Abstract
Cranioplasties are performed to protect the brain and correct cosmetic defects, but there is growing evidence that this procedure may result in neurological improvement. We prospectively studied cranioplasties performed at our hospital over a 5-year period. The National Institute of Health Stroke Scale and Barthel index were recorded prior to and within 72 h after the cranioplasty. A perfusion computed tomography (PCT) and transcranial Doppler sonography (TCDS) were performed prior to and 72 h after the surgery. For the PCT, regions irrigated by the anterior cerebral artery, the middle cerebral artery (MCA), the posterior cerebral artery, and the basal ganglia were selected, as well as the mean values for the hemisphere. The sonography was performed in the sitting and the supine position for the MCA and internal carotid. The velocities, pulsatility index, resistance index, and Lindegaard ratio (LR) were obtained, as well as a variation value for the LR (ΔLR = LR sitting – LR supine). Fifty-four patients were included in the study. Of these, 23 (42.6%) patients presented with objective improvement. The mean cerebral blood flow of the defective side (m-CBF-d) increased from 101.86 to 117.17 mL/100 g/min (p = 0.064), and the m-CBF of the healthy side (m-CBF-h) increased from 128.14 to 145.73 mL/100 g/min (p = 0.028). With regard to the TCDS, the ΔLR was greater on the defective side prior the surgery in those patients who showed improvement (1.295 vs. −0.714; p = 0.002). Cranioplasty resulted in clinical improvement in 40% of the patients, with an increase in the post-surgical CBF. The larger variations in the LR when the patient is moved from the sitting to the supine position might predict the clinical improvement.
Key words: : cranioplasty, Lindegaard ratio, perfusion computed tomography, transcranial doppler sonography, trephined syndrome
Introduction
Decompressive craniectomy (DC) is a lifesaving procedure performed in patients suffering intracranial hypertension (ICHT) refractory to medical treatment. Several underlying conditions, such as traumatic brain injury (TBI), spontaneous subarachnoid hemorrhage, and malignant cerebral infarction, can increase intracranial pressure and cause ICHT. The relative increase in the use of this procedure and the improvement in the survival rate have led to a growing number of patients who might need a cranioplasty.1–5
Classically, the benefits of a cranioplasty were primarily to protect the brain and repair cosmetic defects. However, there are an increasing number of publications suggesting that cranioplasties may result in neurological improvement via increased cerebral blood flow (CBF), and an improvement of cerebrospinal fluid (CSF) dynamics and cerebral metabolism.6–22 Of these, the reported neurological improvement appears to be primarily associated with the CBF improvement. Nevertheless, quantitative assessment of blood flow with, for example, Xenon-enhanced computerized tomography (Xe-CT),9 depicts a “snapshot” of the patient's CBF at a singular moment, but whether those changes are a static process irrespective of time or body posture or rather a dynamic event is unknown.
We have prospectively studied a series of cranioplasties with perfusion computed tomography (PCT) of the head and Doppler ultrasonography in the vertical and horizontal positions in order to identify the changes in cerebral hemodynamics and the short-term neurological improvement, and whether they are related or not.
Methods
Patients
We prospectively studied cranioplasties performed at the 12 de Octubre Hospital in Spain from November 2009 to January 2014. Demographic data (e.g., age, sex, and medical comorbidities) were collected. Patients who underwent DC because they had medically refractory intracranial hypertension due to their underlying pathology were included. Patients who underwent DC for either tumor infiltration of the bone or infection of the bone flap after a scheduled craniotomy were excluded. The defective area was calculated by measuring the largest diameter (D1) and the diameter perpendicular to D1 (D2) inputted into the following ellipse formula: (D1/2 × D2/2 × π). Patients who underwent a bifrontal craniectomy were considered to have two defective sides, whereas the remainder of the patients had one defective side and one healthy side.
A score on the National Institutes of Health Stroke Scale (NIHSS) and the Barthel index were recorded for every patient the week before the cranioplasty and between 24 h and 72 h after the surgery. Objective improvement was defined as an improvement of either at least 1 point in the NIHSS or 5 points in the Barthel index, and it was considered to be a trephined syndrome case. Members of the research team blinded to the Doppler and PCT results performed the assessment in a standardized interview.
Concurrently, subjective improvement was recorded by either asking the patient directly or speaking with his/her caregivers if the patient was unable to answer. To quantify this improvement, the unpleasant symptoms for the patients who were not amenable to objective measure were recorded but the aesthetic improvement was disregarded. The more diverse symptoms (i.e., headaches, dizziness, insomnia, vague discomfort, etc.) were grouped under the term “subjective symptoms.”
Surgical procedures
Swab cultures were taken from the bone during the DC, then the bone flap was stored at −80°C in a tissue bank. The durotomy was either C or X shaped, and the dura was closed with a biological substitute as either an onlay (DuraGen®;) or with stitches (Tutopatch®;) based on the surgeon's preference. For patients undergoing a bifrontal DC, the falx cerebri was sectioned. No biological glues or any other sealants were routinely used.
The cranioplasty was performed a variable amount of time after the DC depending on the amount of time necessary to resolve the underlying pathology that led to the decompression.
The material used for the cranioplasty was the autologous bone flap, unless either the swab cultures were positive or the bone flap deteriorated during harvesting. If the bone flap was unavailable, then either computer-designed polyetheretherketone implants or manually shaped implants constructed from acrylic cement were used. A subgaleal drain was routinely used 24-72 h after the procedure. Antibiotic prophylaxis (2 g of cefazolin) was administered 15-60 min prior to skin incision and 6 h after completion of the surgery. Depending on the surgeon's preference, antibiotic administration may be extended for up to 72 h after the surgery.
The use of a ventriculoperitoneal shunt (VPS) was reserved for patients who present with obvious preoperative ventricular enlargement, and it was performed simultaneously with the cranioplasty. Patients with either a ventricular size at the higher limit of the normal range (Evan's index between 0.3 and 0.4) or subdural fluid collections were operated upon without VPS implantation. In these cases, either a lumbar drain or ventricular puncture was used before or during the procedure to avoid excessive pressure on the brain parenchyma) because in our experience, most of these CSF derangements resolve after the surgery.22 In cases involving a persistent or progressive ventricular dilatation, a shunt was placed during a second operation. To avoid confounding effects of DVP implantation, patients who underwent both a cranioplasty and DVP implantation at the same time were excluded from the analysis.
This study was revised and approved by the ethical committee at the hospital (reference, CEIC n°: 11/083).
PCT technique and measurements
PCT was performed one week before the procedure and within 72 h after the operation. All of the imaging studies were performed on a six-slice spiral CT scanner. PCT consisted of a 25-sec series of two slices during intravenous administration of contrast medium. PCT studied a 2.4-cm slice of brain centered on the third ventricle, obtaining two consecutive 1.2-cm thick images corresponding to regions of the basal ganglia and main cerebral arteries. These brain slices were continuously scanned, acquiring two consecutive 1.2-cm thickness images during each cycle. A total of 10 cycles for each slice were acquired at a rate of one cycle every 2.4 seconds after the intravenous administration of an 80-mL bolus of contrast (Omnipaque 300 mg/mL; General Electric,) into the cubital vein (18-gauge needle) at a flow rate of 4 mL/sec. Image acquisition began 5 sec after the injection of contrast. The acquisition parameters were 120 kvp and 80 mA using a 512 × 512 matrix. All images were analyzed using perfusion software developed by Philips (), which produces quantitative PCT data based on temporal changes in signal intensity during the first pass of a bolus of an iodinated contrast agent. This software relies on the central deconvolution principle to obtain different flow parameters. Deconvolution requires an operator to identify an artery and a vein.
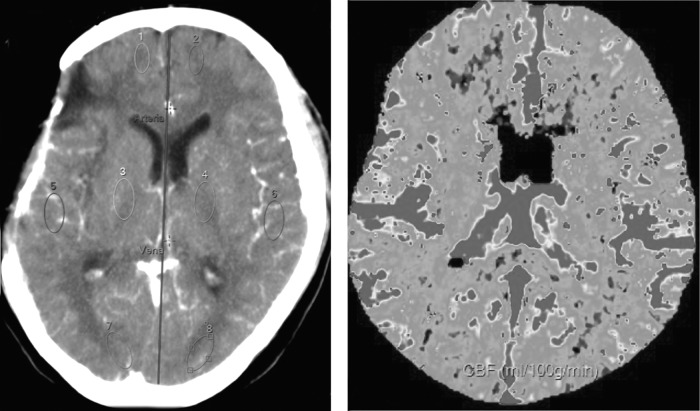
Four regions-of-interest (ROIs) were drawn on each hemisphere corresponding to white matter related to the territory of the major cerebral arteries (anterior, middle, and posterior cerebral arteries [ACA, MCA, and PCA, respectively]), as well as to basal ganglia (BG). Measurements of the mean transient time (MTT), time to peak (TTP), cerebral blood volume (CBV), and cerebral blood flow (CBF) were performed in each ROI. An average value of the four ROIs of each hemisphere was calculated for every measurement (MTT, TTP, CBV and CBF). Each ROI was named according to the cerebral artery responsible for their irrigation (Fig. 1). PCT quantitative data were obtained by two independent neuroradiologists who were unaware of the patients' clinical status.
