Abstract
Significance: Currently, calcific aortic valve disease (CAVD) is only treatable through surgical intervention because the specific mechanisms leading to the disease remain unclear. In this review, we explore the forces and structure of the valve, as well as the mechanosensors and downstream signaling in the valve endothelium known to contribute to inflammation and valve dysfunction. Recent Advances: While the valvular structure enables adaptation to dynamic hemodynamic forces, these are impaired during CAVD, resulting in pathological systemic changes. Mechanosensing mechanisms—proteins, sugars, and membrane structures—at the surface of the valve endothelial cell relay mechanical signals to the nucleus. As a result, a large number of mechanosensitive genes are transcribed to alter cellular phenotype and, ultimately, induce inflammation and CAVD. Transforming growth factor-β signaling and Wnt/β-catenin have been widely studied in this context. Importantly, NADPH oxidase and reactive oxygen species/reactive nitrogen species signaling has increasingly been recognized to play a key role in the cellular response to mechanical stimuli. In addition, a number of valvular microRNAs are mechanosensitive and may regulate the progression of CAVD. Critical Issues: While numerous pathways have been described in the pathology of CAVD, no treatment options are available to avoid surgery for advanced stenosis and calcification of the aortic valve. More work must be focused on this issue to lead to successful therapies for the disease. Future Directions: Ultimately, a more complete understanding of the mechanisms within the aortic valve endothelium will lead us to future therapies important for treatment of CAVD without the risks involved with valve replacement or repair. Antioxid. Redox Signal. 25, 401–414.
Introduction
Calcific aortic valve disease (CAVD) is present in 25–29% of the population aged over 65 years and is associated with a 50% increase in myocardial infarction and cardiovascular death (52, 72, 87, 93, 134). This disease is defined by a pathological change in aortic valve biology, including aortic valve sclerosis, which ultimately leads to significantly lower blood flow from the left ventricle to the systemic vasculature (101). The only treatment option for advanced CAVD is valve replacement or repair (10). The number of hospitalizations and valve implantations is projected to increase substantially over the next few decades, resulting in increased medical burden and risk (136).
CAVD begins with inflammation at the endothelium, leading to ultimate calcium deposition in the valve interstitium (32). The aortic side of the valve (fibrosa) is preferentially calcified, sparing the ventricular side of the tissue. The current hypothesis is that the fibrosa is subjected to oscillatory shear stress, combined with increased mechanical strain, conditions known to increase inflammation and endothelial dysfunction (31, 68, 98). Inflammation at the fibrosa endothelium may result from many systemic factors, including pathological flow, chronic inflammatory disease, or other inflammatory conditions (18, 42). Valve stenosis occurs after initial inflammation, resulting from lipoprotein accumulation, cellular infiltration, and extracellular matrix (ECM) formation (93). Cytokines released by the endothelium promote ECM formation and tissue remodeling in later stages of CAVD, finally leading to calcification within the valve tissue (59, 61).
This review explores the current state of understanding of the aortic valve endothelium, from proteins at the cell surface to transcription factors in the nucleus. While many potential therapeutic targets have been discovered in recent years, nonsurgical treatment options remain elusive. The overall picture of valvular endothelial biology shown in this review points to exciting areas of future research, which may lead to effective treatment and prevention of CAVD.
Aortic Valve Structure and Hemodynamics
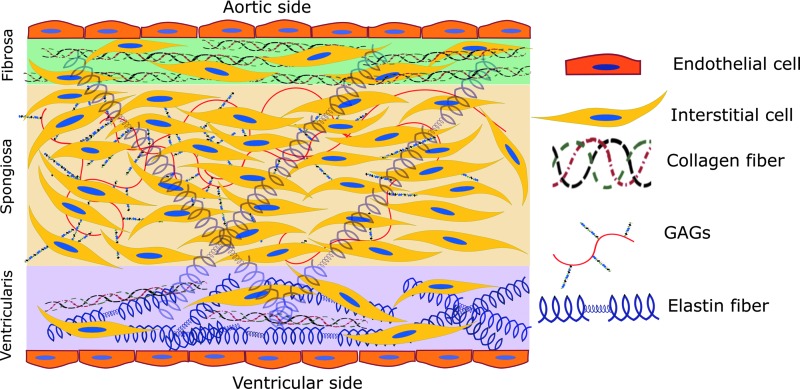
The aortic valve consists of three separate, but highly interactive, layers, which control mechanical and biological function of the tissue. As discussed above, the fibrosa faces the aortic side of the valve, containing endothelial cells at the interface with the blood, and valvular interstitial cells (VICs) below the endothelium, interspersed among a small amount of elastin fibers and circumferential type I and type III fibrillar collagen (Figure 1). The circumferential collagen allows the fibrosa to bear the higher mechanical loads exerted upon it during systole (105). The spongiosa is located between fibrosa and ventricularis and contains VICs, a small amount of elastin fibers, glycosaminoglycans (GAGs), and proteoglycans. The glycosylated components lubricate the fibrosa and ventricularis layers during tissue deformation. Hyaluronan plays a major role in blunting the impact of constant valve movement, binding large amounts of water and forming a foam-like structure in the spongiosa that absorbs energy (109, 132). The ventricularis, at the ventricular side of the valve, is made up of valvular endothelial cells, VICs, collagen, and radially oriented elastin fibers (22, 72, 132). The larger amount of matrix proteins, most especially elastin, within the ventricularis allows for fast and consistent compression during valve opening and closing (109).
FIG. 1.
Aortic valve structure. The fibrosa, facing the aorta, contains mainly collagen with some sparse elastin fibers. Interstitial cells are dispersed throughout the layers of the valve, except in the endothelium. The spongiosa, located in the center, contains the majority of interstitial cells as well as glycosaminoglycans (GAGs) and some elastin fibers. Elastin spans the spongiosa and the ventricularis, the side facing the left ventricle. The ventricularis layer comprises a larger amount of elastin than the others and a small amount of collagen fibers. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The constant movement of the valve affects biological pathways within the valvular endothelium. The endothelial cells experience a wide array of forces, which are integrated into signaling pathways to translate a biological response to increased stress on the tissue. The following discussion gives an overview of those forces, followed by an overview of the known mechanical signaling in the valve endothelium, and how those pathways may change in CAVD.
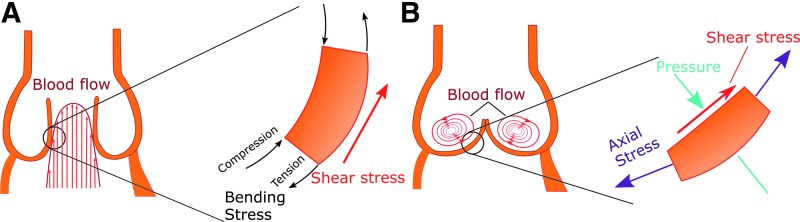
There are three types of forces, summarized in Figure 2, exerted upon the aortic valve:
(i) Pressure: Differential pressures during the dynamic movement of the aortic valve significantly shape the tissue shape and geometry (8). Low diastolic blood pressure in the ventricle versus stable pressure in the aorta causes the valve to close. The physiological transvalvular pressure remains at 80–120 mmHg. In hypertension, the transvalvular pressure may exceed 180 mmHg (121), contributing to degeneration of the valve tissue. According to findings from the SMART study, systolic blood pressure is correlated with calcification in the valve and vasculature, reflecting the contribution of pressure to general cardiovascular calcifications (118). Increased diastolic blood pressure also contributes significantly to aortic valve calcification in the aging population (58), possibly through synergistic effects on shear stress and cyclic stretch. A wide array of studies (13, 57, 62) have indicated the connection between valvular and systemic pressure on aortic valve calcification, indicating the importance of this parameter in the disease.
(ii) Axial and bending stress: Axial stress, perpendicular to the valve face in both the circumferential and radial directions, is critical for prevention of blood regurgitation backward into the ventricle (23). As the aortic valve ages, the highest valvular axial stress is observed near the aortic root, the area at which the first calcium nodules are observed in early valve calcification (111). Thus, a vicious cycle occurs, during which increased axial stress induces valve dysfunction and stenosis, leading to even higher levels of stress on the valve. Valvular bending stress compresses the concave fibrosa layer and increases tension on the convex ventricularis layer, as visualized in Figure 2A (23).
(iii) Shear stress: Shear stress, caused by blood flow parallel to the valve surface, occurs in a cycle due to changing blood flow during the cardiac cycle. The ventricularis experiences unidirectional laminar shear stress as blood is ejected from the ventricle to the aorta. The fibrosa experiences disturbed flow and oscillatory shear stress, as shown in Figure 2B, which have been directly linked to onset and progression of CAVD (6, 40, 104). Shear stress regulates endothelial cell function: the cells sense changes in shear stress to determine systemic changes, such as hypertension. For example, laminar shear stress alters cell morphology and cytoskeletal arrangement, resulting in alignment of endothelial cells in the direction of flow (78).
FIG. 2.
