Abstract
The variations in prevalence levels of two tick-borne rickettsial pathogens, Ehrlichia chaffeensis and Ehrlichia Ewingii, in a periurban environment were evaluated along with their ecological determinants. Tick life stage and sex, month of tick collection, landscape fragmentation, and ecological covariates specific to pasture and woodland sites were considered as explanatory covariates. Questing lone star ticks (Amblyomma americanum) were collected by flagging for an hour once every week during mid-April through mid-August in years 2013 and 2014. A total of 4357 adult and nymphal ticks (woodland = 2720 and pasture = 1637) were collected and assessed for pathogen prevalence by molecular methods. Female A. americanum ticks were more infected with E. chaffeensis than males or nymphs in woodland areas [♂ = 6.05%; ♀ = 12.0%; nymphs = 2.09%] and pastures [♂ = 8.05%; ♀ = 12.03%; nymphs = 3.33%], and the prevalence was influenced by edge density in the landscape. Higher E. ewingii infection was noted among female A. americanum ticks within woodland areas [♂ = 1.89%; ♀ = 2.14%; nymphs = 1.57%], but no such difference was evident in pastures [♂ = 1.03%; ♀ = 1.33%; nymphs = 1.12%]. Prevalence of E. ewingii was influenced by edge contrast index, and the percentage of pasture perimeter that was less than 20 meters from woodland areas. This study elucidates the complexity of tick-borne pathogen ecology and points to the need for further studies on the role of reservoir hosts, particularly that played by small vertebrates, which is not fully understood in the region.
Keywords: : Ehrlichia, geostatistics, rickettsia, surveillance, tick(s)
Introduction
Non-Lyme tick-borne diseases are a growing concern in Midwestern United States with a steady increase in the report frequency and geographic extent in recent years, particularly of human monocytic ehrlichiosis (HME),i human ewingii ehrlichiosis (HEE)i, and Rocky Mountain spotted fever (Raghavan et al. 2014a, 2015, 2016b, Beckham et al. 2015), even while these diseases are considered severely underreported by the region's state health departments.
Many factors must come together for humans and animals to get infected with a tick-borne pathogen, and within endemic areas, it is quite common to observe patchy distributions of high and low incidences of human and animal infections. Unsurprisingly, the prevalence levels of tick pathogens are heterogeneous over geographic space, likely due to factors such as the ability of their hosts to establish populations in different environments, success rates of contact with infected reservoir hosts, and the local host community structure (Ostfeld and Keesing 2000, Halos et al. 2010, Rynkiewicz et al. 2014). It is comprehensible therefore that a better understanding of the variations in tick pathogen prevalence and the factors that contribute to such variations is crucial for implementing sound surveillance and management programs and to understand disease risk for humans and animals.
Two hard ticks in the family Ixodidae, the lone star tick, Amblyomma americanum, and the dog tick, Dermacentor variabilis, are common species in the Midwestern United States (Burg et al. 2001, CDC 2016a, Raghavan et al. 2016b) that are implicated in transmitting several rickettsial pathogens (Dahlgren et al. 2016, CDC 2016b). In this study, we focused on the former, which is an identified biological vector for Ehrlichia chaffeensis and Ehrlichia ewingii, known to cause HME and HEE, respectively, and are also infectious to the canine host. Lone star ticks are also responsible for other diseases in North America (CDC 2016c), most notably the recently described Heartland virus (Savage et al. 2013), and potentially also the Bourbon virus.
Our objective in this study was to evaluate if and which among the different landscape-level ecological factors impact variations in prevalence levels for the two rickettsial pathogens, E. chaffeensis and E. ewingii, among questing A. americanum ticks in a periurban setting in the Midwestern United States. We chose a periurban environment for conducting this study since they represent areas where humans are relatively more likely to come in contact with infected ticks due to proximity to their residences and the potential relevance for public health management. The ultimate event of a tick transmitting a pathogen to a human or a pet animal depends on a set of factors that are different from those that govern pathogen prevalence among ticks, although there will be clear overlaps. Factors that contribute to infection status among ticks (pathogen prevalence) depend on many aspects that are intrinsic to the ticks themselves (e.g., tick life stage, time of emergence, feeding preference) and other extrinsic aspects, including the relative abundance of intermediate and reservoir hosts in the environment and their community structure, which are often studied using their representative habitats and habitat structures as proxies (Halos et al. 2010, Estrada-Peńa et al. 2013). The factors we considered in this study were two land cover types, pasture and woodland, which represent habitats for most hard tick species and their hosts, and different landscape metrics that quantified landscape area, edge metrics, and connectivity that are related to fragmentation, which is widely recognized to amplify vector-borne disease incidences. Host-level influences, sex and life stage of the ticks, and month of tick collection were also considered in addition to the ecological factors.
