Abstract
[Purpose] There have been no investigations into the improvement of activities of daily living among patients suffering from post-stroke depression on admission to convalescent rehabilitation wards in Japan. This study aimed to assess the improvement of activities in daily living in patients with or without post-stroke depression at the time of admission to a convalescent rehabilitation ward. [Subjects and Methods] This retrospective study included 108 stroke patients divided into two groups according to their Geriatric Depression Scale 15-item short form scores. Activities of daily living were assessed using the Functional Independence Measure. The degree of improvement on the Functional Independence Measure was defined as the difference between scores on admission and at discharge. [Results] The Functional Independence Measure gain score was significantly different from the Functional Independence Measure total score. There was a significant interaction between time period and post-stroke depression factors for the Functional Independence Measure total score. A multiple regression analysis revealed a significant association between Geriatric Depression Scale score and Functional Independence Measure total score. [Conclusion] The present study suggests that post-stroke depression has a negative impact on recovery of activities of daily living and on rehabilitation outcomes in a convalescent rehabilitation ward setting.
Key words: Post-stroke depression, Activities of daily living, Convalescent rehabilitation ward
INTRODUCTION
Post-stroke depression (PSD) is one of the symptoms observed in stroke patients. The prevalence of PSD is high, as it is diagnosed in 53% of patients at 3 months and in 42% of patients at 12 months after stroke1). Additionally, PSD has been reported to have a high incidence even 1 month from the onset of stroke2,3,4).
Many reports have acknowledged the impact of PSD. PSD has been associated with increased mortality5,6,7,8,9,10) and reduced social activity11,12,13,14). PSD is the most important reason for impaired quality of life in stroke patients15,16,17). Furthermore, several studies have shown that PSD is associated with decreased activities of daily living (ADL)1,18,19,20,21,22,23,24) and impaired recovery of ADL25,26,27,28,29).
Bhogal et al.4) and Hackett et al.30) suggested that depressive symptoms differ depending on the stage of stroke recovery (e.g., acute, subacute, or chronic) and the living environment (e.g., in a hospital or in the community). However, little is known about the association between PSD and the improvement in ADL among patients in a rehabilitation ward setting. Sinyor et al.18) reported that ADL were worse in depressed patients than in non-depressed patients on admission and at discharge; however, both groups showed similar improvement over the course of rehabilitation in a subacute hospital setting, suggesting that the effect of PSD on improvement in ADL may be minimal. Patients in that previous study, however, did not show an equal level of ADL between groups on admission, and the study did not distinguish between patients with and without PSD on admission. The level of ADL on admission has been reported to be related to improvement in ADL during the hospital stay31, 32). Therefore, to clarify the effect of PSD on improvement in ADL, there needs to be an equivalent level independence in ADL on admission in the PSD group and the non-PSD group. In another study, Chemerinski et al.27) reported that PSD patients showed significantly greater recovery in ADL than non-PSD patients in the subacute hospital setting. In that study, the level of independence in ADL on admission for the PSD and non-PSD groups was equal; however, the study had a small sample size. Finally, there are no reports on the effects of PSD on improvement in various ADL domains (i.e., the motor domain and the cognitive domain).
Therefore, the purpose of this study was to investigate improvement of ADL between admission to and discharge from a convalescent rehabilitation ward in patients with or without PSD who had equal levels of ADL on admission.