Major forces affecting aortic valve. (A) Main forces acting during systole on the valve. The ventricularis is stretched, while the fibrosa is compressed, generating a bending stress in the valve. Blood ejection from the heart exerts unidirectional laminar shear stress on the ventricularis (red arrow). (B) The main forces acting during diastole. Pressure (cyan arrows) is applied to the valve as the ventricle relaxes, and axial stress (purple arrows) prevents regurgitation by sealing the valve. The fibrosa experiences low oscillatory shear stress (red arrow). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Mechanosensors in the Aortic Valve Endothelium
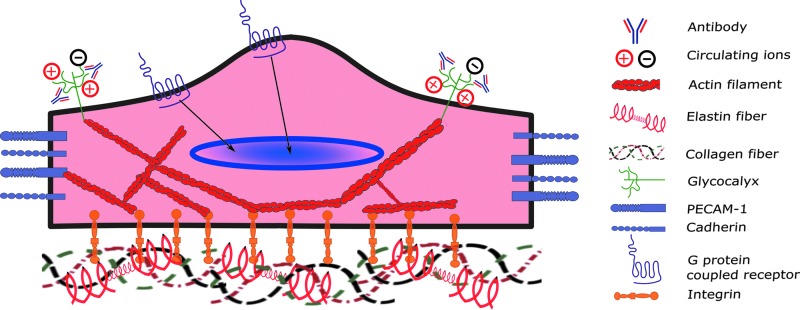
Endothelial cells utilize a variety of sensing mechanisms to respond to the extracellular mechanical environment, including transmembrane proteins, cytoskeletal proteins, networks of sugars, ion channels, or some combination of these. A vast array of studies have been carried out to further understand mechanosensors in the vascular endothelium, including caveolae, glycocalyx, G protein-coupled receptors (GPCRs), integrins, intercellular junctional proteins, ion channels, and tyrosine kinase receptors (119). However, a relatively small body of work has focused on the mechanical sensing of the valvular endothelial cells. In this study, we describe the current state of research into valvular endothelial mechanosensing, highlighting the major players at the cell surface, including integrins, GPCR, and the glycocalyx. Figure 3 shows a representative image of aortic valve endothelial cells and the major mechanosensors discussed in this article.
FIG. 3.
Major mechanosensors in valvular endothelial cells. Integrins bind to extracellular matrix to sense the dynamic mechanical environment surrounding the endothelial cell. Ions and antibodies are trapped by the glycocalyx in the surface of the cell, which translates to various downstream signal transduction pathways. Cell adhesion molecules (CAMs), such as cadherins or PECAM-1, sense changes in the mechanical environment between cells due to shear stress or other mechanical stimuli. These mechanosensors transduce the changes sensed through diverse signaling cascades (represented by black arrows) and through the actin cytoskeleton, which ultimately leads to transcriptional changes. The function of G protein-coupled receptors is not fully known in the valve, but their role in vascular endothelium suggests that they play a role in sensing the cellular mechanical environment and causing transcriptional changes in the nucleus. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Integrins, focal adhesion complexes, and the cytoskeleton
Integrins are a superfamily of transmembrane cell adhesion receptors that have traditionally been known to bind to ligands at the cell surface, within the ECM, and within the cytoplasm. Integrins transduce signals from the extracellular environment to the cell interior; in this way, changes in the concentrations of key signaling molecules in the plasma are communicated to the cell. In addition, integrins have more recently been recognized as sensors of the mechanical environment surrounding the cell, which also results in changes in intracellular signal transduction pathways (139, 147). In addition, integrins receive intracellular signals that regulate their ligand-binding affinity, fine-tuning the communication between the cell membrane and other cellular compartments (117). In coordinating cues from the extracellular environment with intracellular signaling, integrins play an important role in cell adhesion, migration, proliferation, and survival of the cell.
The mechanism by which integrins transmit signals to the cell is dependent on their binding to the cytoskeleton (149). Along with cytoskeletal proteins such as actin, a large number of binding partners colocalize with integrins near the cell surface, forming complexes known as focal adhesions (29, 97). Focal adhesion proteins in these complexes mediate the bidirectional mechanical transduction among the integrins, external stimuli, and the cell (145). Extensive research has found that the integrin–focal adhesion complexes have the ability to sense forces important to the aortic valve, including cyclic stretch (71), shear stress (74), and hypotonic stress (46). The complexes at the focal adhesions are also critical in signaling changes in the cell phenotype in response to mechanical and other cues, including signals for cell and tissue remodeling (51, 112), cell proliferation and apoptosis (112, 135), and cell migration and angiogenesis (74, 127).
Integrins encompass all but one of the families of mechanosensors, but the signals they transmit through focal adhesion complexes are critical for cell phenotype. Other classes transmit signals specific to cell–cell communication and inflammation, and all of the adhesion molecules work in tandem in the valve endothelium to determine the fate of the cell and the entire tissue.
G protein-coupled receptors
Similar to the other transmembrane proteins discussed above, GPCRs bind to a variety of extracellular ligands to activate downstream signaling, namely through G protein-activated signal transduction pathways (65). These receptors have also been shown to be strongly sensitive to changes in flow across the surface of the endothelium (19).
While some work has shown specific methods of subunit recruitment utilized by the GPCR to become activated under mechanical stimuli (30), the role of GPCR in the endothelium has not been fully realized, either in the vasculature or in the valve. Studies by Anger et al. showed that the mechanism by which statins may partially inhibit inflammation in the aortic valve is through GPCRs and their downstream effectors (5). This work demonstrated via array analysis that the extracellular regulated kinase (ERK) activation regulated by GPCRs is highly upregulated in calcified human aortic valve tissue (5). In addition, although the mechanism is unclear, statin therapy inhibits valvular expression of the GPCR regulatory proteins (RGS) (5). While these initial data offer exciting direction for future targeted therapies, new insights have not been delivered for several years. These receptors have known functions in many disease models (28) and thus their potential importance in the valve is clear. More work must be focused here to integrate these mechanical receptors into the lexicon of valvular mechanobiology.
Glycocalyx
The large network of sugars embedded into the cell membrane consists of proteoglycans and glycoproteins, bound to long chains of carbohydrates, called GAGs. The sugars provide a physical barrier between the blood and the endothelial cells (4, 11) and act as a trap where ions and other molecules in the blood may interact with membrane proteins (2). The endothelial glycocalyx significantly changes in shape and height as a result of disturbed flow, possibly leading to changes in its function as a scaffold for signaling molecules (66).
Inflammation also plays a role in the deformation of the glycocalyx, possibly indicating a positive feedback between the oscillatory flow and inflammation and exacerbating proinflammatory signaling (24). Specific studies of the glycocalyx in the valve endothelium show that proinflammatory mediators such as low-density lipoprotein (LDL) and immunoglobulins are more tightly bound to the sugar network in the valves of rabbits fed a high-cholesterol diet (107, 108). The binding of these molecules to the glycocalyx is increased on the fibrosa endothelium, the aortic side of the valve more vulnerable to interstitial calcification. Thorough investigative work by Sarphie (107) indicates the link between shear stress and the composition of the glycocalyx, which may alter the affinity of LDL particles for the cell surface, leading to infiltration and lesion formation. Of note, these studies have not been confirmed or progressed for over a quarter century, leaving a significant gap in our understanding of the glycocalyx and its role in the valve. New technologies may prove useful in defining this role and future therapies, which may change glycocalyx stability and composition. In addition, although the characterization of the glycocalyx has not recently been performed in the valve, its extensive characterization in the vasculature may be easily translated to the valvular environment. With more work focused on this exciting area, we may discover a wide assortment of targets for therapeutic intervention in early stages of valve dysfunction in the future.
Mechanosensitive Genes in Flow-Mediated Valve Biology and Dysfunction
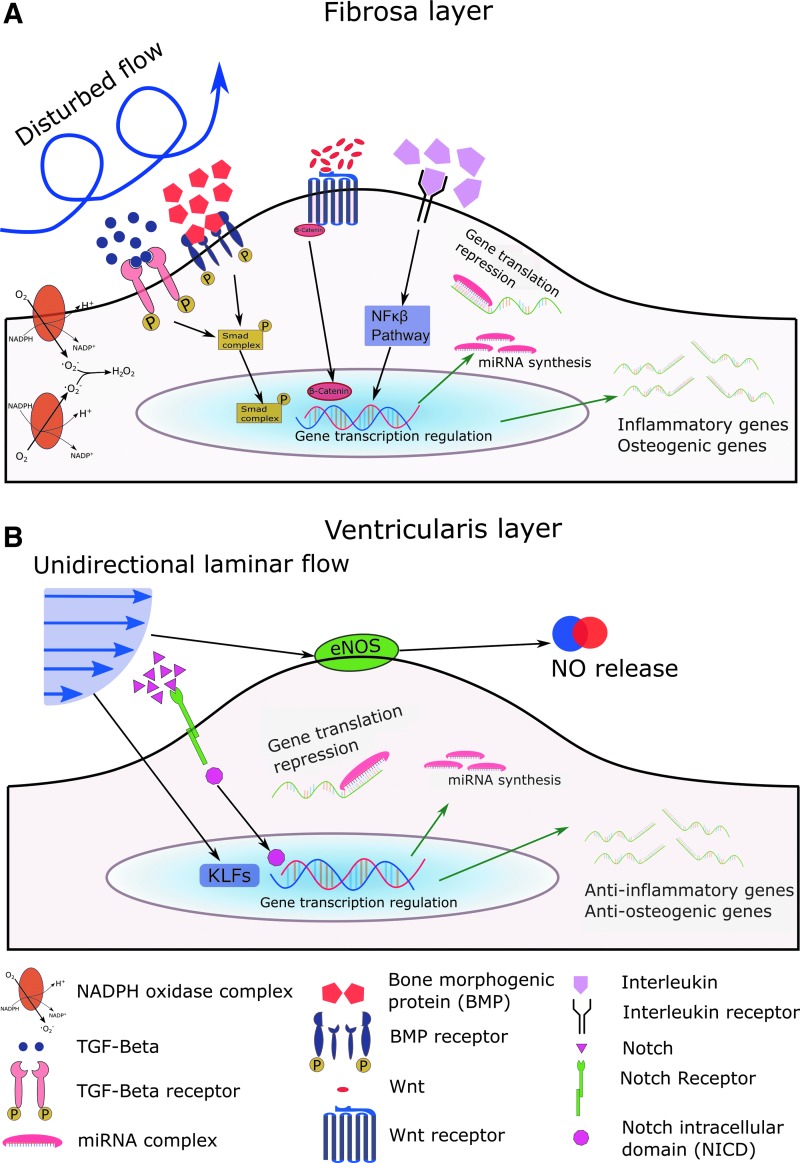
Shear stress is an important regulator of endothelial cell function. The vascular endothelium has been widely studied in this context, and a variety of vascular mechanosensitive genes have been discovered (25, 27, 34, 119). However, there is little known about the role of these mechanosensitive genes in the valvular endothelium. In this section, we review the genes that have been studied in the valvular endothelial cells, including some exciting new opportunities in valve research based on findings in the vasculature. For reference, Figure 4 shows a summary of valvular mechanosensitive genes in both disturbed flow, as seen at the calcifying fibrosa endothelium, and laminar flow as observed at the ventricularis.