Materials and Methods
Study sites and tick sampling
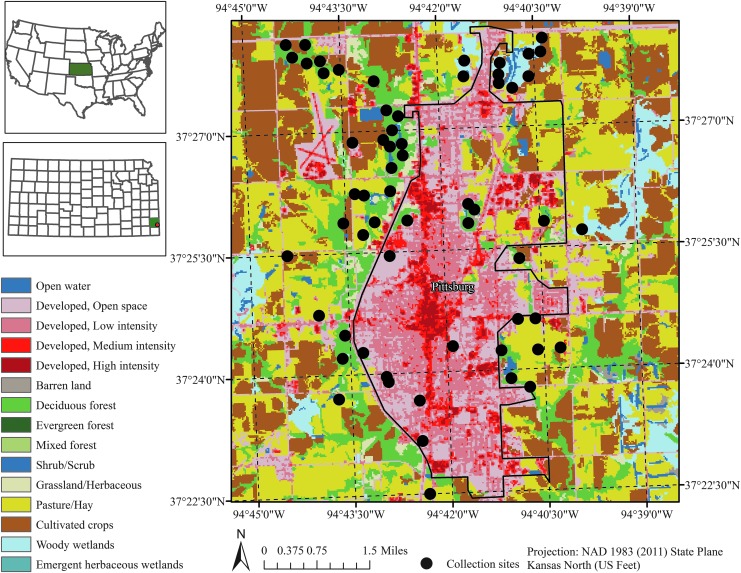
Review of case reports submitted to the Kansas Department of Health and Environment and our own previous investigations on tick-borne diseases in the region (Raghavan et al. 2013, 2014a, 2014b) revealed that counties in the southeastern portion of the state rank high in the number of reported tick-borne disease cases. Study sites for this work were therefore selected around the city of Pittsburg in southeast Kansas located in Crawford County. Sampling sites were selected within the city limits in areas where human interaction with outdoors was expected to be higher. These included recreational areas and areas adjacent to low and medium-intensity residential zones. The land cover was later classified as woodland and pasture types. There were a total of 60 collection sites (Fig. 1) whose locations were recorded using Global Positioning Systems, and ticks were collected by dragging the vegetation in each of these sites for ∼1 h every time except when interrupted by rain on the day of collection or the day before. Collections were done once every week during mid-April through mid-August, coinciding with the peak tick season in the region. Ticks were collected from the same sites for 2 consecutive years in 2013 and 2014 to ensure inclusion of adequate number of ticks for the study. Ticks were labeled and stored in dry ice containers immediately upon their collection and stored at −80°C after transportation to the laboratory until molecular analysis.
FIG. 1.
Map of periurban Pittsburg, Kansas, and collection sites where ticks were sampled for prevalence estimation of three rickettsial ticks.
Explanatory variables
Tick life stage (nymph, adult) and sex (male, female) and the month in which ticks were collected (April, May, June, July, August) were considered as host-level factors. Different fragmentation metrics for the sampling sites were estimated from the 2011 National Land Cover Dataset (Homer et al. 2015) using FRAGSTATS version-4, a spatial analysis program for quantifying landscape pattern metrics (McGarigal et al. 2012), for a buffered area of 1 km. There were a total of five landscape metrics (which included patch density, edge density, and total edge contrast) that were based on landscape composition and two metrics that quantified how the patches are interconnected that potentially allowed tick–host movements. Patch density is calculated as follows:
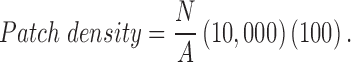 |
where N is the total number of patches in the landscape and A is the total area considered
 . Edge density is calculated as follows:
. Edge density is calculated as follows:
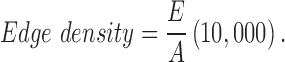 |
where E is the total length 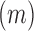 of edge in the landscape and A is the total landscape area
of edge in the landscape and A is the total landscape area 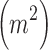 . Total edge contrast index,
. Total edge contrast index,  , is calculated as follows:
, is calculated as follows:
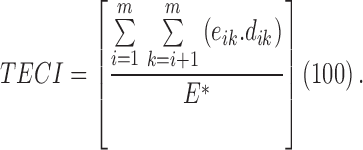 |
where 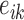 is the total length
is the total length 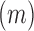 of edge in landscape between patch types
of edge in landscape between patch types 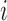 and
and 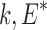 is the total length
is the total length 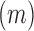 of edge in landscape and
of edge in landscape and 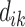 is the dissimilarity between patch types
is the dissimilarity between patch types 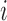 and
and 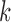 . Patch cohesion index is calculated as follows:
. Patch cohesion index is calculated as follows:
 |
where 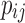 is the perimeter of patch
is the perimeter of patch 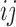 in terms of numbers of cell surfaces,
in terms of numbers of cell surfaces, 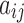 is the area of patch
is the area of patch 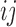 in terms of number of cells, and
in terms of number of cells, and 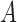 is the total number of cells in the landscape, and connectance index is calculated as follows:
is the total number of cells in the landscape, and connectance index is calculated as follows:
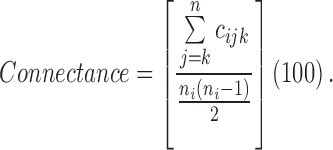 |
where 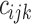 is the joining between patch
is the joining between patch 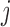 and
and 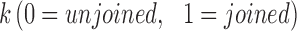 of the corresponding patch type
of the corresponding patch type  , based on a user-specified threshold distance, and
, based on a user-specified threshold distance, and 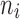 is the number of patches in the landscape of the corresponding patch type.
is the number of patches in the landscape of the corresponding patch type.