SUBJECTS AND METHODS
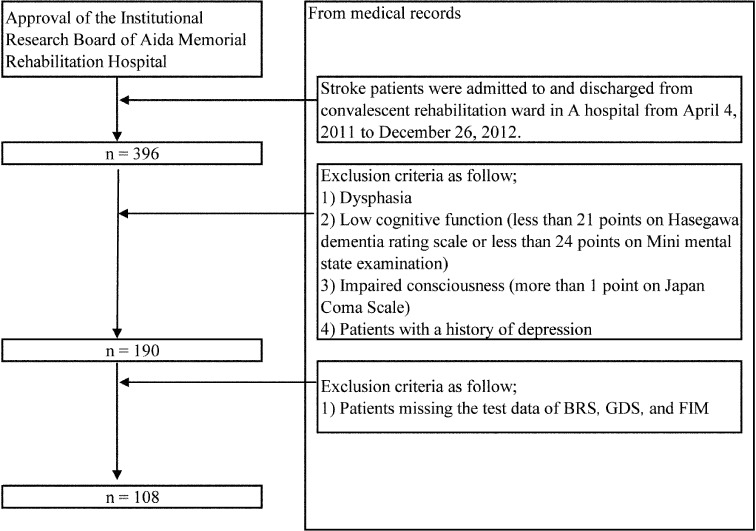
There were 396 stroke patients who were admitted to and discharged from a convalescent rehabilitation hospital ward from April 4, 2011 to December 26, 2012. On the basis of clinical evaluations, 206 patients with dysphasia, low cognitive function (<21 points on the Hasegawa Dementia Rating Scale or <24 points on the Mini Mental State Examination), impaired consciousness (>1 point on the Japanese Coma Scale), or a history of depression were excluded. Another 82 patients were missing data on one or more of following items: the Brunnstrom Recovery Stage (BRS), Geriatric Depression Scale, short version (GDS), and Functional Independence Measure (FIM). Therefore, 108 patients fulfilled the inclusion criteria for enrollment into the study (Fig. 1). This study was approved by the Institutional Research Board of Aida Memorial Rehabilitation Hospital (Approval No., 014). The study used only data collected retrospectively from patients’ medical records. Data were anonymized so that individuals could not be identified. Because of the study’s design, there were no risks, disadvantages, or infringements to individual and/or family rights.
Fig. 1.
Flow chart of participant selection
Information obtained from the medical records included sociodemographic variables (i.e., age and gender), laterality of the lesion, primary disease, whether discharge was to home or to a geriatric facility, medication information, illness duration, length of stay, total units of therapy, units of therapy per day, and BRS. The BRS was assessed for upper limbs, fingers, and lower limbs at the time of admission and at discharge. The BRS is an assessment of the motor recovery of paralytic symptoms. BRS is classified in 6 stages, as follows: (1) flaccidity, (2) synergy development, (3) voluntary synergistic movement, (4) some movements deviating from synergy, (5) independence from basic synergies, and (6) isolated joint movements. One unit of therapy was defined as 20 minutes of rehabilitation. The GDS was used to assess depressive symptoms. Scores were rated on admission. The GDS has scores ranging from 0 to 15, with a score of >6 points indicating a likelihood of depression. The short version of the test used in this study is more convenient and less of a burden on the patient. The GDS does not examine stroke symptoms (e.g., fatigability, constipation, and decreased appetite)33, 34). As a screening test, the GDS is predictive for PSD35) and has a high sensitivity for PSD as diagnosed by a psychiatrist36). ADL were assessed using the FIM. The FIM contains 18 items composed of 13 motor tasks and 5 cognitive tasks. The dimensions assessed by the FIM include eating, grooming, bathing, upper body dressing, lower body dressing, toileting, bladder management, bowel management, bed-to-chair transfer, toilet transfer, shower transfer, locomotion (ambulatory or wheelchair-bound), stairs, comprehension, expression, social interaction, problem solving, and memory. Tasks are rated on a 7-point ordinal scale that ranges from total assistance to complete independence. Scores range from 18 (lowest) to 126 (highest), indicating the level of function. Scores were rated on admission and at discharge. The reliability and validity of the FIM have already been confirmed in stroke patients37).