FIG. 4.
Known and predicted mechanosensitive genes in the aortic valve endothelium under disturbed flow (A) and laminar flow (B). (A) Transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) are mechanosensitive molecules that bind to their respective receptors and activate the Smad signaling cascade by phosphorylating Smad molecules, which ultimately translocate to the nucleus. Wnt molecules bind to frizzled Wnt receptors and cause the release of β-catenin (Wnt canonical pathway), which leads to cytosolic β-catenin accumulation and translocation to the nucleus. Interleukins bind to interleukin receptors and regulate the NF-κβ signaling cascade that alters gene transcription. All these pathways induce inflammatory and osteogenic gene transcription, leading to endothelial dysfunction. Inflammatory miRNAs are also upregulated in disturbed flow, causing gene translation repression of anti-inflammatory genes. NADPH is upregulated in disturbed flow, which induces the generation of reactive oxygen species. (B) Kruppel-like factors (KLFs) are mechanosensitive genes upregulated in laminar flow that translocate to the nucleus and induce anti-inflammatory gene transcription. Notch1 binds to Notch receptors and causes NICD to be released and translocated to the nucleus, thereby promoting gene transcription. Anti-inflammatory miRNAs are upregulated in laminar flow, repressing translation of inflammatory genes. Endothelial nitric oxide synthase (eNOS) is upregulated in laminar flow, which induces nitric oxide generation and release outside of the cell. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Transforming growth factor-β ligand and receptors
The transforming growth factor (TGF)-β family of growth factors regulates a wide variety of cellular responses such as growth, development, immune system regulation, and tissue homeostasis (69). TGF-β signaling is initiated by the binding of TGF-β to TGF-β receptors, type I and type II. These two receptors form a receptor heterocomplex that recruits and phosphorylates R-Smad proteins, causing different signal transduction pathways depending on the Smad protein complexes that have been phosphorylated (53). TGF-β activity increases more than 100-fold in bovine aortic endothelial cells under shear conditions compared with static culture (92), suggesting a role for TGF-β in flow-induced vascular remodeling driven by fluid shear stress in the endothelium.
Villar et al. (125) found that circulating plasma levels of TGF-β1, the most abundant isoform of the TGF-β family, in patients with aortic stenosis were nearly threefold higher than in healthy controls. Yetkin et al. (141) studied patients with tricuspid calcified and stenotic aortic valves and found that the calcified valves presented similarly increased expression of TGF-β1. Furthermore, this work also suggested a link between TGF-β1 and cysteine C, a cysteine protease inhibitor highly expressed in mature osteoblasts, which inhibit bone resorption and appear in osteoblast differentiation (17, 60). These findings suggest the important role of TGF-β in aortic calcification and of TGF-β receptors in aortic valve endothelium as the high levels of this cytokine in blood induce an endothelial cell inflammatory phenotype. TGF-β has also been found to promote endothelial–mesenchymal transition (EMT): ovine aortic valve endothelial cells grown in the presence of TGF-β exhibited higher levels of CD31 (an endothelial cell marker) and α-smooth muscle actin, both EMT markers (96).
The combined evidence from the described studies reflects the importance of TGF-β in the events leading to CAVD. Matrix production and endothelial phenotypic transformation are critical for the events leading to stenosis of the valve, and TGF-β is a critical regulator of these pathologies. In combination with the proteins discussed below, a plethora of targets is available for future therapies.
Bone morphogenetic proteins
Bone morphogenetic proteins (BMPs) are multifunctional growth factors that belong to the TGF-β superfamily (21). These proteins are physiologically observed in bone and they initiate its formation during development and in injury (113). In vascular endothelium, bone morphogenic protein 4 (BMP4) is upregulated by disturbed flow, leading to endothelial inflammation and ultimate dysfunction (20, 113, 114). Additionally, in human atherosclerotic vasculature, both BMP2 and BMP4 expression levels are upregulated (37, 113). Our group has observed endothelial dysfunction and hypertension in mice infused with BMP4 (84), and BMP antagonist treatment protects against atherosclerosis (36, 140). Endothelial cells in the aortic valve may secrete BMPs in response to changes in shear stress (110), and the BMP expression of the interstitial cells may be linked with age-related valvular degeneration (20). Smad 1,5, and 8 are downstream molecules in the BMP signaling pathway and have been associated with onset of aortic stenosis (144). Sucosky et al. (116) studied porcine aortic valve leaflets exposed to physiological and altered shear stress conditions for 48 h ex vivo and found that BMP-4 and TGF-β were increased in pathologically altered conditions, which stimulated endothelial inflammatory response demonstrated by an increase in adhesion molecule expression. These results were validated by Ankeny et al. (6) in human calcified aortic valves and noncalcified aortic valves, which presented higher Smad 1/5/8 phosphorylation and lower BMP antagonists (crossveinless-2/BMPR and noggin) in calcified fibrosa.
These data, in the valve and in other vascular tissues, strongly suggest the importance of the BMP signaling pathway in aortic valve calcification. In designing future therapies for valve stenosis and calcification, the pathological downregulation of BMP antagonists provides an interesting target since this process may contribute to the side-dependent calcification pattern observed in human aortic valves. Any treatment providing some replenishment of physiological levels of these antagonists in the valve might be effective in treatment of valve dysfunction.
Wnt/β-catenin
Wnt proteins are a family of secreted signaling glycoproteins used for short or long-range signaling, which bind to receptors of the Frizzled family to regulate a wide variety of cellular processes such as cell fate determination, motility, stem cell renewal, and cell migration (67, 76, 103). The Wnt/β-catenin pathway (also known as canonical Wnt pathway) is widely known to regulate aortic valve formation and disease (54). β-catenin is a cytosolic protein that is bound to Wnt receptors in the cytosol and is degraded via ubiquitination in the absence of Wnt (1). When Wnt binds to Wnt receptors, β-catenin is stabilized and released to the cytosol, where it accumulates and translocates to the nucleus to activate the transcription of specific genes (76).
Shear stress has been found to regulate the canonical Wnt pathway in endothelial cells, leading to the increased expression of angiopoietin-2, a protein involved in vascular development and repair, in human aortic endothelial cells (HAECs) (73). In patients with symptomatic aortic valve stenosis, modulators of the Wnt signaling pathway, including WIF-1, DKK-1, and sFRP-3, exhibit increased expression in calcified aortic valves, and some modulators positively correlate with valvular calcification, indicating their potential role as biomarkers of aortic valve stenosis (7). Additionally, total levels of β-catenin have been found to be upregulated in human calcified aortic valves (16), calcified valves in a hypercholesterolemic rabbit model (102), and calcified valves in a hypercholesterolemic mouse model (83). This evidence shows the importance of the Wnt canonical pathway in CAVD. Although no evidence has been found directly linking the Wnt/β-catenin pathway and valvular shear stress, the data obtained from HAECs indicate that the abnormal shear stress profile in the fibrosa may regulate this pathway and lead to CAVD.
Notch signaling pathways
Notch is a single transmembrane protein that upon activation, releases a soluble Notch intracellular domain, which translocates to the nucleus and binds to specific sequences in the DNA to regulate gene transcription (9, 39). The Notch signaling pathway plays a critical role in the development and homeostasis of the cardiovascular system (47). While not directly working with the valve endothelium, Theodoris et al. (122) have studied the effect of shear stress in Notch1, a single transmembrane protein of the Notch family, in endothelial cells. Notch1 haploinsufficient (Notch1+/−) and wild-type (Notch1+/+) IPSC-derived ECs were exposed to hemodynamic shear stress. The expression of more than 1000 genes involved in osteogenesis, oxidative stress, and inflammation was dysregulated in the Notch+/− cells, causing an osteogenic and inflammatory phenotype. Interestingly, in the aortic valve, overrepresentation of Notch1 missense variants correlates with increased prevalence of bicuspid aortic valve (BAV) disease in human patients (80). Notch1 in the aortic valve interstitium has been found to repress the activity of Runx2, a transcription regulator of osteoblast cell fate (41), and therefore decreased expression of Notch1 in the aortic valve may lead to accelerated valve calcification. While no direct link is known to relate the disturbed shear stress profile observed at the fibrosa with calcification, the hemodynamic shear stress at the ventricularis may promote Notch1 activity, leading to an antiosteogenic and anti-inflammatory endothelial phenotype and protecting the ventricularis endothelium from dysfunction and calcification.