As in a previous work by Halos et al. (2010), wherein the prevalence variations of different bacterial spp. were transmitted by a hard tick, Ixodes ricinus, we considered the following vegetation factors for the pasture sampling sites in this study: percentage of pasture perimeter with woodland boundaries, the type of major landscape lying outside the pasture (shrub-tree, cultivated land, mixed composition), and percentage of pasture perimeter that was less than 20 meters from woodland areas. For the woodland sampling sites, the factors were the type of forest (deciduous, coniferous, and mixed) and the percentage of forest perimeter with water bodies. These variables were estimated from the NLCD (National Land Cover Database) in ArcGIS.
DNA extraction and PCR amplification
Ticks were first thawed to room temperature and sorted under a compound microscope by month of collection, species, life stage, and sex. Before DNA extraction, the ticks were washed in ethanol to remove surface microbes and collected in sterile microcentrifuge tubes. Total genomic DNA was isolated from each tick using the Wizard genomic DNA isolation kit by following the manufacturer's protocol (Promega, Madison, WI) and the final DNA pellet was resuspended in 100 μL nuclease-free water; DNA concentration was measured by Nano drop and stored at −20°C for PCR detection of rickettsial pathogens. DNA from nymphal pools was extracted following the same protocol mentioned above. Specifically for E. chaffeensis and E. ewingii detection, nested PCR was used targeting to the 16S rRNA gene by following the protocol as described by Dawson et al. (1994) and Yabsley et al. (2002), respectively. Briefly, for the first-round PCR amplification, 5 μL (∼1 μg) DNA from a tick in a 25-μL reaction containing 1.5 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate, and 2.5 U Taq DNA polymerase (Invitrogen, Carlsbad, CA) and 0.2 μM of primers ECC (5′-AGAACGAACGCTGGCGGC AAGCC-3′) and ECB (5′-CGTATTACCGCGGCTGCTGGCA-3′). The expected amplicon size in first-round PCR is 478 bp for Ehrlichia genus. For the nested PCR, 1 μL of primary product was used as template in a 25-μL reaction containing the same PCR components, except for replacing of E. chaffeensis-specific primers, HE1 (5′-CAATTGC′TTATAACCl-lTlJ1GGTrATAAAT-3′) and HE3 (5′-TATAGGTACCGTCATTATCTTCCCTAT-3′), or E. ewingii-specific primers, EE72-ewingii (5′-CAATTCCTAAATAGTCTCTGACTATT-3′) and HE3 (5′-TATAGGTACCGTCATTATCTTCCCTAT-3′). Amplification products were analyzed by electrophoresis in 1.5% agarose. The expected amplicon sizes for E. chaffeensis and E. ewingii are 389 and 407 bp, respectively.
Statistical analyses
Statistical analysis was performed using R-Statistical Software, Version 3.2.2 (R-Core Team 2015). Differences between pathogen prevalence in woodland versus pasture land cover types and the association between the presence of rickettsial DNA and the explanatory variables were modeled in several generalized linear model constructs. To avoid model overfitting, we screened the association of individual explanatory variables with the response variable in several bivariate analyses using a liberal p value of ≤0.2, and only variables that were significant at this level were kept for further analysis. Since spatially explicit variables are often correlated, the presence of multicollinearity among the screened explanatory factors was verified. Explanatory variables with a variance inflation factor ≥10 were considered to indicate multicollinearity and were dropped until all multicollinearity was absent.
The prevalence of infection for individual bacterium was modeled separately. The probability, p, of rickettsial DNA presence in an individual adult tick and pools of 5 nymphal ticks and their absence 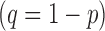 was estimated using a logit function
was estimated using a logit function 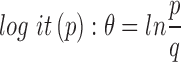 . The
. The 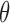 was estimated using maximum likelihood function. Association of explanatory variables with estimated infection prevalence with a bacterium was individually modeled for each bacterium and woodland and pasture sampling locations separately in multivariate models with a backward elimination procedure, starting with a full model that included all the screened variables. The AIC values were used as a guide to select most parsimonious models.
was estimated using maximum likelihood function. Association of explanatory variables with estimated infection prevalence with a bacterium was individually modeled for each bacterium and woodland and pasture sampling locations separately in multivariate models with a backward elimination procedure, starting with a full model that included all the screened variables. The AIC values were used as a guide to select most parsimonious models.
Results
A summary of ticks collected in this study grouped by their life stage and sex and the prevalence of different rickettsial pathogens in the two tick species collected around the city of Pittsburg, Kansas, is present in Table 1. A total of 3237 A. americanum DNAs were analyzed, which included DNAs from 1766 adult ticks and 191 pools of nymphs collected from woodland areas and 1190 DNAs from adults and 90 pools of nymphal ticks collected from pasture sites. Coinfection of A. americanum ticks with E. chaffeensis and E. ewingii was observed among 56 (3.61%) adult females. Additionally, 3 (1.06%) pools of A. americanum nymphs were coinfected with both Ehrlichia spp.