Based on standard a cut-off score of 6 for the GDS at the time of admission, patients were divided into the non-PSD group or the PSD group, and the two groups were compared. The degree of improvement in the FIM (FIM gain) was defined as the difference between the FIM score on admission and at discharge. FIM scores were compared in total (FIM total), by the subscale of motor activities (FIM motor), and by the subscale of cognition (FIM cognition). BRS was analyzed in all patients except those with right or left hemiplegia. χ2 tests were used to compare differences between groups for gender, laterality of the lesion, primary disease, whether discharge was to home or to a geriatric facility, and medication. An independent t-test was used to compare age, illness duration, length of stay, total units of therapy, and units of therapy per day between groups. The Mann-Whitney U-test was used to compare FIM total, FIM motor, FIM cognition, FIM gain scores, and BRS between groups. Analysis of variance (ANOVA) was used to examine the effect of the time period on PSD. FIM total, FIM motor, and FIM cognition scores were analyzed with a repeated-measures two-way ANOVA, with PSD (non-PSD/PSD) and the time period (admission/discharge) being within-subject variables. Factors that affected FIM scores at the time of discharge were examined using multiple regression analysis. Dependent variables were FIM total, FIM motor, and FIM cognition. Explanatory variables were GDS, age, length of stay, illness duration, units of therapy per day, and BRS of the lower limbs on admission. Explanatory variables (length of stay, total units of therapy, BRS of upper limbs and fingers on admission and at discharge, and BRS of lower limbs at discharge) that caused multicollinearity were eliminated. Data were analyzed using the Japanese version of SPSS Statistics for Windows version 20.0 (IBM Corporation, New York, NY, USA). The level of significance was set at p<0.05.
RESULTS
At the time of admission, 45 patients (42%) were classified into the non-PSD group, and 63 patients (58%) were classified into the PSD group. With respect to the sociodemographic variables, none (age, gender, laterality of the lesion, primary disease, discharge to home or to a geriatric facility, medication information, illness duration, length of stay, total units of therapy, units of therapy per day, and BRS) was significantly different between the two groups (Table 1).
Table 1. Demographic data.
| non-PSD group (n=45) | PSD group (n=63) | |
|---|---|---|
| Age, years | 66.3 ± 15.2 | 67.2 ± 14.7 |
| Gender, n | Male: 31 Female: 14 | Male: 39 Female: 24 |
| Laterality of lesion, n | Right: 17 | Right: 20 |
| Left: 24 | Left: 39 | |
| Bilateral: 4 | Bilateral: 4 | |
| Primary disease, n | CH: 14 | CH: 23 |
| CI: 28 | CI: 39 | |
| SAH: 3 | SAH: 1 | |
| Discharge destination, n | Facility: 2 | Facility: 10 |
| Home: 43 | Home: 53 | |
| Medication information (Antidepressant/Ataractic), n |
Nothing: 41 | Nothing: 55 |
| Antidepressant: 1 | Antidepressant: 4 | |
| Ataractic: 3 | Ataractic: 4 | |
| Illness duration, day | 39.8 ± 39.8 | 43.9 ± 13.7 |
| Length of stay, day | 94.6 ± 44.2 | 99.6 ± 43.4 |
| Total units of therapy, units* | 608.4 ± 259.9 | 616.8 ± 235.7 |
| Units of therapy per day, units* | 6.6 ± 1.2 | 6.4 ± 1.0 |
| GDS, score | 3 (0–5) | 9 (6–15) |
| BRS (admission) | ||
| Upper limb, score | 5 (1–6) | 5 (1–6) |
| Hand, score | 4 (1–6) | 5 (1–6) |
| Lower limb, score | 5 (2–6) | 5 (1–6) |
| BRS (discharge) | ||
| Upper limb, score | 5 (1–6) | 5 (1–6) |
| Hand, score | 5 (1–6) | 5 (1–6) |
| Lower limb, score | 6 (2–6) | 5 (2–6) |
Scores are presented as mean ± SD, median (minimum to maximum). *One unit is defined as 20 minutes of rehabilitation. χ2 tests were used to compare differences between groups for gender, laterality of the lesion, primary disease, discharge destination, and medication information. Independent t-tests were used to compare differences between groups for age, illness duration, length of stay, total units of therapy, and units of therapy per day. BRS was compared between groups with the Mann-Whitney U test. BRS: Brunnstrom Recovery Stage; CH: cerebral hemorrhage; CI: cerebral infarction; GDS: Geriatric Depression Scale Short Version; PSD: post-stroke depression; SAH: subarachnoid hemorrhage; SD: standard deviation
When the FIM scores on admission were compared, there was no significant difference between the two groups. When the scores for FIM total (p=0.003), FIM motor (p=0.002), and FIM cognition (p=0.011) at the time of discharge were compared, there were significant differences between the two groups. The PSD group had worse scores than the non-PSD group did. When the FIM gain score was compared between groups, there were significant differences in FIM total (p=0.022) and FIM motor (p=0.035) between the two groups. The PSD group had worse scores than the non-PSD group did. However, there was no significant difference between the two groups in the FIM gain score for cognition (p=0.317) (Table 2).