Nitric oxide signaling and endothelial nitric oxide synthase
Nitric oxide (NO) is a soluble gas with a short half-life (up to 30 s) that is synthetized from the amino acid, L-arginine, by the constitutive calcium–calmodulin-dependent enzyme nitric oxide synthase (NOS) (95), which is considered one of the most importance substances produced in the endothelium as it plays a key role in inflammation, vasodilation, and oxidative stress (142). Decreased NO biosynthesis facilitates vascular inflammation through an increase in lipoprotein oxidation (123).
Endothelial nitric oxide synthase (eNOS) is a shear-sensitive gene, which is upregulated in laminar shear conditions (physiological conditions) and downregulated in low and oscillatory shear conditions (pathophysiological conditions) (94). Bosse et al. (14) studied the role of NO in aortic valve calcification using a coculture system of endothelial cells and aortic valve interstitial cells (AVICs) and found that endothelial cells secrete NO, which is absorbed by AVICs, preventing calcification through regulation of the Notch1 signaling pathway in AVICs. Richards et al. (104) found that in both calcified and noncalcified human aortic valves, the ventricularis side exhibited a threefold higher expression of eNOS compared with the fibrosa. Interestingly, the calcified valves presented lower values of eNOS expression on both sides compared with healthy aortic valves. In the same study, in vitro experiments determined that endothelial secretion of NO decreases myofibroblastic activation, osteoblastic differentiation, and matrix calcification of VICs. El Accaoui et al. (38) have developed a mice model of aortic valve stenosis by knocking down eNOS and found that 30% of these mice had BAVs and that these mice presented fibrosis and calcification at 6 and 18 months of age. To further validate their work, porcine VICs were cocultured with or without valvular endothelial cells and they found that the endothelial cells inhibited profibrotic processes in VICs.
This VIC study shows the importance of eNOS in valvular calcification and provides a clear mechanism linking decreased NO bioavailability to endothelial dysfunction, leading to increased fibrosis and calcification. While NO has been investigated previously as a therapeutic agent, stimulation of endothelial NO production and exogenous NO treatment in the valve still offers an avenue of potential therapy for aortic valve disease.
Reactive oxygen species
Reactive oxygen species (ROS) are partially reduced metabolites of oxygen that possess high oxidizing capabilities and act by damaging DNA and oxidizing lipid and cellular constituents (85). They regulate cell growth, apoptosis, senescence, cell adhesion, and differentiation and are considered key components in inflammatory diseases (43, 120). One of the most important mechanisms of ROS production is the NADPH oxidase complex, which donates an electron to oxygen species in the cell to generate superoxide (12). Both the gene expression and protein levels of NADPH are increased fourfold in oscillatory flow in bovine aortic endothelial cells, which correlates with an increase of oxidized LDL through superoxide (O2—) modification (55).
Under laminar shear stress, ROS production in human umbilical vein endothelial cells (HUVECs) increases initially and decreases back to baseline levels over time, reflecting the complexity of ROS signaling in the endothelium (26). Both superoxide and hydrogen peroxide expression levels are significantly increased in calcified regions of the valves, whereas noncalcified regions, even in stenosed valves, present similar levels of superoxide and hydrogen peroxide as the tissue of healthy valves (82). ROS signaling, integrated with our understanding of NO, provides an interconnected web, linking the pathological signaling pathways of many mechanical genes expressed in the valve. Finding key components of these pathways may be critical for a full picture of the pathological degeneration of the valve.
ROS play a role further downstream of the endothelium: even in early stages of CAVD, VICs exhibit an accumulation of ROS, suggesting a role in disease development (86, 106, 143). Branchetti et al. (15) collected VICs from patients with and without CAVD and found that cells from stenosed patients possessed ROS-induced DNA damage and impaired DNA repair ability, which could be rescued with antioxidant enzyme treatment. In a thorough study showing the direct role of ROS in the interstitium, Das et al. (33) found that treatments decreasing ROS formation, specifically via decreases in the MAPK-TGF-β pathway, resulted in decreased calcium nodule formation.
Throughout the valve, ROS are important contributors to CAVD, both at the endothelial and interstitial cell layers, and changes in the oxidative state of the valve and the valvular endothelium may contribute significantly to the pathological degeneration of the valve. Further studies into specific species and their regulatory enzymes may be invaluable to determine their validity as therapeutic targets for CAVD.
Interleukins
Interleukins are important inflammatory cytokines released from T lymphocytes, macrophages, monocytes, and endothelial cells, which bind to specific cell receptors and play a role in communication with leukocytes, growth, and cell differentiation (3, 64). Interleukins produced in the endothelium play an important role in several diseases such as atherosclerosis, tumor development, and chronic infections (3, 130). Pathological low shear stress facilitates the increased expression of interleukin-6 (IL-6) and interleukin-1 (IL-1) from the endothelium, causing vascular smooth muscle cells to proliferate in early atherosclerotic plaques (115).
The interleukins also play a role in the valve: IL-6 has been found to be involved in the development and progression of aortic valve disease by affecting the EMT (124). In innovative studies, Mahler et al. (77) utilized a three-dimensional collagen gel to culture porcine aortic valve endothelial cells (PAVECs) and found that PAVECs underwent EMT when treated with IL-6 in a dose-dependent manner, via the Akt/nuclear factor-κβ-dependent pathway. The EMT is a critical step in tissue dysfunction, ultimately leading to valve calcification (133), and thus IL-6 is a potent regulator of the process. Moura et al. (89) conducted a study in patients with asymptomatic moderate to severe aortic stenosis treated with or without rosuvastatin, a statin used to reduce total and LDL cholesterol (75), measuring the expression of IL-6 in the patients before and after the statin treatment. Patients treated with rosuvastatin exhibited a slowed progression of aortic valve stenosis and a fivefold decrease in the serum levels of IL-6, indicating reduced inflammation at the endothelial level. Interleukin-1 is expressed in the endothelium of atherosclerotic plaque and it may be linked to atherogenic inflammation (81). In the frame of aortic valve disease, IL-1 receptor antagonist-deficient mice were studied and it was found that these mice presented an increase of aortic valve thickness compared with wild-type mice. T cells of the IL-1 receptor-deficient mouse were analyzed and they expressed much higher levels of tumor necrosis factor (TNF)-α compared with T cells of wild-type mice; these findings suggest that IL-1 may be inducing inflammation in aortic valve endothelial cells through the TNF-α signaling pathway and this inflammation may play a critical role in development of aortic stenosis (56).
From these studies, in combination with those in vitro, it is clear that abnormal shear stress, as experienced at the fibrosa, increases IL-6, and this interleukin, possibly coupled with others, promotes valve calcification. More studies into how the interleukins may be targeted to change the EMT signature profile may shed light on how to alter endothelial phenotype to alleviate valve calcification therapeutically.
Kruppel-like factors
Kruppel-like factors (KLFs) are members of the zinc finger family of transcription factors, which bind to DNA sequences, including CACC-, GC-, or GT- box elements in promoter and enhancer regions (100). In the vascular endothelium, KLF2 has been widely studied; it is upregulated in high laminar unidirectional shear stress (physiological conditions) and downregulated in low oscillatory shear stress (pathophysiological conditions) (35, 128, 137). In extensive in vitro experiments, human aortic valve endothelial cells (HAVECs) from noncalcified aortic valves exhibited higher KLF2 expression in high unidirectional shear stress (20 dynes/cm2, conditions similar to ventricularis) and lower expression in low and oscillatory shear stress (±5 dynes/cm2, conditions similar to fibrosa), reflecting the fact that the ventricularis side shows higher KLF2 expression and an anti-inflammatory phenotype (50). To study inflammatory markers, including KLF2, in detail, Weinberg et al. (129) created a computational model of the aortic valve hemodynamics to model the exact shear profile experienced by the aortic valve. The simulated shear profiles were then applied to HUVECs, and the expression of KLF2 was found to be significantly higher in the cells that experienced the ventricularis shear profile. In microarray studies by our group, porcine aortic valve fibrosa shows decreased expression of KLF2 and KLF4, indicating their importance in valvular endothelium just as in the vasculature (50). These initial studies may lead to important insights for these anti-inflammatory markers in valvular function and endothelial cell phenotype.
KLF4 is another member of the KLF family that when overexpressed, induces anti-inflammatory and antithrombotic factors such as thrombomodulin and endothelial nitric oxide synthase, whereas knockdown of KLF4 induces inflammation (45, 148). More importantly, KLF4 is known to be upregulated in vascular endothelial cells in laminar shear stress compared with oscillatory shear stress (126, 131). So far, the role of this KLF protein in aortic valve calcification is unknown; however, a study conducted by Maleki et al. (79) analyzed patients with BAV, who are known to develop aortic valvular stenosis more rapidly than patients with tricuspid aortic valve (TAV), finding a significant decrease in KLF4 expression in the aortic region near the valve in BAV patients compared with the TAV patients. The authors postulate that the disturbed flow generated by the BAV is the primary contributor to this decreased KLF4 expression. Therefore, we expect that the same abnormal shear profile will be experienced by the valve and would cause a decrease of KLF4 with the corresponding increase in inflammatory endothelial response.