FIG. 1.
Perfusion computed tomography. Left: a slice centered on the third ventricle. Regions of interest are placed over brain parenchyma perfused by the anterior, middle, and posterior cerebral arteries (ACA, MCA, PCA), and basal ganglia (BG). An artery and vein are selected for software processing. Right: Cerebral brain perfusion map obtained with the partial deconvolution paradigm.
Transcranial Doppler sonography
All patients were evaluated by transcranial Doppler sonography (TCDS) 1 week before and within 72 h after cranioplasty. Every study was performed at the patients' bedside with the patient relaxed, and maintenance of a consistent environment was attempted (e.g., room temperature, humidity, visual stimuli, noise, etc.). In every study, both the MCA and internal carotid artery (ICA) were insonated first with the patient in the sitting position; then the patient was placed at the supine position for 5 min, and the measurements were repeated.
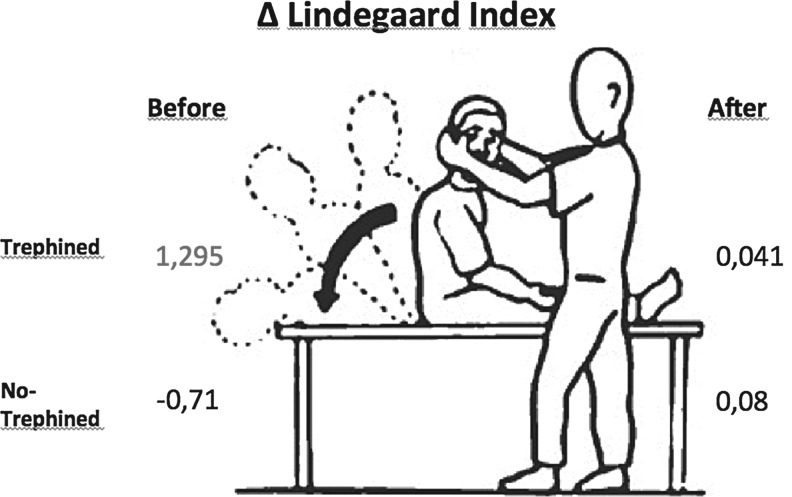
In this study, we recorded TCDS measurements with a hand-held transducer in a range-gated, pulsed-wave mode at a frequency of 2-6 MHz (ProSound Alpha 6, Aloka®;). The MCA was examined through the temporal window at a depth of 45-55 mm using a 300-600 insonation angle with angle correction. We used the lowest setting for sample size and wall filter. Aliasing artifacts were eliminated by optimum baseline correction. After a frozen stable waveform was obtained, the peak systolic velocity (PSV), end diastolic velocity (EDV), and mean blood flow velocity (MV), as well as the pulsatility index (PI = PSV-EDV/MV) and resistance index (RI = PSV-EDV/PSV), which are automatically calculated by the transducer software, were obtained by cursor pointing. The ICA was insonated through the submandibular window at a depth of 40-60 mm, and the PSV, EDV and MV were obtained. The Lindegaard ratio (LR = MCA-MV/ICA-MV) was calculated for both sides in the sitting and supine positions. For every value obtained, a variation value (Δ = variation) was calculated by subtracting the value in the supine position from the value in the sitting position. For example, the LR variation was calculated as follows: ΔLR = LR sitting – LR supine (Fig. 2).
FIG. 2.
Doppler studies are performed in the sitting position, then the patient is positioned supine and the measurements are repeated after 5 min. The Lindegaard ratio (LR) is obtained in each position, and a variation value is calculated by subtracting the supine from the sitting values (ΔLR = LR sitting – LR supine). The ΔLR is greater in the defective side of patients who had neurological improvement. After the surgery, the variation is negligible on both sides.
Statistical analysis
SPSS® statistical software (version 20.0;) was used for the data analysis. All TCD and PCT values are presented as the mean ± standard deviation. An unpaired t-test was used for parametric statistics analysis between quantitative parameters and the presence of objective clinical improvement; if the distribution did not allow the use of the t-test, a non-parametric test, such as the Mann-Whitney U test, was used. Comparisons of the changes between the groups were computed with Student's t-test. Associations with categorical variables were explored using either the χ2 test or the Fisher's exact test (the latter when expected cell sizes were smaller than five). A bivariate correlation was used to investigate the relationship between the NIHSS improvement (as a continuous variable) and the quantitative parameters. Results with p < 0.05 were considered to be statistically significant, whereas p < 0.08 was considered to be a trend. A 95% confidence interval is given when considered appropriate.
A multivariate analysis was performed to identify independent predictors for clinical objective improvement after cranioplasty using a binary logistic regression. Variables with significant p values in univariate analyses, as well as pre-surgical variables, were considered to be independent variables in the multivariate analysis.
Results
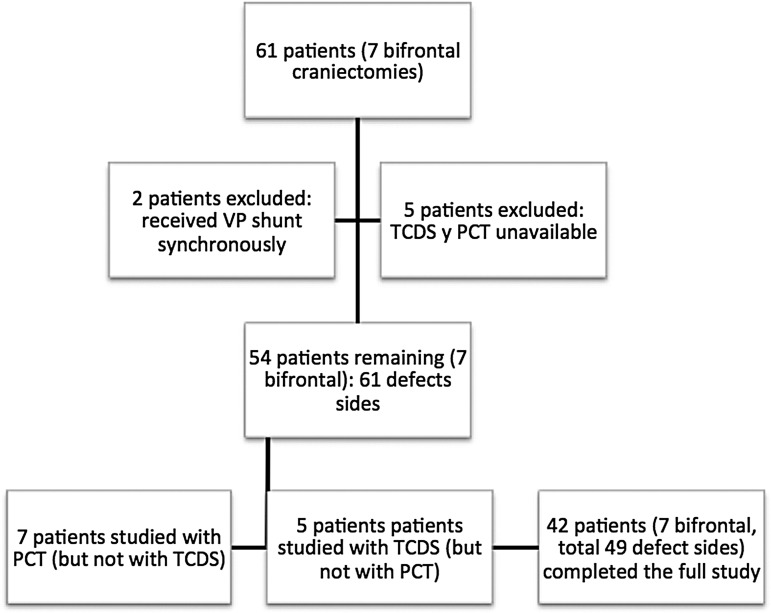
Sixty-one patients (22 women and 39 men) harboring 54 hemicraniectomies and seven bifrontal craniectomies were prospectively analyzed, with a total of 68 defective sides and 54 normal sides. For various reasons (e.g., technical issues, patients or family not providing consent, etc.), seven patients were excluded from the analysis. Of the remaining 54 patients (61 defective sides), 49 completed the full study, five were studied with TCDS but not with PCT, and seven were studied with PCT but not with TCDS (Fig. 3). The 12 patients who did not participate in the full study were unable to so because either the PCT or TCDS were unavailable within the time frame designated for the study.
FIG. 3.
Flowchart of the patients studied. Of the 61 patients operated, 54 were included in the study, with a total of 61 defective sides studied. Of these, seven patients did not undergo TCDS and five did not undergo PCT. VP shunt, ventricle-peritoneal shunt; TCDS, transcranial Doppler sonography; PCT, perfusion computed tomography.
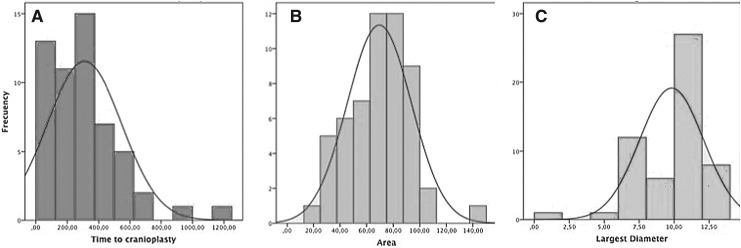
The average size of the bone defect was 69.5 cm2 (standard deviation [SD], 24.5 cm2; median, 73.51 cm2; range, 19.5–149.5 cm2; asymmetry coefficient, 0.23). The average size of the largest diameter was 9.81 cm (SD, 2.29; median, 10.5; asymmetry coefficient, −1.66). The average time elapsed between the DC and cranioplasty was 309 days (SD, 237 days; median, 268 days; range, 25–1217 days; asymmetry coefficient, 1.42; Fig. 4).
FIG. 4.
Histogram of the distribution of the time to cranioplasty (A), area of the defect (B), and largest diameter (C). Time to cranioplasty might appear too long, but the histogram shows that the majority of the patients are grouped on the left side. Similarly, the largest diameter might be confounded by some of the small values, but the majority are grouped on the right tail.