Table 2. Comparison of FIM scores in the PSD group and the non-PSD group.
| non-PSD group (n=45) |
PSD group (n=63) |
|
|---|---|---|
| Admission | ||
| FIM total, score | 76.7 ± 16.7 | 72.4 ± 21.7 |
| FIM motor, score | 50.6 ± 14.0 | 47.5 ± 18.4 |
| FIM cognition, score | 26.1 ± 6.3 | 24.9 ± 5.8 |
| Discharge | ||
| FIM total, score | 110.1 ± 6.7 | 91.2 ± 25.2** |
| FIM motor, score | 80.3 ± 5.4 | 65.4 ± 22.1** |
| FIM cognition, score | 29.7 ± 3.8 | 27.5 ± 4.7* |
| Gain | ||
| FIM total, score | 33.3 ± 12.9 | 26.8 ± 16.1* |
| FIM motor, score | 29.7 ± 11.0 | 24.5 ± 13.4* |
| FIM cognition, score | 3.6 ± 4.7 | 2.3 ± 5.5 |
*p<0.05, **p<0.01
Scores are presented as means ± SD.
FIM scores were analyzed between groups with the Mann-Whitney U test.
FIM: Functional Independence Measure; PSD: post-stroke depression
The ANOVA revealed significant interactions between the time period and PSD factors for FIM total (F [1, 106]=5.07, p=0.030) and FIM motor (F [1, 106]=4.61, p=0.030) (Table 3).
Table 3. Results of a two-way ANOVA in the PSD group (n=63) and the non-PSD group (n=45).
| Time period | Interaction | ||
|---|---|---|---|
| Admission | Discharge | ||
| Mean ± SD | Mean ± SD | ||
| FIM total, score | |||
| non-PSD | 76.7 ± 16.6 | 110.1 ± 14.7 | * |
| PSD | 72.3 ± 21.7 | 99.1 ± 22.2 | |
| FIM cognition, score | |||
| non-PSD | 26.1 ± 6.2 | 29.7 ± 5.1 | |
| PSD | 24.9 ± 5.8 | 27.2 ± 5.6 | |
| FIM motor, score | |||
| non-PSD | 50.6 ± 13.9 | 80.3 ± 11.7 | * |
| PSD | 47.4 ± 18.3 | 71.9 ± 18.5 | |
*p<0.05. ANOVA: analysis of variance; FIM: Functional Independence Measure; PSD: post-stroke depression; SD: standard deviation
The multiple regression analyses for FIM total and FIM motor were significant for GDS, age, and BRS (p<0.05). The multiple regression analysis for FIM cognition was significant for GDS, age, and illness duration (p<0.05). The multiple regression analysis for GDS was significant for FIM total (R2=0.28, β=−0.22, p=0.011), FIM motor (R2=0.30, β=−0.19, p=0.025), and FIM cognition (R2=0.19, β=−0.22, p=0.014) (Table 4).
Table 4. Results of a multivariate regression analysis to identify factors related to FIM score in the patients (n=108).
| Dependent variable | Explanatory variable | ||
|---|---|---|---|
| FIM total | FIM motor | FIM cognition | |
| β | β | β | |
| GDS | −0.22* | −0.19* | −0.22* |
| Age | −0.40** | −0.37** | −0.34** |
| Illness duration | −0.13 | −0.10 | −0.19 |
| Units of therapy of per day | 0.06 | 0.04 | 0.10 |
| BRS (Lower limb, Admission) | 0.29** | 0.38** | −0.08 |
| Adjusted R2 | 0.28** | 0.30 | 0.19** |
*p<0.05, **p<0.01, β: standardized coefficient; BRS: Brunnstrom Recovery Stage; GDS: Geriatric Depression Scale, short version; FIM: Functional Independence Measure
DISCUSSION
ADL improvement was investigated in patients who were categorized into the non-PSD or PSD groups on admission in a convalescent rehabilitation ward setting. In general, the results indicated that PSD decreased both the level of ADL independence at discharge and ADL improvement in stroke patients.
