Ultrasonographic measurement of femoral cartilage thickness may be useful in the early diagnosis of knee osteoarthritis in patients with hypothyroidism.
Key words: Hypothyroidism, Femoral cartilage thickness, Ultrasound
INTRODUCTION
Osteoarthritis (OA) is a chronic musculoskeletal disease characterized by loss of articular cartilage and changes in the subchondral bone. It is becoming increasingly prevalent worldwide because of its association with aging. OA of the knee is a common cause of pain and disability. Several risk factors (e.g., age, female gender, hypothyroidism, race, genetic susceptibility, and obesity) are associated with OA1). Hypothyroidism is a syndrome resulting from thyroid hormone deficiency2). Thyroid hormones might be of key importance in the maintenance of articular cartilage and play a role in the pathogenesis of OA3). Radiographic OA is characterized by osteophytes, sclerosis, and joint space narrowing, which is a result of cartilage erosion and subchondral sclerosis4). Ultrasonography is also used to evaluate the condition of the articular cartilage, and pre-OA evaluation of the knee by ultrasound may have prognostic value5).
The aim of the present study was to investigate the effects of hypothyroidism on femoral cartilage thickness by using ultrasound, which is a valid and reliable method in this regard and has previously been found to be useful in the early diagnosis of knee OA6, 7).
SUBJECTS AND METHODS
Forty patients diagnosed with hypothyroidism and 30 age-, gender-, body mass index (BMI)-, smoking status-, and physical activity-matched healthy subjects were enrolled. The demographic and clinical features of all participants were recorded. Subjects with a history of knee trauma or previous knee surgery; additional (other than hypothyroidism) systemic and/or chronic diseases including diabetes mellitus and rheumatoid arthritis; and any abnormal laboratory results regarding renal, hepatic, thyroid, or parathyroid function were excluded. All subjects were informed about the study procedure, and they provided consent to participate.This study was approved by the local ethics committee of Recep Tayyip Erdogan University Medical School.
The thickness of the femoral articular cartilage was measured using a 7- to 12-MHz linear probe (Eizo Nanao Corporation, Esaote, Italia) while the subjects comfortably sat on the examination table with their knees in maximum flexion, and the probe was placed in the axial plane on the outer edge6, 8). Three mid-point measurements were taken from each knee, from the right lateral condyle (RLC), right intercondylar area (RIA), right medial condyle (RMC), left medial condyle (LMC), left intercondylar area (LIA), and left lateral condyle (LLC) (Fig. 1). The cartilage thickness was interpreted as the distance between the thin hyperechoic line at the synovial space/cartilage interface and the sharp hyperechoic line at the cartilage-bone interface7).
Fig. 1.
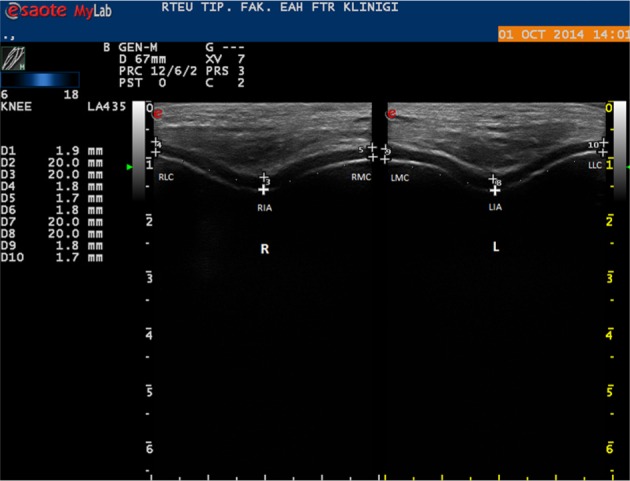
Ultrasonogram (suprapatellaraxial view) demonstrating femoral cartilage measurements
RLC: right lateral condyle; RIA: right intercondylar area; RMC: right medialcondyle; LLC: left lateral condyle; LIA: left intercondylar area; LMC: left medial condyle
All statistical analyses were performed using SPSS version 16.0. Data were expressed as mean ± standard deviation. The normal distribution of continuous variables was tested using the Kolmogorov-Smirnov test. Student’s t-test was used to compare normally distributed data, and the Mann-Whitney U test was used to compare non-normally distributed data. The χ2 test was used to compare qualitative parameters. Statistical significance was set at p<0.05.