Table 1.
Breakdown of Amblyomma americanum Ticks Captured in Woodland and Pasture Sites and the Prevalence Levels of Ehrlichia chaffeensis and Ehrlichia ewingii Among These Ticks Collected Around Periurban Landscape in Pittsburg, Kansas
| Pathogen | |||
|---|---|---|---|
| Land cover |
Tick species Amblyomma americanum n, % |
Ehrlichia chaffeensis n, % (95% CI) |
Ehrlichia ewingii n, % (95% CI) |
| Woodland (n = 2720) | |||
| Adult male | 925, 34.00 | 56, 6.05 (4.60, 7.70) | 11, 1.89 (0.67, 2.12) |
| Adult female | 841, 30.91 | 101, 12.0 (9.98, 14.38) | 18, 2.14 (1.36, 3.36) |
| Nymph poola | 954, 35.07 | 4, 2.09 (0.82, 5.29) | 3, 1.57 (0.54, 4.54) |
| Pasture (n = 1637) | |||
| Adult male | 484, 29.56 | 39, 8.05 (5.95, 10.83) | 5, 1.03 (0.44, 2.39) |
| Adult female | 706, 43.12 | 85, 12.03 (9.84, 14.65) | 8, 1.33, (0.57, 2.31) |
| Nymph poolb | 447, 27.30 | 3, 3.33 (1.15, 9.45) | 1, 1.12 (0.06, 6.97) |
The 95% CI corresponds to the proportions of pathogen prevalence without continuity correction. Estimates included coinfected samples.
One hundred ninety pools of five nymphs and one pool of four nymphs were analyzed.
Eighty-nine pools of five nymphs and one pool of two nymphs were analyzed.
CI, confidence interval.
Results of bacterial infection status for the two ticks and their life stages indicated that a combination of host characteristics, fragmentation, and vegetation characteristics influences pathogen prevalence (Table 2). The infection prevalence of E. chaffeensis among A. americanum ticks was influenced by tick life stage and sex, with adult females in both woodland and pasture sites significantly more infected than adult males and nymphs. Higher edge density was an influential factor for prevalence of E. chaffeensis. For every unit increase in edge density, the odds of a tick or nymphal tick pool turning positive instead of negative by the PCR test increased by 1.98 times. No overall difference in infection prevalence among ticks in woodland versus pasture sites was noted, and no woodland or pasture-related covariate explained the variability in E. chaffeensis prevalence. Higher percentage of pasture perimeter with shrub vegetation selected in the univariate screening did not retain significance in the multivariate logistic model.
Table 2.
Estimated Associations of Host-Level and Ecological Covariates and 95% Confidence Interval Limits of Infection Prevalence by Ehrlichia chaffeensis and Ehrlichia ewingii Among Amblyomma americanum Ticks Collected in Woodland and Pasture Sites in a Periurban Landscape Around Pittsburg, Kansas
| Pathogen/factora |
Coefficient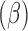
|
Standard error | exp 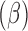
|
p Value | 95% CIb |
|---|---|---|---|---|---|
| E. chaffeensis | |||||
| Stage and sex | 0.118 | 0.0 | — | 0.008** | — |
| Adult female | 0.580 | 0.011 | 1.786 | 0.012* | 1.747, 1.824 |
| Adult male | 0.273 | 0.152 | 1.313 | 0.094 | 0.975, 1.769 |
| Nymph | Reference category | ||||
| Edge density | 0.684 | 0.122 | 1.981 | 0.010* | 1.560, 2.517 |
| % of pasture perimeter with shrub vegetation | 0.341 | 0.214 | 1.406 | 0.104 | 0.924, 2.139 |
| E. ewingii | |||||
| Total edge contrast index | 0.451 | 0.024 | 1.560 | 0.010* | 1.497, 1.645 |
| Patch cohesion | 0.072 | 0.031 | 1.074 | 0.216 | 1.011, 1.141 |
| % of pasture perimeter that was less than 20 meters from woodland areas | 0.315 | 0.014 | 1.370 | 0.031* | 1.011, 1.141 |
Multivariate logistic models for individual pathogens included several covariates screened in the univariate procedure with a liberal p value (≤0.2) in addition to the covariates retained in the reduced final models (presented in the Table 2). The full model for E. chaffeensis included the covariates, patch cohesion and connectance, the full model for E. ewingii included type of major landscape lying outside pasture, and the full model for Rickettsia rickettsii included month of collection and surface area of forest cover within 1-km buffer.
95% confidence limits for the exponential of model covariates.
p < 0.05.
p < 0.01.