In this study, there were no significant differences in ADL between patients with and without PSD on admission, but there were significant differences in ADL at discharge and in the degree of improvement in ADL. These results indicate that there is less of a restorative effect on ADL in PSD patients. In addition, there was a statistically significant interaction between the time period and PSD in FIM scores. This finding supports the idea that PSD is related to ADL recovery and that it adversely affects a patient’s prognosis for ADL improvement in a convalescent rehabilitation ward setting.
Generally, the effects of the gain in ADL score are associated with age and the intensity of rehabilitation38, 39). Therefore, differences in age and intensity of rehabilitation were controlled between PSD patients and non-PSD patients. However, there was a statistically significant difference in the improvement of ADL between the two groups, which also indicated that PSD affects the improvement in ADL. Several studies have also shown that there is impaired recovery of ADL in PSD patients compared with that in non-PSD patients25,26,27), similar to the results shown here in the setting of a convalescent rehabilitation ward.
On the other hand, in the multiple regression analysis, GDS, age, and BRS influenced the ADL at the time of discharge. Other studies have also reported that the ADL of stroke patients were influenced by age and the degree of paralysis of the lower limbs39,40,41). The results of the present study indicate that the level of ADL independence at discharge was related not only to these factors but also to the severity of PSD at admission. Feigin et al.42) investigated the course of stroke-related function and concluded that depression was associated with ADL independence at 5 years after stroke. Accordingly, this study concluded that PSD had a negative impact on recovery of ADL and led to negative outcomes with respect to rehabilitation in a convalescent rehabilitation ward.
In addition, the present results revealed that PSD particularly inhibited FIM motor ADL, which may be due to psychological rather than to physiological mechanisms. For example, depressed patients may be hopeless about the future and thus may be psychologically less motivated to put any effort into rehabilitation or recovery27). In addition, because training may cause mental stress in PSD patients, it is important to train by avoiding mental load and to listen to the complaints of patients43). High-intensity training is needed to promote recovery of ADL44), and such training is often difficult for PSD patients. In addition, depression leads to reduced attention and to an increased risk of falling45, 46). Falling is reportedly associated with reduced ADL47, 48). Therefore, the falling risk induced by PSD may be associated with low independence levels in ADL. However, these issues remain conjectural. There is need for future research.
This study indicated a poor outcome with respect to rehabilitation in patients who had PSD on admission to a convalescent rehabilitation ward. Previous studies have shown that alleviation of depressive symptoms is associated with better functional recovery27, 49). Therefore, early detection of PSD by screening and early treatment may lead to better functional recovery in patients in convalescent rehabilitation wards.
This study has limitations with respect to the nature of the study population. The study sample was only from one convalescent ward, and it excluded patients with aphasia and mild paralysis. Furthermore, this study did not assess the effects of higher brain dysfunction and balance, which are related to ADL and gait ability50,51,52,53,54,55,56,57,58,59,60,61).