The mean age was 41.7 ± 15.53, the NIHSS and Barthel pre-surgical values were 5.77 (± 7.24) and 72.37 (± 30.99), respectively, and the post-surgical values were 5.05 (± 7.31) and 75.93 (± 30.27), respectively. Twenty-three (42.6%) patients presented with objective improvement and 31 (57.4%) with subjective improvement. Of those who improve objectively, 12 (21%) improved 1 point in the NIHSS, eight (14.5%) improved 2 points, one (1.8%) improved 3 points; and two (1.8%) improved 5 points in the Barthel index (without NIHSS improvement). The mean improvement values for the NIHSS and Barthel were 0.5273 and 1,727, respectively. Most of the patients were originally decompressed because of either a head injury (59%) or a spontaneous hemorrhage (16.5%). A detailed description has been reported elsewhere.23
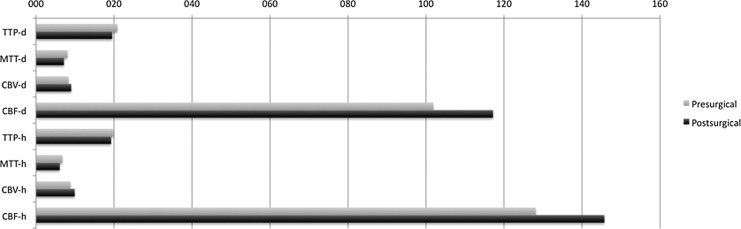
With regard to the PCT measurements, nearly all of the values improved (improvement defined as either a decrease in the TTP and MTT or increasing CBV and CBF; Fig. 5), in both in the defective and normal sides; however, when a Student's t-test was applied, only a few variables were statistically significant: the TTP of the MCA on the defective side decreased from 21.719 to 19.512 sec (p = 0.046), the MTT of the MCA on the defective side decreased from 8.241 to 6.26 sec (p = 0.014), and the CBV of the PCA on the normal side increased from 10.67 to 13.38 mL/100 g (p = 0.026). Additionally, the mean CBF on the defective side (m-CBF-d) increased from 101.86 to 117.17 mL/100 g/min (p = 0.064; trend) and the m-CBF on the healthy side (m-CBF-h) increased from 128.14 to 145.73 mL/100 g/min (p = 0,028; Table 1).
FIG. 5.
Mean values of the time-to-peak (TTP), mean-transition-time (MTT), cerebral-blood-volume (CBV), and cerebral-blood-flow (CBF) of the defective and healthy sides before and after the surgery. There was an increase in the CBF in both sides after the surgery as well as a decrease in the TTP and MTT, along with a small increase of the CBV on both sides.
Table 1.
Perfusion CT
| Defect | Healthy | |||||
|---|---|---|---|---|---|---|
| Pre-surgery | Post-Surgery | p value | Pre-surgery | Post-Surgery | p value | |
| TTP in sec | TTP | TTP | ||||
| ACA | 20.350 | 20.211 | 20.310 | 19.486 | ||
| MCA | 21.719 | 19.512 | p = 0.046 | 19.454 | 18.951 | |
| PCA | 21.285 | 19.972 | 20.438 | 19.760 | ||
| BG | 20.055 | 18.838 | 19.015 | 18.687 | ||
| Mean | 20.836 | 19.520 | 19.812 | 19.221 | ||
| MTT in sec | MTT | MTT | ||||
| ACA | 9.017 | 8.899 | 6.945 | 6.480 | ||
| MCA | 8.241 | 6.260 | p = 0.014 | 6.541 | 5.430 | p = 0.071 |
| PCA | 7.641 | 6.859 | 6.622 | 6.353 | ||
| BG | 7.461 | 6.689 | 6.845 | 6.011 | ||
| Mean | 8.050 | 7.150 | 6.738 | 6.068 | ||
| CBV in mL/100 g | CBV | CBV | ||||
| ACA | 6.537 | 6.521 | 6.989 | 7.338 | ||
| MCA | 10.456 | 10.580 | 10.713 | 11.332 | ||
| PCA | 8.947 | 11.017 | p = 0.079 | 10.678 | 13.382 | p = 0.0026 |
| BG | 7.561 | 8.000 | 6.941 | 7.578 | ||
| Mean | 8.310 | 9.010 | 8.830 | 9.907 | ||
| CBF in mL/100 g/min | CBF | CBF | ||||
| ACA | 71.28 | 90.98 | p = 0.057 | 107.66 | 121.05 | |
| MCA | 119.36 | 136.73 | 151.24 | 168.82 | ||
| PCA | 108.96 | 133.89 | 136.28 | 166.02 | ||
| BG | 109.07 | 108.03 | 117.39 | 127.00 | ||
| Mean | 101.860 | 117.170 | p = 0.064 | 128.140 | 145.730 | p = 0.028 |
Student's t-test for paired data was applied to compare the pre- and post-surgical values; the p values are reported if they were less than 0.08 (trend) or if they were less than 0.05 (statistically significant).
CT, computed tomography; TTP, time to peak; ACA, anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; BG, basal ganglia; MTT, mean transient time; CBV, cerebral blood volume; CBF, cerebral blood flow.
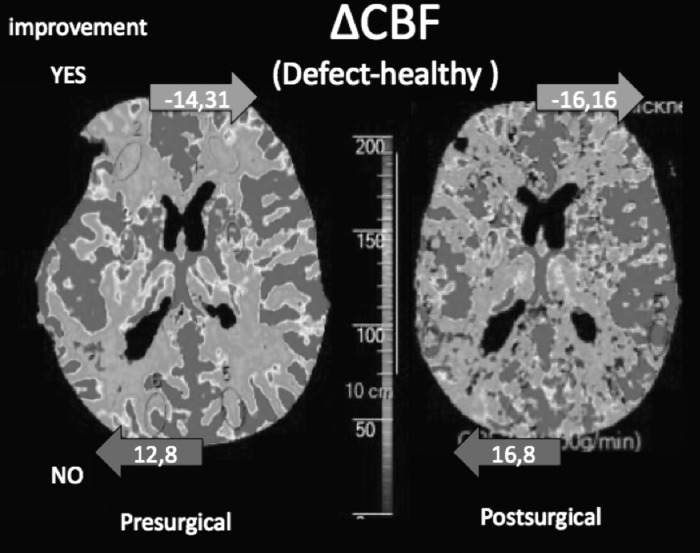
Differences in the PCT between those who improved (trephined) and those who did not are shown in Table 2, compared with an unpaired t-test. It is remarkable that there were no statistically significant differences when the values of the defective side in the pre-surgical examination were compared (trephined vs. untrephined). In the healthy side, the patients who showed improvement appeared to present better perfusion values (pre-surgical m-CBF-h 148.31 vs. 111.32 mL/100 g/min; p = 0.04) and shorter transition times (m-MTT-h 5.43 vs. 7.82 seconds; p = 0.039) than those who did not. In the post-surgical examination, the CBF on the healthy side appeared to be even better in patients who showed improvement, compared with the patients who did not show improvement (173.47 vs. 122.62 mL/100 g/min; p = 0.049). When the difference in the CBF between the healthy and defective sides was calculated (ΔCBF = [m-CBF-d]-[m-CBF-h]), there was a marked difference in favor of the healthy side in patients who showed improvement (-14.31 vs. +12.8 mL/100 g/min; p = 0.049), which was even larger after the surgery (-16.16 vs. +16.8; p = 0.068) although not statistically significant (Fig. 6). The CBF changes ([post-surgical m-CBF] – [pre-surgical m-CBF]) on the defective side of patients who either did or did not show neurological improvement were 11.47 and 7.92 mL/100 g/min, respectively (p = 0.873), and the CBF changes on the healthy side were 26.76 and 13.10 mL/100 g/min, respectively (p = 0.614).
Table 2.