The KLFs are important protective anti-inflammatory mediators in the valvular endothelium. Therapies developed to increase these factors, possibly along with the many other factors discussed in this review, will have a significant impact on the maintenance of physiological valve function and health.
microRNAs
microRNAs (miRNAs) act as suppressors of protein expression by targeting transcription. miRNAs are small nucleotide sequences up to ∼22 bases in length; their binding to the 3′ untranslated region of mRNA leads to the degradation of that mRNA or inhibition of protein translation (44). miRNAs have been implicated in most diseases, either preserving physiological cell function or promoting pathological signaling and dysfunction. In cardiovascular diseases, major miRNAs have been thoroughly cataloged and analyzed; exciting miRNA targets have been found in atherosclerosis (70, 90), heart failure (63), diabetes (88), and hypertension (91).
Despite their characterization in other cardiovascular systems, miRNAs have not been well characterized in the aortic valve. In the interstitium, miRNA-30b was shown to prevent the signaling necessary for interstitial cells to transform into calcifying cells (146). In addition, miRNA-141 targets TGF-β and BMP-2 signaling, which are critical for interstitial osteoblastic differentiation leading to calcification of the tissue (138). Our group has analyzed miRNAs in the endothelium, and we found a novel family of shear-sensitive miRNAs, which change expression under different shear stress conditions (70). As our recent review indicates, the many studies linking disturbed flow to cardiovascular complications provide important information for other diseases involving laminar and oscillatory flow, including aortic valve stenosis and calcification.
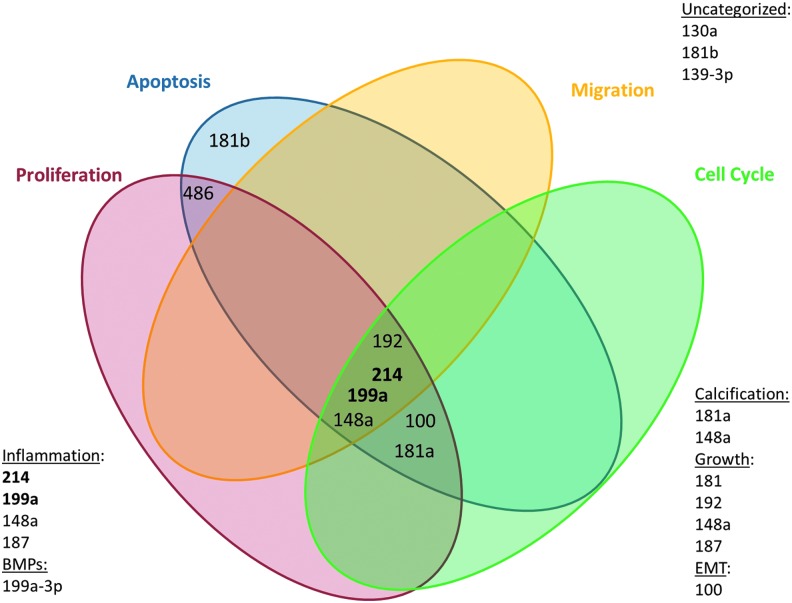
In recent studies, we have performed an HAVEC microarray to find novel miRNAs differentially expressed in the aortic valve endothelium and which are sensitive to disturbed flow (Figure 5). We have discovered many interesting potential targets for therapy, including miRNA-486-5p, which exhibited one of the most dramatic changes in expression between different flow conditions.
FIG. 5.
Discovery of key shear-sensitive microRNAs (miRNAs) in the porcine aortic valve endothelium. Our group performed microarray to determine miRNAs, which are sensitive to laminar versus oscillatory shear stress. Those miRNAs significantly changed by the differential shear stresses were categorized by cell phenotype known to be affected. Previously published data show that many of the miRNAs highlighted in our array are involved in cellular functions important in heart valve disease. Figure modified from Holliday dissertation (49). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In preliminary studies, we have found that miRNA-486-5p in the aortic valve endothelium enhances cell movement in response to shear stress to alter cell phenotype (48, 99). The study conducted in our laboratory (149) showed that Efna1 and Prnd are directly targeted by miR-486-5p. Cell migration was significantly enhanced in HAVECs overexpressing miRNA-486-5p (see Figure 6 for a representation of this potential mechanism). This work reveals the power of microarray to piece together miRNAs, their targets, and ultimate cell fate. However, more work must be performed to confirm these links and their potential in treatment of valve disease. Our group and others have made great strides in the study of miRNAs in the valve. Studies investigating their potential in treatment of valvular dysfunction and stenosis are ongoing and they may reveal insights that provide dramatic benefits for valvular function. These exciting results are only the beginning of our investigation into the role of miRNAs in the aortic valve endothelium; many more potential targets are sure to lead us to a clearer understanding of the disease in the future.
FIG. 6.
Putative mechanism through which miR-486-5p controls cell movement in response to laminar shear stress. Studies by our group via microarray have shown that miR-486-5p is upregulated under laminar flow conditions in human aortic valve endothelial cells and at the porcine ventricularis, which causes an increase in miR-486-5p complex formation and alters a multitude of pathways controlling endothelial cell movement within the tissue. Among the genes affected by miR-486-5p upregulation are Efna1 and Prnd, which are downregulated and may contribute to the changes in endothelial phenotype observed in the microarray. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Future Perspectives in CAVD Research and Treatment
Work in aortic valve mechanics and flow-mediated signaling has rapidly advanced in recent years; however, viable options for nonsurgical treatment of CAVD remain elusive. Future research should translate findings in the vascular endothelium to research in the valve, which is a much clearer example of biomechanical force. The aortic valve is a complex machine and an elegant regulator of systemic blood flow. With the latest technological advances in computer and animal models, we are closer than ever to treating and preventing aortic valve stenosis and calcification.
Abbreviations Used
- AVIC
aortic valve interstitial cell
- BAV
bicuspid aortic valve
- BMP
bone morphogenetic protein
- CAVD
calcific aortic valve disease
- ECM
extracellular matrix
- EMT
endothelial–mesenchymal transition
- eNOS
endothelial nitric oxide synthase
- ERK
extracellular regulated kinase
- GAG
glycosaminoglycan
- GPCR
G protein-coupled receptor
- HAEC
human aortic endothelial cell
- HAVEC
human aortic valve endothelial cell
- HUVEC
human umbilical vein endothelial cell
- IL-1
interleukin-1
- IL-6
interleukin-6
- KLF
Kruppel-like factor
- LDL
low-density lipoprotein
- miRNA
microRNA
- NO
nitric oxide
- NOS
nitric oxide synthase
- PAVEC
porcine aortic valve endothelial cell
- RGS
regulator of G protein signaling
- ROS
reactive oxygen species
- TAV
tricuspid aortic valve
- TGF-β
transforming growth factor-β
- TNF
tumor necrosis factor
- VIC
valvular interstitial cell
References
- 1.Aberle H, Bauer A, Stappert J, Kispert A, and Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16: 3797–3804, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karousou E, Theocharis AD, Pavao MS, Tzanakakis GN, and Karamanos NK. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J 279: 1177–1197, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O'Mahony L, Palomares O, Rhyner C, Quaked N, Schaffartzik A, Van De Veen W, Zeller S, Zimmermann M, and Akdis CA. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol 127: 701–721.e70, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Alphonsus CS. and Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia 69: 777–784, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Anger T, El-Chafchak J, Habib A, Stumpf C, Weyand M, Daniel WG, Hombach V, Hoeher M, and Garlichs CD. Statins stimulate RGS-regulated ERK 1/2 activation in human calcified and stenotic aortic valves. Exp Mol Pathol 85: 101–111, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Ankeny RF, Thourani VH, Weiss D, Vega JD, Taylor WR, Nerem RM, and Jo H. Preferential activation of SMAD1/5/8 on the fibrosa endothelium in calcified human aortic valves—association with low BMP antagonists and SMAD6. PLoS One 6: e20969, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Askevold ET, Gullestad L, Aakhus S, Ranheim T, Tonnessen T, Solberg OG, Aukrust P, and Ueland T. Secreted Wnt modulators in symptomatic aortic stenosis. J Am Heart Assoc 1: e002261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bäck M, Gasser TC, Michel J-B, and Caligiuri G. Biomechanical factors in the biology of aortic wall and aortic valve diseases. Cardiovasc Res 99: 232–241, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron M. An overview of the Notch signalling pathway. Semin Cell Dev Biol 14: 113–119, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Baxley WA. Aortic valve disease. Curr Opin Cardiol 9: 152–157, 1994 [DOI] [PubMed] [Google Scholar]