REFERENCES
- 1.Kauhanen M, Korpelainen JT, Hiltunen P, et al. : Poststroke depression correlates with cognitive impairment and neurological deficits. Stroke, 1999, 30: 1875–1880. [DOI] [PubMed] [Google Scholar]
- 2.Aben I, Verhey F, Strik J, et al. : A comparative study into the one year cumulative incidence of depression after stroke and myocardial infarction. J Neurol Neurosurg Psychiatry, 2003, 74: 581–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aben I, Lodder J, Honig A, et al. : Focal or generalized vascular brain damage and vulnerability to depression after stroke: a 1-year prospective follow-up study. Int Psychogeriatr, 2006, 18: 19–35. [DOI] [PubMed] [Google Scholar]
- 4.Bhogal SK, Teasell R, Foley N, et al. : Lesion location and poststroke depression: systematic review of the methodological limitations in the literature. Stroke, 2004, 35: 794–802. [DOI] [PubMed] [Google Scholar]
- 5.Morris PL, Robinson RG, Andrzejewski P, et al. : Association of depression with 10-year poststroke mortality. Am J Psychiatry, 1993, 150: 124–129. [DOI] [PubMed] [Google Scholar]
- 6.Everson SA, Roberts RE, Goldberg DE, et al. : Depressive symptoms and increased risk of stroke mortality over a 29-year period. Arch Intern Med, 1998, 158: 1133–1138. [DOI] [PubMed] [Google Scholar]
- 7.Williams LS, Ghose SS, Swindle RW: Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am J Psychiatry, 2004, 161: 1090–1095. [DOI] [PubMed] [Google Scholar]
- 8.Boden-Albala B, Litwak E, Elkind MS, et al. : Social isolation and outcomes post stroke. Neurology, 2005, 64: 1888–1892. [DOI] [PubMed] [Google Scholar]
- 9.Kamphuis MH, Kalmijn S, Tijhuis MA, et al. : Depressive symptoms as risk factor of cardiovascular mortality in older European men: the Finland, Italy and Netherlands Elderly (FINE) study. Eur J Cardiovasc Prev Rehabil, 2006, 13: 199–206. [DOI] [PubMed] [Google Scholar]
- 10.Ellis C, Zhao Y, Egede LE: Depression and increased risk of death in adults with stroke. J Psychosom Res, 2010, 68: 545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andersen G: Treatment of uncontrolled crying after stroke. Drugs Aging, 1995, 6: 105–111. [DOI] [PubMed] [Google Scholar]
- 12.Baseman S, Fisher K, Ward L, et al. : The relationship of physical function to social integration after stroke. J Neurosci Nurs, 2010, 42: 237–244. [DOI] [PubMed] [Google Scholar]
- 13.Sienkiewicz-Jarosz H, Milewska D, Bochyńska A, et al. : Predictors of depressive symptoms in patients with stroke—a three-month follow-up. Neurol Neurochir Pol, 2010, 44: 13–20. [DOI] [PubMed] [Google Scholar]
- 14.Hinojosa R, Haun J, Hinojosa MS, et al. : Social isolation poststroke: relationship between race/ethnicity, depression, and functional independence. Top Stroke Rehabil, 2011, 18: 79–86. [DOI] [PubMed] [Google Scholar]
- 15.Aström M, Adolfsson R, Asplund K: Major depression in stroke patients. A 3-year longitudinal study. Stroke, 1993, 24: 976–982. [DOI] [PubMed] [Google Scholar]
- 16.Kauhanen ML, Korpelainen JT, Hiltunen P, et al. : Domains and determinants of quality of life after stroke caused by brain infarction. Arch Phys Med Rehabil, 2000, 81: 1541–1546. [DOI] [PubMed] [Google Scholar]
- 17.Sturm JW, Donnan GA, Dewey HM, et al. : Quality of life after stroke: the North East Melbourne Stroke Incidence Study (NEMESIS). Stroke, 2004, 35: 2340–2345. [DOI] [PubMed] [Google Scholar]
- 18.Sinyor D, Amato P, Kaloupek DG, et al. : Post-stroke depression: relationships to functional impairment, coping strategies, and rehabilitation outcome. Stroke, 1986, 17: 1102–1107. [DOI] [PubMed] [Google Scholar]
- 19.van de Weg FB, Kuik DJ, Lankhorst GJ: Post-stroke depression and functional outcome: a cohort study investigating the influence of depression on functional recovery from stroke. Clin Rehabil, 1999, 13: 268–272. [DOI] [PubMed] [Google Scholar]
- 20.Singh A, Black SE, Herrmann N, et al. : Functional and neuroanatomic correlations in poststroke depression: the Sunnybrook Stroke Study. Stroke, 2000, 31: 637–644. [DOI] [PubMed] [Google Scholar]