Trephined (Improvement) and Perfusion CT
| Pre-surgery | p value | Post-surgery | p value | Pre-surgery | p value | Post-surgery | p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TTP in sec | TTP Defect | TTP Healthy | ||||||||||
| trephined | Yes | No | Yes | No | Yes | No | Yes | No | ||||
| ACA | 20.813 | 19.991 | N.S | 21.651 | 19.177 | N.S | 21.074 | 19.673 | N.S | 19.714 | 19.296 | N.S |
| MCA | 22.976 | 20.776 | N.S | 20.606 | 18.726 | N.S | 19.222 | 19.648 | N.S | 19.245 | 18.706 | N.S |
| PCA | 22.407 | 20.443 | N.S | 21.103 | 19.123 | N.S | 20.707 | 20.214 | N.S | 20.065 | 19.505 | N.S |
| BG | 21.535 | 18.945 | N.S | 19.681 | 18.205 | N.S | 19.054 | 18.982 | N.S | 19.315 | 18.164 | N.S |
| Mean | 21.911 | 20.031 | N.S | 20.471 | 18.808 | N.S | 20.033 | 19.629 | N.S | 19.585 | 18.918 | N.S |
| MTT in sec | MTT Defect | MTT Healthy | ||||||||||
| trephined | Yes | No | Yes | No | Yes | No | Yes | No | ||||
| ACA | 8.743 | 9.221 | N.S | 9.457 | 8.498 | N.S | 5.694 | 7.988 | 0.059 | 6.704 | 6.294 | N.S |
| MCA | 7.998 | 8.424 | N.S | 6.310 | 6.420 | N.S | 5.059 | 7.776 | 0.031 | 5.058 | 5.740 | N.S |
| PCA | 6.912 | 8.188 | N.S | 7.312 | 6.519 | N.S | 6.096 | 7.061 | N.S | 5.959 | 6.683 | N.S |
| BG | 6.855 | 7.916 | N.S | 7.250 | 6.268 | N.S | 4.886 | 8.478 | 0.01 | 5.781 | 6.204 | N.S |
| Mean | 7.536 | 8.436 | N.S | 7.450 | 6.926 | N.S | 5.434 | 7.825 | 0.039 | 5.875 | 6.230 | N.S |
| CBV in mL/100 g | CBV Defect | CBV Healthy | ||||||||||
| trephined | Yes | No | Yes | No | Yes | No | Yes | No | ||||
| ACA | 5.988 | 6.926 | N.S | 7.288 | 5.971 | N.S | 6.986 | 6.992 | N.S | 8.225 | 6.600 | N.S |
| MCA | 10.046 | 10.764 | N.S | 11.620 | 9.834 | N.S | 11.964 | 9.671 | N.S | 14.707 | 8.519 | 0.002 |
| PCA | 10.010 | 8.151 | N.S | 11.900 | 10.354 | N.S | 11.837 | 9.712 | N.S | 13.946 | 12.913 | N.S |
| BG | 8.185 | 7.094 | N.S | 8.668 | 7.499 | N.S | 7.696 | 6.312 | N.S | 8.988 | 6.403 | 0.057 |
| Mean | 8.432 | 8.235 | N.S | 9.808 | 8.414 | N.S | 9.620 | 8.172 | N.S | 11.466 | 8.609 | N.S |
| CBF in mL/100 g/min | CBF Defect | CBF Healthy | ||||||||||
| trephined | Yes | No | Yes | No | Yes | No | Yes | No | ||||
| ACA | 65.338 | 75.699 | N.S | 108.542 | 78.357 | N.S | 111.579 | 104.395 | N.S | 145.251 | 100.888 | N.S |
| MCA | 120.055 | 118.841 | N.S | 136.565 | 136.851 | N.S | 181.115 | 126.344 | 0.031 | 193.855 | 147.953 | N.S |
| PCA | 128.095 | 94.604 | N.S | 162.046 | 112.766 | N.S | 159.674 | 116.778 | N.S | 208.467 | 130.653 | N.S |
| BG | 126.385 | 96.091 | N.S | 109.307 | 107.077 | N.S | 140.907 | 97.787 | 0.024 | 146.257 | 110.958 | N.S |
| Mean | 109.288 | 96.303 | N.S | 128.401 | 108.763 | N.S | 148.318 | 111.326 | 0.04 | 173.474 | 122.612 | 0.049 |
An unpaired t-test was applied and grouped based on either the presence or absence of improvement (trephined yes or no); p values are reported if they were less than 0.08 (trend) or if they were less than 0.05 (statistically significant). The remainder of the values is presented as not significant. There were no differences between trephined and not trephined on the defective side either pre- or post-surgery. On the healthy side, we observed a statistically shorter MTT in patients who showed improvement, as well as higher pre-surgical CBF values and higher postsurgical CBV values compared to patients who were not trephined.
CT, computed tomography; TTP, time to peak; ACA, anterior cerebral artery; N.S., not significant; MCA, middle cerebral artery; PCA, posterior cerebral artery; BG, basal ganglia; MTT, mean transient time; CBV, cerebral blood volume; CBF, cerebral blood flow.
FIG. 6.
Cerebral blood flow (CBF) asymmetry. When the CBF value obtained in the perfusion CT of the healthy side is subtracted from the defective side, a great asymmetry in favor of the healthy side (14.31 mL/100 g/min more in the healthy than in the defective side) is observed in patients who showed neurological improvement, whereas the asymmetry favors the defective side (12.8 mL/100 g/min more in the defective than in the healthy side) in patients who showed no improvement. These findings are more pronounced after the surgery.
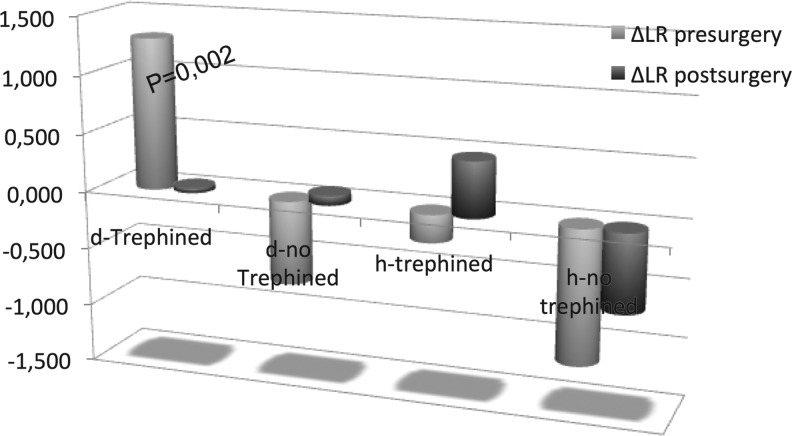
The values obtained from the Doppler studies are shown in Table 3. When comparing the pre- and post-surgical values, the majority of the differences were small, with only few achieving statistical significance. The variation of the mean velocities and the LR when the patient is moved from the sitting to the supine position trended towards a reduction after the surgery, but this was not statistically significant. However, when a t-test is applied (Table 4) to compare each value in patients who showed neurological improvement with those who did not, interesting differences were observed: the ΔLR was significantly higher in the defective side before the surgery in patients who showed improvement (1.295 vs. −0.714; p = 0,002), compared with those who did not (Fig. 7). The ΔLR was much smaller after the surgery, and there was no longer a significant difference in the variation of the LR when the patient was moved from sitting to supine position in either the defective or healthy sides (Fig. 2). The variation in the values of the pre-surgical LR is primarily due to the velocities of the ICA, which presented significantly lower velocities in the sitting position in patients who showed improvement, compared with those who did not (pre-surgical sitting m-ICA-d 14.37 cm/sec vs. 24.22 cm/sec, respectively; p = 0.,007). That difference in the ICA velocity was not observed in either the healthy side prior to surgery or in either side after surgery (Table 4). There were no significant differences in the PI or resistance index of the ICA in either position before or after the surgery.
Table 3.
Doppler, Pre- and Post-Surgery
| Pre-surgery | Post-surgery | p value | Pre-surgery | Post-surgery | p value | |
|---|---|---|---|---|---|---|
| MCA sitting cm/sec | MCA sitting, defect | MCA sitting, healthy | ||||
| Systolic | 71.740 | 64.373 | 63.388 | 75.583 | ||
| Dyastolic | 26.715 | 25.239 | 24.431 | 29.913 | 0.075 | |
| Mean | 43.090 | 38.206 | 37.156 | 43.726 | ||
| MCA Supine cm/sec | MCA supine, defect | MCA Supine, healthy | ||||
| Systolic | 66.000 | 59.146 | 69.695 | 66.408 | ||
| Dyastolic | 26.804 | 23.189 | 0.051 | 28.886 | 25.798 | 0.063 |
| Mean | 40.165 | 35.831 | 41.467 | 39.252 | ||
| ICA sitting cm/sec | ICA sitting, defect | ICA sitting, healthy | ||||
| Systolic | 47.169 | 44.652 | 46.387 | 39.958 | ||
| Dyastolic | 10.828 | 10.650 | 12.205 | 10.443 | ||
| MEAN | 20.242 | 19.495 | 21.257 | 18.055 | 0.067 | |
| ICA supine cm/sec | ICA supine, defect | ICA supine, healthy | ||||
| Systolic | 37.660 | 39.879 | 37.038 | 40.823 | ||
| Dyastolic | 9.833 | 10.462 | 10.798 | 10.245 | ||
| MEAN | 16.822 | 17.905 | 18.025 | 18.583 | ||
| LR | Lindegaard index, defect | Lindegaard index, healthy | ||||
| Sitting | 2.828 | 2.445 | 2.163 | 2.294 | ||
| Supine | 2.731 | 2.381 | 2.899 | 2.512 | ||
| ΔLR (Sitting-Supine) | 0.097 | 0.064 | −0.736 | −0.180 | ||
A t-test for paired data was applied to compare each side with itself before and after the surgery. There was a small decrease in the MCA diastolic velocity on both sides after the surgery. Insignificant p values have been left blank.