RESULTS
Eighty knees of the 40 patients with hypothyroidism (29 females and 11 males) and 60 knees of the 30 healthy subjects (22 females and 8 males) were examined in this study. The demographic characteristics of the participants are shown in Table 1. The mean age of the patients with hypothyroidism was 25.50 ± 3.34 years, while that of the healthy subjects was 24.46 ± 3.15 years (p>0.05). The mean BMI of the former was 26.38 ± 1.38 kg/m2, while that of the latter was 25.45 ± 3.06 kg/m2 (p>0.05). The mean disease duration in the patients with hypothyroidism was 30.32 ± 15.41 months. Gender, smoking status, and physical activity were similar between the groups (p>0.05 for all).
Table 1. Characteristics of study population (mean ± standard deviation).
| Patients (n=40) | Control (n=30) | |
|---|---|---|
| Age (years) | 25.50 ± 3.34 | 24.46 ± 3.15 |
| Gender, n (%) | ||
| Female | 29 (72.5) | 22 (73.3) |
| Male | 11 (27.5) | 8 (26.7) |
| BMI (kg/m2) | 26.38 ± 1.38 | 25.45 ± 3.06 |
| Disease duration (months) | 30.3 ± 15.4 | |
| Physical activity, n (%) | ||
| Physical activity | 34 (85.0) | 25 (83.3) |
| No physical activity | 6 (15.0) | 5 (16.7) |
| Smoking status, n (%) | ||
| Smokers | 7 (17.5) | 6 (20.0) |
| Non-smokers | 33 (82.5) | 24 (80.0) |
*Significant at p<0.05
Table 2 shows the femoral cartilage thickness values for all participants. Patients with hypothyroidism had thinner femoral cartilage than the healthy controls at all measurement sites, but the differences were not statistically significant except in the case of the LMC (p<0.05).
Table 2. Comparison of femoral cartilage thickness (mm) between patients with hypothyroidism and healthy controls (mean ± standard deviation).
| Patients (n=40) | Control (n=30) | |
|---|---|---|
| RLC (mm) | 1.83 ± 0.14 | 1.88 ± 0.08 |
| RIA (mm) | 1.89 ± 0.11 | 1.92 ± 0.09 |
| RMC (mm) | 1.79 ± 0.11 | 1.83 ± 0.08 |
| LLC (mm) | 1.86 ± 0.12 | 1.91 ± 0.08 |
| LIA (mm) | 1.88 ± 0.14 | 1.93 ± 0.10 |
| LMC (mm) | 1.77 ± 0.12* | 1.83 ± 0.08 |
RLC: right lateral condyle; RIA: right intercondylar area; RMC: right medial condyle; LLC: left lateral condyle; LIA: left intercondylar area; LMC: left medial condyle. *Significant at p<0.05
Discussion
Hypothyroidism is known to be associated with OA and inflammatory forms of arthritis and connective tissue diseases, which can cause arthritis9). The clinical findings of hypothyroidism include epiphyseal disgenesis, aseptic necrosis, and viscous non-inflammatory arthropathy particularly affecting the knees, wrist joints, and hands10). The thyroid hormones are essential for endochondral ossification and stimulating the expression of the gene that controls chondrocyte maturation and matrix synthesis3). Further, thyroxine (T4) is a potent stimulator of chondrocyte differentiation11). Mature chondrocytes in the cartilage synthesize type II collagen and proteoglycans, which begin to degradation in early OA. As this degradation progresses, the bony cartilage gets thinner12).
Hypothyroidism is one of the causes of secondary OA13). OA involves the entire joint, including the subchondral bone and articular cartilage, and the prevalence of periarticular soft tissue lesions is high14, 15). Cartilage defects are commonly found on arthroscopy in individuals with knee OA16). In addition to degradation and loss of the articular hyaline cartilage, progressive thickening, remodeling, and sclerosis of the subchondral bone; formation of osteophytes; and chronic inflammation of the synovial membrane are other features of OA17).