- 11.Becker BF, Chappell D, and Jacob M. Endothelial glycocalyx and coronary vascular permeability: the fringe benefit. Basic Res Cardiol 105: 687–701, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Bermejo J. The effects of hypertension on aortic valve stenosis. Heart 91: 280–282, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosse AK, Hans CP, Zhao N, Koenig SN, Huang N, Guggilam A, LaHaye S, Tao G, Lucchesi PA, Lincoln J, Lilly B, and Garg V. Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease. J Mol Cell Cardiol 60: 27–35, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Branchetti E, Sainger R, Poggio P, Grau JB, Patterson-Fortin J, Bavaria JE, Chorny M, Lai E, Gorman RC, Levy RJ, and Ferrari G. Antioxidant enzymes reduce DNA damage and early activation of valvular interstitial cells in aortic valve sclerosis. Arterioscler Thromb Vasc Biol 33: e66–e74, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, and Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol 47: 1707–1712, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Candeliere GA, Rao Y, Floh A, Sandler SD, and Aubin JE. cDNA fingerprinting of osteoprogenitor cells to isolate differentiation stage-specific genes. Nucleic Acids Res 27: 1079–1083, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carabello BA. Introduction to aortic stenosis. Circ Res 113: 179–185, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Chachisvilis M, Zhang YL, and Frangos JA. G protein-coupled receptors sense fluid shear stress in endothelial cells. Proc Natl Acad Sci U S A 103: 15463–15468, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang K, Weiss D, Suo J, Vega JD, Giddens D, Taylor WR, and Jo H. Bone morphogenic protein antagonists are coexpressed with bone morphogenic protein 4 in endothelial cells exposed to unstable flow in vitro in mouse aortas and in human coronary arteries: role of bone morphogenic protein antagonists in inflammation and atherosclerosis. Circulation 116: 1258–1266, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Chen D, Zhao M, and Mundy GR. Bone morphogenetic proteins. Growth Factors 22: 233–241, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Chen JH. and Simmons CA. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res 108: 1510–1524, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Chester AH, El-Hamamsy I, Butcher JT, Latif N, Bertazzo S, and Yacoub MH. The living aortic valve: from molecules to function. Glob Cardiol Sci Pract 2014: 52–77, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chien S. Molecular and mechanical bases of focal lipid accumulation in arterial wall. Prog Biophys Mol Biol 83: 131–151, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Chien S. Mechanotransduction and endothelial cell homeostasis: the wisdom of the cell. Am J Physiol Heart Circ Physiol 292: H1209–H1224, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Chiu JJ, Wung BS, Shyy JYJ, Hsieh HJ, and Wang DL. Reactive oxygen species are involved in shear stress-induced intercellular adhesion molecule-1 expression in endothelial cells. Arterioscler Thromb Vasc Biol 17: 3570–3577, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Chrétien ML, Zhang M, Jackson MR, Kapus A, and Langille BL. Mechanotransduction by endothelial cells is locally generated, direction-dependent, and ligand-specific. J Cell Physiol 224: 352–361, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Christopoulos A. Advances in G protein-coupled receptor allostery: from function to structure. Mol Pharmacol 86: 463–478, 2014 [DOI] [PubMed] [Google Scholar]
- 29.Ciobanasu C, Faivre B, and Le Clainche C. Integrating actin dynamics, mechanotransduction and integrin activation: the multiple functions of actin binding proteins in focal adhesions. Eur J Cell Biol 92: 339–348, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Cuerrier CM, Gagner A, Lebel R, Gobeil F, Jr., and Grandbois M. Effect of thrombin and bradykinin on endothelial cell mechanical properties monitored through membrane deformation. J Mol Recognit 22: 389–396, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Cunningham KS. and Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest 85: 9–23, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Czarny MJ. and Resar JR. Diagnosis and management of valvular aortic stenosis. Clin Med Insights Cardiol 8(Suppl 1): 15–24, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Das D, Holmes A, Murphy GA, Mishra K, Rosenkranz AC, Horowitz JD, and Kennedy JA. TGF-beta1-Induced MAPK activation promotes collagen synthesis, nodule formation, redox stress and cellular senescence in porcine aortic valve interstitial cells. J Heart Valve Dis 22: 621–630, 2013 [PubMed] [Google Scholar]
- 34.Davies PF. Multiple Signaling Pathways in Flow-Mediated Endothelial Mechanotransduction: PYK-ing the Right Location. Arterioscler Thromb Vasc Biol 22: 1755–1757, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Dekker R, van Soest S, Fontijn R, Salamanca S, de Groot P, VanBavel E, Pannekoek H, and Horrevoets A. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2). Blood 100: 1689–1698, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Derwall M, Malhotra R, Lai CS, Beppu Y, Aikawa E, Seehra JS, Zapol WM, Bloch KD, and Yu PB. Inhibition of bone morphogenetic protein signaling reduces vascular calcification and atherosclerosis. Arterioscler Thromb Vasc Biol 32: 613–622, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, and Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol 21: 1998–2003, 2001 [DOI] [PubMed] [Google Scholar]
- 38.El Accaoui RN, Gould ST, Hajj GP, Chu Y, Davis MK, Kraft DC, Lund DD, Brooks RM, Doshi H, Zimmerman KA, Kutschke W, Anseth KS, Heistad DD, and Weiss RM. Aortic valve sclerosis in mice deficient in endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol 306: H1302–H1313, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell 16: 633–647, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Freeman RV. and Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation 111: 3316–3326, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, and Srivastava D. Mutations in NOTCH1 cause aortic valve disease. Nature 437: 270–274, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Gould ST, Srigunapalan S, Simmons CA, and Anseth KS. Hemodynamic and cellular response feedback in calcific aortic valve disease. Circ Res 113: 186–197, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, and Thannickal VJ. NOX enzymes and pulmonary disease. Antioxid Redox Signal 11: 2505–2516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo H, Ingolia NT, Weissman JS, and Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466: 835–840, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, Feinberg MW, Gerszten RE, Edelman ER, and Jain MK. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem 282: 13769–13779, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Hirakawa M, Oike M, Karashima Y, and Ito Y. Sequential activation of RhoA and FAK/paxillin leads to ATP release and actin reorganization in human endothelium. J Physiol 558: 479–488, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hofmann JJ. and Iruela-Arispe ML. Notch signaling in blood vessels: who is talking to whom about what? Circ Res 100: 1556–1568, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Holliday-Ankeny CJ, Ankeny RF, Ferdous Z, Nerem RM, and Jo H. The function of shear-responsive and side-dependent microRNA-486-5p in aortic valve endothelium. Cardiovasc Pathol 22: e50, 2013 [Google Scholar]
- 49.Holliday CJ. Discovery of shear- and side- dependent microRNAs and messenger RNAs in aortic valvular endothelium [Doctoral]. Atlanta, GA: Georgia Institute of Technology, 2012, p. 224 [Google Scholar]
- 50.Holliday CJ, Ankeny RF, Jo H, and Nerem RM. Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am J Physiol Heart Circ Physiol 301: H856–H867, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu HJ, Lee CF, Locke A, Vanderzyl SQ, and Kaunas R. Stretch-induced stress fiber remodeling and the activations of JNK and ERK depend on mechanical strain rate, but not FAK. PLoS One 5: e12470, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu SY, Hsieh IC, Chang SH, Wen MS, and Hung KC. Aortic valve sclerosis is an echocardiographic indicator of significant coronary disease in patients undergoing diagnostic coronary angiography. Int J Clin Pract 59: 72–77, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Huang F. and Chen YG. Regulation of TGF-beta receptor activity. Cell Biosci 2: 9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurlstone AFL, Haramis A-PG, Wienholds E, Begthel H, Korving J, van Eeden F, Cuppen E, Zivkovic D, Plasterk RHA, and Clevers H. The Wnt/[beta]-catenin pathway regulates cardiac valve formation. Nature 425: 633–637, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Hwang J, Ing MH, Salazar A, Lassegue B, Griendling K, Navab M, Sevanian A, and Hsiai TK. Pulsatile versus oscillatory shear stress regulates NADPH oxidase subunit expression: implication for native LDL oxidation. Circ Res 93: 1225–1232, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Isoda K, Matsuki T, Kondo H, Iwakura Y, and Ohsuzu F. Deficiency of interleukin-1 receptor antagonist induces aortic valve disease in BALB/c mice. Arterioscler Thromb Vasc Biol 30: 708–715, 2010 [DOI] [PubMed] [Google Scholar]
- 57.Ivanovic B, Tadic M, and Dincic D. The effects of arterial hypertension on aortic valve stenosis. Vojnosanit Pregl 67: 588–592, 2010 [DOI] [PubMed] [Google Scholar]
- 58.Iwata S, Russo C, Jin Z, Schwartz JE, Homma S, Elkind MSV, Rundek T, Sacco RL, and Di Tullio MR. Higher ambulatory blood pressure is associated with aortic valve calcification in the elderly: a population-based study. Hypertension 61: 55–60, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jian B, Narula N, Li QY, Mohler ER, 3rd, and Levy RJ. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg 75: 457–465; discussion 465–466, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Johansson L, Grubb A, Abrahamson M, Kasprzykowski F, Kasprzykowska R, Grzonka Z, and Lerner UH. A peptidyl derivative structurally based on the inhibitory center of cystatin C inhibits bone resorption in vitro. Bone 26: 451–459, 2000 [DOI] [PubMed] [Google Scholar]