There was no overall difference in E. ewingii infection prevalence among A. americanum ticks collected from woodland versus pasture sites; however, adult females collected from woodland sites were significantly more infected by E. ewingii than adult males and nymphs (p < 0.01). The prevalence of E. ewingii among A. americanum ticks was influenced by edge density and the percentage of pasture perimeter that was less than 20 meters from woodland areas. Higher values of TECI, indicating higher degree of forest fragmentation, and higher percentages of pasture perimeter less than 20 meters from woodland areas increased E. ewingii prevalence. For every unit increase in TECI, the odds of PCR test result being positive was 1.56 times larger than being negative; and the odds of being positive increased by 1.37 times for every unit increase in the percentage of pasture perimeter that was less than 20 meters from woodland areas. None of the pasture vegetation factors considered in the study explained variability in E. ewingii prevalence among A. americanum ticks.
The AIC values for multivariate logistic models for each pathogen differed by ≥10 U, indicating that there were no competing models with different number of variables for each dataset. Therefore, the models with the lowest AIC values were considered desirable for each pathogen.
Discussion
Diseases caused by the two rickettsial pathogens whose prevalence levels were evaluated in this study have known to have increased intensities of infection and spatial distributions in the Midwestern United States, particularly in the states of Kansas, Missouri, Oklahoma, and Arkansas, over the last decade (Raghavan et al. 2014a, 2014b, 2016a, 2016b, Beckham et al. 2015, Hanzlicek et al. 2016). A previous study by Steiert and Gilfoy (2002) conducted in the year 2002 across six counties in southwestern Missouri (in relative close proximity to the sampling locations around the city of Pittsburg in the present study) reported infection prevalence for E. chaffeensis and E. ewingii (Table 3), and Berrada et al. (2011) assessed infection rates of Rickettsia spp. among A. americanum and D. variabilis ticks in Kansas and Missouri (Table 3). Other than these two studies, we are not aware of any published reports that assessed prevalence levels of either pathogen in these four states. Published literature from areas elsewhere in the United States, however, indicates highly varied prevalence levels for the two pathogens over the native ranges of their tick hosts (Table 3). Although it is expected for infection prevalence in ticks to change over space and also time, knowledge of local prevalence is vitally necessary to understand the emerging risk of pathogens to humans and animals.
Table 3.
Infection Prevalence (%) of Ehrlichia chaffeensis and Ehrlichia ewingii, Reported by Previous Studies in the United States
| Pathogen/host | Adult male | Adult female | Nymph pool | Study | State/region where collection was made |
|---|---|---|---|---|---|
| E. chaffeensis | |||||
| A. americanum | 3.8 (10/262) | Irving et al. (2000) | Ohio | ||
| A. americanum | 0.0 (0/151), 0.9 (1/111), 9.3 (12/129) | Whitlock et al. (2000) | Georgia Coast and Barrier Islands | ||
| A. americanum | 9.8 (57/579) | 1.7 | Steiert and Gilfoy (2002) | Missouri | |
| A. americanum | 44/190 (4.6)a | 54/1128 (4.4)b | — | Mixson et al. (2006) | 29 sites in 9 states in the eastern and southeastern United States |
| Dermacentor variabilis | 8/120 (6.7) | 0.6 | Steiert and Gilfoy (2002) | Missouri | |
| E. ewingii | |||||
| A. americanum | 2/217 (0.9) | 1/245 (0.4) | 5/106 (4.7) | Wolf and McPherson (2000) | North Carolina |
| A. americanum | 31/579 (5.4) | 3/115 (0.6) | Steiert and Gilfoy (2002) | Missouri | |
| D. variabilis | 4/120 (3.3) | — | Steiert and Gilfoy (2002) | Missouri | |
Overall prevalence from 29 sites. Prevalence in individual collection sites varied between 0 to 30 in varied number of male A. americanum ticks.
Numbers within parenthesis indicate the number of specimens that tested positive for a pathogen among total number of ticks collected in that study.
In addition to knowing prevalence levels, it is also essential to recognize the ecological forces that determine fluctuations in prevalence so that appropriate preventive measures can be devised. However, most available studies that report infection prevalence do so only according to the sampling locations and tick–host characteristics, while not considering the effects of physical environment such as cover type or fragmentation, which clearly influences prevalence levels and host–pathogen ecology. The present study considered these important issues; and even though the rates at which these pathogens will be identified in future and will fluctuate due to natural variations, the prevalence levels reported here and the landscape-level ecological factors identified will aid in making current public health policies and in research planning. In addition, estimates made here will serve as a baseline for monitoring changes due to anthropogenic influences on tick-borne diseases such as exurbanization and climate change.