- 21.Snaphaan L, van der Werf S, Kanselaar K, et al. : Post-stroke depressive symptoms are associated with post-stroke characteristics. Cerebrovasc Dis, 2009, 28: 551–557. [DOI] [PubMed] [Google Scholar]
- 22.Chatterjee K, Fall S, Barer D: Mood after stroke: a case control study of biochemical, neuro-imaging and socio-economic risk factors for major depression in stroke survivors. BMC Neurol, 2010, 10: 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown C, Hasson H, Thyselius V, et al. : Post-stroke depression and functional independence: a conundrum. Acta Neurol Scand, 2012, 126: 45–51. [DOI] [PubMed] [Google Scholar]
- 24.Lewin A, Jöbges M, Werheid K: The influence of self-efficacy, pre-stroke depression and perceived social support on self-reported depressive symptoms during stroke rehabilitation. Neuropsychol Rehabil, 2013, 23: 546–562. [DOI] [PubMed] [Google Scholar]
- 25.Kotila M, Waltimo O, Niemi ML, et al. : The profile of recovery from stroke and factors influencing outcome. Stroke, 1984, 15: 1039–1044. [DOI] [PubMed] [Google Scholar]
- 26.Parikh RM, Robinson RG, Lipsey JR, et al. : The impact of poststroke depression on recovery in activities of daily living over a 2-year follow-up. Arch Neurol, 1990, 47: 785–789. [DOI] [PubMed] [Google Scholar]
- 27.Chemerinski E, Robinson RG, Kosier JT: Improved recovery in activities of daily living associated with remission of poststroke depression. Stroke, 2001, 32: 113–117. [DOI] [PubMed] [Google Scholar]
- 28.Pohjasvaara T, Vataja R, Leppävuori A, et al. : Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur J Neurol, 2001, 8: 315–319. [DOI] [PubMed] [Google Scholar]
- 29.Espárrago Llorca G, Castilla-Guerra L, Fernández Moreno MC, et al. : Post-stroke depression: an update. Neurologia, 2015, 30: 23–31. [DOI] [PubMed] [Google Scholar]
- 30.Hackett ML, Anderson CS: Predictors of depression after stroke: a systematic review of observational studies. Stroke, 2005, 36: 2296–2301. [DOI] [PubMed] [Google Scholar]
- 31.Koyama T, Matsumoto K, Okuno T, et al. : A new method for predicting functional recovery of stroke patients with hemiplegia: logarithmic modelling. Clin Rehabil, 2005, 19: 779–789. [DOI] [PubMed] [Google Scholar]
- 32.Sonoda S, Saitoh E, Nagai S, et al. : Stroke outcome prediction using reciprocal number of initial activities of daily living status. J Stroke Cerebrovasc Dis, 2005, 14: 8–11. [DOI] [PubMed] [Google Scholar]
- 33.Sugishita M, Asada T: Creating a Geriatric Depression Scale—Short Version-Japanese, GDS-S-J. Jpn J Cogn Neurosci, 2009, 11: 87–90. [Google Scholar]
- 34.Okada K, Yamaguchi S: Post-stroke depression and post-stroke apathy. Gen Rehabil, 2011, 39: 1165–1170. [Google Scholar]
- 35.Lewin-Richter A, Volz M, Jöbges M, et al. : Predictivity of early depressive symptoms for post-stroke depression. J Nutr Health Aging, 2015, 19: 754–758. [DOI] [PubMed] [Google Scholar]
- 36.Agrell B, Dehlin O: Comparison of six depression rating scales in geriatric stroke patients. Stroke, 1989, 20: 1190–1194. [DOI] [PubMed] [Google Scholar]
- 37.Ottenbacher KJ, Hsu Y, Granger CV, et al. : The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil, 1996, 77: 1226–1232. [DOI] [PubMed] [Google Scholar]
- 38.Nagai S, Sonoda S, Miyai I, et al. : Relationship between the intensity of stroke rehabilitation and outcome: a survey conducted by the Kaifukuki Rehabilitation Ward Association in Japan. Jpn J Compr Rehabil Sci, 2011, 2: 77–81. [Google Scholar]
- 39.Watanabe M, Okuyama Y, Nobotachi N, et al. : Relationship between increase in exercise dose and ADL improvement with stratification by age in stroke patients in the Kaifukuki rehabilitation ward. Jpn J Stroke, 2012, 34: 383–390. [Google Scholar]
- 40.Tokunaga M, Yonemura M, Inoue R, et al. : Effects of age on functional independence measure score gain in stroke patients in kaifukuki rehabilitation ward. Jpn J Compr Rehabil Sci, 2012, 3: 32–36. [Google Scholar]
- 41.Alexander MP: Stroke rehabilitation outcome. A potential use of predictive variables to establish levels of care. Stroke, 1994, 25: 128–134. [DOI] [PubMed] [Google Scholar]