Ultrasound is an inexpensive noninvasive method for imaging the musculoskeletal system that is readily accepted by patients. It can be provide information about the joint cartilage, synovitis, periarticular soft tissues, and bony cortical abnormalities in peripheral joints with OA. It seems to be a promising and accurate method for monitoring cartilage response to drug therapy in OA18). Ultrasonography has also enabled quantitative assessment of articular cartilage thickness. Cartilage defects may predict cartilage loss in asymptomatic knee OA and can indicate early OA, which is demonstrated by a loss in the sharpness of the margin19). Lee et al. reported a significant correlation between ultrasonographic measurements and histologic grading of OA femoral condylar cartilage6). A previous study reported that low hemoglobin levels had a negative effect on femoral cartilage thickness as detected by ultrasonography in healthy subjects20). Ultrasound is also useful to monitor the effect of biologic therapy in rheumatoid arthritis and can be used to evaluate both inflammatory and destructive changes21). Tsai et al. suggested that ultrasound could be used to monitor changes in the cartilage of patients with rheumatoid arthritis22), and Tunc et al. revealed that the femoral cartilage is thinner on the hemiparetic side of stroke patients23). The results of the present study show the negative effects of hypothyroidism on the thickness of the distal femoral cartilage. To the best of the authors’ knowledge, this is the first study exhibiting thinning of the distal femoral cartilage in correlation with hypothyroidism.
The present study has some limitations. The sample size was relatively small, and only the thickness and not the volume of the cartilage was measured, although it has been shown that differences in cartilage volume result primarily because of differences in joint surface areas (epiphyseal bone size) rather than cartilage thickness24).
Overall, on the basis of the study findings, patients with hypothyroidism seem to have thinner femoral cartilage. Thus, the presence and severity of femoral cartilage thinning may be useful for early diagnosis of knee OA. Ultrasonography is a noninvasive, cost-effective method to measure the thickness of the articular cartilage, and screening of joint cartilage by ultrasonography may enable early diagnosis of knee OA in patients with hypothyroidism.
REFERENCES
- 1.Malas FU, Kara M, Aktekin L, et al. : Does vitamin D affect femoral cartilage thickness? An ultrasonographic study. Clin Rheumatol, 2014, 33: 1331–1334. [DOI] [PubMed] [Google Scholar]
- 2.Erdogan M, Canataroglu A, Ganidagli S, et al. : Metabolic syndrome prevalence in subclinic and overt hypothyroid patients and the relation among metabolic syndrome parameters. J Endocrinol Invest, 2011, 34: 488–492. [DOI] [PubMed] [Google Scholar]
- 3.Williams GR: Thyroid hormone actions in cartilage and bone. Eur Thyroid J, 2013, 2: 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bay-Jensen AC, Slagboom E, Chen-An P, et al. : Role of hormones in cartilage and joint metabolism: understanding an unhealthy metabolic phenotype in osteoarthritis. Menopause, 2013, 20: 578–586. [DOI] [PubMed] [Google Scholar]
- 5.Ito K, Kimura Y, Tajika A, et al. : Ultrasonographic changes of the knee joint cartilage associated with physical characterization in middle-aged women: 6-month observational survey. J Phys Ther Sci, 2007, 19: 277–282. [Google Scholar]
- 6.Lee CL, Huang MH, Chai CY, et al. : The validity of in vivo ultrasonographic grading of osteoarthritic femoral condylar cartilage: a comparison with in vitro ultrasonographic and histologic gradings. Osteoarthritis Cartilage, 2008, 16: 352–358. [DOI] [PubMed] [Google Scholar]