This study has found wide variations in infection prevalence among the most frequently encountered tick spp. in a relatively small-scale periurban environment, and the influential factors affecting prevalence varied for the two pathogens. Infection prevalence of E. chaffeensis among the different sexes and life stages of A. americanum ticks for the region is not readily available in published literature, but the average prevalence noted in this study for E. chaffeensis among pasture and woodland sites is higher than previous reports from Kansas and other areas (Whitlock et al. 2000, Irving et al. 2000, Steiert and Gilfoy 2002, Mixson et al. 2006) (Table 3). Adult female A. americanum ticks were more infected by E. chaffeensis than adult males and nymphs in both woodland and pasture sites. Due to differences in the length of lifetime, adult ticks may have had more chances to become infected than the younger nymphs, and the noted difference is predictable; however, the reasons for male–female difference are not very clear. Different hypotheses have been proposed for other hard ticks that show a similar tendency, including difference in feeding preferences by the two sexes, and the amount of bloodmeal taken as nymphs from infected hosts (Halos et al. 2010). Laboratory and field evaluation of the causes for differences in male–female infection prevalence for E. chaffeensis is worthy of further consideration as this may help us better understand the host–pathogen interactions.
Infection prevalence of E. chaffeensis in A. americanum ticks was greater with increasing edge density, a measure of landscape fragmentation. At the landscape level, pasture sampling sites that had higher edge density likely indicate ideal habitats for white-tailed deer (Odocoileus virginianus), the primary reservoir host for E. chaffeensis, and a potentially higher contact rate for A. americanum ticks in these habitats. Higher amount of edge also promotes the abundance of small mammals (Bowers and Matter 1997) and higher bird density (Glennon and Potter 1999), many of which may serve as reservoir hosts for E. chaffeensis (Childs and Paddock 2003).
The infection prevalence of E. ewingii among A. americanum ticks in this study was considerably lower compared with E. chaffeensis, which is consistent with previous studies (Wolf et al. 2000, Mixson et al. 2006) and further confirms that the latter bacterial species is more common of the two. Female ticks in woodland sites had higher infections than those that were collected from pastures, but unlike E. chaffeensis prevalence, any male versus female differences were not noted for ticks collected from either cover type for E. ewingii. It is likely that female ticks in woodland areas have higher encounter rates with white-tailed deer, also the primary reservoir host for E. ewingii (Yabsley et al. 2002), and other small mammal reservoir hosts preferred by nymphs (Koch 1981, Kollars et al. 2000) that later molt as females. This proposition is perhaps further supported by two other influential factors that increased E. ewingii prevalence among the ticks, the TECI and the percentage of pasture perimeter that was less than 20 meters from woodland areas. Like edge density, TECI is also an indicator of land cover fragmentation, but in addition to being a metric of landscape composition, this metric also accounts for variations in the types of ground cover (or) vegetation complexity in the landscape. Greater variation in patch types and simultaneous fragmentation has been suggested to increase small vertebrate communities, upon whom subadult Amblyomma ticks are known to feed more commonly (Kollars et al. 2000). Likewise, pasture sites with higher percentages of perimeter length that was less than 20 meters from woodland areas indicate transitional areas on a landscape (ecotone) that are favorable for higher encounter rates with white-tailed deer and other small vertebrate hosts.
Such identification of fragmentation and other landscape properties associated with pathogen prevalence is not only unprecedented but it is also not a common occurrence in published literature. Earlier studies have pointed out the complex interplay between different abiotic (e.g., microclimate, cover type) and biotic factors (presence and abundance of reservoir hosts and intermediate hosts) that affect the phenology of ticks as well as pathogen prevalence (e.g., Estrada-Peńa 2015). Different metrics that were retained in models in this study underscore the ecological complexity and interplay between reservoir hosts, their movement patterns, habitats, vegetation complexity, and microclimate. It is also evident from this study that the prevalence of two rickettsial pathogens is influenced by different landscape metrics (as well as intrinsic factors in the case of E. chaffeensis). For instance, edge density and TECI differ in the way they account for different properties of the landscape, reflecting the fact that the background processes involved among different players are different for individual pathogen prevalence. Such subtle influences need to be quantitatively verified in future studies.
An important limitation in the present study needs to be addressed and that is the resolution (30 meters) of remotely sensed land cover data used in the study. Changes to data resolution will affect how landscape patches are defined and, in turn, the strength of association between pathogen prevalence and explanatory variables. We do feel that the use of NLCD for this study is an appropriate first step, and the use of high-resolution data from aerial photography in future studies may help us better understand the effects of fragmentation on pathogen prevalence. Microclimatic conditions (soil moisture, relative humidity) have long been associated with the phenology (Daniel et al. 1976) and interactions with reservoir hosts (Randolph and Storey 1999) for Ixodes spp. as well as pathogen prevalence (Estrada-Peńa 2009, James 2013); however, remotely sensed data that adequately capture microclimate are seldom obtainable and are usually in coarser resolutions when available. This led us not to evaluate such data in the models. Future studies may benefit by allowing microclimatic data to compete with tick stage, sex, and fragmentation variables in multivariate models since they are undoubtedly important determinants of tick phenology and pathogen persistence.
Conclusions
This study reports infection prevalence of two emerging pathogens in A. americanum ticks in an endemic periurban environment in Midwestern United States. Infection prevalence levels for E. chaffeensis and E. ewingii in the present study are higher than previous reports from both Kansas and other regions in United States;
Different measures of landscape fragmentation (edge density and TECI) determined the prevalence of pathogens considered in this study. Collectively, they point to the role of reservoir hosts and microclimatic conditions in ecotones that may selectively favor pathogen persistence.