MCA, middle cerebral artery; ICA, internal carotid artery; LR, Lindegaard ratio.
Table 4.
Doppler and Trephined
| Pre-surgery | Post-surgery | |||||
|---|---|---|---|---|---|---|
| Trephined | Trephined | |||||
| Yes | No | p value | Yes | No | p value | |
| MCA Sitting cm/sec | MCA sitting, defect | |||||
| Systolic | 76.30 | 68.65 | 66.42 | 62.98 | ||
| Dyastolic | 29.67 | 24.72 | 25.40 | 25.13 | ||
| Mean | 46.78 | 40.59 | 38.21 | 38.21 | ||
| MCA Supine cm/sec | MCA supine, defect | |||||
| Systolic | 54.40 | 73.86 | 62.43 | 56.92 | ||
| Dyastolic | 24.18 | 28.58 | 24.26 | 22.46 | ||
| Mean | 35.28 | 43.47 | 36.97 | 35.06 | ||
| ICA sitting cm/sec | ICA sitting, defect | |||||
| Systolic | 32.88 | 56.85 | 0.008 | 37.98 | 41.16 | |
| Dyastolic | 8.13 | 12.65 | 0.024 | 10.50 | 10.44 | |
| Mean | 14.37 | 24.22 | 0.007 | 16.86 | 18.61 | |
| ICA Supine cm/sec | ICA supine, defect | |||||
| Systolic | 38.79 | 36.89 | 37.98 | 41.16 | ||
| Dyastolic | 10.40 | 9.45 | 10.50 | 10.44 | ||
| Mean | 17.26 | 16.53 | 16.86 | 18.61 | ||
| LR | Lindegaard index, defect | |||||
| Sitting | 3.692 | 2.242 | 0.029 | 2.454 | 2.439 | |
| Supine | 2.397 | 2.957 | 2.413 | 2.358 | ||
| ΔLR(sitting-supine) | 1.295 | −0.714 | 0.002 | 0.041 | 0.080 | |
| Pre-surgery | Post-surgery | |||||
|---|---|---|---|---|---|---|
| Trephined | Trephined | |||||
| Yes | No | P value | Yes | No | P value | |
| MCA Sitting cm/sec | MCA sitting, healthy | |||||
| Systolic | 73.29 | 56.81 | 72.26 | 78.03 | ||
| Dyastolic | 30.94 | 19.89 | 29.05 | 30.55 | ||
| Mean | 45.80 | 33.37 | 41.24 | 46.00 | ||
| MCA Supine cm/sec | MCA supine, healthy | |||||
| Systolic | 65.42 | 74.29 | 62.15 | 69.55 | ||
| Dyastolic | 27.45 | 30.48 | 24.89 | 26.47 | ||
| Mean | 39.77 | 45.79 | 36.09 | 41.97 | ||
| ICA sitting cm/sec | ICA sitting, healthy | |||||
| Systolic | 34.66 | 54.47 | 37.20 | 42.00 | ||
| Dyastolic | 10.64 | 13.42 | 9.91 | 10.84 | ||
| Mean | 17.54 | 24.16 | 17.00 | 19.01 | ||
| ICA Supine cm/sec | ICA supine, healthy | |||||
| Systolic | 30.96 | 41.02 | 0.069 | 33.75 | 46.05 | |
| Dyastolic | 9.90 | 11.47 | 9.52 | 10.78 | ||
| Mean | 15.45 | 20.04 | 16.07 | 20.44 | ||
| LR | Lindegaard index, healthy | |||||
| Sitting | 2.797 | 1.715 | 2.941 | 1.815 | ||
| Supine | 3.032 | 2.853 | 2.558 | 2.479 | ||
| ΔLR(sitting-supine) | −0.235 | −1.138 | 0.472 | −0.664 | ||
Student's t-test was applied to compare patients who improved with those who did not (i.e., trephined yes or no). The LR varied greatly when moving from sitting to supine in the defective side of patients who showed improvement, but there was little to no variation after the surgery. This is primarily due to a lower ICA velocity in the sitting position in patients who showed improvement. When Student's t-test resulted in a p value greater than 0.08, the box was left blank.
MCA, middle cerebral artery; ICA, internal carotid artery; LR, Lindegaard ratio.
FIG. 7.
Variation of the Lindegaard ratio from the sitting to supine positions. The variation was greater prior to surgery in patients defined as trephined (i.e., patients showing improvement after surgery), and the difference was statistically significant when Student's t-test was applied to patients who showed improvement vs patients who did not. d, defective; h, healthy.
A bivariate correlation was used to investigate the relationship between the variation of the NIHSS as a continuous variable and the pre-surgical continuous variables. The ΔLR (r = +0.272; p = 0,037), the pre-surgical m-MTT-h (r = −0,252; p = 0,049) and the pre-surgical m-CBV-h (r = +0.27; p = 0.039) were observed to have a significant correlation with the variation of the NIHSS.
Once these results were obtained, the statistically significant variables were used in a multivariate analysis using a binary logistic regression. Based on our previously published data,23 the size of the craniectomy and early surgery status (i.e., surgery performed within 85 days of the DC) were included in the analysis. Once the variables with no statistically significant associations were removed, the ΔLR, m-MTT-h, early surgery status, and craniectomy size remained in the model. A higher odds ratio (OR) was calculated for the ΔLR, which was 3.098 (1.560-6.153; p = 0 001). The constant of the equation was 1.614, and the coefficients were 0.053 for the DC size, 1.131 for the ΔLR, 4.126 for the early surgery status, and −1.069 for the m-MTT-h (Table 5).
Table 5.
Binary Logistic Regression Analysis Results for Clinical Objective Improvement in 54 Patients
| Objective improvement | |||
|---|---|---|---|
| Variables | OR | 95% CI | p value |
| DC size (c.v.) | 1.054 | 1.011–1.100 | 0.014 |
| ΔLR (c.v.) | 3.098 | 1.56–6.153 | 0.001 |
| Early surgery | |||
| >85 days | 1 | ||
| <85 days | 61.92 | 4.023–963.044 | 0.003 |
| m-MTT-h | 0.343 | 0.172–0.685 | 0.002 |
OR, odds ratio; CI, confidence interval; DC, decompressive craniectomy; c.v., continuous variable; ΔLR, pre-surgical variation of the Lindegaard ratio of the defective side when moving from the sitting so supine position; Early surgery, surgery performed with 85 days of the original decompression; m-MTT-h, mean transition time of the healthy hemisphere obtained with perfusion computed tomography before the surgery.
The probability of improvement in a patient who had an “early operation” with a DC of 71 cm2, a ΔLR of 0.1, and an m-MTT-h of 6.6 sec (which represent the mean values for the quantitative variables) is 92% (p = OR/1+OR; OR = e[a+b1+b2+b3+b4]; R square of Nagelkerke = 0.615. −2Log [likelihood] = 44.697). The Hosmer-Lemeshow goodness of fit test was p = 0.304; therefore, the model is considered to fit well. The area under the curve of the receiver operating characteristic curve derived from the model is 0.897 (0.819-0.976).
Discussion
The trephined syndrome was first described by Grant and Norcross24 in 1939 as a group of vague symptoms, such as headache, dizziness, fatigue, instability, intolerance to vibration, and depression. Those symptoms appeared in patients who underwent a craniectomy and then experienced neurological improvement after a cranioplasty. Later on, multiple terms and names were added, including motor trephined syndrome10 and the sinking flap syndrome25; the commonality between these syndromes was that the patients who had been craniectomized and later presented with subjective, objective, or both types of symptoms showed improvement after the cranioplasty.
The relative increase in the use of the DC has brought along a parallel raise in the number of publications analyzing the complications of this procedure and providing evidence that the trephined syndrome is not an uncommon clinical picture.6–11,14–16,18,20,21,26–42 Several studies19,32,43–46 have reported an incidence of the syndrome of 1–15% and a time latency of 3–6 months from the DC to the onset of the symptoms.
Further, in recent years, we have witnessed a growing number of papers reporting neurological improvement of patients who underwent a cranioplasty or not whether they suffered from a previous worsening6,8,16,18,31,34,47–51 (as originally described by Grant and Norcross).10,11,13,15,25,27,29,33,35,37,39,48 We decided to measure the improvement between 24 h and 72 h after the procedure because these patients might continue to improve despite the absence of the calvarial bone, and we observed a striking 42% objective improvement and 57% subjective improvement.
Different theories have attempted to explain the reason for the improvement of these patients and can be summarized in three categories: CSF derangements,7,8,22,39,52 cerebral perfusion improvement,6,9,10,14,15,18,20,21 and brain metabolism improvement.11 The two latter theories are frequently proposed together because an improvement in cerebral perfusion is thought to lead to improved brain metabolism.