- 7.Yoon CH, Kim HS, Ju JH, et al. : Validity of the sonographic longitudinal sagittal image for assessment of the cartilage thickness in the knee osteoarthritis. Clin Rheumatol, 2008, 27: 1507–1516. [DOI] [PubMed] [Google Scholar]
- 8.Mathiesen O, Konradsen L, Torp-Pedersen S, et al. : Ultrasonography and articular cartilage defects in the knee: an in vitro evaluation of the accuracy of cartilage thickness and defect size assessment. Knee Surg Sports Traumatol Arthrosc, 2004, 12: 440–443. [DOI] [PubMed] [Google Scholar]
- 9.Tagoe CE, Zezon A, Khattri S: Rheumatic manifestations of autoimmune thyroid disease: the other autoimmune disease. J Rheumatol, 2012, 39: 1125–1129. [DOI] [PubMed] [Google Scholar]
- 10.McLean RM, Podell DN: Bone and joint manifestations of hypothyroidism. Semin Arthritis Rheum, 1995, 24: 282–290. [DOI] [PubMed] [Google Scholar]
- 11.Miura M, Tanaka K, Komatsu Y, et al. : Thyroid hormones promote chondrocyte differentiation in mouse ATDC5 cells and stimulate endochondral ossification in fetal mouse tibias through iodothyronine deiodinases in the growth plate. J Bone Miner Res, 2002, 17: 443–454. [DOI] [PubMed] [Google Scholar]
- 12.Doral MN, Donmez G, Atay OA, et al. : Degenerative diseases of joint. Turk J TOTBID, 2007, 6: 56–65. [Google Scholar]
- 13.Flores RH, Hochberg MC: Definition and classifi cation of osteoarthritis. In: Brandt KD, Doherty M, Lohmander LS (eds.), Osteoarthritis, 2nd ed. Oxford University Press, 2003, pp 1–8. [Google Scholar]
- 14.Dieppe P: Subchondral bone should be the main target for the treatment of pain and disease progression in osteoarthritis. Osteoarthritis Cartilage, 1999, 7: 325–326. [DOI] [PubMed] [Google Scholar]
- 15.Cicuttini FM, Wluka AE, Forbes A, et al. : Comparison of tibial cartilage volume and radiologic grade of the tibiofemoral joint. Arthritis Rheum, 2003, 48: 682–688. [DOI] [PubMed] [Google Scholar]
- 16.Brandt KD, Fife RS, Braunstein EM, et al. : Radiographic grading of the severity of knee osteoarthritis: relation of the Kellgren and Lawrence grade to a grade based on joint space narrowing, and correlation with arthroscopic evidence of articular cartilage degeneration. Arthritis Rheum, 1991, 34: 1381–1386. [DOI] [PubMed] [Google Scholar]
- 17.Paton D: Instructional course lectures: the American Academy of Orthopaedic Surgeons. Br J Surg, 1979, 66: 68. [Google Scholar]
- 18.Naredo E, Acebes C, Möller I, et al. : Ultrasound validity in the measurement of knee cartilage thickness. Ann Rheum Dis, 2009, 68: 1322–1327. [DOI] [PubMed] [Google Scholar]
- 19.Myers SL, Dines K, Brandt DA, et al. : Experimental assessment by high frequency ultrasound of articular cartilage thickness and osteoarthritic changes. J Rheumatol, 1995, 22: 109–116. [PubMed] [Google Scholar]
- 20.Unsal FM, Ozturk GT, Kara M, et al.: The relationship between hemoglobin levels and femoral cartilage thickness. Acta Medica (Cordoba), 2014, 3: 19–22. [Google Scholar]
- 21.Iagnocco A, Perella C, Naredo E, et al. : Etanercept in the treatment of rheumatoid arthritis: clinical follow-up over one year by ultrasonography. Clin Rheumatol, 2008, 27: 491–496. [DOI] [PubMed] [Google Scholar]
- 22.Tsai CY, Lee CL, Chai CY, et al. : The validity of in vitro ultrasonographic grading of osteoarthritic femoral condylar cartilage—a comparison with histologic grading. Osteoarthritis Cartilage, 2007, 15: 245–250. [DOI] [PubMed] [Google Scholar]
- 23.Tunç H, Oken O, Kara M, et al. : Ultrasonographic measurement of the femoral cartilage thickness in hemiparetic patients after stroke. Int J Rehabil Res, 2012, 35: 203–207. [DOI] [PubMed] [Google Scholar]
- 24.Faber SC, Eckstein F, Lukasz S, et al. : Gender differences in knee joint cartilage thickness, volume and articular surface areas: assessment with quantitative three-dimensional MR imaging. Skeletal Radiol, 2001, 30: 144–150. [DOI] [PubMed] [Google Scholar]