Evidence for the role of small vertebrate hosts in the ecoepidemiology of rickettsial pathogens can be found throughout the literature; however, infection prevalence of rickettsial pathogens among small vertebrates in the region is not well understood and such research merits further consideration.
Acknowledgments
The authors would like to thank Mike Banfield, Lisa Crevoiserat, Alex Moran, Lindsay Morgan, Alexander Staggs, and Savannah Wilson, Kansas State University, for help with field collection of ticks; Nilima Bhoi, Savannah Wilson, and Justin Manford, Kansas State University, and Vijaya Varma Indukuri, currently at University of Saskatchewan, for help with DNA extraction and PCR analysis; and Mal Hoover, Kansas State University, for technical assistance with manuscript preparation. The authors are deeply grateful to the property owners in the city of Pittsburg, Kansas, who graciously allowed them to conduct field surveys at their lands. This study was supported, in part, by Kansas State Veterinary Diagnostic Laboratory (KSVDL) and the PHS grant number AI070908 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA. This article is a contribution (contribution no. 16-227-J) from the Kansas Agricultural Experiment Station. Publication cost for this article was provided by the K-State Open Access Publishing (KOAPF) program.
Author Disclosure Statement
No competing financial interests exist.
Please refer to the National Notifiable Diseases Surveillance System (NNDSS) for current nomenclature for diseases caused by E. chaffeensis and E. ewingii.
References
- Beckham TR, Bowen RA, Coate EA, Dhariwala MO, et al. . The growing risk of zoonotic & vector-borne diseases conference August 30–31, 2015 Kansas City Convention Center. Vector Borne Zoonotic Dis 2015; 15:453–460 [Google Scholar]
- Berrada ZL, Goethert HK, Cunningham J, Telford SR. Rickettsia rickettsii (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) from Kansas. J Med Entomol 2011; 48:461–467 [DOI] [PubMed] [Google Scholar]
- Bowers MA, Matter SF. Landscape ecology of mammals: relationships between density and patch size. J Mammal 1997; 78:999–1013 [Google Scholar]
- Burg J. Seasonal activity and spatial distribution of host‐seeking adults of the tick Dermacentor variabilis. Med Vet Entomol 2001; 15:413–421 [DOI] [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention (2016a) Geographic distribution of ticks that bite humans. Available at www.cdc.gov/ticks/geographic_distribution.html
- [CDC] Centers for Disease Control and Prevention (2016b). Rocky Mountain spotted fever (RMSF). Available at www.cdc.gov/rmsf/
- [CDC] Centers for Disease Control and Prevention (2016c) Tickborne diseases in the United States. Available at www.cdc.gov/ticks/diseases/
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol 2003; 48:307–337 [DOI] [PubMed] [Google Scholar]
- Dahlgren FS, Paddock CD, Springer YP, Eisen RJ, et al. . Expanding range of Amblyomma americanum and simultaneous changes in the epidemiology of spotted fever group rickettsiosis in the United States. Am J Trop Med Hyg 2016; 94:35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M, Cerný V, Dusbabek F, Honzakova E, et al. , Influence of microclimate on the life cycle of the common tick Ixodes ricinus (L.) in an open area in comparison with forest habitats. Folia Parasitol 1976; 24:149–160 [PubMed] [Google Scholar]
- Dawson JE, Stallknecht DE, Howerth EW, Warner C, et al. . Susceptibility of white-tailed deer (Odocoileus virginianus) to infection with Ehrlichia chaffeensis, the etiologic agent of human ehrlichiosis. J Clin Microbiol 1994; 32:2725–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Peńa A. Tick-borne pathogens, transmission rates and climate change. Front Biosci 2009; 14:2674–2687 [DOI] [PubMed] [Google Scholar]
- Estrada-Peńa A. Ticks as vectors: taxonomy, biology and ecology. Rev Sci Tech 2015; 34:53–65 [DOI] [PubMed] [Google Scholar]
- Estrada-Peńa A, Gray JS, Kahl O, Lane RS, et al. . Research on the ecology of ticks and tick-borne pathogens—methodological principles and caveats. Front Cell Infect Microbiol 2013; 3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon MJ, Porter WF. Using satellite imagery to assess landscape-scale habitat for wild turkeys. Wildlife Soc Bull 1999:646–653 [Google Scholar]
- Halos L, Bord S, Cotté V, Gasqui P, et al. . Ecological factors characterizing the prevalence of bacterial tick-borne pathogens in Ixodes ricinus ticks in pastures and woodlands. Appl Environ Microbiol 2010; 76:4413–4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzlicek GA, Raghavan RK, Ganta RR, Anderson GA. Bayesian space-time patterns and climatic determinants of bovine anaplasmosis. PLoS One. 2016; 11:e0151924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homer CG, Dewitz JA, Yang L, Jin S, et al. . Completion of the 2011 National Land Cover Database for the conterminous United States-representing a decade of land cover change information. Photogramm Eng Remote Sensing 2015; 81:345–354 [Google Scholar]