- 61.Kaden JJ, Dempfle CE, Grobholz R, Tran HT, Kilic R, Sarikoc A, Brueckmann M, Vahl C, Hagl S, Haase KK, and Borggrefe M. Interleukin-1 beta promotes matrix metalloproteinase expression and cell proliferation in calcific aortic valve stenosis. Atherosclerosis 170: 205–211, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Kaden JJ. and Haghi D. Hypertension in aortic valve stenosis—a Trojan horse. Eur Heart J 29: 1934–1935, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Kalozoumi G, Yacoub M, and Sanoudou D. MicroRNAs in heart failure: small molecules with major impact. Glob Cardiol Sci Pract 2014: 79–102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanda T. and Takahashi T. Interleukin-6 and cardiovascular diseases. Jpn Heart J 45: 183–193, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Katritch V, Cherezov V, and Stevens RC. Structure-function of the G protein-coupled receptor superfamily. Annu Rev Pharmacol Toxicol 53: 531–556, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kolářová H, Ambrůzová B, Švihálková Šindlerová L, Klinke A, and Kubala L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediators Inflamm 2014: 694312, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komiya Y. and Habas R. Wnt signal transduction pathways. Organogenesis 4: 68–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ku DN, Giddens DP, Zarins CK, and Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arterioscler Thromb Vasc Biol 5: 293–302, 1985 [DOI] [PubMed] [Google Scholar]
- 69.Kubiczkova L, Sedlarikova L, Hajek R, and Sevcikova S. TGF-beta—an excellent servant but a bad master. J Transl Med 10: 183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar S, Kim CW, Simmons RD, and Jo H. Role of flow-sensitive microRNAs in endothelial dysfunction and atherosclerosis: mechanosensitive athero-miRs. Arterioscler Thromb Vasc Biol 34: 2206–2216, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lehoux S, Esposito B, Merval R, and Tedgui A. Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation 111: 643–649, 2005 [DOI] [PubMed] [Google Scholar]
- 72.Leopold JA. Cellular mechanisms of aortic valve calcification. Circ Cardiovasc Interv 5: 605–614, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li R, Beebe T, Jen N, Yu F, Takabe W, Harrison M, Cao H, Lee J, Yang H, Han P, Wang K, Shimizu H, Chen J, Lien C-L, Chi NC, and Hsiai TK. Shear stress–activated wnt-angiopoietin-2 signaling recapitulated vascular repair in zebrafish embryos. Arterioscler Thromb Vasc Biol 34: 2268–2275, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li S, Butler P, Wang Y, Hu Y, Han DC, Usami S, Guan JL, and Chien S. The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. Proc Natl Acad Sci U S A 99: 3546–3551, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luvai A, Mbagaya W, Hall AS, and Barth JH. Rosuvastatin: a review of the pharmacology and clinical effectiveness in cardiovascular disease. Clin Med Insights Cardiol 6: 17–33, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacDonald BT, Tamai K, and He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell 17: 9–26, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahler GJ, Farrar EJ, and Butcher JT. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler Thromb Vasc Biol 33: 121–130, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malek AM. and Izumo S. Mechanism of endothelial cell shape change and cytoskeletal remodeling in response to fluid shear stress. J Cell Sci 109 (Pt 4): 713–726, 1996 [DOI] [PubMed] [Google Scholar]
- 79.Maleki S, Bjorck HM, Folkersen L, Nilsson R, Renner J, Caidahl K, Franco-Cereceda A, Lanne T, and Eriksson P. Identification of a novel flow-mediated gene expression signature in patients with bicuspid aortic valve. J Mol Med (Berl) 91: 129–139, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McKellar SH, Tester DJ, Yagubyan M, Majumdar R, Ackerman MJ, and Sundt TM., 3rd Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J Thorac Cardiovasc Surg 134: 290–296, 2007 [DOI] [PubMed] [Google Scholar]
- 81.Merhi-Soussi F, Kwak BR, Magne D, Chadjichristos C, Berti M, Pelli G, James RW, Mach F, and Gabay C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc Res 66: 583–593, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Miller JD, Chu Y, Brooks RM, Richenbacher WE, Pena-Silva R, and Heistad DD. Dysregulation of antioxidant mechanisms contributes to increased oxidative stress in calcific aortic valvular stenosis in humans. J Am Coll Cardiol 52: 843–850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller JD, Weiss RM, Serrano KM, Castaneda LE, Brooks RM, Zimmerman K, and Heistad DD. Evidence for active regulation of pro-osteogenic signaling in advanced aortic valve disease. Arterioscler Thromb Vasc Biol 30: 2482–2486, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miriyala S, Gongora Nieto MC, Mingone C, Smith D, Dikalov S, Harrison DG, and Jo H. Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation 113: 2818–2825, 2006 [DOI] [PubMed] [Google Scholar]
- 85.Mittal M, Siddiqui MR, Tran K, Reddy SP, and Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal 20: 1126–1167, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mody N, Parhami F, Sarafian TA, and Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med 31: 509–519, 2001 [DOI] [PubMed] [Google Scholar]
- 87.Mohler ER, Sheridan MJ, Nichols R, Harvey WP, and Waller BF. Development and progression of aortic valve stenosis: atherosclerosis risk factors—a causal relationship? A clinical morphologic study. Clin Cardiol 14: 995–999, 1991 [DOI] [PubMed] [Google Scholar]
- 88.Moura J, Borsheim E, and Carvalho E. The role of micrornas in diabetic complications-special emphasis on wound healing. Genes (Basel) 5: 926–956, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moura LM, Ramos SF, Zamorano JL, Barros IM, Azevedo LF, Rocha-Gonçalves F, and Rajamannan NM. Rosuvastatin affecting aortic valve endothelium to slow the progression of aortic stenosis. J Am Coll Cardiol 49: 554–561, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nazari-Jahantigh M, Egea V, Schober A, and Weber C. MicroRNA-specific regulatory mechanisms in atherosclerosis. J Mol Cell Cardiol 2014. [Epub ahead of print]; DOI: 10.1016/j.yjmcc.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 91.Neves VJ, Fernandes T, Roque FR, Soci UP, Melo SF, and de Oliveira EM. Exercise training in hypertension: role of microRNAs. World J Cardiol 6: 713–727, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ohno M, Cooke JP, Dzau VJ, and Gibbons GH. Fluid shear stress induces endothelial transforming growth factor beta-1 transcription and production. Modulation by potassium channel blockade. J Clin Invest 95: 1363–1369, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Otto CM, Lind BK, Kitzman DW, Gersh BJ, and Siscovick DS. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N Engl J Med 341: 142–147, 1999 [DOI] [PubMed] [Google Scholar]
- 94.Ozawa N, Shichiri M, Iwashina M, Fukai N, Yoshimoto T, and Hirata Y. Laminar shear stress up-regulates inducible nitric oxide synthase in the endothelium. Hypertens Res 27: 93–99, 2004 [DOI] [PubMed] [Google Scholar]
- 95.Palmer RM, Ashton DS, and Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature 333: 664–666, 1988 [DOI] [PubMed] [Google Scholar]
- 96.Paranya G, Vineberg S, Dvorin E, Kaushal S, Roth SJ, Rabkin E, Schoen FJ, and Bischoff J. Aortic valve endothelial cells undergo transforming growth factor-β-mediated and non-transforming growth factor-β-mediated transdifferentiation in vitro. Am J Pathol 159: 1335–1343, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Parsons JT, Martin KH, Slack JK, Taylor JM, and Weed SA. Focal adhesion kinase: a regulator of focal adhesion dynamics and cell movement. Oncogene 19: 5606–5613, 2000 [DOI] [PubMed] [Google Scholar]
- 98.Peiffer V, Sherwin SJ, and Weinberg PD. Does low and oscillatory wall shear stress correlate spatially with early atherosclerosis? A systematic review. Cardiovasc Res 99: 242–250, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Platt MO, Xing Y, Jo H, and Yoganathan AP. Cyclic pressure and shear stress regulate matrix metalloproteinases and cathepsin activity in porcine aortic valves. J Heart Valve Dis 15: 622–629, 2006 [PubMed] [Google Scholar]
- 100.Prosdocimo DA, Sabeh MK, and Jain MK. Kruppel-like factors in muscle health and disease. Trends Cardiovasc Med 25: 278–287, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, Simmons CA, Masters KS, Mathieu P, O'Brien KD, Schoen FJ, Towler DA, Yoganathan AP, and Otto CM. Calcific aortic valve disease: not simply a degenerative process: a review and agenda for research from the national heart and lung and blood institute aortic stenosis working groupexecutive summary: calcific aortic valve disease—2011 update. Circulation 124: 1783–1791, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rajamannan NM, Subramaniam M, Stock SR, Stone NJ, Springett M, Ignatiev KI, McConnell JP, Singh RJ, Bonow RO, and Spelsberg TC. Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolaemic aortic valve. Heart 91: 806–810, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rao TP. and Kühl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res 106: 1798–1806, 2010 [DOI] [PubMed] [Google Scholar]
- 104.Richards J, El-Hamamsy I, Chen S, Sarang Z, Sarathchandra P, Yacoub MH, Chester AH, and Butcher JT. Side-specific endothelial-dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling. Am J Pathol 182: 1922–1931, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104a.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, and Turner MB. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation 125: e2–e220, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sacks MS, Schoen FJ, and Mayer JE. Bioengineering challenges for heart valve tissue engineering. Annu Rev Biomed Eng 11: 289–313, 2009 [DOI] [PubMed] [Google Scholar]