- 42.Feigin VL, Barker-Collo S, Parag V, et al. ASTRO study group: Auckland Stroke Outcomes Study. Part 1: Gender, stroke types, ethnicity, and functional outcomes 5 years poststroke. Neurology, 2010, 75: 1597–1607. [DOI] [PubMed] [Google Scholar]
- 43.Terai S, Miyamoto H, Nabeshima A: Comparative study of patient discharge disposition in a convalescent rehabilitation unit: an analysis of cerebrovascular disorder and disuse syndrome cases. Jpn J Rehabil Med, 2008, 45: 236–241. [Google Scholar]
- 44.Chen CC, Heinemann AW, Granger CV, et al. : Functional gains and therapy intensity during subacute rehabilitation: a study of 20 facilities. Arch Phys Med Rehabil, 2002, 83: 1514–1523. [DOI] [PubMed] [Google Scholar]
- 45.Jørgensen L, Engstad T, Jacobsen BK: Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke, 2002, 33: 542–547. [DOI] [PubMed] [Google Scholar]
- 46.Langhorne P, Stott DJ, Robertson L, et al. : Medical complications after stroke: a multicenter study. Stroke, 2000, 31: 1223–1229. [DOI] [PubMed] [Google Scholar]
- 47.Cho K, Lee G: Impaired dynamic balance is associated with falling in post-stroke patients. Tohoku J Exp Med, 2013, 230: 233–239. [DOI] [PubMed] [Google Scholar]
- 48.Cho K, Yu J, Rhee H: Risk factors related to falling in stroke patients: a cross-sectional study. J Phys Ther Sci, 2015, 27: 1751–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gainotti G, Antonucci G, Marra C, et al. : Relation between depression after stroke, antidepressant therapy, and functional recovery. J Neurol Neurosurg Psychiatry, 2001, 71: 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cho KH, Lee JY, Lee KJ, et al. : Factors related to gait function in post-stroke patients. J Phys Ther Sci, 2014, 26: 1941–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nam CW, Lee JH, Cho SH: The effect of non-elastic taping on balance and gait function in patients with stroke. J Phys Ther Sci, 2015, 27: 2857–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim YW, Moon SJ: Effects of treadmill training with the eyes closed on gait and balance ability of chronic stroke patients. J Phys Ther Sci, 2015, 27: 2935–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi JU, Kang SH: The effects of patient-centered task-oriented training on balance activities of daily living and self-efficacy following stroke. J Phys Ther Sci, 2015, 27: 2985–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bae YH, Ko Y, Ha H, et al. : An efficacy study on improving balance and gait in subacute stroke patients by balance training with additional motor imagery: a pilot study. J Phys Ther Sci, 2015, 27: 3245–3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hahn J, Shin S, Lee W: The effect of modified trampoline training on balance, gait, and falls efficacy of stroke patients. J Phys Ther Sci, 2015, 27: 3351–3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park KH, Kim DY, Kim TH: The effect of step climbing exercise on balance and step length in chronic stroke patients. J Phys Ther Sci, 2015, 27: 3515–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SJ, Cho HY, Kim YL, et al. : Effects of stationary cycling exercise on the balance and gait abilities of chronic stroke patients. J Phys Ther Sci, 2015, 27: 3529–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hyun KH, Cho HY, Lim CG: The effect of knee joint Mulligan taping on balance and gait in subacute stroke patients. J Phys Ther Sci, 2015, 27: 3545–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim K, Lee DK, Jung SI: Effect of coordination movement using the PNF pattern underwater on the balance and gait of stroke patients. J Phys Ther Sci, 2015, 27: 3699–3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fujita T, Sato A, Yamamoto Y, et al. : Relationship between dressing and motor function in stroke patients: a study with partial correlation analysis. J Phys Ther Sci, 2015, 27: 3771–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oh SI, Kim JK, Park SY: The effects of prism glasses and intensive upper limb exercise on hemineglect, upper limb function, and activities of daily living in stroke patients: a case series. J Phys Ther Sci, 2015, 27: 3941–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]