PCT
Much attention has been paid to the changes experienced in the brain perfusion, and somehow linked to it to the brain metabolism, after the cranioplasty. We have summarized it in Table 6.9–11,13,14,18,21,29,53,54 Suzuki and colleagues10 studied for the first time six patients who went through a cranioplasty with a primitive form of perfusion CT 1–3 weeks before and 1 week after the surgery. They measured the changes in the peak intensity reached in each one of four predefined ROIs on each hemisphere, and the “washout” defined as the intensity reached 12 sec after the peak value; and they repeated the study after the surgery. They found a 2% and 20% increase in the peak value after the surgery on the defect and healthy sides, respectively, and a 13% mean increase in the washout value. These changes were more marked in the temporal and frontal lobes of the healthy side. Five of their patients showed a clinical improvement, but they did not correlate the perfusion changes with the clinical improvement. Maekawa and colleagues9 studied eight patients with Xe-CT and found an improvement in five defect sides and three healthy ones. Winkler and colleagues11 studied 13 patients with TCD and positron emission tomography (PET) scan. In the PET, they found an increase in the fludeoxyglucose (18 F) uptake of 12 and 4% after the cranioplasty in the defect and healthy sides, respectively. They also found that the asymmetry of glucose uptake decreases from 35 to 28% after the surgery, and correlated it with the clinical findings: 10 out of 12 PET performed showed improved their perfusion and eventually, all of them showed clinical improvement.
Table 6.
Studies on Cranioplasty and Brain Perfusion
| Author | Year | Number of patients | Modality | Conclusion |
|---|---|---|---|---|
| Suzuki and colleagues | 1993 | 6 | PCT | ↑ 2 and 20% in defect and healthy sides |
| Maekawa and colleagues | 1999 | 8 | Xe-CT | ↑ In 4 out 8 defect and 3 out 8 healthy sides |
| Winkler and colleagues | 2000 | 13 | PET scan | ↑ glucose intake by 12% in defect and by 4% in healthy sides |
| Agner and colleagues | 2002 | 1 | Xe-CT | ↑ 125% after surgery |
| Isago and colleagues | 2004 | 1 | Xe-CT | ↑ 2-fold and 1.5-fold on each side |
| Sakamoto and colleagues | 2006 | 1 | PCT | ↑ From 23.1 to 37.4 mL/100 g/sec and from 31.8 to 41.6 mL/100 g/sec in the defect and healthy sides, respectively |
| Won and colleagues | 2008 | 27 | PCT | ↑ From 39.1 to 44.7 mL/100 g/sec and from 42.9 to 47.2 mL/100 g/sec in the defect and healthy sides, respectively. |
| Decaminada and colleagues | 2008 | 10 | PCT | Slight ↑ in ` in defect side |
| Kemmling and colleagues | 2010 | 1 | PMR | ↑ of CBV and CBF. and ↓ MTT defect after surgery |
| Sarubbo and colleagues | 2014 | 6 | PCT | ↑ in CBV and CBF. and ↓ in TTP in defect side at 7 days |
| Paredes and colleagues | 2015 | 49 | PCT | ↑ From 101.86 to 117.17 mL/100 g/sec and from 128.14 to 145.73 mL/100 g/sec in the defect and healthy sides, respectively |
PCT, perfusion computed tomography; Xe-CT, Xenon-enhanced computed tomography; PET, positron emission tomography; CBV, cerebral blood volume; CBF cerebral blood flow; TTP, time to peak; PMR, perfusion magnetic resonance.
Won and colleagues21 published one of the largest series of patients studied with PCT, reporting 27 patients studied before and 14 days after the surgery. Although poorly detailed on the PCT acquisition data, they found an increase of the mean values from 39.1 to 44.7 mL/100 g/min and from 42.9 to 47.2 mL/100 g/min in the defect and healthy sides, respectively. Even though it was not specified, it can be extracted from their tables that 21 out of the 27 patients improved in some degree. Decaminada and colleagues53 studied 10 cases with PCT, finding an increase in CBV and CBF values, and a decrease in TTP. Sarubbo and colleagues54 studied six cases with PCT before and 7 days and 3 months after the surgery. The found an increase in the CBV and CBF values, and a decrease in the MTT at 7 days of the surgery. Those changes were lesser at 3 months, suggesting that changes in cerebral hemodynamics might not be constant over time.
We present, to the best of our knowledge, the largest series of cranioplasties studied with PCT. We performed the PCT at a constant time after the surgery to avoid dynamic changes of the CBF over time to mislead our conclusions. We found a significant increase of the mean CBF from 101.86 to 117.17 mL/100 g/min and from 128.14 to 145.73 mL/100 g/min in the defect and healthy sides. We also found an increase in the CBV, and a decrease in the MTT and TTP values (Table 1). Most of the previous reports assumed that the clinical improvement witnessed was secondary to the perfusion improvement found with the different techniques, but we found no proper statistical analysis on this respect on the bibliography. When we tried to correlate our findings in the perfusion CT with the presence of clinical improvement, we found a poor correlation. Although we did find a greater increase in the CBF in those who improved (even more noticeable in the healthy side), it was by far not statistically significant. This might be due to the relative small number of our series, or because an improvement in the CBF is something that happens in most of the patients undergoing a cranioplasty (as actually shown in our results) whether they improve clinically with the procedure or not.
TCDS
Less widespread is the use of the TCDS to study these patients. A summary of the studies is depicted in Table 7.6,11,15,20,21 In 2000, Winkler and colleagues11 investigated CBF reactivity with transcranial color duplex in 13 patients, and measured the differences with the postural changes. They observed that the ICA velocity of the defective side decreases significantly in the sitting position, and it did not happen after the surgery. They concluded that cranioplasty appears to affect postural blood flow regulation and reported a profound improvement of both the ipsilateral and contralateral cerebral vascular reserve capacity. Erdogan and colleagues6 used TCDS and discovered that before cranioplasty, all of the velocities ipsilateral to the cranial defect were significantly low, whereas the velocities were near normal on the contralateral side. The low ipsilateral velocities increased and reached normal levels after cranioplasty. Kuo and colleagues15 studied 13 patients with TCDS prior to and 2 weeks after the cranioplasty, measuring blood velocity in all of the main intracranial arteries in the supine position. They also measured the Glasgow Coma Scale score, muscle strength, and the Barthel index. They observed a significant clinical improvement and an increase in the velocities in all arteries, although only the MCA of the healthy side presented with a statistically significant increase. Won and colleagues21 used TCDS, PCT, and echocardiograms to study 27 patients undergoing a cranioplasty; although the study was poorly detailed regarding the timing, posture, or conditions under which the measurements were made, in this relatively large series they observed a significant decrease of blood velocity in both sides, as well as in both MCA and ICA after the surgery. In a more recent paper, Song and colleagues20 studied 43 cranioplasties with relatively small defects (mean diameter, 7.54 cm), using TCDS in the supine position both prior to and 7–12 days after the surgery and compared the changes in the early (less than 12 weeks) versus late (more than 12 weeks elapsed since DC) surgery groups. They found that the MCAs on both sides of the early group increased significantly their velocities after the surgery, whereas only the MCA on the defective side in the late group showed any increases.
Table 7.
Studies on Cranioplasty and Transcranial Doppler
| Author | Year | Number of patients | Conclusion |
|---|---|---|---|
| Winkler and colleagues | 2000 | 13 | ↑ MCA velocities after surgery↓ variation in ICA velocity with posture changes. after surgery |
| Erdogan and colleagues | 2003 | 18 | ↑ PSV in all main intracerebral arteries after surgery |
| Kuo and colleagues | 2004 | 13 | ↑ MCA velocity in healthy side after surgery |
| Won and colleagues | 2008 | 27 | ↓ Both MCA and ICA after the surgery |
| Song and colleagues | 2013 | 43 | ↑ MCA velocity. Greater increase in those operated earlier |
| Paredes and colleagues | 2015 | 47 | ↑ variation in Lindegaard ratio in the defect side in those who will improve. No such a difference after the surgery. |
MCA, middle cerebral artery; ICA, internal cerebral artery; PSV, peak systolic velocity.
We have observed marked changes on in the LR when the patient is moved from the sitting to supine position in those patients who showed improvement after the surgery. Previous studies reported that changes in the blood velocity of healthy subjects when they change positions are temporary and return to baseline after 3-8 min.55 This abnormality or lack of vascular regulation could explain why the classic trephined syndrome usually presents months after the original DC, since these neurologically impaired patients might need those months to recover enough to spend longer periods in a vertical position, and then suffer from a lack of vascular regulation. Why only some patients suffer from this lack of autoregulation is yet to be elucidated.