- Irving R, Pinger R, Vann C, Olesen J, et al. . Distribution of Ehrlichia chaffeensis (Rickettsiales: Rickettsiaeceae) in Amblyomma americanum in southern Indiana and prevalence of E. chaffeensis-reactive antibodies in white-tailed deer in Indiana and Ohio in 1998. J Med Entomol 2000; 37:595–600 [DOI] [PubMed] [Google Scholar]
- James M, Bowman A, Forbes K, Lewis F, et al. . Environmental determinants of Ixodes ricinus ticks and the incidence of Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis, in Scotland. Parasitology 2013; 140:237–246 [DOI] [PubMed] [Google Scholar]
- Koch H. Suitability of birds and mammals as hosts for immature stages of the lone star tick, Amblyomma americanum (Acari: Ixodidae). J Med Entomol 1981; 18:93–98 [DOI] [PubMed] [Google Scholar]
- Kollars TM, Jr., Oliver JH, Jr., Durden LA, Kollars PG. Host associations and seasonal activity of Amblyomma americanum (Acari: Ixodidae) in Missouri. J Parasitol 2000; 86:1156–1159 [DOI] [PubMed] [Google Scholar]
- McGarigal K, Cushman SA, Ene E. 2012. FRAGSTATS v4: Spatial Pattern Analysis Program for Categorical and Continuous Maps. Computer software program produced by the authors at the University of Massachusetts, Amherst. Available at www.umass.edu/landeco/research/fragstats/fragstats.html
- Mixson TR, Campbell SR, Gill JS, Ginsberg HS, et al. . Prevalence of Ehrlichia, Borrelia, and Rickettsial agents in Amblyomma americanum (Acari: Ixodidae) collected from nine states. J Med Entomol 2006; 43:1261–1268 [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Keesing F. Biodiversity series: the function of biodiversity in the ecology of vector-borne zoonotic diseases. Can J Zool 2000; 78:2061–2078 [Google Scholar]
- R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: Available at www.R-project.org/ [Google Scholar]
- Raghavan RK, Almes K, Goodin DG, Harrington JA Jr., et al. . Spatially heterogeneous land cover/land use and climatic risk factors of tick-borne feline cytauxzoonosis. Vector Borne Zoonotic Dis 2014b; 14:486–495 [DOI] [PubMed] [Google Scholar]
- Raghavan RK, Goodin DG, Hanzlicek GA, Zolnerowich G, et al. . Maximum entropy-based ecological niche model and bio-climatic determinants of Lone Star Tick (Amblyomma americanum) niche. Vector Borne Zoonotic Dis 2016a; 16:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan RK, Goodin DG, Neises D, Anderson GA, et al. . Hierarchical Bayesian spatio-temporal analysis of climatic and socio-economic determinants of Rocky Mountain spotted fever. PLoS One 2016b; 11:e0150180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan RK, Harrington J, Jr., Anderson GA, Hutchinson JS, et al. . Environmental, climatic, and residential neighborhood determinants of feline tularemia. Vector Borne Zoonotic Dis 2013; 13:449–456 [DOI] [PubMed] [Google Scholar]
- Raghavan RK, Neises D, Goodin DG, Andresen DA, et al. . Bayesian spatio-temporal analysis and geospatial risk factors of human monocytic ehrlichiosis. PLoS One 2014a; 9:e100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph SE, Storey K. Impact of microclimate on immature tick-rodent host interactions (Acari: Ixodidae): implications for parasite transmission. J Med Entomol 1999; 36:741–748 [DOI] [PubMed] [Google Scholar]
- Rynkiewicz EC, Clay K. Tick community composition in Midwestern US habitats in relation to sampling method and environmental conditions. Exp Appl Acarol 2014; 64:109–119 [DOI] [PubMed] [Google Scholar]
- Savage HM, Godsey MS, Lambert A, Panella NA, et al. . First detection of Heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg 2013; 89:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiert JG, Gilfoy F. Infection rates of Amblyomma americanum and Dermacentor variabilis by Ehrlichia chaffeensis and Ehrlichia ewingii in southwest Missouri. Vector Borne Zoonotic Dis 2002; 2:53–60 [DOI] [PubMed] [Google Scholar]
- Whitlock J, Fang Q, Durden L, Oliver J. Prevalence of Ehrlichia chaffeensis (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) from the Georgia coast and barrier islands. J Med Entomol 2000; 37:276–280 [DOI] [PubMed] [Google Scholar]
- Wolf L, McPherson T, Harrison B, Engber B, et al. . Prevalence of Ehrlichia ewingii in Amblyomma americanum in North Carolina. J Clin Microbiol 2000; 38:2795–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabsley MJ, Varela AS, Tate CM, Dugan VG, et al. . Ehrlichia ewingii infection in white-tailed deer (Odocoileus virginianus).(Research). Emerg Infect Dis 2002; 8:668–672 [DOI] [PMC free article] [PubMed] [Google Scholar]