- 106.Sage AP, Tintut Y, and Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol 7: 528–536, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sarphie TG. A cytochemical study of the surface properties of aortic and mitral valve endothelium from hypercholesterolemic rabbits. Exp Mol Pathol 44: 281–296, 1986 [DOI] [PubMed] [Google Scholar]
- 108.Sarphie TG. Interactions of IgG and beta-VLDL with aortic valve endothelium from hypercholesterolemic rabbits. Atherosclerosis 68: 199–212, 1987 [DOI] [PubMed] [Google Scholar]
- 109.Schoen FJ. Aortic valve structure-function correlations: role of elastic fibers no longer a stretch of the imagination. J Heart Valve Dis 6: 1–6, 1997 [PubMed] [Google Scholar]
- 110.Seya K, Yu Z, Kanemaru K, Daitoku K, Akemoto Y, Shibuya H, Fukuda I, Okumura K, Motomura S, and Furukawa K. Contribution of bone morphogenetic protein-2 to aortic valve calcification in aged rat. J Pharmacol Sci 115: 8–14, 2011 [DOI] [PubMed] [Google Scholar]
- 111.Singh R, Strom JA, Ondrovic L, Joseph B, and VanAuker MD. Age-related changes in the aortic valve affect leaflet stress distributions: implications for aortic valve degeneration. J Heart Valve Dis 17: 290–298; discussion 299, 2008 [PubMed] [Google Scholar]
- 112.Sokabe M, Naruse K, Sai S, Yamada T, Kawakami K, Inoue M, Murase K, and Miyazu M. Mechanotransduction and intracellular signaling mechanisms of stretch-induced remodeling in endothelial cells. Heart Vessels Suppl 12: 191–193, 1997 [PubMed] [Google Scholar]
- 113.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, and Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res 95: 773–779, 2004 [DOI] [PubMed] [Google Scholar]
- 114.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, and Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem 278: 31128–31135, 2003 [DOI] [PubMed] [Google Scholar]
- 115.Sterpetti AV, Cucina A, Morena AR, Di Donna S, D'Angelo LS, Cavalarro A, and Stipa S. Shear stress increases the release of interleukin-1 and interleukin-6 by aortic endothelial cells. Surgery 114: 911–914, 1993 [PubMed] [Google Scholar]
- 116.Sucosky P, Balachandran K, Elhammali A, Jo H, and Yoganathan A. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol 29: 254–260, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takada Y, Ye X, and Simon S. The integrins. Genome Biol 8: 215, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Takx RAP, Zanen P, Leiner T, van der Graaf Y, and de Jong PA. The interdependence between cardiovascular calcifications in different arterial beds and vascular risk factors in patients at high cardiovascular risk. Atherosclerosis 238: 140–146, 2015 [DOI] [PubMed] [Google Scholar]
- 119.Tarbell JM, Shi ZD, Dunn J, and Jo H. Fluid mechanics, arterial disease, and gene expression. Annu Rev Fluid Mech 46: 591–614, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thannickal VJ. and Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279: L1005–L1028, 2000 [DOI] [PubMed] [Google Scholar]
- 121.Then KL. and Rankin JA. Hypertension: a review for clinicians. Nurs Clin North Am 39: 793–814, 2004 [DOI] [PubMed] [Google Scholar]
- 122.Theodoris CV, Li M, White MP, Liu L, He D, Pollard KS, Bruneau BG, and Srivastava D. Human disease modeling reveals integrated transcriptional and epigenetic mechanisms of NOTCH1 haploinsufficiency. Cell 160: 1072–1086, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tousoulis D, Kampoli AM, Tentolouris C, Papageorgiou N, and Stefanadis C. The role of nitric oxide on endothelial function. Curr Vasc Pharmacol 10: 4–18, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Towler DA. Molecular and cellular aspects of calcific aortic valve disease. Circ Res 113: 198–208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Villar AV, Cobo M, Llano M, Montalvo C, Gonzalez-Vilchez F, Martin-Duran R, Hurle MA, and Nistal JF. Plasma levels of transforming growth factor-beta1 reflect left ventricular remodeling in aortic stenosis. PLoS One 4: e8476, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Villarreal G, Jr., Zhang Y, Larman HB, Gracia-Sancho J, Koo A, and García-Cardeña G. Defining the regulation of KLF4 expression and its downstream transcriptional targets in vascular endothelial cells. Biochem Biophys Res Commun 391: 984–989, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang JG, Miyazu M, Matsushita E, Sokabe M, and Naruse K. Uniaxial cyclic stretch induces focal adhesion kinase (FAK) tyrosine phosphorylation followed by mitogen-activated protein kinase (MAPK) activation. Biochem Biophys Res Commun 288: 356–361, 2001 [DOI] [PubMed] [Google Scholar]
- 128.Wang N, Miao H, Li Y-S, Zhang P, Haga JH, Hu Y, Young A, Yuan S, Nguyen P, Wu C-C, and Chien S. Shear stress regulation of Krüppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun 341: 1244–1251, 2006 [DOI] [PubMed] [Google Scholar]
- 129.Weinberg EJ, Mack PJ, Schoen FJ, García-Cardeña G, and Kaazempur Mofrad MR. Hemodynamic environments from opposing sides of human aortic valve leaflets evoke distinct endothelial phenotypes in vitro. Cardiovasc Eng 10: 5–11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weisman D, Hakimian E, and Ho GJ. Interleukins, inflammation, and mechanisms of Alzheimer's disease. In: Vitamins & Hormones. Waltham (MA): Gerald L. Academic Press, 2006, pp. 505–530 [DOI] [PubMed] [Google Scholar]
- 131.White S, Hayes E, Lehoux S, Jeremy J, Horrevoets A, and Newby A. Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress. J Cell Physiol 226: 2841–2848, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wiltz D, Arevalos CA, Balaoing LR, Blancas AA, Sapp MC, Zhang X, and Grande-Allen KJ. Extracellular matrix organization, structure, and function. In: Calcific Aortic Valve Disease. Rijeka, Croatia: Intech, 2013, pp. 3–30 [Google Scholar]
- 133.Wirrig EE. and Yutzey KE. Conserved transcriptional regulatory mechanisms in aortic valve development and disease. Arterioscler Thromb Vasc Biol 34: 737–741, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.This reference has been deleted.
- 135.Wu CC, Li YS, Haga JH, Kaunas R, Chiu JJ, Su FC, Usami S, and Chien S. Directional shear flow and Rho activation prevent the endothelial cell apoptosis induced by micropatterned anisotropic geometry. Proc Natl Acad Sci U S A 104: 1254–1259, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yacoub MH. and Takkenberg JJ. Will heart valve tissue engineering change the world? Nat Clin Pract Cardiovasc Med 2: 60–61, 2005 [DOI] [PubMed] [Google Scholar]
- 137.Yamamoto K, Protack CD, Kuwahara G, Tsuneki M, Hashimoto T, Hall MR, Assi R, Brownson KE, Foster TR, Bai H, Wang M, Madri JA, and Dardik A. Disturbed shear stress reduces Klf2 expression in arterial-venous fistulae in vivo. Physiol Rep 3: pii:, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yanagawa B, Lovren F, Pan Y, Garg V, Quan A, Tang G, Singh KK, Shukla PC, Kalra NP, Peterson MD, and Verma S. miRNA-141 is a novel regulator of BMP-2–mediated calcification in aortic stenosis. J Thorac Cardiovasc Surg 144: 256–262.e2, 2012 [DOI] [PubMed] [Google Scholar]
- 139.Yang B. and Rizzo V. Shear stress activates eNOS at the endothelial apical surface through 1 containing integrins and caveolae. Cell Mol Bioeng 6: 346–354, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, and Bostrom KI. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res 107: 485–494, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yetkin E, Tchaikovski V, Erdil N, Alan S, and Waltenberger J. Increased expression of cystatin C and transforming growth factor beta-1 in calcific aortic valves. Int J Cardiol 176: 1252–1254, 2014 [DOI] [PubMed] [Google Scholar]
- 142.Ying L. and Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res 67: 1407–1410, 2007 [DOI] [PubMed] [Google Scholar]
- 143.Yip CY. and Simmons CA. The aortic valve microenvironment and its role in calcific aortic valve disease. Cardiovasc Pathol 20: 177–182, 2011 [DOI] [PubMed] [Google Scholar]
- 144.Yu Z, Seya K, Daitoku K, Motomura S, Fukuda I, and Furukawa K-I. Tumor necrosis factor-α accelerates the calcification of human aortic valve interstitial cells obtained from patients with calcific aortic valve stenosis via the BMP2-Dlx5 pathway. J Pharmacol Exp Ther 337: 16–23, 2011 [DOI] [PubMed] [Google Scholar]
- 145.Zebda N, Dubrovskyi O, and Birukov KG. Focal adhesion kinase regulation of mechanotransduction and its impact on endothelial cell functions. Microvasc Res 83: 71–81, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang M, Liu X, Zhang X, Song Z, Han L, He Y, and Xu Z. MicroRNA-30b is a multifunctional regulator of aortic valve interstitial cells. J Thorac Cardiovasc Surg 147: 1073–1080.e2, 2014 [DOI] [PubMed] [Google Scholar]
- 147.Zhao F, Li L, Guan L, Yang H, Wu C, and Liu Y. Roles for GP IIb/IIIa and alphavbeta3 integrins in MDA-MB-231 cell invasion and shear flow-induced cancer cell mechanotransduction. Cancer Lett 344: 62–73, 2014 [DOI] [PubMed] [Google Scholar]
- 148.Zhou G, Hamik A, Nayak L, Tian H, Shi H, Lu Y, Sharma N, Liao X, Hale A, Boerboom L, Feaver RE, Gao H, Desai A, Schmaier A, Gerson SL, Wang Y, Atkins GB, Blackman BR, Simon DI, and Jain MK. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest 122: 4727–4731, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ziegler WH, Gingras AR, Critchley DR, and Emsley J. Integrin connections to the cytoskeleton through talin and vinculin. Biochem Soc Trans 36: 235–239, 2008 [DOI] [PubMed] [Google Scholar]