Limitations
Although we have presented, to the best of our knowledge, the largest series of patients undergoing a cranioplasty and studied by PCT and TCDS, there are obvious limitations to this study. Although a DC was performed on every patient because of underlying ICHT, this group of patients suffers from a heterogeneous range of pathologies, comprising primarily of vascular and traumatic pathologies. The size of the craniectomy and the time elapsed from the DC varies greatly among the patients, and although this was incorporated in the multivariate analysis, this wide variability limits the generalization of our findings. Our clinical assessment was detailed, but no specific standardized measurement of neurocognitive impairment was made; therefore, some clinical correlations could have been missed with the tests performed. Also, the lack of a control group makes it difficult to conclude that the neurological improvement observed is due only to the cranioplasty. Although the assessment was performed blind to the PCT and TCDS results, it was unavoidably not blinded for the patient undergoing the operation, which could be a potential source of bias. Neither the PCT nor TCDS was performed with an arterial CO2 pressure assessment, which could serve as a potential confounder. Both studies were performed 1 week before the surgery, which is a short but not negligible period; thus, some hemodynamic changes could occur during that period irrespectively of the performance of the surgery. Finally, the size of the patient cohort is still small and might affect the statistical analysis.
Conclusion
Cranioplasty in patients who underwent a craniectomy due to medically refractory raised intracranial pressure is a procedure that might produce an objective clinical improvement in up to 40% of patients. The CBV and CBF values increased in both the defective and healthy sides after the surgery, and the MTT and TTP values decreased on both sides, as well. These changes appear to be more noticeable in patients who showed neurological improvement, but this effect was not statistically significant. There is a marked asymmetry of the CBF between the hemispheres, with greater flow observed on the healthy side of patients who improved after the cranioplasty.
The LR was much higher in the sitting position in the defective side of patients who showed improvement, but this difference vanished after surgery. This high value is primarily due to the low ICA velocity in the sitting position rather than a high MCA velocity.
In our series, the presence of larger craniectomies, the cranioplasty being performed within 85 days of the DC, the shorter MTT in the healthy side, and greater variations in the LR when the patients are moved from the sitting to supine position were able to predict the occurrence of clinical improvement after the cranioplasty. Larger prospective studies are necessary to confirm and generalize these findings.
Acknowledgments
This research was jointly financed by ISCIII and FEDER European institutions; with the FIS project number: PI14/01457
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Honeybul S. and Ho K.M. (2014). Decompressive craniectomy for severe traumatic brain injury: the relationship between surgical complications and the prediction of an unfavourable outcome. Injury 45, 1332–1339 [DOI] [PubMed] [Google Scholar]
- 2.Sinha S., Raheja A., Garg M., Moorthy S., Agrawal D., Gupta D.K., Satyarthee G.D., Singh P.K., Borkar S.A., Gurjar H., Tandon V., Pandey R.M., and Sharma B.S. (2015). Decompressive craniectomy in traumatic brain injury: a single-center, multivariate analysis of 1,236 patients at a tertiary care hospital in India. Neurol. India 63, 175–183 [DOI] [PubMed] [Google Scholar]
- 3.Bor-Seng-Shu E., Figueiredo E.G., Amorim R.L., Teixeira M.J., Valbuza J.S., de Oliveira M.M., and Panerai R.B. (2012). Decompressive craniectomy in traumatic brain injury: a single center, multivariate analysis of 1,236 patients at a tertiary care hospital in Indiapressive craniectomy: a meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury. J. Neurosurg. 117, 589–596 [DOI] [PubMed] [Google Scholar]
- 4.Hofmeijer J., Amelink G.J., Algra A., van Gijn J., Macleod M.R., Kappelle L.J., and van der Worp H.B; HAMLET investigators. (2006). Hemicraniectomy after middle cerebral artery infarction with life-threatening Edema trial (HAMLET). Protocol for a randomised controlled trial of decompressive surgery in space-occupying hemispheric infarction. Trials 7, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vahedi K., Vicaut E., Mateo J., Kurtz A., Orabi M., Guichard J.P., Boutron C., Couvreur G., Rouanet F., Touze E., Guillon B., Carpentier A., Yelnik A., George B., Payen D., and Bousser M.G.; DECIMAL Investigators. (2007). Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke 38, 2506–2517 [DOI] [PubMed] [Google Scholar]
- 6.Erdogan E., Duz B., Kocaoglu M., Izci Y., Sirin S., and Timurkaynak E. (2003). The effect of cranioplasty on cerebral hemodynamics: evaluation with transcranial Doppler sonography. Neurol. India 51, 479–481 [PubMed] [Google Scholar]
- 7.Fodstad H., Ekstedt J., and Friden H. (1979). CSF hydrodynamic studies before and after cranioplasty. Acta neurochir. Suppl. 28, 514–518 [PubMed] [Google Scholar]
- 8.Fodstad H., Love J.A., Ekstedt J., Friden H., and Liliequist B. (1984). Effect of cranioplasty on cerebrospinal fluid hydrodynamics in patients with the syndrome of the trephined. Acta Neurochir. (Wien) 70, 21–30 [DOI] [PubMed] [Google Scholar]
- 9.Maekawa M., Awaya S., and Teramoto A. (1999). [Cerebral blood flow (CBF) before and after cranioplasty performed during the chronic stage after decompressive craniectomy evaluated by xenon-enhanced computerized tomography (Xe-CT) CBF scanning]. No Shinkei Geka 27, 717–722 [PubMed] [Google Scholar]
- 10.Suzuki N., Suzuki S., and Iwabuchi T. (1993). Neurological improvement after cranioplasty. Analysis by dynamic CT scan. Acta Neurochir (Wien) 122, 49–53 [DOI] [PubMed] [Google Scholar]
- 11.Winkler P.A., Stummer W., Linke R., Krishnan K.G., and Tatsch K. (2000). The influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. Neurosurg. Focus 8, e9. [DOI] [PubMed] [Google Scholar]
- 12.Gadde J., Dross P., and Spina M. (2012). Syndrome of the trephined (sinking skin flap syndrome) with and without paradoxical herniation: a series of case reports and review. Del. Med. J 84, 213–218 [PubMed] [Google Scholar]
- 13.Isago T., Nozaki M., Kikuchi Y., Honda T., and Nakazawa H. (2004). Sinking skin flap syndrome: a case of improved cerebral blood flow after cranioplasty. Ann. Plast. Surg. 53, 288–292 [DOI] [PubMed] [Google Scholar]
- 14.Kemmling A., Duning T., Lemcke L., Niederstadt T., Minnerup J., Wersching H., and Marziniak M. (2010). Case report of MR perfusion imaging in sinking skin flap syndrome: growing evidence for hemodynamic impairment. BMC Neurol. 10, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo J.R., Wang C.C., Chio C.C., and Cheng T.J. (2004). Neurological improvement after cranioplasty—analysis by transcranial doppler ultrasonography. J. Clin. Neurosci. 11, 486–489 [DOI] [PubMed] [Google Scholar]
- 16.Mokri B. (2010). Orthostatic headaches in the syndrome of the trephined: resolution following cranioplasty. Headache 50, 1206–1211 [DOI] [PubMed] [Google Scholar]
- 17.Nalbach S.V., Ropper A.E., Dunn I.F., and Gormley W.B. (2012). Craniectomy-associated Progressive Extra-Axial Collections with Treated Hydrocephalus (CAPECTH): redefining a common complication of decompressive craniectomy. J. Clin. Neurosci. 19, 1222–1227 [DOI] [PubMed] [Google Scholar]
- 18.Sakamoto S., Eguchi K., Kiura Y., Arita K., and Kurisu K. (2006). CT perfusion imaging in the syndrome of the sinking skin flap before and after cranioplasty. Clin. Neurol. Neurosurg. 108, 583–585 [DOI] [PubMed] [Google Scholar]
- 19.Sarov M., Guichard J.P., Chibarro S., Guettard E., Godin O., Yelnik A., George B., Bousser M.G., and Vahedi K. (2010). Sinking skin flap syndrome and paradoxical herniation after hemicraniectomy for malignant hemispheric infarction. Stroke 41, 560–562 [DOI] [PubMed] [Google Scholar]
- 20.Song J., Liu M., Mo X., Du H., Huang H., and Xu G.Z. (2013). Beneficial impact of early cranioplasty in patients with decompressive craniectomy: evidence from transcranial Doppler ultrasonography. Acta Neurochir. (Wien). 156,193–198 [DOI] [PubMed] [Google Scholar]
- 21.Won Y.D., Yoo D.S., Kim K.T., Kang S.G., Lee S.B., Kim D.S., Hahn S.T., Huh P.W., Cho K.S., and Park C.K. (2008). Cranioplasty effect on the cerebral hemodynamics and cardiac function. Acta Neurochir. Suppl. 102, 15–20 [DOI] [PubMed] [Google Scholar]
- 22.Paredes I., Cicuendez M., Delgado M.A., Martinez-Perez R., Munarriz P.M., and Lagares A. (2011). Normal pressure subdural hygroma with mass effect as a complication of decompressive craniectomy. Surg. Neurol. Int. 2, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paredes I., Castano-Leon A.M., Munarriz P.M., Martinez-Perez R., Cepeda S., Sanz R., Alen J.F., and Lagares A. (2015). Cranioplasty after decompressive craniectomy. A prospective series analyzing complications and clinical improvement. Neurocirugia 26, 115–125 [DOI] [PubMed] [Google Scholar]
- 24.Grant F.C. and Norcross N.C. (1939). Repair of cranial defects by cranioplasty. Ann. Surg. 110, 488–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamaura A. and Makino H. (1977). Neurological deficits in the presence of the sinking skin flap following decompressive craniectomy. Neurol. Med. Chir. (Tokyo) 17, 43–53 [DOI] [PubMed] [Google Scholar]
- 26.Stula D. (1982). The problem of the “sinking skin-flap syndrome” in cranioplasty. J. Maxillofac. Surg. 10, 142–145 [DOI] [PubMed] [Google Scholar]
- 27.Ng D. and Dan N.G. (1997). Cranioplasty and the syndrome of the trephined. J. Clin. Neurosci. 4, 346–348 [DOI] [PubMed] [Google Scholar]
- 28.Dujovny M., Agner C., and Aviles A. (1999). Syndrome of the trephined: theory and facts. Crit. Rev. Neurosurg. 9, 271–278 [DOI] [PubMed] [Google Scholar]
- 29.Agner C., Dujovny M., and Gaviria M. (2002). Neurocognitive assessment before and after cranioplasty. Acta Neurochir. (Wien) 144, 1033–1040 [DOI] [PubMed] [Google Scholar]
- 30.Moreira-Gonzalez A., Jackson I.T., Miyawaki T., Barakat K., and DiNick V. (2003). Clinical outcome in cranioplasty: critical review in long-term follow-up. J. Craniofac. Surg. 14, 144–153 [DOI] [PubMed] [Google Scholar]
- 31.Bijlenga P., Zumofen D., Yilmaz H., Creisson E., and de Tribolet N. (2007). Orthostatic mesodiencephalic dysfunction after decompressive craniectomy. J Neurol Neurosurg. Psychiatry 78, 430–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akins P.T. and Guppy K.H. (2008). Sinking skin flaps, paradoxical herniation, and external brain tamponade: a review of decompressive craniectomy management. Neurocrit. Care 9, 269–276 [DOI] [PubMed] [Google Scholar]
- 33.Stiver S.I., Wintermark M., and Manley G.T. (2008). Reversible monoparesis following decompressive hemicraniectomy for traumatic brain injury. J Neurosurg 109, 245–254 [DOI] [PubMed] [Google Scholar]
- 34.Joseph V. and Reilly P. (2009). Syndrome of the trephined. J. Neurosurg. 111, 650–652 [DOI] [PubMed] [Google Scholar]
- 35.Stelling H., Graham L., and Mitchell P. (2011). Does cranioplasty following decompressive craniectomy improve consciousness? Br. J. Neurosurg. 25, 407–409 [DOI] [PubMed] [Google Scholar]
- 36.Araujo Junior A.S., Arlant P.A., Salvestrini A., Jr., Altieri C.E., Santos J.G., Pinto L.F., Fazzito M.M., Lee H.W., and Godoy L.F. (2013). Asymmetric optic nerve sheath diameter as an outcome factor following cranioplasty in patients harboring the ‘syndrome of the trephined’. Arq. Neuropsiquiatr. 71, 963–966 [DOI] [PubMed] [Google Scholar]
- 37.Honeybul S., Janzen C., Kruger K., and Ho K.M. (2013). The impact of cranioplasty on neurological function. Br. J. Neurosurg. 27, 636–641 [DOI] [PubMed] [Google Scholar]
- 38.Huang Y.H., Lee T.C., Yang K.Y., and Liao C.C. (2013). Is timing of cranioplasty following posttraumatic craniectomy related to neurological outcome? Int. J. Surg. 11, 886–890 [DOI] [PubMed] [Google Scholar]
- 39.Lin C.H., Yang J.T., Wang T.C., Lin M.H., Cheng W.C., and Lee M.H. (2013). Is preoperative brain midline shift a determinant factor for neurological improvement after cranioplasty? J. Formos. Med. Assoc. 114, 577–182 [DOI] [PubMed] [Google Scholar]
- 40.Piedra M.P., Ragel B.T., Dogan A., Coppa N.D., and Delashaw J.B. (2013). Timing of cranioplasty after decompressive craniectomy for ischemic or hemorrhagic stroke. J. Neurosurg. 118, 109–114 [DOI] [PubMed] [Google Scholar]
- 41.Coelho F., Oliveira A.M., Paiva W.S., Freire F.R., Calado V.T., Amorim R.L., Neville I.S., de Andrade A.F., Bor-Seng-Shu E., Anghinah R., and Teixeira M.J. (2014). Comprehensive cognitive and cerebral hemodynamic evaluation after cranioplasty. Neuropsychiatr. Dis. Treat. 10, 695–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narro-Donate J.M., Huete-Allut A., Escribano-Mesa J.A., Rodriguez-Martinez V., Contreras-Jimenez A., and Masegosa-Gonzalez J. (2014). [Paradoxical transtentorial herniation, extreme trephined syndrome sign: a case report.]. Neurocirugia 26, 95–99 [DOI] [PubMed] [Google Scholar]
- 43.Honeybul S. (2010). Complications of decompressive craniectomy for head injury. J. Clin. Neurosci. 17, 430–435 [DOI] [PubMed] [Google Scholar]
- 44.Stiver S.I. (2009). Complications of decompressive craniectomy for traumatic brain injury. Neurosurg. Focus 26, E7. [DOI] [PubMed] [Google Scholar]
- 45.Honeybul S. and Ho K.M. (2011). Long-term complications of decompressive craniectomy for head injury. J. Neurotrauma 28, 929–935 [DOI] [PubMed] [Google Scholar]
- 46.Yang X.F., Wen L., Shen F., Li G., Lou R., Liu W.G., and Zhan R.Y. (2008). Surgical complications secondary to decompressive craniectomy in patients with a head injury: a series of 108 consecutive cases. Acta Neurochir. (Wien) 150, 1241–1247 [DOI] [PubMed] [Google Scholar]
- 47.Janzen C., Kruger K., and Honeybul S. (2012). Syndrome of the trephined following bifrontal decompressive craniectomy: implications for rehabilitation. Brain Inj. 26, 101–105 [DOI] [PubMed] [Google Scholar]
- 48.Chalouhi N., Teufack S., Fernando Gonzalez L., Rosenwasser R.H., and Jabbour P.M. (2012). An extreme case of the syndrome of the trephined requiring the use of a novel titanium plate. Neurologist 18, 423–425 [DOI] [PubMed] [Google Scholar]
- 49.Liang W., Xiaofeng Y., Weiguo L., Gang S., Xuesheng Z., Fei C., and Gu L. (2007). Cranioplasty of large cranial defect at an early stage after decompressive craniectomy performed for severe head trauma. J. Craniofac. Surg. 18, 526–532 [DOI] [PubMed] [Google Scholar]
- 50.Nakamura T., Takashima T., Isobe K., and Yamaura A. (1980). Rapid neurological alteration associated with concave deformity of the skin flap in a craniectomized patient. Case report. Neurol. Med. Chir. (Tokyo) 20, 89–93 [DOI] [PubMed] [Google Scholar]
- 51.Romero F.R., Zanini M.A., Ducati L.G., and Gabarra R.C. (2013). Sinking skin flap syndrome with delayed dysautonomic syndrome—an atypical presentation. Int. J. Surg. Case Rep. 4, 1007–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magnaes B. (1976). Body position and cerebrospinal fluid pressure. Part 1: clinical studies on the effect of rapid postural changes. J. Neurosurg. 44, 687–697 [DOI] [PubMed] [Google Scholar]
- 53.Decaminada N., Pernter P., Imondi A., and Tomassini A. (2008). CT Perfusion evaluation of cerebral haemodynamics before and after cranioplasty. Neuroradiol. J. 21, 459–471 [DOI] [PubMed] [Google Scholar]
- 54.Sarubbo S., Latini F., Ceruti S., Chieregato A., d'Esterre C., Lee T.Y., Cavallo M., and Fainardi E. (2014). Temporal changes in CT perfusion values before and after cranioplasty in patients without symptoms related to external decompression: a pilot study. Neuroradiology 56, 237–243 [DOI] [PubMed] [Google Scholar]
- 55.Schwarz S., Georgiadis D., Aschoff A., and Schwab S. (2002). Effects of body position on intracranial pressure and cerebral perfusion in patients with large hemispheric stroke. Stroke 33, 497–501 [DOI] [PubMed] [Google Scholar]